Abstract
The stimulation of fluid and electrolyte secretion in salivary cells results in ionic changes that promote rapid increases in the activity of the Na,K-ATPase. In many cell systems, there are conflicting findings concerning the regulation of the phosphorylation of the Na,K-ATPase α subunit, which is the catalytic moiety. Initially, we investigated the phosphorylation sites on the α1 subunit in native rat parotid acinar cells using tandem mass spectrometry and identified two new phosphorylation sites (Ser222, Ser407), three sites (Ser217, Tyr260, Ser47) previously found from large scale proteomic screens, and two sites (Ser23, Ser16) known to be phosphorylated by PKC. Subsequently, we used phospho-specific antibodies to examine the regulation of phosphorylation on Ser23 and Ser16 and measured changes in ERK phosphorylation in parallel. The G-protein-coupled muscarinic receptor mimetic carbachol, the phorbol ester phorbol 12-myristate 13-acetate, the Ca2+ ionophore ionomycin, and the serine/threonine phosphatase inhibitor calyculin A increased Ser23 α1 phosphorylation. Inhibition of classical PKC proteins blocked carbachol-stimulated Ser23 α1 subunit phosphorylation but not ERK phosphorylation, which was blocked by an inhibitor of novel PKC proteins. The carbachol-initiated phosphorylation of Ser23 α1 subunit was not modified by ERK or PKA activity. The Na,K-ATPase inhibitor ouabain reduced and enhanced the carbachol-promoted phosphorylation of Ser23 and Ser16, respectively, the latter because ouabain itself increased Ser16 phosphorylation; thus, both sites display conformational-dependent phosphorylation changes. Ouabain-initiated phosphorylation of Ser16 α1 was not blocked by PKC inhibitors, unlike carbachol- or phorbol 12-myristate 13-acetate-initiated phosphorylations, suggesting that this site was also a substrate for a kinase other than PKC.
Keywords: ERK; Na,K-ATPase; Protein Kinase A (PKA); Protein Kinase C (PKC); Protein Phosphorylation; Salivary Gland; Ouabain
Introduction
The Na,K-ATPase, or sodium pump, is an important ion transport protein that maintains the electrochemical gradient across the plasma membrane of eukaryotic cells. It is an integral plasma membrane protein that transports three Na+ ions for every two K+ ions, an event that consumes one molecule of ATP. As such, it participates in fluid and electrolyte secretion and cell volume regulation and is part of a network of ion transporters that regulate cellular ionic changes during basal, stimulated, and pathological conditions. The basic functional unit of the Na,K-ATPase protein consists of an α subunit responsible for the catalytic activity, a glycosylated β subunit, and in some cells, an FXYD protein. There are multiple isoforms of α and β subunits (1) and different FXYD proteins (2).
Although it has been known for decades that the Na,K-ATPase activity is regulated by the intracellular and extracellular ionic composition, regulation can also occur by the phosphorylation of the α subunit by various kinases (for review, see Ref. 3). The β subunit and FXYD proteins may also play regulatory roles under some conditions. Various studies demonstrated that Ser943, a site on the α subunit C terminus, was a target for PKA phosphorylation, and Ser16 and Ser23 on the N terminus were identified as PKC phosphorylation sites (4–7). In addition to contrasting results obtained from different species and differences between in vitro and intact cells (below), investigators have used different numbering of the α subunit amino acids because the first five are cleaved during biosynthesis and production of the mature protein. Thus, Ser16 and Ser23 are alternatively identified as Ser11 and Ser18 in some published studies (see Ref. 8). For consistency, here we use Ser16 and Ser23, even when published literature used the latter set of numbered sites.
There are conflicting studies concerning the effects of different kinases on the α subunit phosphorylation and Na,K-ATPase activity. The phosphorylation of the α subunit by PKC on Ser23 and unspecified sites stimulated the Na,K-ATPase activity in intact cells (9, 10), in some cases due to its enhanced insertion into the plasma membrane (11). Alternatively, the PKC-mediated phosphorylation of α produced a reduction in Na,K-ATPase activity due to its endocytosis and internalization (12–14). In some studies, the α subunit was preferentially phosphorylated by members of the classical PKC family (cPKC:2 α, β, γ) when compared with novel PKC family members (nPKC: δ, ϵ, θ) (11, 15). However, both cPKC (PKCβI) and nPKC (PKCδ) proteins were reported to regulate α subunit phosphorylation (16), and PKCζ, an atypical PKC family member, also phosphorylated the α subunit on Ser23 (13).
In addition to PKC, other kinases were reported to phosphorylate the α subunit and regulate Na,K-ATPase activity. The phosphorylation of the α subunit on Tyr10 increased Na,K-ATPase activity in insulin-treated cells (17), and insulin also promoted an ERK-dependent phosphorylation of the α subunit on Ser/Thr sites and a subsequent increase in Na,K-ATPase activity due to its insertion into the plasma membrane (18). In contrast, ERK contributed positively to Na,K-ATPase activity in intact cells and in vitro in a manner that did not rely on changes in Na,K-ATPase insertion into the plasma membrane (19). Relative increases of Na,K-ATPase activity in intact cells by PKA/cAMP were linearly related to the relative increases in the α subunit phosphorylation (20). In comparison, the phosphorylation of the α subunit on Ser943 by PKA increased the PKC-dependent inhibition of Na,K-ATPase activity in isolated membranes (21). In addition to the sites mentioned above, additional sites on serine, tyrosine, and threonine residues have been identified, and a total of at least 20 phosphorylation sites are reported at databases such as PhosphoSitePlus.
Phosphorylation of the α subunit on the C and N termini may be differentially regulated by the conformation of the α subunit, although the particular regulation may vary for in vitro and in situ findings. In studies with purified rat kidney Na,K-ATPase, the phosphorylation of the α subunit by PKC was increased and the phosphorylation by PKA was decreased by the presence of the Na,K-ATPase inhibitor ouabain (22). In contrast, in intact rat kidney cells, ouabain blocked the phosphorylation of the α subunit by PKC (23).
The Na,K-ATPase plays an important role in regulating Na+ and K+ transport during the initiation of fluid secretion by neurotransmitters in salivary glands, including parotid, submandibular, and sublingual glands. Activation of the G-protein-coupled M3 muscarinic acetylcholine receptor (M3R) produces the second messengers inositol 1,4,5-trisphosphate and diacylglycerol, which release intracellular Ca2+ stores and increase PKC activity, respectively. Activation of the M3R by acetylcholine or the muscarinic mimetic carbachol promotes the elevation of [Ca2+]i and the opening of Ca2+-sensitive K+ and Cl− channels and increases Na+ entry via the Na-K-2Cl cotransporter and other ion transporters (for review, see Ref. 24). Consequently, carbachol increases the Na,K-ATPase activity as much as 8-fold in parotid acinar cells (25). Inhibition of the Na,K-ATPase with ouabain blocks the increases in fluid secretion. In an early study, carbachol promoted a transient increase in the phosphorylation of the Na,K-ATPase α subunit in metabolically labeled rat submandibular gland cells (26). PKA and cAMP increased the α subunit phosphorylation in basolateral membrane vesicles purified from rat parotid glands, and it was suggested that protein kinase A anchoring protein 150 (27) plays an important role in promoting the phosphorylation of the α subunit by PKA (28). In addition to the phosphorylation of the α subunit by PKA, recent studies indicate that modulation of the Na,K-ATPase activity by PKA in cardiac cells involved glutathionylation of the β subunit (29).
