Abstract
Remodeling of the fibronectin matrix occurs during a variety of pathological and regenerative processes. Cellular generated tensional forces can alter the secondary and tertiary structure of the fibronectin matrix and regulate the exposure of cryptic activities that directly impact cell behavior. In the present study, we evaluated the effect of the partially unfolded Type III fibronectin module, FnIII-1c, on gene expression in dermal fibroblasts. Microarray and PCR analysis indicated that the addition of FnIII-1c to human dermal fibroblasts induced the expression of several inflammatory genes including the cytokines, IL-8 and TNF-α. ELISA analysis indicated that the increased gene expression was accompanied by the secretion of IL-8 and TNF-α protein. FnIII-1c-induced gene expression was preceded by increased phosphorylation of IκB kinase (IKK) and IκBα as well as the nuclear translocation of NFκB. PCR and ELISA analysis showed that inhibition of the NFκB signaling pathway completely blocked the induction of IL-8 and TNF-α. Blocking antibodies to Toll-like receptor 4 inhibited both the activation of the NFκB signaling pathway as well as cytokine expression in response to FnIII-1c. These data suggest that fibronectin matrix remodeling can induce the expression of cytokines by stromal cells present in the tissue microenvironment.
Keywords: Cytokine Induction, Fibronectin, Inflammation, NF-kappa B, Toll-like Receptors (TLR)
Introduction
Fibronectin is found in the extracellular matrix of most tissues where it functions to support basic cellular processes including cell adhesion, migration, survival, and growth. Fibronectin is a modular protein consisting of independently folded domains, Types I, II, and III, which represent regions of amino acid sequence homology (1). Polymerized fibronectin within the extracellular matrix is subjected to tensional forces generated by the cells that expose cryptic activities within the molecule, including motogenic activity (2), and binding sites for integrins (3–5) and growth factors (6).
Recent conformational studies using intramolecular FRET have indicated that unfolding of fibronectin Type III (FnIII)2 modules within the extracellular matrix occurs in response to cell-generated contractile forces and that the modules refold when contractility is inhibited (7). Unfolding and refolding of FnIII modules in response to cellular forces suggest that cells have the ability to inactivate molecular recognition sites present within the fibronectin matrix while exposing new sites buried within the secondary structure. Very little is known about the cryptic activities buried within the secondary structure of FnIII modules. The best studied FnIII module with respect to cryptic activities is the first Type III module, FnIII-1. Unfolding of FnIII-1 has been implicated in the assembly of soluble fibronectin into tissue matrix (8–10), in the regulation of angiogenesis (11–14), and in the control of local vasodilation within skeletal muscle (15). These studies all point to a role for FnIII-1 unfolding in the regulation of the biologic properties of matrix fibronectin.
Steered molecular dynamics has shown that a peptide representing the C-terminal two-thirds of the FnIII-1 module, FnIII-1c, recapitulates a stable intermediate structure that is predicted to occur during the unfolding of FnIII-1 (16). We show that when added to dermal fibroblast cells, FnIII-1c causes an immediate increase in the expression of several inflammatory genes, including IL-8 and TNF-α, indicating that cryptic activity within the FnIII-1 module modulates the tissue inflammatory response. This study provides evidence for a previously unrecognized link between fibronectin matrix remodeling and the regulation of the innate immune system.
EXPERIMENTAL PROCEDURES
Gene Profiling
Human dermal fibroblasts were cultured in DMEM (Invitrogen) containing 10% fetal bovine serum (HyClone, Logan, UT) and treated with fibronectin modules as described previously (17). Total RNA was isolated from cells using the RT2 qPCR-Grade RNA isolation kit. The MyiQ cycler system (Bio-Rad Laboratories) was used for real-time PCR detection. The RT2 First Strand kit was used to convert 1 μg of RNA into first strand cDNA according to the manufacturer's protocol (SABiosciences, Frederick, MD) and applied to the PCR array (the Human Cancer PathwayFinderTM RT2 ProfilerTM). The data were analyzed by using the Excel-based PCR array data analysis templates provided by the manufacturer. The RT2 qPCR Primer Assays for IL-8 and TNF-α were from Qiagen (Valencia, CA), and qPCRs were performed according to the manufacturer's instructions. IL-8 and TNF-α were measured using the BD OptEIATM set using human IL-8 or TNF-α according to the manufacturer's instructions (BD Biosciences). Triplicates were set for each condition.
