Hu et al. demonstrate that systemic delivery of a replication competent oncolytic adenovirus encoding TGF-β fused with human immunoglobulin Fc fragment results in significant inhibition of tumor growth and osteolytic bone destruction in a nude mouse model. The authors show that although a replication deficient adenovirus has some effects on tumor growth and tumor-induced hypercalcemia, these therapeutic effects are significantly weaker than those shown by the replication-competent adenovirus vector.
Abstract
We have investigated whether systemic delivery of an oncolytic adenovirus, Ad.sTβRFc, expressing the soluble form of transforming growth factor-β receptor II fused with human immunoglobulin Fc fragment (sTGFβRIIFc), could inhibit breast cancer bone metastasis in a mouse model. MDA-MB-231 (human breast cancer) cells were inoculated into the left heart ventricles of nude mice. Once the skeletal tumors were visible by X-rays, mice were intravenously injected with either buffer, Ad.sTβRFc, Ad(E1−).sTβRFc (a replication-deficient adenovirus expressing sTGFβRIIFc), or Ad.luc2 (a replicating adenovirus expressing firefly luciferase gene). On days 2 and 7 after viral injections, viral replication and sTGFβRIIFc expression were detected in the skeletal tumors in Ad.sTβRFc-treated group; only viral replication in Ad.luc2 group, and sTGFβRIIFc expression in the Ad(E1−).sTβRFc group, were detected. To examine the therapeutic effects, buffer or various viral vectors were administered on days 4 and 7 after intracardiac injection of MDA-MB-231 cells. On day 28, X-ray radiography showed a highly significant reduction in lesion size by Ad.sTβRFc, a significant reduction by Ad.luc2, and some reduction by Ad(E1−).sTβRFc. Goldner's trichrome and hematoxylin–eosin staining of the bone sections revealed a significant reduction of tumor burden in the Ad.sTβRFc group, but not in the Ad(E1−).sTβRFc or Ad.luc2 group. There were significant reductions in free calcium levels by Ad.sTβRFc, Ad(E1−).sTβRFc, and Ad.luc2; however, only in the Ad.sTβRFc group were calcium levels reduced to the normal values. These results suggest that concomitant viral replication and sTGFβRIIFc production are important to inhibit bone metastasis and osteolysis, and that Ad.sTβRFc could be developed for targeting breast cancer bone metastases.
Introduction
Nearly 200,000 women are diagnosed with breast cancer, resulting in approximately 40,000 deaths, each year in the United States (American Cancer Society, 2010). During the advanced stage of breast cancer, the majority of patients develop bone metastases, which is a major cause of morbidity and mortality in these patients. Thus, there is an urgent need to develop novel therapies for the treatment of bone metastases of breast cancer. Our laboratory has been interested in developing recombinant adenoviruses for the treatment of breast cancer (Katayose et al., 1995; Seth, 1999; Seth et al., 2006; Wang et al., 2006; Hu et al., 2010). Although oncolytic adenoviruses have been developed in many laboratories, their application in targeting bone metastasis has not been described (McCormick, 2005; Crompton and Kirn, 2007). The literature suggests that aberrant activation of transforming growth factor (TGF)-β pathways are associated with breast cancer bone metastases (Kang et al., 2005; Steeg, 2006; Shipitsin et al., 2007; Akhtari et al., 2008; Padua and Massague, 2009), and that downregulation of TGF-β signaling in the tumor cells is considered a viable approach to control bone metastasis (Yin et al., 1999; Yang et al., 2002; Iyer et al., 2005; Korpal et al., 2009; Serganova et al., 2009). Human breast cancer cells expressing a dominant negative transforming growth factor-β receptor II (TGF-βRII; lacking the kinase domain) or transgenic mice expressing soluble TGFβRII–Fc fusion protein (sTGFβRIIFc) have also been shown to reduce the incidence of metastases in mice (Yin et al., 1999; Yang et al., 2002), and life-long exposure to sTGFβRIIFc in transgenic mice did not cause any apparent harmful effect to the mice (Yang et al., 2002). To combine the capacity of oncolytic viruses to kill cancer cells, and the function of sTGFβRIIFc to inhibit TGF-β signaling, we have developed Ad.sTβRFc, an oncolytic adenovirus expressing sTGFβRIIFc (Seth et al., 2006). Ad.sTβRFc is derived from an adenoviral mutant, dl01/07, that has two deletions in the E1A region, amino acids 4–25 and amino acids 111–123. The resultant E1 proteins are not able to bind with p300 or Rb proteins and cannot induce S-phase progression in primary cells. Therefore, dl01/07 is attenuated for viral replication in normal cells, but can replicate in cancer cells regardless of their genetic background (Howe et al., 2000). We have previously shown that human breast tumor cells (cell line MDA-MB-231) possesses nearly 10,000 adenoviral receptors per cells, and are easily infectable by human adenoviruses (Seth et al., 1996). Ad.sTβRFc can replicate in the MDA-MB-231 cell line (Seth et al., 2006), which harbors a missense mutation in the p53 gene, in codon 280, changing arginine to lysine (Lacroix et al., 2006). Infection of MDA-MB-231 cells with Ad.sTβRFc produces sTGFβRIIFc and inhibits TGF-β signaling, and its direct administration into subcutaneous tumors in nude mice results in significant inhibition of tumor growth (Seth et al., 2006).
