It is believed that the accumulation of neurotoxic amyloid-β (Aβ) plays a central role in the onset and progression of Alzheimer's disease (AD). In this study, Movsesyan and colleagues examine the efficacy of a DNA-based anti-Aβ vaccine in reducing AD pathology in a mouse model of the disease.
Abstract
It has been demonstrated that an active vaccination strategy with protein- or DNA-based epitope vaccines composed of the immunodominant self B cell epitope of amyloid-β42 (Aβ42) and a non-self T helper (Th) cell epitope is an immunotherapeutic approach to preventing or treating Alzheimer's disease (AD). As a DNA-based epitope vaccine, we used a plasmid encoding three copies of Aβ1–11 and Th cell epitope, PADRE (p3Aβ1–11-PADRE). We have previously reported that three copies of component of complement C3d (3C3d) acts as a molecular adjuvant significantly enhancing immune responses in wild-type mice of the H2b haplotype immunized with p3Aβ1–11-PADRE. Here, we tested the efficacy of p3Aβ1–11-PADRE and the same vaccine fused with 3C3d (p3Aβ1–11-PADRE-3C3d) in a transgenic (Tg) mouse model of AD (Tg2576) of the H2bxs immune haplotype. The overall responses to both vaccines were very weak in Tg2576 mice despite the fact that the 3C3d molecular adjuvant significantly enhanced the anti-Aβ response to 3Aβ1–11-PADRE. Importantly, generation of low antibody responses was associated with the strain of amyloid precursor protein Tg mice rather than with a molecular adjuvant, as a p3Aβ1–11-PADRE-3C3d vaccine induced significantly higher antibody production in another AD mouse model, 3xTg-AD of the H2b haplotype. Finally, this study demonstrated that low concentrations of antibodies generated by both DNA vaccines were not sufficient for the reduction of Aβ pathology in the brains of vaccinated Tg2576 animals, confirming previous reports from preclinical studies and the AN-1792 clinical trials, which concluded that the concentration of anti-Aβ antibodies may be essential for the reduction of AD pathology.
Introduction
Plasmid DNA represents an attractive platform for the development of vaccines against a variety of pathogens as well as for human diseases, including cancer, autoimmune disorders, and Alzheimer's disease (AD). Several characteristics make naked DNA a desirable route for vaccination: simplicity in manipulation, induction of antigen-specific T and B cell responses similar to those elicited by live attenuated platforms, ease of rapid large-scale production and formulation, long shelf life, and the fact that they are more temperature-stable than conventional vaccines. Although DNA vaccines have generally been very effective mostly in preclinical studies, current results imply that they could also be promising in large animals (Fynan et al., 1993; Robinson et al., 1993; Donnelly and Ulmer, 1999; Powell, 2004) and even in humans (MacGregor et al., 1998; Boyer et al., 1999, 2000; Conry et al., 2002; Epstein et al., 2004; Miller et al., 2005) if a right delivery system and molecular adjuvants are used (Babiuk et al., 2002; Otten et al., 2004; Scheerlinck et al., 2004; Luxembourg et al., 2007). In fact, promising results have been reported in Phase I and Phase II trials for malaria, tuberculosis, and HIV, the three main diseases that have defied the development of effective vaccines to date (MacGregor et al., 1998; Le et al., 2000; Rottinghaus et al., 2003).
Having this data in mind, several years ago, we suggested DNA vaccination as a strategy for generating an AD vaccine (Ghochikyan et al., 2003; Cribbs and Agadjanyan, 2005). AD is a devastating disease that currently affects about 5.3 million people in the United States (http://www.alz.org), and is the most common form of dementia, exhibiting progressive memory loss and general cognitive decline. Current data suggest that the accumulation of neurotoxic amyloid-β (Aβ) may play a central role in the onset and progression of AD (Hardy and Higgins, 1992). Accordingly, many therapies for AD are aimed at reducing the level of Aβ in the brain and/or blocking the assembly of Aβ peptide into pathological forms that disrupt cognitive function (Haass and Selkoe, 2007). Thus, a potentially powerful strategy is immunotherapy with anti-Aβ antibody because it can facilitate the reduction of pathological forms of Aβ in the brains of mice (Schenk et al., 1999; Bard et al., 2000; Janus et al., 2000; Morgan et al., 2000; Dodart et al., 2002) and humans (Nicoll et al., 2003, 2006; Ferrer et al., 2004; Masliah et al., 2005a; Patton et al., 2006; Boche et al., 2007; Nitsch and Hock, 2007; Holmes et al., 2008).
However, the first immunotherapy clinical trial was halted because a subset of individuals immunized with the Aβ42 peptide formulated in T helper (Th) 1-type adjuvant developed aseptic meningoencephalitis (Hock et al., 2003; Nicoll et al., 2003, 2006; Orgogozo et al., 2003; Ferrer et al., 2004; Bayer et al., 2005; Masliah et al., 2005b; Patton et al., 2006; Holmes et al., 2008). It was suggested that these adverse events in the central nervous system were associated with autoreactive T cells specific to the T cell epitope in Aβ, the conventional adjuvant, and/or the reformulation of the vaccine with polysorbate 80 (Schenk, 2002; Nicoll et al., 2003; Ferrer et al., 2004; Holmes et al., 2008). In addition, there was only a low percentage of elderly participants who responded to the AN-1792 vaccine by producing titers ≥1:2,200 of anti-Aβ antibodies (Gilman et al., 2005; Patton et al., 2006). To avoid problems associated with active immunization of elderly AD patients, we previously suggested an active vaccination strategy using peptide- or DNA-based epitope vaccines (Agadjanyan et al., 2005; Cribbs and Agadjanyan, 2005; Mamikonyan et al., 2007; Petrushina et al., 2007; Movsesyan et al., 2008a,b). More specifically, we proposed a DNA epitope vaccine expressing three copies of the immunodominant self B cell epitope (Aβ1–11) and a non-self Th cell epitope, PADRE (p3Aβ1–11-PADRE) instead of the whole Aβ42 antigen (Ghochikyan et al., 2003). Of note, to augment the humoral immune response, we fused this DNA epitope vaccine with molecular adjuvants: either the macrophage-derived chemokine (MDC) or three copies of C3d component of complement (3C3d). Although we have already tested the efficacy of plasmid MDC-3Aβ1–11-PADRE in the 3xTg-AD mouse model of AD, the reduction of AD-like pathology has not been previously assessed in transgenic mice immunized with plasmid encoding 3Aβ1–11-PADRE-3C3d. Thus, in this study we tested the immunological and therapeutic efficacy of a DNA epitope vaccine encoding 3Aβ1–11-PADRE-3C3d in a Tg2576 mouse model of AD (Hsiao et al., 1996). We demonstrated that this vaccine was capable of eliciting only low titers of Aβ-specific antibodies in Tg2576 mice. More importantly, this study demonstrated that low concentrations of antibody are not sufficient for the reduction of AD-like pathology in this mouse model of AD. Of note, the low titers of antibodies were associated with the Tg2576 AD mouse model, rather than with the molecular adjuvant, as 3Aβ–11-PADRE-3C3d induced stronganti-Aβ antibody responses in another AD mouse model (3xTg-AD).