Extraction and Immunoblotting
For preparation of nuclear extracts, cells were washed three times with ice-cold PBS and scraped on ice in 200 μl of lysis buffer (10 mm HEPES, 5 mm KCl, 1.5 mm MgCl2, 1 mm NaF, 1 mm Na3VO4, and 0.1% Nonidet P-40, pH 7.9). Cell lysates were passed through a 24-gauge needle six times and centrifuged at 4 °C at 3000 × g for 5 min. The supernatant was centrifuged at 21,000 × g for 15 min at 4 °C, and the supernatant was designated as the cytosolic fraction. The nuclear pellet was gently washed with lysis buffer, and nuclear proteins were extracted by resuspending the pellet in 50 μl of nuclear extraction buffer (20 mm HEPES, 400 mm NaCl, 1.5 mm MgCl2, 1 mm NaF, 1 mm Na3VO4, and 20% glycerol, pH 7.9). Resuspended nuclear pellets were centrifuged at 4 °C at 20,000 × g for 15 min, and the supernatant was collected as nuclear extract. Preparation of whole cell lysate and immunoblot analyses were performed as described previously (17). All lysate buffers contained one tablet of Complete protease inhibitor per 10 ml (Roche Diagnostics). Rabbit monoclonal antibodies against NFκB, phospho-IκBα, and phospho-IKKα/β were used at 1:1000 (Cell Signaling Technology, Beverly, MA). Rabbit polyclonal antibodies against IκBα, lamin A/C, and FAK (Santa Cruz Biotechnology, Santa Cruz, CA) were used at 1:1000. Goat anti-rabbit or goat anti-mouse HRP (Bio-Rad Laboratories) was used at 1:10,000. Rabbit polyclonal antibody against β-actin (Sigma-Aldrich) was used at 1:2000. The inhibitors of NFκB signaling, PS-1145 (Sigma-Aldrich) and BAY 11-7082 (Calbiochem), were dissolved in dimethyl sulfoxide (DMSO) and used as described in the legend for Fig. 3. The blocking antibodies to human TLR4 and TLR2 were obtained from R&D Systems (Minneapolis, MN).
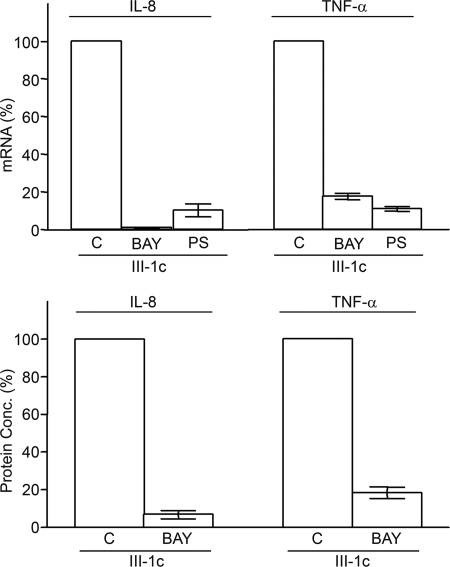
FIGURE 3.
Induction of IL-8 and TNF-α by FnIII-1c is dependent on NFκB. Monolayers of human dermal fibroblasts were serum-starved overnight and then pretreated with 10 μm BAY11-7082 (BAY) or 10 μm PS-1145 (PS) for 2 h prior to the addition of 10 μm FnIII-1c in DMEM. Changes in the amount of mRNA (upper) or protein (lower) for either IL-8 or TNFα in response to FnIII-1c were monitored at 2 h by RT-PCR or at 4 h by ELISA. The mRNA level in response to FnIII-1c in the absence of inhibitor (control, C) was analyzed relative to the housekeeping gene, β-actin, and the value was set at 100%. The effect of inhibitors on IL-8 and TNF-α protein secretion was expressed relative to protein secretion following FnIII-1c treatment (set as 100%). Bars indicate S.E. of the mean for triplicate samples. Protein Conc., protein concentration.