In the present study, we have examined whether systemic administration of Ad.sTβRFc could target breast cancer bone metastases in a nude mouse model. Our results presented here indicate that intravenous delivery of Ad.sTβRFc produced viral replication and sTGFβRIIFc in skeletal tumors and resulted in significant inhibition of tumor growth and osteolytic bone destruction. Ad(E1−).sTβRFc, a replication-deficient adenovirus expressing sTGFβRIIFc; and Ad.luc2, a replicating virus expressing the firefly luciferase gene, had some effects on tumor growth and tumor-induced hypercalcemia; but the therapeutic effects were weaker than that of Ad.sTβRFc. These findings suggest that both viral replication and sTGFβRIIFc production are critical in mediating the in vivo effects of Ad.sTβRFc in inhibiting bone metastases, and that Ad.sTβRFc can be developed for the treatment of breast cancer bone metastases.
Materials and Methods
Cell lines and viruses
HEK293 (American Type Culture Collection [ATCC], Manassas, VA) and MDA-MB-231 cells (Yin et al., 1999) were maintained as described earlier (Katayose et al., 1995; Yin et al., 1999). Ad.sTβRFc (Seth et al., 2006) is a replicating adenovirus in which the cytomegalovirus (CMV) promoter drives the sTGFβRIIFc gene cloned in the E3 region, while retaining the adenovirus death protein sequence. Ad.luc2 is identical to Ad.sTβRFc except that the luc2 gene is cloned in the E3 region. Ad(E1−).sTβRFc (Hu et al., 2010) is a nonreplicating virus expressing the CMV promoter driving sTGFβRIIFc in the E3 region. All adenoviral vectors were amplified in HEK293 cells and purified as described earlier (Katayose et al., 1995).
Breast cancer bone metastasis model and radiography
Five-week-old female athymic nu/nu mice were purchased from Charles River Laboratories (Wilmington, MA). All animal experimental procedures were approved by the Institutional Animal Care and Use Committee at NorthShore University HealthSystem (Evanston, IL). MDA-MB-231 cells were trypsinized, washed twice, and resuspended in phosphate-buffered saline (PBS) to a final concentration of 1.5 × 105 cells in 100 μl of PBS. Animals were anesthetized with ketamine–xylazine and positioned ventral side up. MDA-MB-231 cells were inoculated into the left ventricle (day 0) by percutaneous injection, using a 27-gauge needle (100 μl per mouse), as previously described (Yin et al., 1999). To evaluate the effects of viral vectors, viruses were injected via the tail vein (2 × 108 plaque-forming units [PFU] in 0.1 ml of buffer) on day 4, followed by a second dose (1 × 108 PFU in 0.1 ml of buffer) on day 7. Osteolytic lesions were analyzed by radiography, using an LX-60 system with digital camera (Faxitron X-Ray, Lincolnshire, IL). Mice were imaged in a prone position on day 28. The osteolytic lesion area was quantified with ImageJ software (National Institutes of Health [NIH], Bethesda, MD). Mice were anesthetized and blood was collected by cardiac aspiration on day 28 before euthanasia.
