Abstract
Through dispersal, deer mice (Peromyscus maniculatus) enter peridomestic settings (e.g., outbuildings, barns, cabins) and expose humans and other deer mouse populations to Sin Nombre virus (SNV). In June 2004, research on deer mouse dispersal was initiated at 2 locations in Montana. During the course of the study, over 6000 deer mouse movements were recorded, and more than 1000 of these movements were classified as dispersal movements. More than 1700 individual deer mice were captured and tested for SNV, revealing an average SNV antibody prevalence of approximately 11%. Most of the dispersing and antibody-positive individuals were adult males. Among the few subadult dispersing mice discovered during the study, none were seropositive for SNV. Our results suggest that dispersal rates are higher in high abundance populations of deer mice and that during peak times of dispersal, human exposure to SNV, which commonly occurs in peridomestic settings, could increase.
Key Words: Dispersal, Resident, Hantavirus, Deer mouse, Sylvan, Antibody, Zoonotic, Sin Nombre virus
Introduction
Sin Nombre Virus (SNV; family Bunyaviridae, genus Hantavirus) is the principal etiologic agent of hantavirus pulmonary syndrome (HPS) in the United States (Nichol et al. 1993). Considerable data on the ecology of the principal host of SNV, the deer mouse (Peromyscus maniculatus), have been collected in long-term studies conducted on sylvan populations in the western United States (Mills et al. 1999, Douglass et al. 2001).
Descriptive data concerning sylvan population dynamics and habitat associations are crucial for understanding disease dynamics and devising ways to protect human health. However, most human infections occur in peridomestic settings (Armstrong et al. 1995, Kuenzi et al. 2001). Therefore, it is important to determine relationships between sylvan and peridomestic populations of deer mice. Because sylvan deer mouse populations are ultimately the source via dispersal of peridomestic deer mice, an understanding of the factors that lead to their dispersal is crucial. However, few data are available concerning deer mouse dispersal (Bowman et al. 2002) and none examine the relationship between dispersal and SNV infection.
The lack of dispersal/SNV data has, in part, resulted from the difficulty in measuring dispersal (Lidicker 1975). Nonetheless, some knowledge of dispersal among deer mouse populations exists (Fairbairn 1977, 1978, Stickel 1946, Stickel 1968, Sullivan 1976). Deer mice can move long distances (Rehmeier et al. 2004), and they can move rapidly into peridomestic areas, especially when resident mice have been removed (Douglass et al. 2003). In patchy habitats, they regularly disperse from patch to patch through less favorable habitats (Fairbairn 1978, Sullivan 1976, Stickel 1968, Sheppe 1965). Dispersal of deer mice is driven by individual behavior, social structure, population densities, and changing patterns of resource availability (Fairbairn 1978).
Further, as deer mice enter peridomestic settings, dispersal influences the spread of zoonotic diseases such as SNV to humans. For example, after an El Ni§o event, substantial increases in the deer mouse population in the Four Corners area of the United States (i.e., the point where Colorado, Utah, Arizona, and New Mexico converge), followed by significant dispersal (of mice and subsequently virus) into peridomestic habitats, likely led to the 1993 SNV outbreak in that region. Hantaviruses can also go extinct for a few months to several years in local populations (Mills et al. 1999, Kuenzi et al. 1999, 2007, Douglass et al. 2001) or until such time as dispersing mice from adjacent populations reintroduce the virus. A viral-induced change in behavior could also, potentially, affect dispersal in deer mice. Klein et al. (2004) found that infection with Seoul virus caused rats (Rattus norvegicus) to become aggressive, and Douglass et al. (2007) found evidence that SNV increased aggressive interaction in deer mice as they seroconverted. Such aggression may lead to dispersal of infected animals.
This study was initiated to gather data on SNV antibody prevalence in dispersing sylvan deer mice. Our specific objectives were to: (1) describe the physical characteristics (e.g., wounds, age, and breeding condition) of dispersing deer mice in sylvan systems, and (2) investigate the possibility that infection may increase dispersal (possibly through increased aggression) in deer mice.