Preparation of the Recombinant Fibronectin Modules
All recombinant His-tagged fibronectin modules, III-1c, III-13, and III-10n, were prepared and purified as described previously (13, 17, 18). Briefly, PCR products were obtained by amplification of human fibronectin cDNA and cloned into bacterial expression vectors pQE-70 (III-1c and III-13) and pQE-30 (III-10n) in-frame with a bacterial His6 tag (Qiagen, Inc., Valencia, CA). Recombinant proteins were assayed for the presence of endotoxin using the limulus amebocyte lysate assay, QCL-1000 (Lonza, Walkersville, MD). Levels of contaminating endotoxin were similar for all preparations of recombinant proteins. All recombinant FnIII modules contained a similar level of endotoxin, ∼0.25 endotoxin units/nmol of protein.
RESULTS
FnIII-1c Induces Expression of Inflammatory Genes
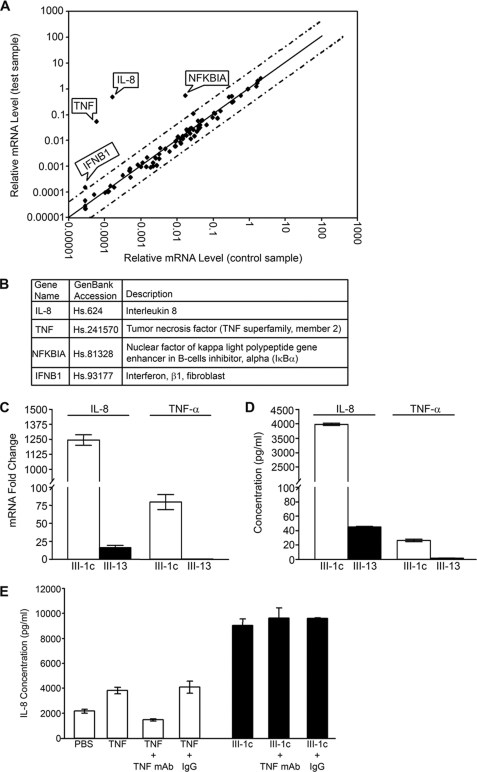
When added to monolayers of human dermal fibroblasts, FnIII-1c altered the expression of several genes as detected on the microarray (Fig. 1A). Four genes were identified that differed from basal expression by more than 3-fold. These were key genes associated with the inflammatory response, IL-8, TNF-α, inhibitor of NFκB (IκBa), and interferon β1 (INFβ1) (Fig. 1B). Based on their strong response to FnIII-1c, the genes for IL-8 and TNF-α were chosen for further analysis. As shown in Fig. 1C, the increased expression of IL-8 and TNF-α in response to FnIII-1c was confirmed by real-time PCR. When normalized against the housekeeping gene, β-actin, expression levels of IL-8 were increased greater than 1000-fold, whereas TNF-α mRNA increased 80-fold. Expression of mRNA for IL-8 and TNF-α was largely unaffected by the control fibronectin module, FnIII-13. Four hours after the addition of FnIII-1c, IL-8 and TNF-α proteins were detected in the conditioned medium by ELISA at nm and pm concentrations, respectively (Fig. 1D). The addition of the FnIII-13 module, which served as a negative control, had little effect on either IL-8 or TNF-α secretion. PBS treatment gave similar results to those observed with FnIII-13 (data not shown), indicating that IL-8 and TNF-α expression remained at baseline when cells were treated with III-13. As TNF-α is known to be a potent inducer of IL-8 expression, we asked whether the induction of IL-8 by FnIII-1c might be secondary to the FnIII-1c-dependent induction of TNF-α. The experiment was therefore repeated in the presence of neutralizing antibody to TNF-α. As shown in Fig. 1E, neutralizing antibody to TNF-α could completely inhibit IL-8 secretion in response to exogenously added TNF-α but had no effect on the secretion of IL-8 in response to FnIII-1c. The data in Fig. 1 suggest that FnIII-1c directly induces expression of both IL-8 and TNF-α in human dermal fibroblasts.
FIGURE 1.