Bone histology and histomorphometry
Hind limbs of mice were collected on euthanasia on day 28. The left or right hind limb of each mouse, picked randomly, was fixed in 10% neutral buffered formalin for 48 hr and decalcified in 10% EDTA for 2 weeks. After decalcification, tissues were processed and embedded in paraffin wax for sectioning. Longitudinal, midsagittal sections (thickness, 3.5 μm) from the tibia and femur were obtained. Tissue sections were stained with hematoxylin and eosin (H&E) or Goldner's trichrome stain and prepared for histomorphometric analysis (Yin et al., 2003). Images were captured with a DMLB microscope (Leica Microsystems, Wetzlar, Germany) equipped with a charge-coupled device (CCD) camera (Roper Scientific, Tucson, AZ) and analyzed with IPLab image acquisition software (BD Biosciences, San Jose, CA). Tumor burden per hind limb was calculated at the tibia and femur, using sections (magnification, × 25) stained with H&E or Goldner's trichrome stain.
Viral replication and sTGFβRIIFc expression in vivo
Animals bearing skeletal tumors as revealed by radiography were injected with viral vectors (2 × 108 PFU in 0.1 ml of buffer, day 0). Hind limbs and blood were collected on days 2 and 7. Immunohistochemical analysis was performed on decalcified paraffin-embedded bone sections for adenoviral hexon protein and sTGFβRIIFc expression. Antigen retrieval was performed with proteinase K (Dako, Carpinteria, CA). Primary antibody against adenovirus 5 hexon (Chemicon, Temecula, CA) or IgG Fcγ fragment (Jackson ImmunoResearch, West Grove, PA) was used to detect hexon and sTGFβRIIFc respectively. Immunodetection was conducted with a 3,3′-diaminobenzidine (DAB) substrate kit (Invitrogen, Carlsbad, CA) and counterstained with hematoxylin.
Measurements of blood calcium and sTGFβRIIFc levels
Serum calcium levels were measured with a QuantiChrom calcium assay kit (BioAssay Systems, Hayward, CA). Blood sTGFβRIIFc levels were determined by enzyme-linked immunosorbent assay (ELISA), using antibodies against the human IgG Fcγ fragment (Jackson ImmunoResearch) as described earlier (Hu et al., 2010).
Statistical analysis
Results are expressed as means ± SE. The two-tailed t test was performed for group comparisons, using Prism software version 5 (GraphPad Software, San Diego, CA). p < 0.05 was considered significant.
Results and Discussion
Systemic administration of Ad.sTβRFc results in replication and simultaneous production of sTGFβRIIFc in vivo
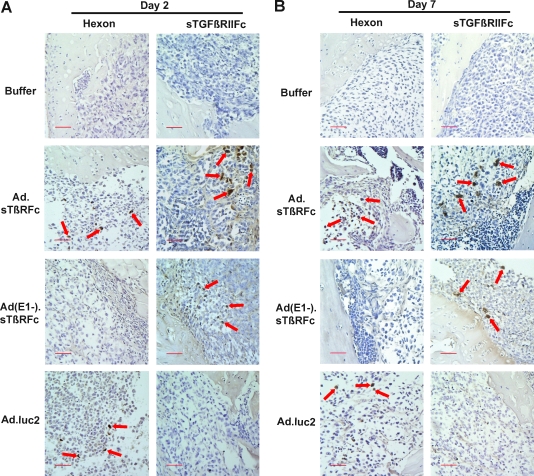
To examine Ad.sTβRFc replication and Ad.sTβRFc-mediated sTGFβRIIFc production in vivo, nude mice bearing skeletal tumors were injected with buffer, Ad.sTβRFc, Ad(E1−).sTβRFc, or Ad.luc2. After 2, 5, and 7 days of various treatments, hind limbs were harvested and bone sections were analyzed for hexon and sTGFβRIIFc expression by immunohistochemical staining. Figure 1A and B shows the results obtained from day 2 and day 7 samples, respectively. In animals that received buffer, no hexon- or sTGFβRIIFc-positive cells were detected (Fig. 1A and B). For animals that received Ad.sTβRFc, a number of hexon- and sTGFβRIIFc-positive brown tumor cells were present in the tumors. Administration of Ad(E1−).sTβRFc resulted in sTGFβRIIFc production, whereas no hexon-positive cells were detected in the tumors. Animals that received Ad.luc2 produced hexon-expressing cells, but did not produce sTGFβRIIFc (Fig. 1A and B). These results indicate that intravenous injection of Ad.sTβRFc and Ad.luc2 viruses results in virus uptake and replication in skeletal tumors; and intravenous injection of Ad.sTβRFc and Ad(E1−).sTβRFc produces sTGFβRIIFc for 7 days after vector administration.