Materials and Methods
Mice
Three- to four-month-old Tg2576 (B6/SJL F1 background, H2bxs haplotype) and 3–4-month-old 3xTg-AD (H2b haplotype) mice were bred and provided by the facility associated with the Alzheimer's Disease Research Center (ADRC) at the University of California, Irvine (UCI). Animals were housed at a temperature- and light-cycle- controlled animal facility at the Institute for Memory Impairments and Neurological Disorders, UCI. All procedures were performed according to the UCI Institutional Animal Care and Use Committee (IACUC) approved protocols and in accordance with guidelines of the National Institutes of Health for the proper care of laboratory animals.
Generation of plasmids
The generation of plasmids tested in the current study has been previously described (Movsesyan et al., 2008b). Plasmids encoding 3Aβ1–11-PADRE-3C3d, 3Aβ1–11-PADRE, and MDC-3Aβ1–11-PADRE were purified by using a Qiagen plasmid maxi kit (Qiagen, Valencia, CA). The purity and concentration of DNA were measured by DNA gel electrophoresis and optical density readings at 260/280 nm.
Immunizations of animals and detection of antibodies
Three- to four-month-old female Tg2576 mice were divided into two treatment groups: p3Aβ1–11-PADRE-3C3d (n = 9) and p3Aβ1–11-PADRE (n = 9). An additional group of naïve (nonimmunized, n = 9) Tg2576 mice of the same gender was kept in the same conditions throughout the entire experiment. Experimental groups received two initial biweekly immunizations and followed up with seven consecutive immunizations at 1.5-month intervals (total of nine injections) using gene gun. Mice were terminated at the age of 17–18 months 10 days after the last immunization. Gene gun immunizations (10 μg per mouse per immunization) were performed exactly as we had reported in prior studies (Ghochikyan et al., 2003; Movsesyan et al., 2008a,b). Anti-Aβ antibody concentrations and antibody isotypes were detected by ELISA exactly as previously described (Cribbs et al., 2003; Ghochikyan et al., 2003; Movsesyan et al., 2008a).
We also performed additional gene gun immunizations of 3–4-month-old Tg2576 and 3xTg-AD female mice with pMDC-3Aβ1–11-PADRE and p3Aβ1–11-PADRE-3C3d (10 μg per mouse for both vaccines, n = 6 per each group). Mice were immunized three times with biweekly intervals. Sera were collected on day 10 after the last immunization, and anti-Aβ antibody response was measured by ELISA.
Biochemical and immunohistochemical analysis of mouse brains
Brains of mice were collected for neuropathological analysis exactly as previously described (Petrushina et al., 2007). In brief, Tg2576 mice from all three groups were anesthetized with Nembutal 150 mg/kg and transcardially perfused with ice-cold PBS. Each mouse's skullcap was surgically removed; following this the brain was removed and bisected sagittally. The left hemisphere was snap-frozen and reserved for biochemical analysis, whereas the right hemisphere was fixed in phosphate-buffered 4% paraformaldehyde at +4°C for 36 hr for further immunohistochemical analysis.
The quantification of the levels of both soluble and insoluble (biochemical analysis) Aβ40 and Aβ42 in the brain was performed as described previously (Petrushina et al., 2007; Movsesyan et al., 2008a,b). In brief, after the extraction of the soluble fraction, protein concentration was determined using the BCA Protein Assay Kit (Pierce, Rockford, IL), and samples were adjusted to an equal concentration and analyzed using Biosource ELISA kits (Invitrogen, Carlsbad, CA). The insoluble fractions were neutralized and analyzed using Biosource ELISA kits (Invitrogen).
Immersion-fixed hemibrains were coronally sectioned at 50 μm and used for immunohistochemical analyses exactly as previously described (Petrushina et al., 2007; Movsesyan et al., 2008a). To detect Aβ deposits, the 6E10 monoclonal antibody (1:1,000; Signet, Dedham, MA) was used on sections pretreated earlier with 90% formic acid for 4 min. To quantify the number of Aβ plaques, six images of the cortical area and six images of hippocampal areas per mouse were taken using a 20× objective with a Sony high-resolution CCD video camera (XC-77) and manually counted. In addition, NIH image analysis was performed using NIH 1.59b5 software. The threshold intensity was manually set and kept constant. Quantification was performed by a single examiner blind to the identity of the samples.
Statistical analysis
All statistical parameters (mean, standard deviation, significant difference) were calculated using Prism 3.03 software (GraphPad Software, Inc., San Diego, CA). Statistically significant differences were examined using a t test or analysis of variance (one-way ANOVA) with Tukey's multiple comparisons posttest (p < 0.05 was considered significantly different).