In the present studies, we examine the regulation of the α1 subunit phosphorylation in intact rat parotid acinar cells. In view of some of the conflicting results from previous studies of the α subunit phosphorylation, we focused on identifying sites of phosphorylation on α1, determining the role of PKC in α1 phosphorylation, examining whether other kinases affect α1 phosphorylation by PKC, and determining whether conformational changes in α1 affect its phosphorylation.
EXPERIMENTAL PROCEDURES
Materials
Carbamylcholine (C4382) and isoproterenol (I5627) were purchased from Sigma. Phorbol 12-myristate 13-acetate (PMA, 524400) was from Calbiochem, and forskolin (CN-100) was from Enzo Life Sciences. Polyclonal rabbit ERK2 (SC-154), mouse monoclonal ERK2 (SC-1647), and mouse monoclonal α1 (SC-21712) were purchased from Santa Cruz Biotechnology. The following antibodies were purchased from Cell Signaling Technology: phospho-Thr202/Tyr204-ERK1/2 (9101), phospho-Na,K-ATPase α1 (Ser23) (4006), phospho-Na,K-ATPase α1 (Ser16) (4020), and polyclonal rabbit Na,K-ATPase α1 (3010). The mouse monoclonal antibody a6F developed by Douglas M. Fambrough was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD, National Institutes of Health, and maintained by the University of Iowa, Department of Biology, Iowa City, IA. Secondary antibodies used for the Odyssey infrared imaging system were IRDye 800-conjugated anti-rabbit IgG (Rockland Immunochemicals, 611-632-122) and Alexa Fluor 680 anti-mouse IgG (Invitrogen A-21058). Anti-rabbit IgG (AP307P) and anti-mouse IgG (AP124P) secondary antibodies used for Western blotting using film were obtained from Millipore. All other chemicals were reagent grade or better.
Salivary Cell Preparations and Solutions
Parotid acinar cells were prepared from male Sprague-Dawley rats (Charles River Laboratories, Kingston, NY, 150–200 g) as described previously (25). Cells were suspended in Solution A (116.4 mm NaCl, 5.4 mm KCl, 1 mm NaH2PO4, 25 mm Na-HEPES, 1.8 mm CaCl2, 0.8 mm MgCl2, 5 mm sodium butyrate, 5.6 mm glucose, pH 7.4). All experiments were performed at 37 °C. Acinar cells were suspended using a magnetic flea to stir the cells in a water-jacketed chamber. Par-C10 cells were grown to near confluence at 37 °C in DMEM-F12 (1:1) medium containing 2.5% fetal bovine serum (FBS) and supplements similar to those specified elsewhere (30).
LC/MS/MS Tandem Mass Spectrometry
For all mass spectrometry (MS) experiments, Na,K-ATPase α1 subunit immunoprecipitates were separated using SDS-PAGE, the gel was stained with Coomassie Blue and destained, and the α1 subunit band was excised. Samples were subjected to reduction with 10 mm dithiothreitol (DTT) for 30 min, alkylation with 55 mm iodoacetamide for 45 min, and in-gel digestion with l-1-tosylamido-2-phenylethyl chloromethyl ketone-modified trypsin or chymotrypsin overnight at pH 8.3 (50 mm ammonium bicarbonate) followed by reversed-phase microcapillary/tandem mass spectrometry (LC/MS/MS). LC/MS/MS was performed using an EASY-nLC splitless nanoflow HPLC (Proxeon Biosciences) with a self-packed 75-μm inner diameter × 15-cm C18 column coupled to an LTQ-Orbitrap XL mass spectrometer (Thermo Scientific) in the data-dependent acquisition and positive ion mode at 300 nl/min with one full MS-Fourier transform scan followed by six MS/MS spectra. MS/MS spectra collected via collision-induced dissociation in the ion trap were searched against the concatenated target and decoy (reversed) single entry α1-Na,K-ATPase and full Swiss-Prot protein databases using Sequest (Proteomics Browser software, Thermo Scientific) with differential modifications for Ser/Thr/Tyr phosphorylation (+79.97) and the sample processing artifacts Met oxidation (+15.99), deamidation of Asn and Gln (+0.984), and Cys alkylation (+57.02). Phosphorylated and unphosphorylated peptide sequences were identified whether they initially passed the following Sequest scoring thresholds against the target database: 1+ ions, Xcorr ≥ 2.0 Sf ≥ 0.4, p ≥ 5; 2+ ions, Xcorr ≥ 2.0, Sf ≥ 0.4, p ≥ 5; 3+ ions, Xcorr ≥ 2.60, Sf ≥ 0.4, p ≥ 5 against the target protein database, where Xcorr represents Sequest cross-correlation score, and Sf represents Sequest final score. Passing MS/MS spectra were manually inspected to make sure that all b- and y-fragment ions aligned with the assigned sequence and modification sites. Determination of the exact sites of phosphorylation was aided using FuzzyIons and GraphMod, and phosphorylation site maps were created using the ProteinReport software (Proteomics Browser software suite, Thermo Scientific). False discovery rates of peptide hits (phosphorylated and unphosphorylated) were estimated below 1.50% based on reversed database hits.
Titanium Dioxide (TiO2) Phosphopeptide Enrichment
Half of the digested peptide pool was reserved for enrichment with the Phos-trap phosphopeptide Enrichment Kit (PerkinElmer Life Sciences) containing TiO2-coated magnetic beads according to the vendor's protocol. Briefly, peptide mixtures containing phosphopeptides were acidified with Binding Buffer and incubated with 20 μl of 20× TiO2 magnetic beads diluted in 180 μl of HPLC grade water for 1 h at room temperature with continuous shaking in a room temperature incubator followed by washing three times with Binding Buffer and one time with Washing Buffer. Phosphopeptides were then incubated with 35 μl of Basic Elution buffer with continuous shaking. Elution Buffer was then transferred to a 12 × 32-mm autosampler vial with 50 μl of HPLC A Buffer (0.1% formic acid), and the final solution was concentrated to 5 μl using a SpeedVac prior to injection via LC/MS/MS.