Materials and Methods
Two study sites were established near Cascade (46° 59.3′ N, 111° 35.3′ W, 1408 m AMS) and Polson (47° 38.4′ N, 114° 20.7′ W, 823 m AMS), Montana. The Cascade study site is grassland habitat (derived from dominant plant form/species), and the Polson site is sagebrush (Artemisia tridentata) habitat. Each site had preexisting pairs of trapping grids (100 traps, 1 ha in size) created for longitudinal hantavirus studies (Douglass et al. 1996). The paired grids at each location were approximately 550 m (Cascade) and 840 m (Polson) from each other (Fig. 1). Distances between grids were dictated by the distance between the preexisting grids. We used the preexisting grids because pairs were in similar habitats and had demonstrated large annual fluctuations in deer mouse abundance since 1994.
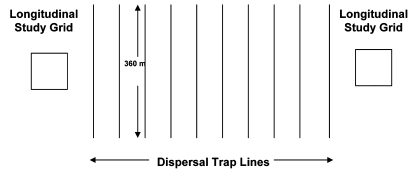
FIG. 1.
Trapping grid and dispersal trap line distribution. Twenty-five live traps were placed at 15 m intervals along each dispersal trap line. Grids were comprised of 100 traps each evenly spaced over 1 ha. Trap lines were spaced at 50 and 76 m intervals at Cascade and Polson, respectively. Areas covered by dispersal trap lines were approximately 14 ha at Cascade and 25 ha at Polson, Montana, from June 2004 through October 2005.
Trapping between preexisting grids provided a minimum estimate of dispersal from each grid. The intergrid arrays each consisted of 10 evenly spaced parallel lines of traps placed perpendicular to the grids (Fig. 1). Trap lines were set 50 m apart at Cascade and 76 m apart at Polson; each was 360 m in length. Twenty-five Sherman nonfolding, aluminum live traps (8 × 9 23 cm; H.B. Sherman Trap Co., Tallahassee, FL) with 15 m spacing between traps were placed along each trap line. Trap stations were marked with surveying flags and were assigned Universal Transverse Mercator (UTM) coordinates using a global positioning (GPS) unit. These dispersal arrays covered approximately 14 ha at Cascade and 25 ha at Polson.
Sampling on all grids and dispersal arrays was conducted monthly at the Cascade study site (June 2004 through October 2005) and during the snow-free months at Polson (June through October 2004 and April through October 2005). Traps were baited with peanut butter and oatmeal and provided with polyester bedding. Trapping was conducted on 3 consecutive nights during each monthly sampling period at Cascade and Polson. Rodents on the dispersal arrays were sampled approximately 7 to 10 days after the preexisting paired grids had been sampled each month. Trapping and processing were conducted according to safety recommendations provided by Mills et al. (1995). Each morning animals were processed and released at the site of capture. Rodents were ear tagged using metal tags (National Band and Tag Co., Newport, KY), and their species, sex, body mass, reproductive condition (males: testes scrotal or abdominal; females: vagina nonperforate or perforate, pubic symphysis closed or open, and nipples normal or enlarged), and presence of scars or wounds were recorded. The age of the deer mice was inferred based on the following weight categories: ≥18 g were considered adults, 14–17 g were considered subadults, and ≤13 g were considered juveniles (Douglass et al. 1996). From June 2004 through October 2005, rodents were trapped during 12,750 trap nights at Cascade and 9000 trap nights at Polson on longitudinal grids and dispersal arrays.
Our definition of dispersal is based partly on a study of an unmanipulated population of white-footed mice (Peromyscus leucopus), which defines dispersers as mice that moved a minimum of 75 m (approximately one home range diameter) (Stickel 1968; Krohne et al. 1984). Additionally, animals had to demonstrate continuous directional movement, ≥75 m from capture to capture, regardless of the time frame. For example, if an animal was captured and upon release the animal moved 150 m (recaptured the next day) and then was recaptured again 2 days later (after its initial capture) back at the original trap site, the movement would not be considered a dispersal event. Such movements were considered short-term exploratory movements by a resident individual.