FIG. 1.
Viral vector-mediated hexon and sTGFβRIIFc (soluble form of transforming growth factor-β receptor II fused with human immunoglobulin Fc fragment) expression in skeletal tumors. Mice with established bone metastases were injected intravenously with Ad.sTβRFc, Ad(E1−).sTβRFc, or Ad.luc2 (2 × 108 PFU in 0.1 ml), or with buffer, on day 0 (two or three mice per group per time point). Hind limb bones were sectioned and probed with anti-hexon or anti-human Fc antibodies on day 2 (A) and on day 7 (B) and photographed (original magnification, × 200). Brown cells in the tumor area indicate hexon (left) or sTGFβRIIFc expression (right). Arrows indicate tumor cells expressing hexon or sTGFβRIIFc. Scale bars: 50 μm. (C) Blood was collected on day 2 or day 7, and analyzed for sTGFβRIIFc expression by ELISA.
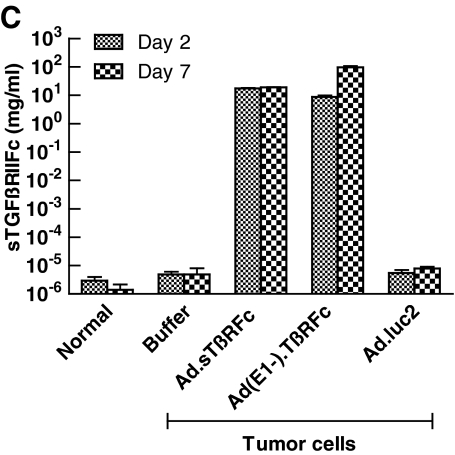
Amounts of sTGFβRIIFc present in blood samples obtained from day 2 and 7 treatments were determined by ELISA. Sera derived from day 2 treatment showed sTGFβRIIFc at 17.46 ± 0.56 and 8.76 ± 1.07 mg/ml in Ad.sTβRFc- and Ad(E1−).sTβRFc-treated groups, respectively (Fig. 1C). sTGFβRIIFc levels on day 7 were 18.95 ± 0.40 and 96.78 ± 10.04 mg/ml in sera derived from the Ad.sTβRFc and Ad(E1−).sTβRFc groups, respectively. Mice from all other groups had less than 8 ng of sTGFβRIIFc per milliliter on day 2 or 7 (Fig. 1C). The large amounts of sTGFβRIIFc in the blood in Ad.sTβRFc- and Ad(E1−).sTβRFc-treated animals is probably due to protein production from tumors as well as other organs such as liver; systemic administration of adenoviruses is known to result in viral uptake by the liver (Waddington et al., 2008).
Intravenous injection of Ad.sTβRFc into nude mice inhibits bone metastases
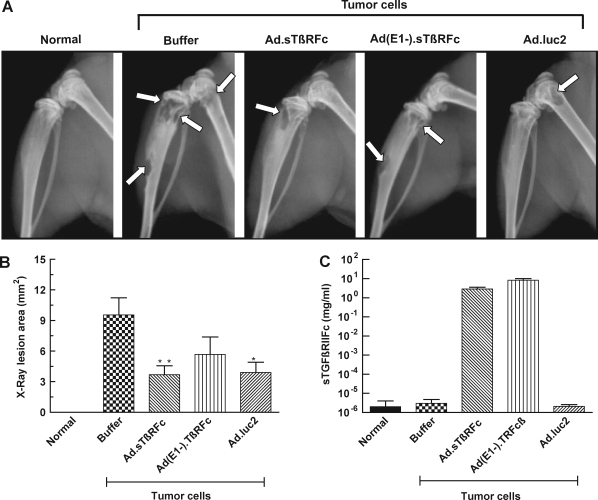
The effect of Ad.sTβRFc, Ad(E1−).sTβRFc, and Ad.luc2 in inhibiting bone metastases was examined by X-ray radiography on day 28 after cell inoculation. A normal hind leg bone, along with a typical bone bearing tumors (marked with arrows), from each of the groups is shown in Fig. 2A. Animals that received tumor cells followed by buffer showed bone with large radiolucent lytic tumor lesions. Bones from Ad.sTβRFc-, Ad(E1−).sTβRFc-, and Ad.luc2-treated groups had smaller lesions. Lesion areas in groups treated with buffer, Ad.sTβRFc, Ad(E1−).sTβRFc, and Ad.luc2 were 9.55 ± 1.67, 3.69 ± 0.88, 5.68 ± 1.70, and 3.91 ± 1.00 mm2, respectively (Fig. 2B). There was a highly significant difference in lesion size between the buffer and Ad.sTβRFc groups (p = 0.0066), and significant differences between the buffer and Ad.luc2 (p = 0.0106) groups. Although some effect of Ad(E1−).sTβRFc (p = 0.1247) was observed on tumor size, the inhibition of tumor growth was not statistically significant. In three day 28 bone samples picked randomly from each group, we were not able to detect hexon or sTGFβRIIFc in any of the vector treatment groups (data not shown), indicating that transient nature of adenoviral replication and gene expression is effective in mediating antitumor responses.