Western Blot Analysis and Immunoprecipitation
At the end of the treatment period, native rat parotid acinar cells suspended at ∼0.5–1 mg/ml were lysed and cleared of insoluble proteins by sedimentation at 15,000 × g for 15 min at 4 °C. For samples subjected to Western blot analysis without immunoprecipitation, cells were lysed in ice-cold Lysis buffer (137 mm NaCl, 20 mm Tris base, pH 7.5, 1 mm EGTA, 1 mm EDTA, 10% (v/v) glycerol, 1% (v/v) IGEPAL) containing the following reagents: 1 mm vanadate, 4.5 mm sodium pyrophosphate, 47.6 mm NaF, 9.26 mm β-glycerophosphate, 0.5 mm dithiothreitol, 2 mg/ml leupeptin, 2 mg/ml pepstatin, 2 mg/ml aprotinin, and 2 mg/ml 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride. The cleared supernatants were diluted with 5× Laemmli sample buffer, heated for 30 min at 37 °C, and stored at −20 °C prior to electrophoresis. For rat parotid acinar cell and Par-C10 cell samples subjected to α1 immunoprecipitation for Western blot analysis, cells were lysed in the following Immunoprecipitation buffer: 20 mm Tris-HCl (pH 7.4), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 0.5% IGEPAL, 0.5% SDS, 0.5 mm dithiothreitol, 20 mm NaF, 1 mm sodium vanadate, and the same mix of phosphatase inhibitors in the Lysis buffer. The lysate was cleared as above, and a6F antibody (∼20 μg/ml) and a mixture of protein A- and protein G-Sepharose beads was added overnight (unless stated otherwise). The beads were collected by sedimentation, washed three times in PBS, 1% IGEPAL solution, and then heated for 30 min at 37 °C in Laemmli sample buffer. All samples were separated using SDS-polyacrylamide gel electrophoresis with an 8% separating gel and a 3% stacking gel. Proteins were transferred to nitrocellulose, and immunoblots were probed overnight with various antibodies according to the supplier's specifications. When probing immunoblots for α1 subunit, lysates were probed using either mouse monoclonal α1 (SC-21712) or rabbit polyclonal α1 (3010); immunoprecipitates (and lysates on the same blot) were probed using rabbit α1. Proteins were visualized using chemiluminescence reagents and x-ray film. Alternatively, in some experiments, proteins were visualized and quantified by direct infrared fluorescence using an Odyssey imaging system (LI-COR Biosciences) as reported previously (31).
For α1 subunit immunoprecipitates subjected to mass spectrometry analysis, cells were suspended at ∼3–6 mg/ml and lysed in Immunoprecipitation buffer, and a6F antibody (∼40 μg/ml) and a mixture of protein A- and protein G-Sepharose beads were added overnight. The collected beads were washed and treated as above and subjected to SDS-PAGE as described above for mass spectrometry.
Quantification of Protein Phosphorylation
The phosphorylation status of proteins visualized on film was quantified by densitometry using the NIH ImageJ software program. For each sample, the phosphoproteins were normalized to total protein level (ERK or α1 subunit) to account for gel loading/transfer variations. Blots were probed for phosphoproteins, stripped, and reprobed for total proteins. The phosphorylations for the various conditions were normalized to the phosphorylation under basal control (non-stimulated, no inhibitors) conditions as indicated. In experiments using the Odyssey imaging system, blots were simultaneously probed for phosphoproteins and total protein levels using polyclonal antibodies and mouse monoclonal antibodies, respectively, and fluorescent anti-rabbit and anti-mouse antibodies were used for visualization and subsequent quantification.
Data Analysis
Values were calculated as the mean ± S.E. of n number of independent experiments (each n from a different cell preparation). The differences between the basal/control and the experimental samples were evaluated using a Student's t test. All Western blot experiments were performed at least three different times. Representative blots are shown in each figure. For each experiment to be analyzed using Western blotting techniques and/or the Odyssey system, multiple (duplicate or triplicate) cell samples were collected for each condition, and the average of the values obtained within each individual experiment was treated as n = 1.
RESULTS
Identification of Sites of α1 Subunit Phosphorylation by Mass Spectrometry
The main isoform of the α subunit of the Na,K-ATPase in rat parotid acinar cells is α1 (31, 32). Initially, we used LC/MS/MS to identify phosphorylation sites on α1 obtained from immunoprecipitating the protein from rat parotid cells, and we also attempted to determine differences in phosphorylation status in cells exposed to different stimuli. We identified seven different phosphorylation sites, of which six were on serine residues and one was on tyrosine. These are listed in Table 1, along with the surrounding sequence, database scoring information about the identification, and putative kinases responsible for phosphorylation. Two sites (Ser222, Ser407) appear to be novel ones that have not yet been reported. Two sites (Ser47, Ser217) were previously identified by mass spectrometry in several published proteomic screens, and the tyrosine phosphorylation site (Tyr260) was identified in many proteomic studies, most of them internal studies by Cell Signaling Technologies. Two sites (Ser16, Ser23) have been identified as sites phosphorylated by PKC and are the subject of various studies (5, 7, 8, 11, 14, 42).
TABLE 1.
Tandem mass spectrometry identification of phosphorylation sites on Na,K-ATPase α1 subunit in rat parotid acinar cells
Samples were obtained prepared using α1-immunopreciptations followed by proteolysis and LC/MS/MS analyses. Shown are conditions for which each site was identified and putative kinases that are known or predicted to phosphorylate specific sites. The abbreviations used are: CK2, casein kinase 2; IR, insulin receptor; IGF1R, IGF1 receptor; ATMK, ataxia telangiectasia mutated kinase; B, basal; I, isoproterenol; C, carbachol; CA, calyculin A; P, PMA; pool, B+C+I combined; Charge, charge state of peptide ion; Δmass, difference between experimental peptide mass and known sequence; Sf, Sequest final score; MH+, protonated molecular mass; Xcorr, Sequest cross-correlation score; ΔCn, Xcorr difference between the top ranked and next best sequence; Sp, Sequest preliminary score; rank, top hit from protein database. Accession number was AAB81285 for all sequences. All sequences were ranked No. 1 as the top hit from the protein database.
| Sequence | Site | Charge | Δmass | Sf | MH+ | Xcorr | ΔCn | Sp | Condition detected | Kinase |
|---|---|---|---|---|---|---|---|---|---|---|
| ppm | ||||||||||
| YEPAAVpSEHGDKK | Ser-16 | 2 | −2.0 | 0.93 | 1511 | 3.98 | 0.41 | 744 | P + CA, pool | PKC |
| YEPAAVSEHGDKKpSK | Ser-23 | 2 | −0.4 | 0.92 | 1726 | 3.94 | 0.63 | 389 | P + CA | PKC |
| VDNSpSLTGESEPQTR | Ser-217 | 2 | −1.5 | 0.9 | 1700 | 4.31 | 0.05 | 1004 | B, C, I, P | CK2 |
| VDNSSLTGEpSEPQTR | Ser-222 | 2 | −2.2 | 0.86 | 1700 | 3.68 | 0.01 | 1114 | B, pool | CK2 |
| GIVVpYTGDR | Tyr-260 | 2 | −1.3 | 0.84 | 1060 | 2.22 | 0.24 | 999 | Pool | IR, IGF1R |
| DNQIHEADTTENQpSGVSFDK | Ser-407 | 3 | 0.3 | 0.77 | 2315 | 3.16 | 0.19 | 922 | Pool | |
| EVSMDDHKLpSLDELHR | Ser-47 | 3 | 1.9 | 0.55 | 2004 | 2.81 | 0.32 | 270 | C, P + CA | ATMK,PKA |
In addition to trypsin, we used chymotrypsin for digestion of the α subunit protein, and in some studies, we also utilized TiO2 to enrich digestions for phosphopeptides (33, 34). In several experiments, after not finding any phosphorylation sites on the α1 subunit, we pooled samples obtained from cells treated with different agents to increase the amount of protein subjected to analysis. Ultimately, using LC/MS/MS, we were unable to clearly demonstrate quantitative changes in phosphorylation due to stimulation of the cells with various agents, including carbachol, isoproterenol, and PMA, because phosphorylation levels were generally of low stoichiometry and peptide signals are sometimes suppressed by the addition of phosphate groups (35). Consequently, we decided to use commercially available phospho-antibodies to evaluate the regulation of α1 subunit phosphorylation.