One blood sample per month was obtained from the retro-orbital sinus of each deer mouse using a heparinized capillary tube. Samples were stored on dry ice and then transferred to a −70°C freezer until they were tested for antibody against SNV at the Montana Department of Public Health and Human Services or the Centers for Disease Control and Prevention by enzyme immunoassay (Feldman et al. 1993). No blood was collected during the first month of the study (June 2004) while we developed other field protocols.
Statistical analysis was performed using Microsoft Access, Microsoft Excel, and SPSS (version 13). An enumeration technique described by Chitty and Phipps (1966) was used to calculate the minimum number of deer mice known alive (MNA) and the minimum number infected with SNV (MNI) during each trap session. This calculation provided an estimate of population size. Statistical comparisons of dispersal and characteristics of dispersing mice were performed with linear or logistic (i.e., drop-in-deviance tests) regression model comparisons (Ramsey and Schafer 2002) and χ2 analysis (Zar 1996). Tests were considered statistically significant if p ≤ 0.05.
Results
A total of 2267 individual small mammals, representing 2 families and 5 species, were captured (Table 1). The overall trap success was 34.8 individuals/100 trap nights (13.0 individuals/100 trap nights at Cascade and 65.4 individuals/100 trap nights at Polson).
Table 1.
Number and Prevalence of Rodent Species Captured at Cascade (June 2004 through October 2005) and Polson (June through October 2004 and April through October 2005), Montana
| Location | Deer mice (Peromyscus maniculatus) (n) | Mountain vole (Microtus montanus) and meadow vole (Microtus pennsylvanicus) (n) | Western jumping mice (Zapus princeps) (n) | Long-tailed vole (Microtus longicaudus) (n) | Total (n) |
|---|---|---|---|---|---|
| Cascade | 372 | 44 | 7 | 1 | 413 |
| (species prevalence = 92%; seroprevalence = 12.1%) | (species prevalence = 6%) | (species prevalence = 2%) | (species prevalence <1%) | 413 | |
| Polson | 1375 | 479 | — | — | 1854 |
| (species prevalence = 74%; seroprevalence = 10.6%) | (species prevalence = 26%) |
In June 2004 (the first month of data collection), the deer mouse populations at Cascade and Polson (dispersal trap lines only) had MNAs of 20 and 54 individuals, respectively (Fig. 2). After the initial trapping period, the deer mouse population at Polson quickly increased, and by October 2004, the MNA at Polson was more than 320 deer mice. Consequently, recruitment at Polson was also highest (mean monthly recruitment [immigration + natality] = 22% ± 7.0%) during the first summer of trapping (June through September 2004). Population numbers remained high through the following spring, summer, and fall, eventually reaching a peak MNA of 419 individuals. In comparison, on the Cascade dispersal grids the population increased the first summer (MNA from 20 to 68 animals by November) and then stayed relatively constant for the remainder of the sampling periods. Deer mouse recruitment at Cascade was highest (17% ± 1.1%) during the September through November 2004 and August through September 2005 trapping sessions.
FIG. 2.
Minimum number of deer mice known to be alive (MNA) and minimum number infected (MNI) compared with seroprevalence for monthly trap sessions at (A) Cascade and (B) Polson, Montana, from June 2004 through October 2005.
For the first summer and fall seasons at Polson, the seroprevalence averaged <3% (± 0.6%) or an MNI of 3 to 5 deer mice per trapping session (Fig. 2). In contrast, the first summer and fall estimates for seroprevalence at Cascade ranged from 9% to 23%, or an MNI of 5 to 7 deer mice per trapping session. However, by the following spring and summer, the MNI estimated at Polson was 3 to 4 times higher than that at Cascade (Fig. 2).