FIG. 2.
Effect of systemic administration of Ad.sTβRFc on bone metastasis. MDA-MB-231 cells were inoculated into the left cardiac ventricle on day 0. Buffer, Ad.sTβRFc, Ad(E1−).sTβRFc, or Ad.luc2 was injected via the tail vein on days 4 and 7. On day 28, radiographs and blood were obtained from nine mice in each group. (A) Representative radiographs from a normal mouse and mice injected with tumor cells followed by buffer, Ad.sTβRFc, Ad(E1−).sTβRFc, or Ad.luc2. (B) Average lesion area per mouse on day 28 is plotted (n = 9 mice in each group). p values for comparison with the buffer group are shown (**p < 0.01; *p < 0.05). (C) Blood levels of sTGFβRIIFc on day 28 for each group are shown (n = 9 mice in each group).
Blood analysis of sTGFβRIIFc by ELISA showed high levels of sTGFβRIIFc (2.89 ± 0.67 and 8.33 ± 1.67 mg/ml from Ad.sTβRFc- and Ad(E1−).sTβRFc-treated groups, respectively) (Fig. 2C). These results suggest that Ad.luc2 replication in the absence of sTGFβRIIFc production can have antitumor effects; sTGFβRIIFc production from Ad(E1−).sTβRFc in the absence of viral replication can have some, but not significant, antitumor effects. However, simultaneous sTGFβRIIFc production and viral replication by Ad.sTβRFc indicates that it is the most effective vector in inhibiting tumor growth.
Intravenous injection of Ad.sTβRFc into nude mice results in a reduction of tumor burden and inhibition of osteolytic bone destruction
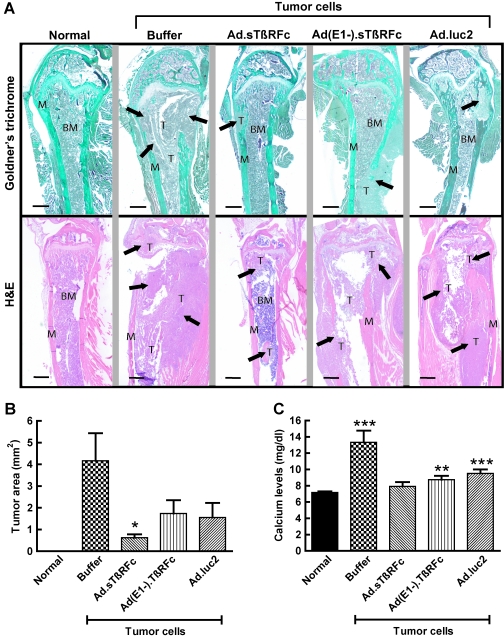
The effect of Ad.sTβRFc on tumor burden and osteolytic bone destruction was examined by Goldner's trichrome or H&E staining of bone sections. Histology showed that normal femur and tibia had a distinct morphology with bone matrix and bone marrow (Fig. 3A). Femur and tibia from animals that had received tumor cells and buffer had eroded bone matrix and lost bone integrity; and tumor cells had replaced the bone marrow and invaded the adjacent tissue by day 28 after tumor cell inoculation (Fig. 3A), confirming the osteolytic bone destruction in this tumor model. However, the animals that received tumor cells and Ad.sTβRFc, Ad(E1−).sTβRFc, or Ad.luc2 had weaker tumor growth and relatively normal bone morphologies (Fig. 3A). Tumor burdens at the bone metastatic sites were evaluated by examining stained bone slices from the various experimental groups. Tumor sizes in the groups treated with buffer, Ad.sTβRFc, Ad(E1−).sTβRFc, and Ad.luc2 were 4.17 ± 1.27, 0.63 ± 0.16, 1.74 ± 0.61, and 1.56 ± 0.67 mm2, respectively (Fig. 3B). These results indicate a significant reduction of tumor burden by Ad.sTβRFc (p = 0.0138), and some reduction by Ad(E1−).sTβRFc (p = 0.1046) and Ad.luc2 (p =0.0881).