Results and Discussion
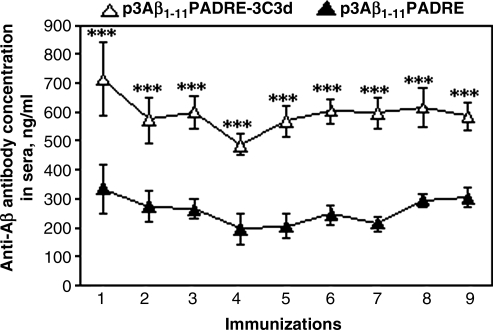
To investigate the potency of the 3C3d molecular adjuvant, we immunized 3–4-month-old Tg2576 mice with p3Aβ1–11-PADRE-3C3d or p3Aβ1–11-PADRE. It has been shown that as Tg2576 animals age, Aβ is altered beginning at the age of 6–7 months (Hsiao et al., 1996; Kawarabayashi et al., 2001). Therefore, immunization started at a young age is intended to prevent Aβ deposition in the brains and slow disease progression by the age of 17–18 months. The first two immunizations (initiated at the age of 3–4 months) were performed biweekly followed by booster injections at 1.5-month intervals (a total of nine). Mice were killed at the age of 17–18 months 10 days after the last immunization. Blood was collected following each immunization, and the humoral immune response was evaluated in sera. DNA vaccine, p3Aβ1–11-PADRE, induced a detectable antibody response to Aβ42 in all of the mice after the first immunization (333.24 ± 84.2 ng/ml; Fig. 1). The fusion of this vaccine with the 3C3d molecular adjuvant significantly enhanced the humoral immune response, reaching 711.86 ± 125.7 ng/ml (Fig. 1). After the booster injection with the epitope vaccine candidates, p3Aβ1–11-PADRE and p3Aβ1–11-PADRE-3C3d, Tg2576 mice generated 273.15 ± 54.7 ng/ml and 571.76 ± 78.9 ng/ml of anti-Aβ antibodies, respectively (Fig. 1). Subsequent booster injections of the experimental animals did not increase the concentrations of anti-Aβ antibody and maintained the low levels of antibodies generated after the second immunization with p3Aβ1–11-PADRE-3C3d and p3Aβ1–11-PADRE. However, the difference in the strength of generated humoral immune responses in both groups after all injections was statistically significant (Fig. 1).
FIG. 1.
3Aβ1–11-PADRE epitope vaccine with and without 3C3d molecular adjuvant induced low titers of anti-Aβ antibodies in Tg2576 mice. Preventive immunizations of Tg2576 mice (initiated at 3–4 months of age and carried up to 17–18 months) with p3Aβ1–11-PADRE induced poor production of anti-Aβ antibodies. Addition of 3C3d molecular adjuvant to p3Aβ1–11-PADRE plasmid (p3Aβ1–11-PADRE-3C3d) significantly enhanced humoral immune response (***p < 0.001); however, the strength of the humoral immune response in both immunized groups was low.
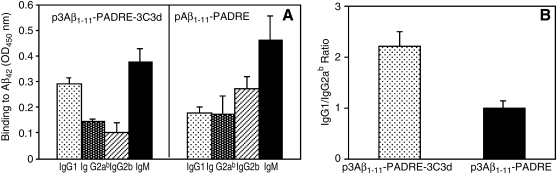
Next, to analyze the isotype profile of the humoral immune response, we detected the subclass of antibodies (IgG1, IgG2ab, IgG2b, IgM). Three immunizations of Tg2576 mice with p3Aβ1–11-PADRE-3C3d induced an increased production of IgM, a substantial amount of IgG1, and low levels of both IgG2ab and IgG2b (Fig. 2A). Meanwhile, the isotype profile in the group of mice immunized with p3Aβ1–11-PADRE looked different. There was a substantial amount of both IgG1 and IgG2ab isotypes and high production of the IgM isotype. In addition, we detected a considerably high level of the IgG2b isotype (Fig. 2A). We measured the IgG1/IgG2ab ratio, which is an indirect indicator of a Th1- or Th2-type immune response (Snapper and Paul, 1987; Finkelman et al., 1990; Hasbold et al., 1999). The IgG1/IgG2ab ratio in both groups of Tg2576 mice immunized with p3Aβ1–11-PADRE-3C3d (2.02 ± 0.3) or p3Aβ1–11-PADRE (1.02 ± 0.15) was greater than 1 (Fig. 2B). Thus, we did not observe significant differences in the type of humoral response generated after vaccination with p3Aβ1–11-PADRE-3C3d versus p3Aβ1–11-PADRE.
FIG. 2.
Isotype profile of Aβ-specific antibodies generated in transgenic Tg2576 mice. (A) Immunizations of Tg2576 mice with DNA epitope vaccines (p3Aβ1–11-PADRE-3C3d or p3Aβ1–11-PADRE) induce production of IgG1, IgG2ab, and IgG2b isotypes. Interestingly, a high production of IgM isotypes was detected in both groups of experimental mice. (B) Calculation of the IgG1/IgG2ab ratio determined a slight Th2-biased humoral immune response in the group immunized with p3Aβ1–11-PADRE, whereas addition of 3C3d molecular adjuvant induced a stronger Th2-type of immune response (group p3Aβ1–11-PADRE-3C3d).