We recorded 6302 deer mouse movements (4592 at Polson and 1701 at Cascade). At Polson, 15.3% (n = 703) were considered dispersal. Mean movement length at this site was 155.0 m (± 110.7 m) for dispersing mice and 22.3 m (± 31.8 m) for residents (nondispersing mice) (Table 2). At Cascade, 19.5% (n = 332) of the 1701 movements were considered dispersal activities. Dispersal movements at Cascade averaged 136.3 m (± 73.6 m) compared with 29.0 m (± 30.7 m) for residents (Table 2). At Polson and Cascade, seroprevalence for all individuals tested on the dispersal grids was 10% and 12%, respectively. Monthly seroprevalence at Cascade was generally between 10% and 20%, with a minimum of 3% in October 2005 and maximum of 23% in September 2004 (Fig. 2). From June through October 2004, seroprevalence at Polson had a mean of 2.5% (± 1.29%) and 11% (± 1.72%) from April through October 2005. Males accounted for a high percentage of antibody-positive individuals at Cascade (65%) and Polson (68%).
Table 2.
Characteristics of Nondisperstng and Dispersing Deer Mice at Cascade (June 2004 through October 2005) and Polson (June through October 2004 and April through October 2005), Montana
| |
Cascade |
Polson |
||||
|---|---|---|---|---|---|---|
| Characteristic | Nondispersal | Dispersal | χ2nondispersal vs. dispersal | Nondispersal | Dispersal | χ2nondispersal vs. dispersal |
| Sex | ||||||
| % Male (n) | 55.6 (761/1369) | 71.7 (238/332) | 34.8 (p < 0.001) | 51.8 (2014/3891) | 61.0 (428/701) | 24.05 (p < 0.001) |
| % Female (n) | 44.4 (608/1369) | 28.3 (94/332) | 48.2 (1877/3891) | 38.9 (273/701) | ||
| In breeding condition | ||||||
| 1st and/or 2nd captures | ||||||
| Males | ||||||
| % Yes (n) | 37.0 (369/998) | 53.7 (110/205) | 24.4 (p < 0.001) | 42.1 (891/2114) | 61.1 (206/337) | 108.7 (p < 0.001) |
| % No (n) | 63.0 (629/998) | 46.3 (95/205) | 57.9 (1223/2114) | 38.9 (131/337) | ||
| Females | ||||||
| % Yes (n) | 39.4 (553/1404) | 48.9 (92/188) | 7.16 (p < 0.01) | 41.8 (1804/4312) | 52.6 (285/542) | 25.9 (p < 0.001) |
| % No (n) | 60.6 (851/1404) | 51.1 (96/188) | 7.16 (p < 0.01) | 58.2 (2508/4312) | 47.4 (257/542) | |
| Age (1st and 2nd captures) | ||||||
| % Subadult (n) | 16.1 (260/1599) | 7.8 (24/307) | 16.0 (p < 0.001) | 12.0 (511/4263) | 9.1 (57/627) | 5.02 (p < 0.025) |
| % Adult (n) | 83.7 (1399/1599) | 92.2 (283/307) | 88.0 (3752/4263) | 90.9 (570/627) | ||
| Acquisition of scars between | ||||||
| 1st and 2nd captures | ||||||
| Both sexes | 6.4 (88/1375) | 8.7 (28/322) | 2.3 (p < 0.25) | 8.7 (337/4333) | 13.1 (92/7020) | 15.8 (p < 0.001) |
| Males | 68 (651/975) | 81 (179/221) | 243.8 (p < 0.0001) | 58 (2128/3668) | 68 (430/632) | 26.1 (p < 0.001) |
| Mean ± SD distances traveled (m) | 29.0 ± 30.7 | 136.3 ± 73.6 | 22.3 ± 31.8 | 155.0 ± 110.7 | ||
We found a correlation (r = +0.70, p < 0.001) between average MNA for the combined longitudinal grids (4 total) at both sites and the number of dispersal movements on dispersal arrays (Fig. 3). However, variable results were found on grids tested separately (Cascade: grid 10, n = 17, r = +0.477, p = 0.053; grid 11, n = 17, r = +0.599, p = 0.011; Polson: grid 4, n = 12, r = 0.094, p = 0.769; grid 5, n = 12, r = 0.063, p = 0.866). Unfortunately for this analysis, large fluctuations in MNA demonstrated at both sites since 1994 did not occur during the current period of study. Polson accounted for the highest MNAs and number of dispersal events and Cascade the lowest MNAs and number of dispersal events.
FIG. 3.