Effects of Various Stimuli on α1 Subunit Phosphorylation
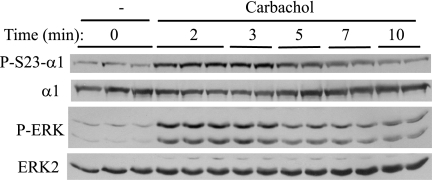
Because M3R stimulation produces a rapid and large increase in Na,K-ATPase activity in parotid acinar cells (25), we examined the effects of the muscarinic ligand carbachol on α1 phosphorylation. We also measured changes in ERK phosphorylation as a representative signaling molecule because carbachol also rapidly activates ERK in parotid acinar cells. Carbachol increased Ser23 α1 subunit phosphorylation within 2 min, and the phosphorylation declined after peaking at 2–3 min (Fig. 1). The carbachol-induced phosphorylation of ERK also displayed a similarly transient time course, as we recently reported (36).
FIGURE 1.
Time course of phosphorylation of ERK and Ser23 α1 subunit in rat parotid acinar cells exposed to carbachol. Cells were treated with carbachol (10−5 m) for times up to 10 min. Western blots of cell lysates were probed as indicated. α1 subunit Ser23 (P-S23-α1) and ERK (P-ERK) phosphorylation occurred very rapidly and then declined.
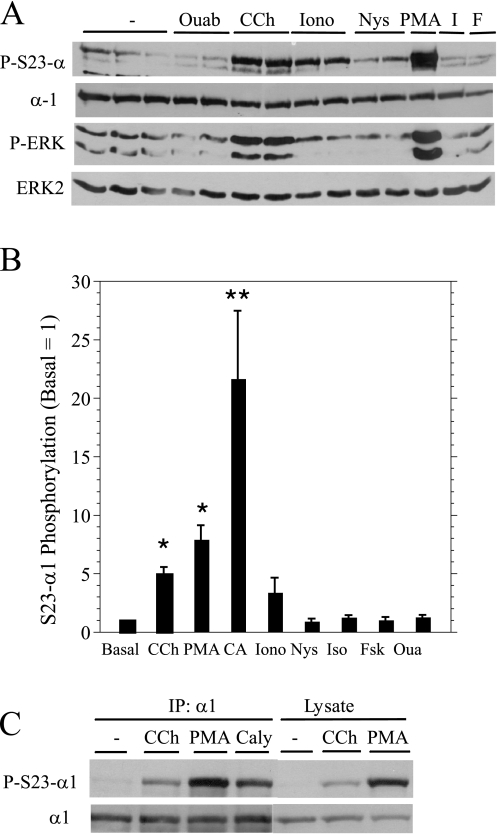
We also examined changes in Ser23 α1 subunit and ERK phosphorylation by other agents that affect the Na,K-ATPase activity and fluid and protein secretion. Not surprisingly, the phorbol ester PMA, an activator of PKC, increased Ser23 α1 subunit and ERK phosphorylations (Fig. 2A), consistent with the phosphorylation of both proteins being dependent on PKC. Ionomycin, a divalent ionophore that elevates intracellular Ca2+ and stimulates fluid secretion and Na,K-ATPase activity by opening Ca2+-sensitive K+ and Cl− channels, increased Ser23 α1 subunit but not ERK phosphorylation. In contrast, nystatin, a monovalent cationophore that increases the activity of the Na,K-ATPase in parotid acinar cells by increasing the intracellular Na+/K+ ratio (25), did not increase Ser23 α1 subunit or ERK phosphorylation. Ouabain, a cardiac glycoside that binds to the α subunits of the Na,K-ATPase and inhibits its activity, also did not increase Ser23 α1 subunit or ERK phosphorylation. Neither Ser23 α subunit nor ERK phosphorylation was increased by isoproterenol (2–5 min), which activates the adenylyl cyclase-coupled parotid β-adrenergic receptor, or by forskolin (2–12 min), which directly activates adenylyl cyclase. Isoproterenol and forskolin stimulate PKA downstream of adenylyl cyclase, promoting protein exocytosis in parotid acinar cells. The relative effects of all of these agents on Ser23 α1 subunit phosphorylation are shown in Fig. 2B, along with that of calyculin A, a serine/threonine phosphatase inhibitor that promoted the largest increase. Notably, immunoblots obtained using cell lysates were similar to those found using α1 subunit immunoprecipitations (Fig. 2C), confirming that the protein recognized by the Ser23 phospho-specific antibody was indeed α1.
FIGURE 2.
Effects of various agents on ERK and Ser23 α1 subunit phosphorylation in parotid acinar cells. A, Western blots of cells treated as described in B. Cell lysates were probed as indicated. Ouab, ouabain; CCh, carbachol; Iono, ionomycin; Nys, nystatin; I, isoproterenol; F, forskolin. P-S23-α1, α1 subunit Ser23 phosphorylation; P-ERK, ERK phosphorylation. B, the phosphorylation of the α1 subunit on Ser23 (S23-Alpha1) was quantified relative to basal conditions. Cells were treated with the following agents: ouabain (Oua, 1 mm, 3 min; n = 5), carbachol (10−5 m, 2 min; n = 12), ionomycin (10−6 m, 2 min; n = 3), nystatin (10−4 m, 2 min; n = 3), PMA (100 nm, 2 min; n = 10), isoproterenol (Iso, 10−5 m, 2 min; n = 6), forskolin (Fsk, 10 μm, 2–12 min; n = 6), calyculin A (CA, 100 nm, 10 min; n = 4). *, p < 0.01 and **, p < 0.05 versus basal. C, Western blot of cell lysates and α1 subunit immunoprecipitates (IP: α1, 4 h). Cells were treated as described in B, except for calyculin A (Caly, 100 nm, 5 min).
cPKC Inhibition Blocks Phosphorylation of Ser23 α1 Subunit but Not ERK
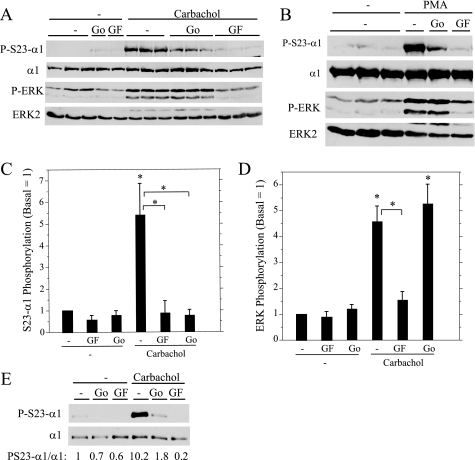
Because the α1 subunit is directly phosphorylated on Ser23 by PKC in various cells, we examined the effects of PKC inhibitors on α1 phosphorylation promoted by carbachol and PMA in parotid acinar cells. We compared the inhibitory effects of Go6976, an inhibitor that blocks cPKC family members, with those of GF109203X, a PKC inhibitor that blocks both cPKC and nPKC family members. In parallel, we examined the phosphorylation of ERK, which is also dependent on PKC in rat parotid acinar cells (37). Both PKC inhibitors substantially blocked Ser23 α1 subunit phosphorylation by carbachol (Fig. 3A) and PMA (Fig. 3B), suggesting that a cPKC protein was largely responsible for phosphorylating α1 subunit on this site. In contrast, Go6976 was ineffective at blocking ERK phosphorylation, indicating that ERK phosphorylation is dependent on nPKC proteins in parotid acinar cells. The different relative effects of the two PKC inhibitors on the α1 subunit (Fig. 3C) and ERK (Fig. 3D) phosphorylation indicate that different PKC family members are upstream of ERK phosphorylation relative to those responsible for α1 phosphorylation.