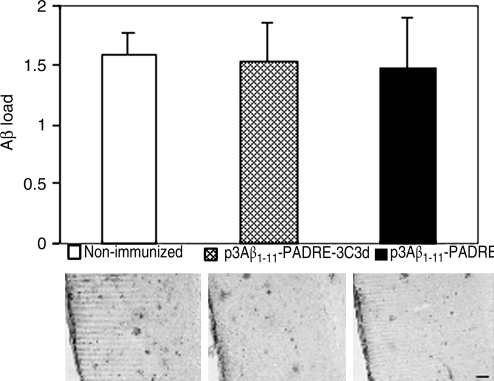
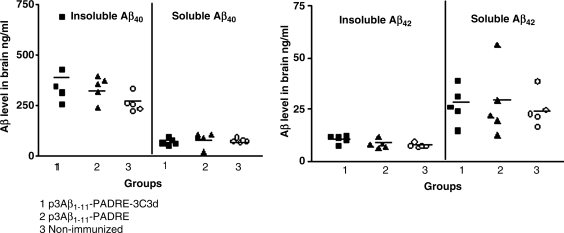
To study the effect of the tested DNA vaccines on neuropathology, two groups of immunized animals and nonimmunized control mice were killed at the age of 17–18 months and their brain sections were used for immunohistochemical analysis. Imaging analysis showed the same level of immunoreactivity to 6E10 in both experimental groups as well as in the nonimmunized control group (Fig. 3). Even though immunization with the epitope vaccine with or without the molecular adjuvant was not effective in reducing the amyloid burden in the brains of immunized animals, we still decided to determine whether there were changes in the levels of both soluble and insoluble Aβ40/42. The results revealed no statistically significant changes in the SDS-soluble and SDS-insoluble (formic acid) extracts for both Aβ40 and Aβ42 between the experimental and the control groups (Fig. 4). These findings indicate that the titer of antibodies generated in Tg2576 mice in response to immunization with DNA epitope vaccines (p3Aβ1–11-PADRE-3C3d and p3Aβ1–11-PADRE) was not sufficient to affect the amyloid clearance in the brains of these mice.
FIG. 3.
Immunizations with either vaccine candidate did not change Aβ plaque load in the brains of Tg2576 mice. Analysis of Aβ plaque load was performed on brain sections of transgenic mice terminated at 17–18 months of age. Preventive immunizations did not affect plaque deposition in the brains of mice from both vaccinated groups. Representative staining images of the distribution of Aβ plaques stained with 6E10 monoclonal antibody in the cortex of nonimmunized and p3Aβ1–11-PADRE-3C3d- and p3Aβ1–11-PADRE-immunized mice are presented. Scale bar: 200 μm at 4× magnification.
FIG. 4.
Biochemical analysis of brain tissues revealed no changes in soluble and insoluble Aβ levels in both immunized and nonimmunized groups of Tg2576 mice. Low titers of anti-Aβ antibodies generated after immunization with p3Aβ1–11-PADRE-3C3d or p3Aβ1–11-PADRE were not sufficient for inhibition of both soluble and insoluble Aβ40 and Aβ42 peptide levels in the brain. Analysis of neuropathological changes was performed in the brains of vaccinated and control (nonimmunized) mice at the age of 17–18 months.
Previously, we reported positive correlation between the concentration of anti-Aβ antibody and the reduction of insoluble, cerebral Aβ plaques in Tg2576 mice immunized with a peptide-based epitope vaccine (Petrushina et al., 2007). Our current data with Tg2576 mice vaccinated with p3Aβ1–11-PADRE-3C3d and p3Aβ1–11-PADRE support these results. It is worth mentioning that the data from clinical trials also reported a positive correlation between levels of anti-Aβ antibodies and the reduction of Aβ pathology (Nicoll et al., 2003; Ferrer et al., 2004; Bayer et al., 2005; Gilman et al., 2005). Future clinical trials with active and passive vaccination may shed more light on the connection between antibody titers, pathology, and the neurological outcome.
Thus, neither p3Aβ1–11-PADRE nor p3Aβ1–11-PADRE-3C3d induced a strong anti-Aβ antibody response in Tg2576 mice. Although the 3C3d molecular adjuvant fused with a DNA epitope vaccine enhanced humoral immune response significantly (Fig. 1), it still induced very low responses in these mice. It is well known that a major function of C’ is the opsonization of antigens/immune complexes. This is mediated by the covalent attachment of activated C3d and C3dg fragments of C’ to the antigen, which links the innate and the adaptive immune responses by targeting the immunogen to specific C’ receptors type 1 (CD35) and type 2 (CD 21) (Carroll, 1998; Fearon and Carroll, 2000). CD21 is expressed on mature B cells as a noncovalent complex with CD19 and functions as a specialized membrane adaptor protein for antigen-specific B cell receptors (BCR) (Ahearn et al., 1996; Croix et al., 1996). The simultaneous engagement of BCR and CD21/CD19 by the antigen-C3d complex dramatically reduces the BCR:antigen affinity threshold, significantly prolongs BCR residency in lipid rafts, and induces much stronger B cell activation (Carter and Fearon, 1992; Mongini et al., 1997; Cherukuri et al., 2001). The immunization of mice with recombinant hen egg lysozyme fused with 3C3d dramatically enhanced antibody formation (Dempsey et al., 1996). DNA vaccines encoding various antigens fused with 3C3d also increased antibody responses (Ross et al., 2000, 2001; Mitchell et al., 2003; Tong et al., 2006). We also reported that p3Aβ1–11-PADRE induced strong anti-Aβ antibody response in C57Bl/6 mice, and that 3C3d significantly enhanced this response (Movsesyan et al., 2008b). However, here we demonstrated that although the fusion of p3Aβ1–11-PADRE with 3C3d enhanced the humoral immune response, both vaccines (p3Aβ1–11-PADRE and p3Aβ1–11-PADRE-3C3d) induced only weak immune responses in the Tg2576 mouse model of AD. One idea is that this low response could be associated with differences in the immune haplotype of C57Bl/6 (H2b) versus Tg2576 (H2bxs) mice or with some other immune functions linked to the transgenic characteristics of the mice. The hyporesponsiveness of Tg2576 mice to Aβ42 due to the induction of tolerance to self T cell epitope was demonstrated earlier by different groups including ours (Monsonego et al., 2001; Petrushina et al., 2003). Here we demonstrated for the first time that Tg2576 animals also do not respond to immunizations with a vaccine composed of foreign T cell epitope and self-B cell epitope, Aβ1–11.