Total number of dispersal movements correlated with MNA by trapping session for 4 individual and combined longitudinal study grids (grids 10 and 11 at Cascade and grids 4 and 5 at Polson). Data were collected monthly from June 2004 through October 2005 in Central and Western Montana.
Gender of dispersing deer mice was biased toward males compared with nondispersing mice (Table 2). For resident deer mice at Polson and Cascade, females accounted for 48.2% and 44.4% (n = 1877 and 608) of the movements, and males produced 51.8% and 55.6% (n = 2014 and 761) of the movements (Table 2). In contrast, for dispersing mice, males produced 61.0% and 71.7% (n = 428 and 238) of the dispersal movements recorded at Polson and Cascade, compared with females that produced 38.9% and 28.3% (n = 273 and 94) of the movements (Table 2).
The proportion of dispersing mice in breeding condition was higher than resident mice (Table 2). A smaller percentage of resident females were reproductively active (41.2%; 41.8% at Polson and 39.4% at Cascade) compared with 51.6% (52.6% at Polson and 48.9% at Cascade) of dispersing females (Table 2). More dispersing females (10.4%) than resident females (7.2%) became reproductively active between monthly capture intervals. Males were more likely to be in breeding condition than females. Approximately 58.3% (61.1% at Polson and 53.7% at Cascade) of dispersing and 40.5% (42.1% at Polson and 37.0% at Cascade) of resident male mice were re-productively active at time of capture (Table 2). Approximately 11.5% of dispersing (13.2% at Polson and 8.4% at Cascade) and 5.0% of resident (5.3% at Polson and 4.3% at Cascade) male mice became reproductively active at the subsequent capture.
Adult mice (≥18 g) comprised most of the resident and dispersing individuals. Approximately 88.0% and 83.7% of the residents at Polson and Cascade were adults at first capture (Table 2). However, 90.9% and 92.2% of dispersing mice were adults at first capture (Table 2).
Mice commonly acquire scars or wounds through aggressive interactions with each other, which is believed to be a possible transmission route for hantaviruses. The prevalence of scars (at both captures) for dispersers and residents at Polson was nearly equal, at 48% (n = 338) for dispersers and 46% (n = 1791) for residents. A higher proportion of mice that dispersed acquired scars than did residents. At Cascade, resident female mice without scars had acquired scars by their next capture 6.4% (n = 88) of the time; similarly, 8.7% (n = 28) of dispersing female mice showed scars at second capture. In comparison, 8.7% (n = 337) of Polson resident female mice with no scars at the first capture had acquired scars by their next capture. Approximately 13.1% (n = 92) of dispersing female mice initially having no scars acquired scars by their second capture.
The proportion of dispersing males acquiring scars in successive captures was higher than that of resident males (Table 2). Male deer mice constituted 58% (n = 2128) and 68% (n = 651) of the total resident mice at Polson and Cascade that had acquired scars by successive captures. At both Polson and Cascade, 68% (n = 430) and 81% (n = 179) of male dispersing deer mice had acquired scars by subsequent captures.
Because the characteristics of dispersing deer mice were similar to those of deer mice with anti-SNV antibodies (biased toward adult, breeding males with scars; Douglass et al. 2001), we used logistic regression to attempt to control for these similar characteristics to determine how dispersal was affected by the presence of SNV antibodies. We used logistic regression model comparisons to test for the association of: (1) the odds of being seropositive based on gender, (2) the odds of being male or female and having SNV, after accounting for mass (age), and (3) the odds of an animal dispersing and having SNV, after accounting for gender and mass. Logistic regression indicated a statistically significant correlation between gender (in favor of males) and seroprevalence for SNV (Polson, p < 0.001, df = 1; Cascade, p < 0.001, df = 1). After accounting for mass, males were also more likely to be seropositive than females (Polson, p < 0.001, df = 1; Cascade, p < 0.001, df = 1). However, results at each study site indicated little association between dispersal and being seropositive for SNV (Polson, p = 0.583, df = 1; Cascade, p = 0.532, df = 1).