FIG. 3.
Effect of systemic administration of Ad.sTβRFc on tumor burden and tumor cell-induced bone destruction. (A) Normal bone of mice that received tumor cells (day 0) followed by buffer, Ad.sTβRFc, Ad(E1−).sTβRFc, or Ad.luc2 was collected on day 28. Longitudinal, midsagittal sections (thickness, 3.5 μm) from the tibia and femur were obtained and processed for Goldner's trichrome staining or hematoxylin and eosin (H&E) staining. Representative photographs of femur (Goldner's trichrome staining) or tibia (H&E staining) from each group are shown (original magnification, × 25). In the Goldner's trichrome sections, bone matrix (M) stains green, bone marrow (BM) stains dark purple, and tumor cells (T) stain purple–gray. Arrows are directed toward tumors present in the various bone samples shown. Scale bars: 500 μm. (B) Tumor burden in hind limbs was measured by quantitative histomorphometry (original magnification, ×25); data were obtained from longitudinal, midsagittal sections from nine bone samples of each experimental group. Three randomly selected longitudinal sections were examined for each of the bone samples. p values for comparison with the buffer treatment group are shown in (B) (*p < 0.05). (C) Serum calcium levels were measured (n = 9 mice in each group). p values for comparison with the normal mouse group are shown in (C) (***p < 0.001; **p < 001).
Given that hypercalcemia is an indicator of osteolytic bone destruction, the effect of Ad.sTβRFc on free calcium levels in the blood was examined. Basal calcium levels in normal sera were 7.18 ± 0.13 mg/dl. Mice that received tumor cells followed by buffer had significantly higher calcium levels: 13.33 ± 1.43 mg/dl (p = 0.0006). In Ad.sTβRFc-treated animals, calcium levels were reduced to 7.91 ± 0.54 mg/dl (p = 0.0028, buffer group vs. Ad.sTβRFc group). There was also a significant reduction in calcium levels in the Ad(E1−).sTβRFc group [8.74 ± 0.49 mg/dl, p = 0.0079, Ad(E1−).sTβRFc group vs. buffer group] and the Ad.luc2 group (9.51 ± 0.49 mg/dl, p = 0.0227, Ad.luc2 group vs. buffer group) (Fig. 3C). Interestingly, whereas in the animals that received Ad.sTβRFc calcium levels were similar to those observed in normal mice (p = 0.2099), in mice that received Ad(E1−).sTβRFc or Ad.luc2 calcium levels were significantly higher than normal (p = 0.0074 and 0.0003, respectively) (Fig. 3C). These results indicate that systemic administration of Ad.sTβRFc, Ad(E1−).sTβRFc, and Ad.luc2 inhibited bone resorption, resulting in the inhibition of calcium release; a combination of viral replication and sTGFβRIIFc production from Ad.sTβRFc was much more effective in mediating the inhibitory effects on bone metastases and reducing free calcium levels.