FnIII-1c induces expression of inflammatory genes in human dermal fibroblasts. A, monolayers of human dermal fibroblasts were serum-starved overnight before treatment with 20 μm FnIII-1c or PBS in 0.1% BSA/DMEM for 2 h. Expression profile of genes was performed using the Human Cancer PathwayFinders PCR array. Dotted lines indicate a 3-fold change in baseline. B, four inflammatory genes, as designated by flags in panel A, were up-regulated greater than 3-fold in response to FnIII-1c and listed in the table. C, fibroblasts were treated with 20 μm FnIII-1c or the control module FnIII-13 in 0.1% BSA/DMEM after 2 h. The cells were lysed, and mRNA for IL-8 and TNF-α was measured by RT-qPCR. D, conditioned medium from cells treated with either FnIII-1c or FnIII-13 was collected after 4 h, and IL-8 and TNF-α were measured by ELISA. E, fibroblasts were treated with TNF-α (0.5 ng/ml) or FNIII-1c (20 μm) in the presence of 2 μg/ml TNF-neutralizing antibody or control IgG for 4 h. Conditioned medium was collected, and IL-8 was measured by ELISA. Error bars indicate S.E. for triplicate samples.
The Induction of Inflammatory Genes by FnIII-1c Is Dependent on NFκB
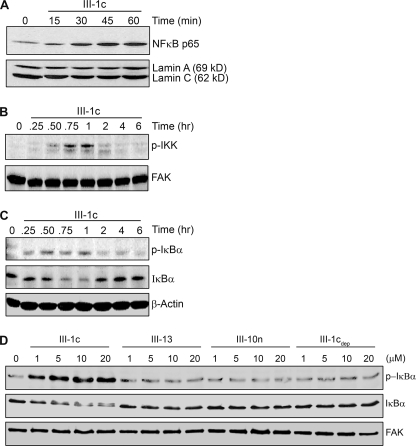
Induction of inflammatory gene expression is often regulated by the NFκB family of transcription factors. Activation of NFκB is characterized by the translocation of the NFκB complex to the nucleus. Such activation of NFκB by FnIII-1c was demonstrated by Western blotting of nuclear extracts from FnIII-1c-treated cells. Fig. 2A shows the accumulation of the p65/rel A subunit of the NFκB transcription complex in the nucleus. Nuclear NFκB was detected within 15–30 min of the addition of FnIII-1c with peak amounts seen within an hour. Blots were also probed for the presence of nuclear lamins to verify equal loading of nuclear lysates. Nuclear translocation of NFκB was not seen in control cells treated with either PBS or FnIII-13 (data not shown). These data indicate that the addition of FnIII-1c to human dermal fibroblasts results in the rapid activation of the NFκB transcription complex. Similar results were observed using mouse embryo fibroblasts null for fibronectin, indicating that activation of NFκB by FnIII-1c did not depend on fibronectin (data not shown).
FIGURE 2.
FnIII-1c activates the NFκB signaling pathway in human dermal fibroblasts. A, monolayers of human fibroblasts were serum-starved overnight and then treated with the designated amounts of FnIII-1c, FnIII-13, or FnIII-10n in 0.1% BSA/DMEM either for 1 h or for the designated time. Control cells (0) received PBS. The nuclear fraction was isolated and analyzed by Western blot for the presence of the NFκB protein p65/rel A. The membranes were then stripped and reprobed with an antibody against nuclear lamin A/C as loading control. B–D, the cytosolic fraction (B and C) or the total cell lysate (D) was electrophoresed and immunoblotted using antibodies against phosphorylated IKK (p-IKK) (B), phosphorylated IκBα (p-IκBα), or total IκBα (IκBα) (C and D). The membranes were then stripped and reprobed with antibodies against FAK or β-actin as loading control. Blots shown are representative of one experiment performed on three separate occasions.