Discussion
Because sylvan mice that disperse are more likely than resident sylvan mice to enter peridomestic localities, understanding the characteristics of the dispersing population and the reasons for such dispersal is important for understanding human exposure to SNV and other rodent-borne diseases. Although what causes animals to disperse is debatable, one might reasonably assume that breeding activity, availability of resources such as food, age structure, sex ratios, escape from predation, and establishment of home ranges are strong influences (Krebs 1996, Lidicker 1975).
In a dispersal study of an unmanipulated population of white-footed mice, Krohne et al. (1984) found that males disperse more than females, while Root et al. (1999) recorded similar results of adult male deer mice moving greater distances than females and, at 1 of their 2 trap locations, subadult males moving greater distances than adult females. In contrast, Metzgar's (1979) ratios for dispersing adult males to females were nearly equal (0.9:1.0). The differences between Metzgar's (1979) findings and the results of other studies, including ours, could be related to discrepancies in dispersal definitions and trapping methods.
Our data demonstrated a propensity for adult male deer mice to disperse; in fact, males were almost twice as likely as females to disperse. Fairbairn (1977, 1978) found similar results, although juvenile, subadult, and adult male dispersal varied depending on the season.
One might reasonably assume that subadult mice would constitute the majority of dispersers primarily because the subadults in many species tend to be subordinate and do not hold territories. Further, the abundance of subadult dispersers seemingly should be highest during the peak breeding season for deer mice in Montana, which is late spring (May) through fall (September) (Douglass et al. 2001). However, we captured only a small number of dispersing subadult deer mice, none of which had antibodies to SNV (Table 2), and we found no significant difference in subadult dispersal movements between breeding and nonbreeding seasons. Thus, our results show that, for both female and male deer mice, adult individuals have the strongest tendency to disperse (Table 2), and they are also more likely to be seropositive.
Population density also affects deer mouse dispersal patterns (Lidicker 1975). Increasing population density causes animals to search for new areas in which they will find less competition. In locations with denser mouse populations (e.g., Polson) individuals are more likely to disperse than in places with lower density populations (e.g., Cascade). Such was the case in our study when data from all 4 longitudinal grids were combined (Fig. 3). However, we did not find a correlation between MNAs and the number of dispersal events within individual grids. Other factors besides abundance could have affected dispersal at these 2 locations. More trapping sessions with large population changes within grids will help determine the within-grid effects of density on dispersal.
Greater deer mouse dispersal increases susceptibility to SNV because mice that move longer distances likely increase their chance of intermingling with SNV-positive individuals (Root et al. 1999). Our data support these findings and show that adult male deer mice are most likely to disperse and are most likely to carry SNV. However, it is also important to understand that dispersing and infected mice shared similar physical characteristics. Because of the similarity in characteristics between dispersing mice and SNV-positive mice, the relationship between infection and dispersal was not clear.
Because adult male deer mice have higher infection rates of SNV compared with females (Root et al. 1999, Douglass et al. 2001), males are more likely to transmit SNV as they disperse. Furthermore, during the breeding season (March through October), SNV transmission could intensify because of increased aggressive interactions between male mice. In spite of males being more aggressive and more likely to disperse, we found little association between dispersal and being seropositive for SNV.
Although not yet demonstrated in mice, fighting is one potential method by which rats circulate hantavirus among populations (Hinson et al. 2004). Antibody-positive (both dispersing and resident) deer mice were more likely to have scars or wounds than antibody-negative individuals, a pattern which, according to some researchers, may be partly explained by behavioral changes caused by SNV infection. Klein et al. (2004) demonstrated that hantavirus infection led to elevated aggression in male Norway rats (Rattus norvegicus). If the onset of SNV infection causes elevated aggressive behavior in its host, this could explain why seropositive individuals have a higher percentage of scars or wounds. Conclusions by Klein et al. (2004) suggest that animals that are seropositive for hantavirus and have yet to sustain discernable scars or wounds should display an increase in scarring upon subsequent captures. And, in fact, such is the case in Montana (Douglass et al. 2007). However, we were not able to demonstrate that dispersal was related to SNV antibody status.