Previously we reported that the same p3Aβ1–11-PADRE vaccine fused with another molecular adjuvant, MDC, was effective in generating high concentrations of anti-Aβ antibodies in a wild-type (C57Bl/6) and triple transgenic mouse model of AD (Movsesyan et al., 2008a). Thus, to understand the underlying reason of such low efficacy of p3Aβ1–11-PADRE-3C3d in Tg2576 mice here, we compared immune responses generated in Tg2576 and 3xTg-AD mouse models of AD immunized with the same vaccine, which was fused with either MDC or 3C3d molecular adjuvants. Results presented in Table 1 demonstrated that both pMDC-3Aβ1–11-PADRE and p3Aβ1–11-PADRE-3C3d vaccines induced significantly stronger immune responses in 3xTg-AD mice than in the Tg2576 mouse model of AD (p < 0.01 and p < 0.05, respectively). It has been reported that T cell epitope, PADRE, is recognized by MHC class II molecules of H2b mice very well (Alexander et al., 2000). So to exclude the possibility that loss of the H2b haplotype during breeding could be responsible for the low immune responses in Tg2576 mice of the H2bxs immune haplotype, we immunized both mouse models of AD, which have different genetic backgrounds, with the peptide-based epitope vaccine, 2Aβ1–11-PADRE, synthesized as a multiple antigenic peptide (2Aβ1–11-PADRE-MAP). 3xTg-AD mice of the H2b haplotype responded to this vaccine very well. More importantly, immunizations with the same peptide-based epitope vaccine induced strong antibody production in Tg2576 mice as well (Table 1). Although this response is slightly lower than in 3xTg-AD mice, this difference was not statistically significant. Of note, SJL mice of the H2s haplotype practically did not respond to p3Aβ1–11-PADRE fused with 3C3d (data not shown). These results argue that our colony of Tg2576 mice did not lose the H2b haplotype during breeding. Further analyses are needed to understand why the immunogenicity of the DNA-based, but not of the peptide-based, epitope vaccine is impaired in Tg2576 mice. One of the mechanisms possibly responsible for this outcome may be the induction of B cell tolerance due to a high level of Aβ in the serum of Tg2576 mice, which could be broken by multiple B cell epitopes in the lysine branch of the MAP peptide.
Table 1.
Concentration of Anti-Aβ Antibody in AD Mouse Models Immunized with DNA and Peptide Based Epitope Vaccines
| |
Anti-Aβ antibody concentration (μg/ml) |
|
|---|---|---|
| Immunogen | Tg2576 (H2bxs) | 3xTg-AD (H2b) |
| pMDC-3Aβ1-11-PADRE | 1.42 ± 1.45 | 11.95 ± 5.49 |
| p3Aβ1-11-PADRE-3C3d | 2.43 ± 1.68 | 21.14 ± 17.79 |
| 2Aβ1-11-PADRE-MAP peptide/Quil A | 141.95 ± 151.3 | 223.8 ± 65.1 |
In sum, this study revealed a low potency of the DNA epitope vaccine in Tg2576 mice and confirmed our previously reported data that low concentrations of anti-Aβ antibodies are not sufficient for the reduction of AD-like pathology.
Acknowledgments
This work was supported by funding from NIH: AG-20241 (D.H.C. and M.G.A.), AG-00538 (D.H.C.), NS-50895 (M.G.A. and D.H.C.), NS-057395 (M.G.A.), Alzheimer's Association IIRG 07-28314 (A.G.). N.M. was supported by NIA training grant AG00096. Transgenic animals and amyloid peptide were provided by the UCI Alzheimer's Disease Research Center (ADRC) under the NIH/NIA P50 AG16573 grant.
Author Disclosure Statement
All authors have nothing to disclose.
References
- Agadjanyan M.G. Ghochikyan A. Petrushina I. Vasilevko V. Movsesyan N. Mkrtichyan M. Saing T. Cribbs D.H. Prototype Alzheimer's disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J. Immunol. 2005;174:1580–1586. doi: 10.4049/jimmunol.174.3.1580. [DOI] [PubMed] [Google Scholar]
- Ahearn J.M. Fischer M.B. Croix D. Goerg S. Ma M. Xia J. Zhou X. Howard R.G. Rothstein T.L. Carroll M.C. Disruption of the Cr2 locus results in a reduction in B-1a cells and in an impaired B cell response to T-dependent antigen. Immunity. 1996;4:251–262. doi: 10.1016/s1074-7613(00)80433-1. [DOI] [PubMed] [Google Scholar]
- Alexander J. del Guercio M.F. Maewal A. Qiao L. Fikes J. Chesnut R.W. Paulson J. Bundle D.R. DeFrees S. Sette A. Linear PADRE T helper epitope and carbohydrate B cell epitope conjugates induce specific high titer IgG antibody responses. J. Immunol. 2000;164:1625–1633. doi: 10.4049/jimmunol.164.3.1625. [DOI] [PubMed] [Google Scholar]
- Babiuk S. Baca-Estrada M.E. Foldvari M. Storms M. Rabussay D. Widera G. Babiuk L.A. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine. 2002;20:3399–3408. doi: 10.1016/s0264-410x(02)00269-4. [DOI] [PubMed] [Google Scholar]
- Bard F. Cannon C. Barbour R. Burke R.L. Games D. Grajeda H. Guido T. Hu K. Huang J. Johnson-Wood K. Khan K. Kholodenko D. Lee M. Lieberburg I. Motter R. Nguyen M. Soriano F. Vasquez N. Weiss K. Welch B. Seubert P. Schenk D. Yednock T. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- Bayer A.J. Bullock R. Jones R.W. Wilkinson D. Paterson K.R. Jenkins L. Millais S.B. Donoghue S. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology. 2005;64:94–101. doi: 10.1212/01.WNL.0000148604.77591.67. [DOI] [PubMed] [Google Scholar]
- Boche D. DeBeer S. Cox A. Wilkinson D. Holmes C. Neal J. Love S. Esiri M. Bridges L. Weller R. Nicoll J.A. Evidence of a transient increase in cerebral amyloid angiopathy after Abeta42 immunization in human Alzheimer's disease. 8th International Conference “Alzheimer's and Parkinson's Disease: Progress and New Perspectives”; Salzburg, Austria. 2007. [Google Scholar]