FIGURE 3.
Effect of PKC inhibitors on Ser23 α1 subunit (P-S23-α1) and ERK (P-ERK) phosphorylation. A and B, rat parotid acinar cells were treated for 10 min with vehicle (−), Go6976 (Go, 1 μm), and GF109203X (GF, 10 μm) followed by carbachol (10−5 m, 2 min) or PMA (100 nm, 2 min). Cell lysates were probed by Western blot as indicated. C and D, the phosphorylation of Ser23 α1 subunit (C) and ERK (D) in parotid acinar cells was quantified relative to basal conditions (no inhibitors). For Ser23 α1 (S23-α1) phosphorylation, n = 5–6, *, p < 0.05 versus basal or paired as indicated. For ERK phosphorylation, n = 5, *, p < 0.01 versus basal or paired as indicated. E, Par-C10 cells were treated with vehicle (−), Go6976 (1 μm, 20 min), and GF109203X (10 μm, 20 min) followed by carbachol (10−4 m, 5 min). α1 subunit immunoprecipitates were probed by Western blot as indicated and quantified relative to basal (no inhibitor).
We also examined changes in α1 subunit phosphorylation in Par-C10 cells, an immortalized rat parotid acinar cell line that also expresses α1, although at levels much less than those found in native parotid acinar cells (31). Both PKC inhibitors also blocked α1 phosphorylation on Ser23 in carbachol-treated Par-C10 cells (Fig. 3E), similar to the effects noted in freshly isolated rat parotid acinar cells.
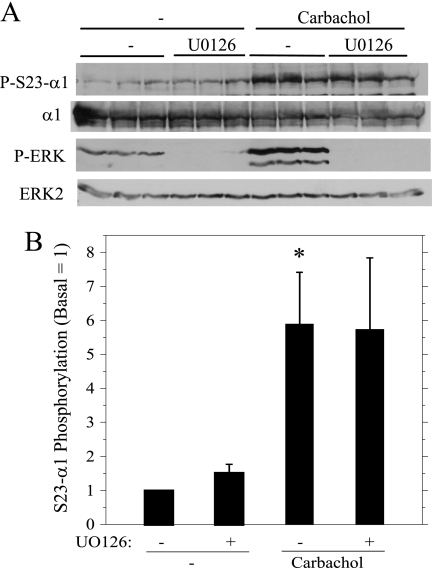
Although the data indicated that the stimulation of ERK and α1 subunit phosphorylation was due to distinct PKC family members, we examined the possibility that ERK activity contributed to α1 phosphorylation on Ser23. Treatment of cells with U0126, an inhibitor of MEK, the kinase immediately upstream of ERK, blocked both the basal and the carbachol-stimulated ERK phosphorylation but did not affect the carbachol-promoted α1 subunit phosphorylation on Ser23 (Fig. 4), consistent with the lack of a contribution of ERK to this phosphorylation in parotid acinar cells.
FIGURE 4.
Effects of ERK inhibition on Ser23 α1 subunit (P-S23-α1) and ERK (P-ERK) phosphorylation. Parotid acinar cells were treated for 10 min with vehicle (−) and the MEK inhibitor UO126 (10 μm) followed by carbachol (10−5 m, 2 min). A, cell lysates were probed on Western blots as indicated. UO126 completely blocked ERK phosphorylation. B, the phosphorylation of α1 subunit on Ser23 (S23-Alpha1) was quantified relative to basal conditions (without UO126). n = 4, *, p < 0.05 versus basal (no additions).
Protein Kinase A Does not Affect Ser23 α1 Subunit Phosphorylation
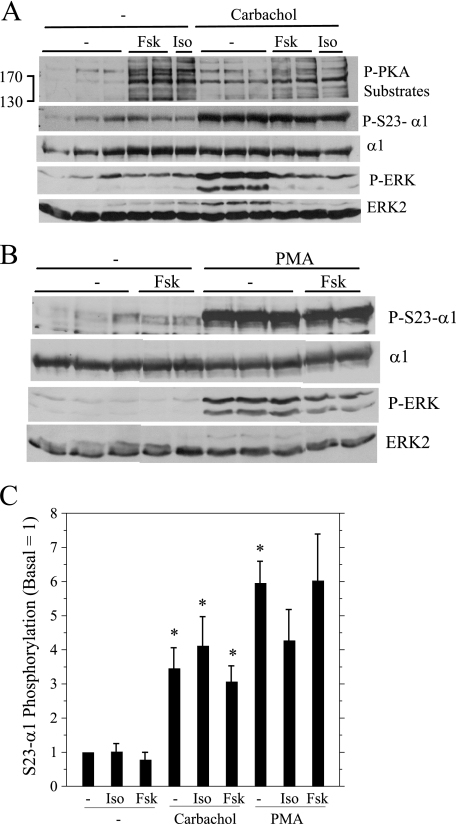
It was reported that phosphorylation of α on Ser943 by PKA increased the PKC-promoted inhibition of Na,K-ATPase activity in vitro, perhaps by enhancing the PKC-dependent phosphorylation of α1 (21). Therefore, we examined whether PKA affected Ser23 phosphorylation by pretreating cells with isoproterenol and forskolin prior to activating PKC with carbachol and PMA (Fig. 5). Isoproterenol and forskolin, which both promote cAMP production, increased PKA activity, as indicated in immunoblots probed with an antibody that recognizes substrate proteins phosphorylated by PKA. Neither of the PKA activators reduced the carbachol- or PMA-promoted phosphorylation of α1 subunit on Ser23. In contrast, the PKA activators blocked the increases in ERK phosphorylation promoted by either carbachol or PMA, similar to our recent report that PKA inhibits PKC-dependent ERK activation by these and other stimuli by >60% (36). The different effects of PKA on ERK (inhibition) and Ser23 α1 subunit (no effect) phosphorylation is also consistent with the phosphorylation of these two proteins being downstream of different PKC families (see “Discussion”).
FIGURE 5.
Effects of PKA activation (P-PKA substrates) on the Ser23 α1 subunit (P-S23-α1) and ERK phosphorylation (P-ERK). Parotid acinar cells were treated with isoproterenol (Iso, 10−5 m, 1 min) and forskolin (Fsk, 10 μm, 10 min) prior to carbachol (10−5 m, 2 min) and PMA (100 nm, 2 min). A and B, cell lysates were analyzed by Western blot as indicated. C, the phosphorylation of α1 subunit on Ser23 (S23 Alpha) was quantified relative to basal conditions (no additions). n = 4 (carbachol) or 3 (PMA). *, p < 0.05 versus basal (no additions).