In the absence of stimulation, NFκB is localized to the cytoplasm in complex with its inhibitor, IκB. The canonical pathway controlling the activation of NFκB requires the IKK-dependent phosphorylation and degradation of IκB. To determine the upstream activators of FnIII-1c-mediated nuclear translocation of NFκB, lysates from FnIII-1c-treated cells were analyzed by Western blot for the activation of IKK and IκBα. Phosphorylated IKK and IκBα were detected in cell lysates within 15–30 min of treatment with FnIII-1c (Fig. 2, B and C). Immunoblotting for total IκBα revealed a loss of IκBα protein at the 45-min and 1-h time points, consistent with its degradation. IκBα protein returned to basal levels between 2 and 4 h. This increase in IκBα protein is consistent with the microarray data showing that the IκBα gene is induced by FnIII-1c treatment within 2 h (Fig. 1A). Dose-response experiments showed that phosphorylation of the NFκB inhibitor, IκBα, was seen in response to 1 μm FnIII-1c, with maximal stimulation seen at 5 μm (Fig. 2D). The increase in phosphorylation of IκBα was accompanied by a loss of IκBα protein, consistent with its degradation. The control Fn Type III modules, FnIII-13 and FnIII-10n, had no effect on IκBα phosphorylation or degradation. Buffer, which had been previously depleted of FnIII-1c (20 μm) by nickel affinity, also had no effect on phosphorylation of IκBα, indicating that the activation of NFκB signaling by FnIII-1c was not due to contaminants in the preparation. FnIII-10n represents a predicted unfolded intermediate of the III-10 module (19) and suggests that the activation of NFκB signaling is not an activity common to all unfolded FnIII modules.
To determine whether the activation of NFκB was required for the FnIII-1c-induced expression of IL-8 or TNF-α, two inhibitors of NFκB signaling, BAY 11-8072 and PS-1145, were compared for their effects on FnIII-1c-mediated gene expression. As shown in Fig. 3, pretreatment of cells with BAY 11-8072 nearly completely inhibited the expression of IL-8 and TNF-α mRNA (upper) and protein (lower) in response to FnIII-1c treatment. Another inhibitor of NFκB signaling, PS-1145, was as effective as BAY 11-8072 in preventing expression of IL-8 and TNF-α mRNA. These data indicate that activation of NFκB is required for the induction of IL-8 and TNF-α gene expression by FnIII-1c.
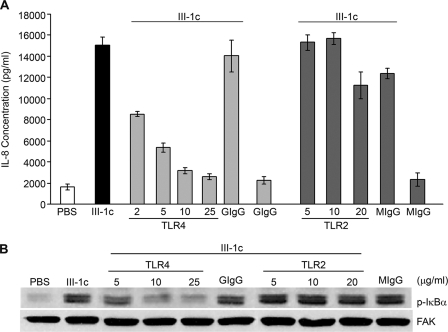
Toll-like receptors (TLRs) represent a family of transmembrane receptors that are involved in the regulation of the innate immune system. TLRs are found on a variety of cell types, and TLR2 and TLR4 have been reported to activate the NFκB-dependent expression of inflammatory cytokines. To determine whether either of these receptors was involved in the FnIII-1c-dependent activation of NFκB signaling, cells were pretreated with blocking antibodies to either TLR2 or TLR4. As shown in Fig. 4, preincubation of cells with blocking antibody to TLR4 completely inhibited the expression of IL-8 (A) and the phosphorylation of IκBα (B) in response to 10 μm FnIII-1c. Dose-response experiments indicated that statistically significant increases in IL-8 expression were seen at 600 nm FnIII-1c, whereas maximal effects were seen at 5–10 μm (data not shown). Inhibition of protein expression and NFκB signaling by anti-TLR4 antibody was dose-dependent, and maximal inhibition was seen between 5 and 10 μg/ml IgG. Blocking antibody to TLR2 had no effect on either IL-8 expression or IκB phosphorylation in response to FnIII-1c. Control immunoglobulin had no effect on FnIII-1c-induced IL-8 expression or IκB phosphorylation. The data indicate a role for TLR4 in induction of cytokine expression by FnIII-1c.
FIGURE 4.
Induction of NFκB signaling and expression of IL-8 by FnIII-1c is dependent on TLR4. Human dermal fibroblasts were serum-starved overnight and pretreated with increasing concentrations of antibodies to human TLR4, TLR2, or 20 μg/ml control mouse IgG2B (MIgG) or 25 μg/ml control goat IgG (GIgG) for 30 min prior to the addition of 10 μm FnIII-1c. A, after a 4-h treatment with FnIII-1c, IL-8 present in the conditioned medium was analyzed by ELISA. Bars indicate S.E. of the mean for triplicate samples. B, after a 45-min treatment with FnIII-1c, cell lysates were analyzed for activation of NFκB signaling by Western blotting for phosphorylated IκBα. FAK served as loading control. p-IκBα, phosphorylated IκBα.