- Boyer J.D. Chattergoon M.A. Ugen K.E. Shah A. Bennett M. Cohen A. Nyland S. Lacy K.E. Bagarazzi M.L. Higgins T.J. Baine Y. Ciccarelli R.B. Ginsberg R.S. MacGregor R.R. Weiner D.B. Enhancement of cellular immune response in HIV-1 seropositive individuals: a DNA-based trial. Clin. Immunol. 1999;90:100–107. doi: 10.1006/clim.1998.4616. [DOI] [PubMed] [Google Scholar]
- Boyer J.D. Cohen A.D. Vogt S. Schumann K. Nath B. Ahn L. Lacy K. Bagarazzi M.L. Higgins T.J. Baine Y. Ciccarelli R.B. Ginsberg R.S. MacGregor R.R. Weiner D.B. Vaccination of seronegative volunteers with a human immunodeficiency virus type 1 env/rev DNA vaccine induces antigen-specific proliferation and lymphocyte production of beta-chemokines. J. Infect. Dis. 2000;181:476–483. doi: 10.1086/315229. [DOI] [PubMed] [Google Scholar]
- Carroll M.C. The role of complement and complement receptors in induction and regulation of immunity. Annu. Rev. Immunol. 1998;16:545–568. doi: 10.1146/annurev.immunol.16.1.545. [DOI] [PubMed] [Google Scholar]
- Carter R.H. Fearon D.T. CD19: lowering the threshold for antigen receptor stimulation of B lymphocytes. Science. 1992;256:105–107. doi: 10.1126/science.1373518. [DOI] [PubMed] [Google Scholar]
- Cherukuri A. Cheng P.C. Pierce S.K. The role of the CD19/CD21 complex in B cell processing and presentation of complement-tagged antigens. J. Immunol. 2001;167:163–172. doi: 10.4049/jimmunol.167.1.163. [DOI] [PubMed] [Google Scholar]
- Conry R.M. Curiel D.T. Strong T.V. Moore S.E. Allen K.O. Barlow D.L. Shaw D.R. LoBuglio A.F. Safety and immunogenicity of a DNA vaccine encoding carcinoembryonic antigen and hepatitis B surface antigen in colorectal carcinoma patients. Clin. Cancer Res. 2002;8:2782–2787. [PubMed] [Google Scholar]
- Cribbs D.H. Agadjanyan M.G. Immunotherapy for Alzheimer's disease: potential problems and possible solutions. Curr. Immunol. Rev. 2005;1:139–155. [Google Scholar]
- Cribbs D.H. Ghochikyan A. Vasilevko V. Tran M. Petrushina I. Sadzikava N. Babikyan D. Kesslak P. Kieber-Emmons T. Cotman C.W. Agadjanyan M.G. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol. 2003;15:505–514. doi: 10.1093/intimm/dxg049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croix D.A. Ahearn J.M. Rosengard A.M. Han S. Kelsoe G. Ma M. Carroll M.C. Antibody response to a T-dependent antigen requires B cell expression of complement receptors. J. Exp. Med. 1996;183:1857–1864. doi: 10.1084/jem.183.4.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey P.W. Allison M.E. Akkaraju S. Goodnow C.C. Fearon D.T. C3d of complement as a molecular adjuvant: bridging innate and acquired immunity. Science. 1996;271:348–350. doi: 10.1126/science.271.5247.348. [DOI] [PubMed] [Google Scholar]
- Dodart J.C. Bales K.R. Gannon K.S. Greene S.J. DeMattos R.B. Mathis C. DeLong C.A. Wu S. Wu X. Holtzman D.M. Paul S.M. Immunization reverses memory deficits without reducing brain Abeta burden in Alzheimer's disease model. Nat. Neurosci. 2002;5:452–457. doi: 10.1038/nn842. [DOI] [PubMed] [Google Scholar]
- Donnelly J.J. Ulmer J.B. DNA vaccines for viral diseases. Braz. J. Med. Biol. Res. 1999;32:215–222. doi: 10.1590/s0100-879x1999000200010. [DOI] [PubMed] [Google Scholar]
- Epstein J.E. Charoenvit Y. Kester K.E. Wang R. Newcomer R. Fitzpatrick S. Richie T.L. Tornieporth N. Heppner D.G. Ockenhouse C. Majam V. Holland C. Abot E. Ganeshan H. Berzins M. Jones T. Freydberg C.N. Ng J. Norman J. Carucci D.J. Cohen J. Hoffman S.L. Safety, tolerability, and antibody responses in humans after sequential immunization with a PfCSP DNA vaccine followed by the recombinant protein vaccine RTS,S/AS02A. Vaccine. 2004;22:1592–1603. doi: 10.1016/j.vaccine.2004.01.031. [DOI] [PubMed] [Google Scholar]
- Fearon D.T. Carroll M.C. Regulation of B lymphocyte responses to foreign and self-antigens by the CD19/CD21 complex. Annu. Rev. Immunol. 2000;18:393–422. doi: 10.1146/annurev.immunol.18.1.393. [DOI] [PubMed] [Google Scholar]
- Ferrer I. Rovira M.B. Guerra M.L.S. Rey M.J. Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer's disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman F.D. Holmes J. Katona I.M. Urban J.F. Beckmann M.P. Park L.S. Schooley K.A. Coffman R.L. Mossmann T.R. Paul W.E. Lymphokine control of in vivo immunoglobulin isotype selection. Annu. Rev. Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- Fynan E.F. Webster R.G. Fuller D.H. Haynes J.R. Santoro J.C. Robinson H.L. DNA vaccines: protective immunizations by parenteral, mucosal, and gene-gun inoculations. Proc. Natl. Acad. Sci. U.S.A. 1993;90:11478–11482. doi: 10.1073/pnas.90.24.11478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghochikyan A. Vasilevko V. Petrushina I. Tran M. Sadzikava N. Babikyan D. Movsesyan N. Tian W. Ross T.M. Cribbs D.H. Agadjanyan M.G. Generation and characterization of the humoral immune response to DNA immunization with a chimeric β-amyloid-interleukin-4 minigene. Eur. J. Immunol. 2003;33:3232–3241. doi: 10.1002/eji.200324000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S. Koller M. Black R.S. Jenkins L. Griffith S.G. Fox N.C. Eisner L. Kirby L. Rovira M.B. Forette F. Orgogozo J.M. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology. 2005;64:1553–1562. doi: 10.1212/01.WNL.0000159740.16984.3C. [DOI] [PubMed] [Google Scholar]