Ouabain Blocks Ser23 and Increases Ser16 α1 Subunit Phosphorylation
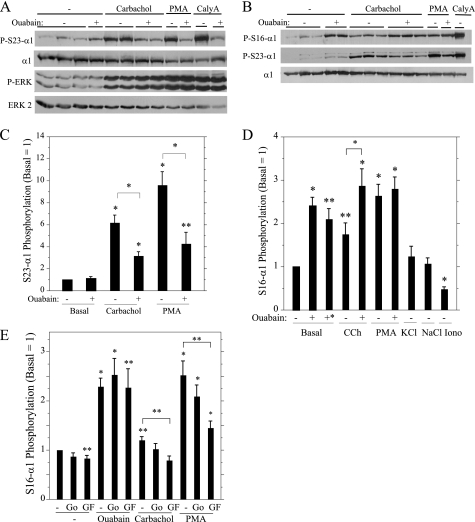
Because the phosphorylation of the α subunit by different kinases is affected by its conformation, we examined the effect of ouabain on the phosphorylation of α1 on Ser23 promoted by various agents. In these experiments, the Na,K-ATPase activity of intact parotid acinar cells was inhibited by ouabain, and then cells were exposed to other phosphorylation-promoting agents. Notably, ouabain substantially blocked the increases in Ser23 α1 subunit phosphorylation initiated by exposure of the cells to carbachol, PMA, and calyculin (Fig. 6, A and C). This suggests that the availability of Ser23 for PKC phosphorylation is reduced when ouabain induces a change in the conformation of α1 subunit. In contrast, ouabain did not block increases in ERK phosphorylation by various stimuli; moreover, we reported previously that ouabain increased the phosphorylation of ERK by submaximal (e.g. 10−6 m) concentrations of carbachol (38).
FIGURE 6.
Effects of ouabain on α1 subunit phosphorylation on Ser23 and Ser16. In A–D, rat parotid acinar cells were treated for 1 min with vehicle (−) or 1 mm ouabain and subsequently treated with carbachol (CCh, 10−5 m, 2 min), PMA (100 nm, 2 min), or calyculin A (CalyA,100 nm, 10 min). Ouabain treatment without other agents was for 3 min, except that cells also were exposed for 15 min (+*) in D. In D, cells were exposed to 20 mm KCl, 20 mm NaCl, and 10−6 m ionomycin (Iono) (all for 3 min). A and B, cell lysates were analyzed by Western blot as indicated. P-S23-α1, α1 subunit Ser23 phosphorylation; P-S16-α1, α1 subunit Ser16 phosphorylation; P-ERK, ERK phosphorylation. C and D, the phosphorylation of α subunit on Ser23 (C, S23-Alpha1) and Ser16 (D, S16-Alpha1) was quantified relative to basal conditions (no ouabain). n = 3–11. *, p < 0.01, and **, p < 0.05 versus basal (no additions) or for paired (+ ouabain) as indicated. In E, rat parotid acinar cells were treated for 10 min with vehicle (−), Go6976 (Go, 1 μm), and GF109203X (10 μm) followed by ouabain (1 mm, 3 min), carbachol (10−5 m, 2 min), or PMA (100 nm, 2 min). n = 5–7. *, p < 0.01 and **, p < 0.05 versus basal (no additions) or for paired (+ GF109203X (GF)) as indicated.
Although ouabain did not affect the basal phosphorylation of α1 subunit on Ser23, the exposure of cells to ouabain produced an increase in Ser16 phosphorylation (Fig. 6B). Carbachol and PMA produced much more modest relative increases in the phosphorylation of the α1 subunit on Ser16 (Fig. 6D) than on Ser23 (Fig. 6C). Pretreatment of cells with ouabain for 1 min enhanced the increase in Ser16 phosphorylation promoted by carbachol, increasing it to the level stimulated by PMA, which was not enhanced further by ouabain (Fig. 6D). Because ouabain depolarizes rat parotid acinar cells and elevates [Ca2+]i in some cells (although not in parotid acinar cells (38)), we compared the stimulatory effect of ouabain on α1 subunit Ser16 phosphorylation with that of increasing the extracellular KCl concentration by 20 mm, which depolarizes parotid acinar cells as much as does 1 mm ouabain3, and with that of ionomycin, a Ca2+ ionophore that increases the [Ca2+]i. The depolarizing effects of KCl did not produce an increase in α1 subunit Ser16 phosphorylation, and ionomycin promoted a decrease, not an increase, in this phosphorylation (Fig. 6D). Not surprisingly, a 20 mm increase in the extracellular NaCl concentration, an experimental control to mimic the extracellular osmotic change of KCl, also did not affect Ser16 phosphorylation.
We also examined the PKC dependence of changes in the Ser16 phosphorylation of α1 subunit by various stimuli. The increases promoted by carbachol and PMA were significantly blocked by GF109203X but not by Go6976 (Fig. 6E). These results are different from the similar inhibitory effects of both PKC inhibitors on the increases in Ser23 phosphorylation promoted by carbachol and PMA (Fig. 3C). Moreover, the increases in Ser16 phosphorylation in cells exposed to ouabain were not blocked by either PKC inhibitor. These results suggest that there are multiple mechanisms by which the α1 subunit is phosphorylated on Ser16.
DISCUSSION
The results of this study demonstrate the following characteristics of the phosphorylation of the α1 subunit of the Na,K-ATPase in rat parotid acinar cells. 1) Carbachol, PMA, and calyculin A increase its phosphorylation on Ser23; 2) in contrast to the phosphorylation of ERK by nPKC proteins, the carbachol-initiated phosphorylation of α1 subunit on Ser23 is largely dependent on cPKC proteins; 3) PKA and ERK do not affect the carbachol-initiated phosphorylation of α1 subunit on Ser23; and 4) the Na,K-ATPase inhibitor ouabain reduces the stimulus-dependent phosphorylation on Ser23 and promotes the stimulus-independent phosphorylation on Ser16.
Using mass spectrometry, we identified several novel phosphorylation sites (Ser222 and Ser407) on the α1 subunit in addition to other known phosphorylated sites that have been found in large proteomic screens (see Table 1), which can be accessed from the PhosphoSite database. Due to the abundance of lysine residues around Ser23, we did not see this site phosphorylated until we used chymotrypsin for digestion and consolidated the phosphorylated peptides using TiO2. Consequently, we focused our studies on using phospho-specific α1 antibodies for Ser23 and Ser16, the two sites previously demonstrated to be phosphorylated directly by PKC.