DISCUSSION
Chronic inflammation is associated with and a major contributor to the progression of a number of diseases including organ fibrosis and cancer (20). A common feature of these pathologies is a change in tissue mechanics resulting from tissue stiffening and loss of compliance (21–23). Recent data have now shown that increased tissue rigidity is associated with the loss of fibronectin secondary structure due to unfolding of Fn Type III modules (24, 25). Our data show that the addition of the partially unfolded intermediate of FnIII-1, FnIII-1c, to human dermal fibroblasts results in the NFκB-dependent induction of several inflammatory genes, particularly the cytokines IL-8 and TNF-α. The present studies point to the unfolded FnIII domains and their associated signaling pathways as potential targets for therapies directed at controlling chronic inflammation.
Expression of cytokine genes in response to FnIII-1c occurs subsequent to the TLR4-dependent activation of NFκB, suggesting that unfolded FnIII-1 may be a ligand for TLR4-containing receptor complexes. Previous studies have implicated TLR4, which is best known as the receptor for bacterial lipopolysaccharide (LPS), in mediating signaling from several endogenous matrix-derived molecules including hyaluronic acid (26), tenascin (27), and the extra domain A (EDA) module of fibronectin (28). However, whether these endogenous molecules are true TLR4 ligands has been controversial. It has been proposed that rather than serve as direct ligands for TLRs, matrix-derived molecules generated in response to tissue damage may function as pathogen binding molecules that facilitate the presentation of exogenous pathogens such as LPS to the cell (29). However, recent studies have provided evidence that distinct TLR4 receptor complexes may be assembled in response to different ligands (27, 30). Similarly, we have found that although FnIII-1c is a potent inducer of nm levels of IL-8, we were unable to induce IL-8 expression in dermal fibroblasts with LPS.3 The ability of FnIII-1c to activate NFκB through TLR4 on dermal fibroblasts suggests a new role for TLR4 as a receptor for force-responsive elements within the fibronectin matrix.
It is thought that chronic inflammation occurs through an intrinsic pathway secondary to genetic mutations in pro-inflammatory signaling circuits or through an extrinsic pathway secondary to pathogen-mediated infections (31). Our data suggest an alternative mechanism for the activation of chronic inflammation that would occur secondarily to changes in the mechanical properties of tissues. Such a mechanism may have important implications for therapies designed to treat disorders where tissue stiffening accompanies disease progression including tumorigenesis, chronic obstructive pulmonary disease, and liver cirrhosis. These diseases are all associated with and exacerbated by increased inflammatory responses. Our studies suggest that changes in the secondary structure of the fibronectin matrix promotes the activation of the innate immune system and may be part of a feed-forward mechanism leading to chronic inflammation.
This study was supported by, in whole or in part, by National Institutes of Health Grant CA-69612 (to P. J. M.-L.).
R. You, M. Zheng, and P. J. McKeown-Longo, unpublished observations.
- FnIII
- fibronectin Type III
- IKK
- IκB kinase
- FAK
- focal adhesion kinase
- TLR
- Toll-like receptor
- qPCR
- quantitative PCR.