- Haass C. Selkoe D.J. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- Hardy J.A. Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hasbold J. Hong J.S. Kehry M.R. Hodgkin P.D. Integrating signals from IFN-gamma and IL-4 by B cells: positive and negative effects on CD40 ligand-induced proliferation, survival, and division-linked isotype switching to IgG1, IgE, and IgG2a. J. Immunol. 1999;163:4175–4181. [PubMed] [Google Scholar]
- Hock C. Konietzko U. Streffer J.R. Tracy J. Signorell A. Müller-Tillmanns B. Lemke U. Henke K. Moritz E. Garcia E. Wollmer M.A. Umbricht D. de Quervain D.J. Hofmann M. Maddalena A. Papassotiropoulos A. Nitsch R.M. Antibodies against beta-amyloid slow cognitive decline in Alzheimer's disease. Neuron. 2003;38:547–554. doi: 10.1016/s0896-6273(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Holmes C. Boche D. Wilkinson D. Yadegarfar G. Hopkins V. Bayer A. Jones R.W. Bullock R. Love S. Neal J.W. Zotova E. Nicoll J.A. Long-term effects of Abeta42 immunisation in Alzheimer's disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Hsiao K. Chapman P. Nilsen S. Eckman C. Harigaya Y. Younkin S. Yang F. Cole G. Correlative memory deficits, Aß elevation, and amyloid plaques in transgenic mice. Science. 1996;274:99–102. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Janus C. Pearson J. McLaurin J. Mathews P.M. Jiang Y. Schmidt S.D. Chishti M.A. Horne P. Heslin D. French J. Mount H.T. Nixon R.A. Mercken M. Bergeron C. Fraser P.E. St George-Hyslop P. Westaway D. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T. Younkin L.H. Saido T.C. Shoji M. Ashe K.H. Younkin S.G. Age-dependent changes in brain, CSF, and plasma amyloid (beta) protein in the Tg2576 transgenic mouse model of Alzheimer's disease. J. Neurosci. 2001;21:372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T.P. Coonan K.M. Hedstrom R.C. Charoenvit Y. Sedegah M. Epstein J.E. Kumar S. Wang R. Doolan D.L. Maguire J.D. Parker S.E. Hobart P. Norman J. Hoffman S.L. Safety, tolerability and humoral immune responses after intramuscular administration of a malaria DNA vaccine to healthy adult volunteers. Vaccine. 2000;18:1893–1901. doi: 10.1016/s0264-410x(99)00407-7. [DOI] [PubMed] [Google Scholar]
- Luxembourg A. Evans C.F. Hannaman D. Electroporation-based DNA immunisation: translation to the clinic. Expert Opin. Biol. Ther. 2007;7:1647–1664. doi: 10.1517/14712598.7.11.1647. [DOI] [PubMed] [Google Scholar]
- MacGregor R.R. Boyer J.D. Ugen K.E. Lacy K.E. Gluckman S.J. Bagarazzi M.L. Chattergoon M.A. Baine Y. Higgins T.J. Ciccarelli R.B. Coney L.R. Ginsberg R.S. Weiner D.B. First human trial of a DNA-based vaccine for treatment of human immunodeficiency virus type 1 infection: safety and host response. J. Infect. Dis. 1998;178:92–100. doi: 10.1086/515613. [DOI] [PubMed] [Google Scholar]
- Mamikonyan G. Necula M. Mkrtichyan M. Ghochikyan A. Petrushina I. Movsesyan N. Mina E. Kiyatkin A. Glabe C. Cribbs D.H. Agadjanyan M.G. Anti-Abeta 1–11 antibody binds to different beta-amyloid species, inhibits fibril formation, and disaggregates preformed fibrils, but not the most toxic oligomers. J. Biol. Chem. 2007;282:22376–22386. doi: 10.1074/jbc.M700088200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E. Hansen L. Adame A. Crews L. Bard F. Lee C. Seubert P. Games D. Kirby L. Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005a;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- Masliah E. Rockenstein E. Adame A. Alford M. Crews L. Hashimoto M. Seubert P. Lee M. Goldstein J. Chilcote T. Games D. Schenk D. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005b;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Miller A.M. Ozenci V. Kiessling R. Pisa P. Immune monitoring in a phase 1 trial of a PSA DNA vaccine in patients with hormone-refractory prostate cancer. J. Immunother. 2005;28:389–395. doi: 10.1097/01.cji.0000165353.19171.41. [DOI] [PubMed] [Google Scholar]
- Mitchell J.A. Green T.D. Bright R.A. Ross T.M. Induction of heterosubtypic immunity to influenza A virus using a DNA vaccine expressing hemagglutinin-C3d fusion proteins. Vaccine. 2003;21:902–914. doi: 10.1016/s0264-410x(02)00539-x. [DOI] [PubMed] [Google Scholar]
- Mongini P.K. Vilensky M.A. Highet P.F. Inman J.K. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2) J. Immunol. 1997;159:3782–3791. [PubMed] [Google Scholar]
- Monsonego A. Maron R. Zota V. Selkoe D.J. Weiner H.L. Immune hyporesponsivness to amyloid beta-peptide in amyloid precursor protein transgenic mice: implications for the pathogenesis and treatment of Alzheimer's disease. Proc. Nat. Acad. Sci. U.S.A. 2001;98:10273–10278. doi: 10.1073/pnas.191118298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. Diamond D.M. Gottschall P.E. Ugen K.E. Dickey C. Hardy J. Duff K. Jantzen P. DiCarlo G. Wilcock D. Connor K. Hatcher J. Hope C. Gordon M. Arendash G.W. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature. 