Carbachol and PMA produced a rapid increase in the phosphorylation of the α1 subunit on Ser23. By comparing the effects of two PKC inhibitors (Go6976, G203109X) that had different inhibitory selectivities, we demonstrated that a member(s) of the cPKC family (α, β, γ) was responsible for the majority of phosphorylation on Ser23. The contrasting effects of ionomycin (increase) and nystatin (no effect) on α1 subunit Ser23 phosphorylation indicate that increases in the phosphorylation on this site are not secondary to increases in Na,K-ATPase activity, which these agents produce via changes in monovalent (nystatin) and divalent (ionomycin) cations. Although ERK phosphorylation induced by carbachol is largely PKC-dependent, it was not blocked by Go6976, which is selective for cPKC proteins. The same was true for PMA-promoted ERK phosphorylation. These data clearly demonstrate that carbachol activates different PKC families and that these have different functional roles downstream of the M3R activation in parotid cells. The parotid β-adrenergic receptor largely does not increase PKC activity, so it was not surprising that the β-receptor ligand isoproterenol did not increase α1 subunit phosphorylation on Ser23.
Because there is literature regarding cross-talk between ERK- and PKC-dependent α subunit phosphorylation (8) (see the Introduction), we examined the effect of UO126, a MEK inhibitor that blocks ERK phosphorylation, on the carbachol-initiated Ser23 α1 phosphorylation. The ineffectiveness of UO126 in blocking this phosphorylation indicates that ERK does not have an impact on the PKC-dependent phosphorylation of α1 subunit on Ser23 in parotid acinar cells. Similarly, we did not find any cross-talk between PKA and Ser23 α1 phosphorylation. Because isoproterenol and forskolin block PKC-dependent ERK phosphorylation (36) but not PKC-dependent Ser23 α1 phosphorylation, these results are a second demonstration (in addition to the PKC inhibitor studies) that different PKC families promote ERK and α1 phosphorylation. These findings also indicate that PKA blocks the phosphorylation of a protein downstream of nPKC activation (ERK) but not downstream of cPKC activation (α1 subunit).
Although ouabain increased the PKC-dependent phosphorylation of purified rat kidney α in vitro (22), it was not entirely surprising that ouabain reduced the stimulus (carbachol, PMA, calyculin A)-dependent phosphorylation of α1 subunit on Ser23 in parotid acinar cells because ouabain blocked the phorbol ester-initiated phosphorylation of α1 in intact rat kidney cells (23). One can imagine that the binding of the Na,K-ATPase inhibitor and the subsequent change in the conformation of the α1 subunit alters the accessibility of a PKC phosphorylation site. However, it was surprising that ouabain increased the phosphorylation of α1 on Ser16 in a PKC-independent fashion. These effects of ouabain were not secondary to [Ca2+]i elevation or depolarization of the cells. This suggests that Ser16 becomes more accessible for phosphorylation, perhaps involving the inhibition of a phosphatase, when the Na,K-ATPase is inhibited in the absence of a stimulus that increases PKC activity. This still begs the question of the identity of the kinase that is upstream of the ouabain-promoted phosphorylation of Ser16. Notably, the increases of the phosphorylation of α1 on Ser16 by carbachol and PMA were PKC-dependent; however, these phosphorylations appeared to be due to nPKCs, a different family from that which promoted the phosphorylation of α1 subunit on Ser23 by these stimuli.
The effects of PKC on Na,K-ATPase activity are varied, and this may be due to the fact that there are four α isoforms that may be differentially regulated (reviewed in Refs. 1 and 3). Sottejeau et al. (39) recently reported that an isoform-specific region in α1, a dileucine motif that is conserved among mammalian α1 proteins but not other α isoforms, affected the trafficking and PKC-dependent activation of Na,K-ATPase activity. They suggest that the two leucines may be part of a recognition motif for adapter proteins that affects the internalization of the Na,K-ATPase, thereby acting as an additional regulatory site that, along with the PKC phosphorylation sites on the α1 subunit, affect the expression and functional activity of the Na,K-ATPase. Because some agents produce multiple changes in α1 subunit phosphorylation, in some cases, it can be difficult to conclude that specific phosphorylation sites are causal for changes in Na,K-ATPase activity. The PKC inhibitor GF109203X blocked insulin-promoted increases in Na,K-ATPase activity and decreases in Ser23 α1 subunit phosphorylation in mouse corneal endothelial cells (40). These observations led investigators to suggest that the insulin receptor tyrosine kinase promoted a PKC-mediated activation of Ser/Thr phosphatase, although contributions from tyrosine phosphorylation were not assessed in these studies. In contrast, in response to a protein tyrosine phosphatase inhibitor, human renal carcinoma cells displayed a decrease in Na,K-ATPase activity that was attributed to decreases in α1 subunit Ser/Thr phosphorylation and/or increases in its tyrosine phosphorylation (41).
In studies examining the phosphorylation of human αl, which has a glycine instead of a serine at position 23, the phosphorylation on Ser16 promoted either stimulation or inhibition of Na,K-ATPase activity depending on the PKC family member that mediated this phosphorylation. In addition, stimuli that increased the phosphorylation of both Ser16 and Ser23 on rat α1 subunit promoted the recruitment of the Na,K-ATPase to the plasma membrane, but this enzyme was internalized by endocytosis when it was phosphorylated only on Ser23 (42). Our findings demonstrated that several conditions (ouabain; PMA in the presence of Go6976) imposed on rat parotid acinar cells would establish yet a different end point: the phosphorylation of α1 mainly on Ser16 but not Ser23.
In summary, we have identified multiple phosphorylation sites on the α1 subunit of the Na,K-ATPase in native rat parotid acinar cells, which initiate fluid and electrolyte secretion leading to saliva formation. These sites include two that appear to be novel and others previously detected mainly by large proteomic studies. Activation of the M3R and PKC rapidly increases the phosphorylation of the α1 subunit on Ser23 and Ser16, and these phosphorylations are differentially modified by inhibition of the Na,K-ATPase with ouabain. It remains to be determined whether the phosphorylation of multiple N-terminal sites on the parotid α1 subunit promotes its insertion into or removal from the basolateral membrane, events that are among the fates of the phosphorylated Na,K-ATPase in other cellular systems, and to determine the localization changes of α1 in response to modulating these phosphorylations.
Acknowledgments
We thank William Lannon for excellent technical assistance and Xuemei Yang for excellent assistance with the mass spectrometry experiments.
This work was supported, in whole or in part, by National Institutes of Health Grants DE-10877 (to S. P. S.), 5P30CA006516-43 (to J. M. A.), and 1P01CA120964-01A (J. M. A.).
S. P. Soltoff, unpublished studies.
- cPKC
- classical PKC family
- nPKC
- novel PKC family
- M3R
- muscarinic M3 receptor
- PMA
- phorbol 12-myristate 13-acetate.