The consequences of these findings to human health risks are significant, especially where deer mouse populations are at high levels. Deer mice tend to disperse more at places with higher densities and the types of deer mice dispersing are those most likely to be antibody positive. Dispersal of deer mice carrying SNV is important because dispersing individuals are most likely to migrate into peridomestic locations where humans come in contact with SNV. Combining experimental approaches with sylvan studies is required to further our understanding of the deer mouse–SNV system as well as help to predict future epizootic outbreaks.
Acknowledgments
We thank the private ranch owner at Cascade and the Confederated Salish and Kootenai Tribes at Polson for unlimited access to their properties. Arlene Alvarado, Farrah Arneson, Karoun Bagamian, Jessica Bertoglio, Jonnae Lumsden, and Amy Skypala provided valuable assistance in the field. Dr. Mike Minnick and Dr. Brian Steele also provided significant logistical support for fieldwork and data analysis. Financial support was provided by the National Institutes for Health (NIH) grant P20RR16455-05 from the INBRE-BRIN program and the U.S. Centers for Disease Control and Prevention, Atlanta, GA, through cooperative agreement US3/CCU813599.
References
- Armstrong LR. Zaki SR. Goldoft MH. Todd RL, et al. Hantavirus pulmonary syndrome associated with entering or cleaning rarely used rodent-infested structures. J Infect Dis. 1995;172:1166. doi: 10.1093/infdis/172.4.1166. [DOI] [PubMed] [Google Scholar]
- Bowman J. Jochen AG. Jaeger JAG. Fahrig L. Dispersal distance of mammals is proportional to home range size. Ecology. 2002;83:2049–2055. [Google Scholar]
- Chitty D. Phipps E. Seasonal changes in survival in mixed populations of two species of vole. J Anim Ecol. 1966;35:313–331. [Google Scholar]
- Douglass RJ. Van Horne R. Coffin KW. Zanto SN. Hantavirus in Montana deer mouse populations: preliminary results. J Wildl Dis. 1996;32:527–530. doi: 10.7589/0090-3558-32.3.527. [DOI] [PubMed] [Google Scholar]
- Douglass RJ. Wilson T. Semmens WY. Zanto SN, et al. Longitudinal studies of Sin Nombre virus in deer mouse dominated ecosystems of Montana. Am J Trop Med Hyg. 2001;65:33–41. doi: 10.4269/ajtmh.2001.65.33. [DOI] [PubMed] [Google Scholar]
- Douglass RJ. Kuenzi AJ. Williams CY. Douglass SJ. Removing deer mice from buildings: potential effects on risk of human exposure to Sin Nombre virus. Emerg Infect Dis. 2003;9:390–392. doi: 10.3201/eid0903.020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass RJ. Calisher CH. Wagoner KD. Mills JN. Sin Nombre virus infection of deer mice in Montana: characteristics of newly infected mice, incidence, and temporal pattern of infection. J Wildl Dis. 2007;43:12–22. doi: 10.7589/0090-3558-43.1.12. [DOI] [PubMed] [Google Scholar]
- Fairbairn DJ. The spring decline in deer mice: death or dispersal? Can J Zool. 1977;55:84–92. [Google Scholar]
- Fairbairn DJ. Dispersal of deer mice, Peromyscus maniculatus: proximal causes and effects on fitness. Oecologia. 1978;32:171–193. doi: 10.1007/BF00366070. [DOI] [PubMed] [Google Scholar]
- Feldman H. Sanchez A. Morzunov S. Spiropoulou C, et al. Utilization of autopsy tissue RNA for the synthesis of the nucleocapsid antigen of a novel highly lethal hantavirus. Virus Res. 1993;30:351–367. doi: 10.1016/0168-1702(93)90101-r. [DOI] [PubMed] [Google Scholar]
- Hinson ER. Shone SM. Zink MC. Glass GE, et al. Wounding: the primary mode of Seoul virus transmission among male Norway rats. Am J Trop Med Hyg. 2004;70:310–317. [PubMed] [Google Scholar]