2000;408:982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- Movsesyan N. Ghochikyan A. Mkrtichyan M. Petrushina I. Davtyan H. Olkhanud P.B. Head E. Biragyn A. Cribbs D.H. Agadjanyan M.G. Reducing AD-like pathology in 3xTg-AD mouse model by DNA epitope vaccine—a novel immunotherapeutic strategy. PLoS One. 2008a;3:e21–e24. doi: 10.1371/journal.pone.0002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movsesyan N. Mkrtichyan M. Petrushina I. Ross T.M. Cribbs D.H. Agadjanyan M.G. Ghochikyan A. DNA epitope vaccine containing complement component C3d enhances anti-amyloid-beta antibody production and polarizes the immune response towards a Th2 phenotype. J. Neuroimmunol. 2008b;205:57–63. doi: 10.1016/j.jneuroim.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll J.A. Wilkinson D. Holmes C. Steart P. Markham H. Weller R.O. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat. Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- Nicoll J.A. Barton E. Boche D. Neal J.W. Ferrer I. Thompson P. Vlachouli C. Wilkinson D. Bayer A. Games D. Seubert P. Schenk D. Holmes C. Abeta species removal after Abeta42 immunization. J. Neuropathol. Exp. Neurol. 2006;65:1040–1048. doi: 10.1097/01.jnen.0000240466.10758.ce. [DOI] [PubMed] [Google Scholar]
- Nitsch R.M. Hock C. Immunotherapy against beta-amyloid in Alzheimer's disease. 8th International Conference “Alzheimer's and Parkinson's Disease: Progress and New Perspectives”; Salzburg, Austria. 2007. [Google Scholar]
- Orgogozo J.M. Gilman S. Dartigues J.M. Laurent B. Puel M. Kirby L.C. Jouanny P. Dubois B. Eisner L. Flitman S. Michel B.F. Boada M. Frank A. Hock C. Subacute meningoencephalitis in a subset of patients with AD after Abeta42 immunization. Neurology. 2003;61(1):46–54. doi: 10.1212/01.wnl.0000073623.84147.a8. [DOI] [PubMed] [Google Scholar]
- Otten G. Schaefer M. Doe B. Liu H. Srivastava I. zur Megede J. O'Hagan D. Donnelly J. Widera G. Rabussay D. Lewis M.G. Barnett S. Ulmer J.B. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine. 2004;22:2489–2493. doi: 10.1016/j.vaccine.2003.11.073. [DOI] [PubMed] [Google Scholar]
- Patton R.L. Kalback W.M. Esh C.L. Kokjohn T.A. Van Vickle G.D. Luehrs D.C. Kuo Y.M. Lopez J. Brune D. Ferrer I. Masliah E. Newel A.J. Beach T.G. Castano E.M. Roher A.E. Amyloid-beta peptide remnants in AN-1792-immunized Alzheimer's disease patients: a biochemical analysis. Am. J. Pathol. 2006;169:1048–1063. doi: 10.2353/ajpath.2006.060269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrushina I. Tran M. Sadzikava N. Ghochikyan A. Vasilevko V. Agadjanyan M.G. Cribbs D.H. Importance of IgG2c isotype in the immune response to beta-amyloid in APP/Tg mice. Neurosci. Lett. 2003;338:5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- Petrushina I. Ghochikyan A. Mktrichyan M. Mamikonyan G. Movsesyan N. Davtyan H. Patel A. Head E. Cribbs D.H. Agadjanyan M.G. Alzheimer's disease peptide epitope vaccine reduces insoluble but not soluble/oligomeric A{beta} species in amyloid precursor protein transgenic mice. J. Neurosci. 2007;27:12721–12731. doi: 10.1523/JNEUROSCI.3201-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell K. DNA vaccines—back in the saddle again? Nat. Biotechnol. 2004;22:799–801. doi: 10.1038/nbt0704-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson H.L. Hunt L.A. Webster R.G. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine. 1993;11:957–960. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- Ross T.M. Xu Y. Bright R.A. Robinson H.L. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat. Immunol. 2000;1:127–131. doi: 10.1038/77802. Comment in: Nat. Immunol. 2000 Aug;1(2):102–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross T.M. Xu Y. Green T.D. Montefiori D.C. Robinson H.L. Enhanced avidity maturation of antibody to human immunodeficiency virus envelope: DNA vaccination with gp120-C3d fusion proteins. AIDS Res. Hum. Retroviruses. 2001;17:829–835. doi: 10.1089/088922201750252025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottinghaus S.T. Poland G.A. Jacobson R.M. Barr L.J. Roy M.J. Hepatitis B DNA vaccine induces protective antibody responses in human non-responders to conventional vaccination. Vaccine. 2003;21:4604–4608. doi: 10.1016/s0264-410x(03)00447-x. [DOI] [PubMed] [Google Scholar]
- Scheerlinck J.P. Karlis J. Tjelle T.E. Presidente P.J. Mathiesen I. Newton S.E. In vivo electroporation improves immune responses to DNA vaccination in sheep. Vaccine. 2004;22:1820–1825. doi: 10.1016/j.vaccine.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Schenk D. Opinion: Amyloid-beta immunotherapy for Alzheimer's disease: the end of the beginning. Nat. Rev. Neurosci. 2002;3:824–828. doi: 10.1038/nrn938. [DOI] [PubMed] [Google Scholar]
- Schenk D. Barbour R. Dunn W. Gordon G. Grajeda H. Guido T. Hu K. Huang J. Johnson-Wood K. Khan K. Kholodenko D. Lee M. Liao Z. Lieberburg I. Motter R. Mutter L. Soriano F. Shopp G. Vasquez N. Vandevert C. Walker S. Wogulis M. Yednock T. Games D. Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse [see comments] Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- Snapper C.M. Paul W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science. 1987;236:944–947. doi: 10.1126/science.3107127. [DOI] [PubMed] [Google Scholar]
- Tong T. Fan H. Tan Y. Xiao S. Ling J. Chen H. Guo A. C3d enhanced DNA vaccination induced humoral immune response to glycoprotein C of pseudorabies virus. Biochem. Biophys. Res. Commun. 2006;347:845–851. doi: 10.1016/j.bbrc.2006.05.091. [DOI] [PubMed] [Google Scholar]