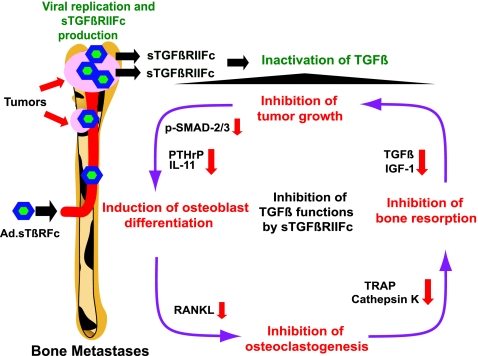
In conclusion, the work presented here shows that systemic administration of Ad.sTβRFc can inhibit bone metastases and osteolytic bone destruction in a breast cancer model. On the basis of the results presented here, we envision the following model of Ad.sTβRFc action on bone metastases (Fig. 4). The systemic delivery of Ad.sTβRFc will result in the uptake of the virus and its replication in tumor cells at the bone site. Ad.sTβRFc-mediated production of sTGFβRIIFc will target TGF-β, inhibiting TGF-β signaling in the various cellular components involved in the “vicious cycle” at the tumor–bone environment (Yin et al., 1999; Iyer et al., 2005; Kingsley et al., 2007; Akhtari et al., 2008; Korpal et al., 2009; Serganova et al., 2009). Although Ad(E1−).sTβRFc (a replication-deficient virus expressing sTGFβRIIFc) and Ad.luc2 (a replicating virus expressing a marker gene) can individually provide some antitumor responses and inhibit osteolysis, simultaneous viral replication and sTGFβRIIFc production is most effective in inhibiting tumor growth and osteolytic bone destruction (Fig. 4). Certainly, further work is needed to examine each of the proposed steps in detail; it is quite intriguing that systemic delivery of an oncolytic adenovirus can be developed as a novel approach to control breast cancer bone metastases.
FIG. 4.
Schematic representation of Ad.sTβRFc-mediated inhibition of bone metastases and osteolytic bone destruction. See text for details. IGF-1, insulin-like growth factor-1; IL-11, interleukin-11; PTHrP, parathyroid hormone-related protein; RANKL, receptor activator of nuclear factor-κB ligand; sTGFβRIIFc, soluble form of transforming growth factor-β receptor II fused with human immunoglobulin Fc fragment; TGF-β, transforming growth factor-β; TRAP, tartrate-resistant acid phosphatase.
Acknowledgments
The authors thank Dr. Janardan Khandekar for support, and Dr. Tamas Jilling for help in acquiring microscopy images. This work was supported by NIH grants R01CA69158 (T.G.) and R01CA127380 (P.S.).
Author Disclosure Statement
No competing financial interests exist for any of the authors.
References
- Akhtari M. Mansuri J. Newman K.A. Guise T.M. Seth P. Biology of breast cancer bone metastasis. Cancer Biol. Ther. 2008;7:3–9. doi: 10.4161/cbt.7.1.5163. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. Cancer Facts & Figures. 2010. http://www.cancer.org/docroot/STT/stt_0.asp. [Aug;2010 ]. http://www.cancer.org/docroot/STT/stt_0.asp
- Crompton A.M. Kirn D.H. From ONYX-015 to armed vaccinia viruses: The education and evolution of oncolytic virus development. Curr. Cancer Drug Targets. 2007;7:133–139. doi: 10.2174/156800907780058862. [DOI] [PubMed] [Google Scholar]
- Howe J.A. Demers G.W. Johnson D.E. Neugebauer S.E. Perry S.T. Vaillancourt M.T. Faha B. Evaluation of E1-mutant adenoviruses as conditionally replicating agents for cancer therapy. Mol. Ther. 2000;2:485–495. doi: 10.1006/mthe.2000.0206. [DOI] [PubMed] [Google Scholar]
- Hu Z. Robbins J.S. Pister A. Zafar M.B. Zhang Z.W. Gupta J. Lee K.J. Neuman K. Yun C.O. Guise T. Seth P. A modified hTERT promoter-directed oncolytic adenovirus replication with concurrent inhibition of TGFβ signaling for breast cancer therapy. Cancer Gene Ther. 2010;17:235–243. doi: 10.1038/cgt.2009.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer I. Wang Z.-G. Akhtari M. Zhao W. Seth P. Targeting TGF β signaling for cancer therapy. Cancer Biol. Ther. 2005;4:e33–e38. doi: 10.4161/cbt.4.3.1566. [DOI] [PubMed] [Google Scholar]
- Kang Y. He W. Tulley S. Gupta G.P. Serganova I. Chen C.R. Manova-Todorova K. Blasberg R. Gerald W.L. Massague J. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc. Natl. Acad. Sci. U.S.A. 2005;102:13909–13914. doi: 10.1073/pnas.0506517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayose D. Gudas J. Nguyen H. Srivastava S. Cowan K.H. Seth P. Cytotoxic effects of adenovirus-mediated wild-type p53 protein expression in normal and tumor mammary epithelial cells. Clin. Cancer Res. 1995;1:889–897. [PubMed] [Google Scholar]