- Klein SL. Zink MC. Glass GE. Seoul virus infection increases aggressive behavior in male Norway rats. Anim Behav. 2004;67:421–429. [Google Scholar]
- Krebs CJ. Population cycles revisited. J Mammal. 1996;77:8–24. [Google Scholar]
- Krohne DT. Dubbs BA. Baccus R. An analysis of dispersal in an unmanipulated population of Peromyscus leucopus. Am Midl Nat. 1984;112:146–156. [Google Scholar]
- Kuenzi AJ. Morrison ML. Swann DE. Hardy PC, et al. A longitudinal study of Sin Nombre virus prevalence in rodents in southeastern Arizona. Emerg Infect Dis. 1999;5:113–117. doi: 10.3201/eid0501.990113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuenzi AJ. Douglass RJ. White D., Jr Bond CW, et al. Antibody to Sin Nombre virus in rodents associated with peridomestic habitats in west central Montana. Am J Trop Med Hyg. 2001;64:137–146. doi: 10.4269/ajtmh.2001.64.137. [DOI] [PubMed] [Google Scholar]
- Kuenzi AJ. Morrison ML. Madhav NK. Mills JN. Brush mouse (Peromyscus boylii) population dynamics and hantavirus infection during a warm, drought period in southern Arizona. J Wildl Dis. 2007;43:675–683. doi: 10.7589/0090-3558-43.4.675. [DOI] [PubMed] [Google Scholar]
- Lidicker WZ., Jr . The role of dispersal in the demography of small mammals. In: Golley FB, editor; Petrusewicz K, editor; Ryszkowski L, editor. Small Mammals. London: Cambridge University Press; 1975. pp. 103–128. [Google Scholar]
- Metzgar LH. Dispersal patterns in a Peromyscus population. J Mammal. 1979;60:129–145. [Google Scholar]
- Mills JN. Childs JE. Ksiazek JG. Peters CJ, et al. Methods for Trapping and Sampling Small Mammals for Virologic Testing. Atlanta, GA: U.S. Centers for Disease Control and Prevention; 1995. [Google Scholar]
- Mills JN. Yates TL. Ksiazek TG. Peters CJ, et al. Long-term studies of hantavirus reservoir populations in the southwestern United States: rationale, potential, and methods. Emerg Infect Dis. 1999;5:95–101. doi: 10.3201/eid0501.990111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol ST. Spiropoulou CF. Morzunov S. Rollin PE, et al. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Ramsey FL. Schafer DW. The Statistical Sleuth: A Course in Methods of Data Analysis. 2nd. Pacific Grove, CA: Duxbury (of Thomson Learning); 2002. pp. 564–608. [Google Scholar]
- Rehmeier RL. Kaufman GA. Kaufman DW. Long-distance movements of the deer mouse in tall grass prairie. J Mammal. 2004;85:562–568. [Google Scholar]
- Root JJ. Calisher CH. Beaty BJ. Relationships of deer mouse movement, vegetative structure, and prevalence of infection with Sin Nombre virus. J Wildl Dis. 1999;35:311–318. doi: 10.7589/0090-3558-35.2.311. [DOI] [PubMed] [Google Scholar]
- Sheppe W. Island populations and gene flow in the deer mouse, Peromyscus leucopus. Evolution. 1965;19:480–495. [Google Scholar]
- Stickel LF. The source of animals moving into a depopulated area. J Mamm. 1946;27:301–307. [PubMed] [Google Scholar]
- Stickel LF. Dispersal of the old field mouse. In: King JA, editor. The Biology of Peromyscus, Spec. Publ. No. 2. Provo, UT: American Society of Mammalogists; 1968. pp. 373–411. [Google Scholar]
- Stickel LF. King JA. The Biology of Peromyscus, Spec. Publ. No. 2. Provo, UT: American Society of Mammalogists; 1968. Home range and travels; pp. 373–411. [Google Scholar]
- Sullivan TP. Master's thesis. Vancouver, Canada: University of British Columbia; 1976. Demography and dispersal in island and mainland populations of the deer mouse, Peromyscus maniculatus. [Google Scholar]
- Zar JH. Biostatistical Analysis. 3rd. Englewood Cliffs, NJ: Prentice-Hall, Inc.; 1996. [Google Scholar]