REFERENCES
- 1.Meighan C. M., Schwarzbauer J. E. (2008) Curr. Opin. Cell Biol. 20, 520–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vakonakis I., Staunton D., Ellis I. R., Sarkies P., Flanagan A., Schor A. M., Schor S. L., Campbell I. D. (2009) J. Biol. Chem. 284, 15668–15675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mao Y., Schwarzbauer J. E. (2005) J. Cell Sci. 118, 4427–4436 [DOI] [PubMed] [Google Scholar]
- 4.Shinde A. V., Bystroff C., Wang C., Vogelezang M. G., Vincent P. A., Hynes R. O., Van De Water L. (2008) J. Biol. Chem. 283, 2858–2870 [DOI] [PubMed] [Google Scholar]
- 5.Miura S., Kamiya S., Saito Y., Wada S., Hayashi R., Taira J., Kodama H., Yajima H., Ueki M., Fukai F. (2007) Biol. Pharm. Bull. 30, 891–897 [DOI] [PubMed] [Google Scholar]
- 6.Mitsi M., Forsten-Williams K., Gopalakrishnan M., Nugent M. A. (2008) J. Biol. Chem. 283, 34796–34807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith M. L., Gourdon D., Little W. C., Kubow K. E., Eguiluz R. A., Luna-Morris S., Vogel V. (2007) PLoS Biol. 5, e268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hocking D. C., Sottile J., McKeown-Longo P. J. (1994) J. Biol. Chem. 269, 19183–19187 [PubMed] [Google Scholar]
- 9.Morla A., Zhang Z., Ruoslahti E. (1994) Nature 367, 193–196 [DOI] [PubMed] [Google Scholar]
- 10.Zhong C., Chrzanowska-Wodnicka M., Brown J., Shaub A., Belkin A. M., Burridge K. (1998) J. Cell Biol. 141, 539–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasqualini R., Bourdoulous S., Koivunen E., Woods V. L., Jr., Ruoslahti E. (1996) Nat. Med. 2, 1197–1203 [DOI] [PubMed] [Google Scholar]
- 12.Yi M., Sakai T., Fassler R., Ruoslahti E. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 11435–11438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambesi A., Klein R. M., Pumiglia K. M., McKeown-Longo P. J. (2005) Cancer Res. 65, 148–156 [PubMed] [Google Scholar]
- 14.Ambesi A., McKeown-Longo P. J. (2009) Mol. Cancer Res. 7, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hocking D. C., Titus P. A., Sumagin R., Sarelius I. H. (2008) Circ. Res. 102, 372–379 [DOI] [PubMed] [Google Scholar]
- 16.Gao M., Craig D., Lequin O., Campbell I. D., Vogel V., Schulten K. (2003) Proc. Natl. Acad. Sci. 100, 14784–14789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.You R., Klein R. M., Zheng M., McKeown-Longo P. J. (2009) Matrix Biol. 28, 101–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein R. M., Zheng M., Ambesi A., Van De Water L., McKeown-Longo P. J. (2003) J. Cell Sci. 116, 4663–4674 [DOI] [PubMed] [Google Scholar]
- 19.Gee E. P., Ingber D. E., Stultz C. M. (2008) PLoS ONE 3, e2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.López-Novoa J. M., Nieto M. A. (2009) EMBO Mol. Med. 1, 303–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butcher D. T., Alliston T., Weaver V. M. (2009) Nat. Rev. Cancer 9, 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells R. G. (2008) Hepatology 47, 1394–1400 [DOI] [PubMed] [Google Scholar]
- 23.Suki B., Majumdar A., Nugent M. A., Bates J. H. T. (2007) Drug Discov. Today Dis. Models 4, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antia M., Baneyx G., Kubow K. E., Vogel V. (2008) Farady Discuss. 139, 229–249; discussion 309–325, 419–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubow K. E., Klotzsch E., Smith M. L., Gourdon D., Little W. C., Vogel V. (2009) Integr. Biol. (Camb.) 1, 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Taylor K. R., Trowbridge J. M., Rudisill J. A., Termeer C. C., Simon J. C., Gallo R. L. (2004) J. Biol. Chem. 279, 17079–17084 [DOI] [PubMed] [Google Scholar]
- 27.Midwood K., Sacre S., Piccinini A. M., Inglis J., Trebaul A., Chan E., Drexler S., Sofat N., Kashiwagi M., Orend G., Brennan F., Foxwell B. (2009) Nat. Med. 15, 774–780 [DOI] [PubMed] [Google Scholar]
- 28.Okamura Y., Watari M., Jerud E. S., Young D. W., Ishizaka S. T., Rose J., Chow J. C., Strauss J. F., 3rd (2001) J. Biol. Chem. 276, 10229–10233 [DOI] [PubMed] [Google Scholar]
- 29.Erridge C. (2010) J. Leukocyte Biol. 87, 989–999 [DOI] [PubMed] [Google Scholar]
- 30.Taylor K. R., Yamasaki K., Radek K. A., Di Nardo A., Goodarzi H., Golenbock D., Beutler B., Gallo R. L. (2007) J. Biol. Chem. 282, 18265–18275 [DOI] [PubMed] [Google Scholar]
- 31.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. (2009) Carcinogenesis 30, 1073–1081 [DOI] [PubMed] [Google Scholar]