REFERENCES
- 1.Blanco G., Mercer R. W. (1998) Am. J. Physiol. 275, F633–F650 [DOI] [PubMed] [Google Scholar]
- 2.Geering K. (2008) Curr. Opin. Nephrol. Hypertens. 17, 526–532 [DOI] [PubMed] [Google Scholar]
- 3.Therien A. G., Blostein R. (2000) Am. J. Physiol. Cell Physiol. 279, C541–C566 [DOI] [PubMed] [Google Scholar]
- 4.Chibalin A. V., Vasilets L. A., Hennekes H., Pralong D., Geering K. (1992) J. Biol. Chem. 267, 22378–22384 [PubMed] [Google Scholar]
- 5.Beguin P., Beggah A. T., Chibalin A. V., Burgener-Kairuz P., Jaisser F., Mathews P. M., Rossier B. C., Cotecchia S., Geering K. (1994) J. Biol. Chem. 269, 24437–24445 [PubMed] [Google Scholar]
- 6.Pedemonte C. H., Pressley T. A., Lokhandwala M. F., Cinelli A. R. (1997) J. Membr. Biol. 155, 219–227 [DOI] [PubMed] [Google Scholar]
- 7.Feschenko M. S., Sweadner K. J. (1995) J. Biol. Chem. 270, 14072–14077 [DOI] [PubMed] [Google Scholar]
- 8.Feschenko M. S., Sweadner K. J. (1997) J. Biol. Chem. 272, 17726–17733 [DOI] [PubMed] [Google Scholar]
- 9.Carranza M. L., Féraille E., Favre H. (1996) Am. J. Physiol. 271, C136–C143 [DOI] [PubMed] [Google Scholar]
- 10.Féraille E., Carranza M. L., Buffin-Meyer B., Rousselot M., Doucet A., Favre H. (1995) Am. J. Physiol. 268, C1277–C1283 [DOI] [PubMed] [Google Scholar]
- 11.Efendiev R., Bertorello A. M., Pressley T. A., Rousselot M., Féraille E., Pedemonte C. H. (2000) Biochemistry 39, 9884–9892 [DOI] [PubMed] [Google Scholar]
- 12.Yudowski G. A., Efendiev R., Pedemonte C. H., Katz A. I., Berggren P. O., Bertorello A. M. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 6556–6561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lecuona E., Trejo H. E., Sznajder J. I. (2007) J. Bioenerg. Biomembr. 39, 391–395 [DOI] [PubMed] [Google Scholar]
- 14.Chibalin A. V., Ogimoto G., Pedemonte C. H., Pressley T. A., Katz A. I., Féraille E., Berggren P. O., Bertorello A. M. (1999) J. Biol. Chem. 274, 1920–1927 [DOI] [PubMed] [Google Scholar]
- 15.Kazanietz M. G., Caloca M. J., Aizman O., Nowicki S. (2001) Arch. Biochem. Biophys. 388, 74–80 [DOI] [PubMed] [Google Scholar]
- 16.Asghar M., Hussain T., Lokhandwala M. F. (2003) Am. J. Physiol. Renal Physiol. 285, F1100–F1107 [DOI] [PubMed] [Google Scholar]
- 17.Féraille E., Carranza M. L., Gonin S., Béguin P., Pedemonte C., Rousselot M., Caverzasio J., Geering K., Martin P. Y., Favre H. (1999) Mol. Biol. Cell 10, 2847–2859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Khalili L., Kotova O., Tsuchida H., Ehrén I., Féraille E., Krook A., Chibalin A. V. (2004) J. Biol. Chem. 279, 25211–25218 [DOI] [PubMed] [Google Scholar]
- 19.Michlig S., Mercier A., Doucet A., Schild L., Horisberger J. D., Rossier B. C., Firsov D. (2004) J. Biol. Chem. 279, 51002–51012 [DOI] [PubMed] [Google Scholar]
- 20.Kiroytcheva M., Cheval L., Carranza M. L., Martin P. Y., Favre H., Doucet A., Féraille E. (1999) Kidney Int. 55, 1819–1831 [DOI] [PubMed] [Google Scholar]
- 21.Cheng X. J., Höög J. O., Nairn A. C., Greengard P., Aperia A. (1997) Am. J. Physiol. 273, C1981–C1986 [DOI] [PubMed] [Google Scholar]
- 22.Feschenko M. S., Sweadner K. J. (1994) J. Biol. Chem. 269, 30436–30444 [PubMed] [Google Scholar]
- 23.Carranza M. L., Féraille E., Favre H. (1996) Am. J. Physiol. Cell Physiol. 271, C136–C143 [DOI] [PubMed] [Google Scholar]
- 24.Melvin J. E., Yule D., Shuttleworth T., Begenisich T. (2005) Annu. Rev. Physiol. 67, 445–469 [DOI] [PubMed] [Google Scholar]
- 25.Soltoff S. P., McMillian M. K., Cantley L. C., Cragoe E. J., Jr., Talamo B. R. (1989) J. Gen. Physiol. 93, 285–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collins S. A., Pon D. J., Sen A. K. (1987) Biochim. Biophys. Acta 927, 392–401 [DOI] [PubMed] [Google Scholar]
- 27.Higashida H., Hoshi N., Zhang J. S., Yokoyama S., Hashii M., Jin D., Noda M., Robbins J. (2005) Neurosci Res. 51, 231–234 [DOI] [PubMed] [Google Scholar]
- 28.Kurihara K., Nakanishi N., Amano O., Yamamoto M., Iseki S. (2003) Biochem. Pharm. 66, 239–250 [DOI] [PubMed] [Google Scholar]
- 29.White C. N., Liu C. C., Garcia A., Hamilton E. J., Chia K. K., Figtree G. A., Rasmussen H. H. (2010) J. Biol. Chem. 285, 13712–13720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Turner J. T., Redman R. S., Camden J. M., Landon L. A., Quissell D. O. (1998) Am. J. Physiol. 275, C367–C374 [DOI] [PubMed] [Google Scholar]
- 31.Soltoff S. P., Hedden L. (2008) Am. J. Physiol. Cell Physiol. 295, C590–C599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurihara K., Nakanishi N., Amano O., Tonosaki K. (2008) Arch. Oral Biol. 53, 593–604 [DOI] [PubMed] [Google Scholar]
- 33.Sano A., Nakamura H. (2004) Anal. Sci. 20, 565–566 [DOI] [PubMed] [Google Scholar]
- 34.Larsen M. R., Thingholm T. E., Jensen O. N., Roepstorff P., Jørgensen T. J. (2005) Mol. Cell. Proteomics 4, 873–886 [DOI] [PubMed] [Google Scholar]
- 35.Asara J. M., Allison J. (1999) J. Am. Soc. Mass Spectrom. 10, 35–44 [DOI] [PubMed] [Google Scholar]
- 36.Soltoff S. P., Hedden L. (2010) J. Biol. Chem. 285, 13337–13348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bradford M. D., Soltoff S. P. (2002) Biochem. J. 366, 745–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plourde D., Soltoff S. P. (2006) Am. J. Physiol. Cell Physiol. 290, C702–C710 [DOI] [PubMed] [Google Scholar]
- 39.Sottejeau Y., Belliard A., Duran M. J., Pressley T. A., Pierre S. V. (2010) Biochemistry 49, 3602–3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatou S., Yamada M., Akune Y., Mochizuki H., Shiraishi A., Joko T., Nishida T., Tsubota K. (2010) Invest. Ophthalmol. Vis. Sci. 51, 3935–3942 [DOI] [PubMed] [Google Scholar]
- 41.El-Beialy W., Galal N., Deyama Y., Yoshimura Y., Suzuki K., Tei K., Totsuka Y. (2010) J. Membr Biol. 233, 119–126 [DOI] [PubMed] [Google Scholar]
- 42.Efendiev R., Pedemonte C. H. (2006) J. Am. Soc. Nephrol. 17, 31–38 [DOI] [PubMed] [Google Scholar]