- Kingsley L.A. Fournier P.G. Chirgwin J.M. Guise T.A. Molecular biology of bone metastasis. Mol. Cancer Ther. 2007;6:2609–2617. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- Korpal M. Yan J. Lu X. Xu S. Lerit D.A. Kang Y. Imaging transforming growth factor-β signaling dynamics and therapeutic response in breast cancer bone metastasis. Nat. Med. 2009;15:960–966. doi: 10.1038/nm.1943. [DOI] [PubMed] [Google Scholar]
- Lacroix M. Toillon R.A. Leclercq G. p53 and breast cancer, an update. Endocr. Relat. Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- McCormick F. Future prospects for oncolytic therapy. Oncogene. 2005;24:7817–7819. doi: 10.1038/sj.onc.1209064. [DOI] [PubMed] [Google Scholar]
- Padua D. Massague J. Roles of TGFβ in metastasis. Cell Res. 2009;19:89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- Serganova I. Moroz E. Vider J. Gogiberidze G. Moroz M. Pillarsetty N. Doubrovin M. Minn A. Thaler H.T. Massague J. Gelovani J. Blasberg R. Multimodality imaging of TGFβ signaling in breast cancer metastases. FASEB J. 2009;23:2662–2672. doi: 10.1096/fj.08-126920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P., editor. Adenoviruses: Basic Biology to Gene Therapy. R.G. Landes; Austin, TX: 1999. pp. 1–314. [Google Scholar]
- Seth P. Brinkmann U. Schwartz G.N. Katayose D. Gress R. Pastan I. Cowan K. Adenovirus-mediated gene transfer to human breast tumor cells: An approach for cancer gene therapy and bone marrow purging. Cancer Res. 1996;56:1346–1351. [PubMed] [Google Scholar]
- Seth P. Wang Z.G. Pister A. Zafar M.B. Kim S. Guise T. Wakefield L. Development of oncolytic adenovirus armed with a fusion of soluble transforming growth factor-β receptor II and human immunoglobulin Fc for breast cancer therapy. Hum. Gene Ther. 2006;17:1152–1160. doi: 10.1089/hum.2006.17.1152. [DOI] [PubMed] [Google Scholar]
- Shipitsin M. Campbell L.L. Argani P. Weremowicz S. Bloushtain-Qimron N. Yao J. Nikolskaya T. Serebryiskaya T. Beroukhim R. Hu M. Halushka M.K. Sukumar S. Parker L.M. Anderson K.S. Harris L.N. Garber J.E. Richardson A.L. Schnitt S.J. Nikolsky Y. Gelman R.S. Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Steeg P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- Waddington S.N. McVey J.H. Bhella D. Parker A.L. Barker K. Atoda H. Pink R. Buckley S.M. Greig J.A. Denby L. Custers J. Morita T. Francischetti I.M. Monteiro R.Q. Barouch D.H. Van Rooijen N. Napoli C. Havenga M.J. Nicklin S.A. Baker A.H. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Wang Z.G. Zhao W. Ramachandra M. Seth P. An oncolytic adenovirus expressing soluble transforming growth factor-β type II receptor for targeting breast cancer: In vitro evaluation. Mol. Cancer Ther. 2006;5:367–373. doi: 10.1158/1535-7163.MCT-05-0125. [DOI] [PubMed] [Google Scholar]
- Yang Y.A. Dukhanina O. Tang B. Mamura M. Letterio J.J. Macgregor J. Patel S.C. Khozin S. Liu Z.Y. Green J. Anver M.R. Merlino G. Wakefield L.M. Lifetime exposure to a soluble TGF-β antagonist protects mice against metastasis without adverse side effects. J. Clin. Invest. 2002;109:1607–1615. doi: 10.1172/JCI15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.J. Selander K. Chirgwin J.M. Dallas M. Grubbs B.G. Wieser R. Massague J. Mundy G.R. Guise T.A. TGF-β signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J. Clin. Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J.J. Mohammad K.S. Kakonen S.M. Harris S. Wu-Wong J.R. Wessale J.L. Padley R.J. Garrett I.R. Chirgwin J.M. Guise T.A. A causal role for endothelin-1 in the pathogenesis of osteoblastic bone metastases. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10954–10959. doi: 10.1073/pnas.1830978100. [DOI] [PMC free article] [PubMed] [Google Scholar]