Abstract
There is increasing evidence that thiazolidinediones (TZDs), antidiabetic compounds that are synthetic ligands for the peroxisome proliferator-activated receptor γ (PPARγ), have cardiovascular effects through as yet poorly defined mechanisms. We tested the effect of two TZD class drugs, rosiglitazone and pioglitazone, on human aortic smooth muscle cell (SMC) expression of insulin-like growth factor-1 receptor (IGF-1R). Both TZDs dose dependently up-regulated IGF-1R protein levels (rosiglitazone, 10 μmol/liter, 67% increase, n = 4, p < 0.01; pioglitazone, 10 μmol/liter, 41% increase, n = 4, p < 0.01) and increased IGF-1R signaling activity (36% increase in Akt phosphorylation). However, the endogenous PPARγ ligand, 15-deoxy-Δ12,14-prostaglandin J2, dose dependently reduced IGF-1R (10 μmol/liter, 80% decrease, n = 4, p < 0.01), and overexpression of PPARγ using an adenovirus likewise reduced IGF-1R (50% decrease versus SMC infected with control adenovirus), suggesting a PPARγ-independent action of TZDs. All three PPARγ ligands (rosiglitazone, pioglitazone, and 15-deoxy-Δ12,14-prostaglandin J2), however, did not change IGF-1R mRNA levels, indicating that their effects were posttranscriptional. Use of bicistronic constructs revealed that TZD induction of IGF-1R translation occurred via internal ribosomal entry. To examine the potential physiological relevance of TZD up-regulation of IGF-1R, we determined the effect of rosiglitazone on oxidized LDL (oxLDL)-induced apoptosis. 20 μmol/liter of rosiglitazone reduced oxidized LDL-induced apoptosis by 40% and neutralizing antibody to IGF-1R (αIR3) counteracted this rescue, suggesting the rosiglitazone survival effect was, at least in part, mediated by IGF-1R. In conclusion, TZDs markedly up-regulate SMC IGF-1R expression and signaling, likely via a PPARγ-independent mechanism. This novel action of TZDs may play an important role in their cardiovascular effects.
Keywords: Apoptosis, Insulin-like Growth Factor (IGF), Oxidative Stress, Receptors, Smooth Muscle, Translation Regulation, Thiazolidinediones, Peroxisome Proliferator-activated Receptor γ
Introduction
Thiazolidinediones (TZDs)2 are insulin-sensitizing drugs used in patients with type 2 diabetes mellitus. Over the past decade, it has been suggested that TZDs could have anti-atherosclerotic effects (1–4), although human studies thus far have not been conclusive with regard to potential benefits (5–7), and one meta-analysis has suggested that rosiglitazone increased the risk of myocardial infarction (8). PPARγ agonists including TZDs have been found to reduce atherosclerosis in rodent models such as LDL receptor-null mice and apolipoprotein E-deficient mice (9–12). TZDs inhibit proliferation and migration of cultured vascular smooth muscle cells (SMCs), which may contribute to their potential beneficial effect on atherogenesis (13–15).
Insulin-like growth factor-1 (IGF-1), a growth factor found in the circulation and also produced locally in multiple tissues including the vasculature, has pleiotropic effects on vascular cells including SMCs (reviewed in Refs. 16 and 17). For instance, IGF-1 is a mitogen for vascular SMCs and could be involved in the restenotic process after mechanical vascular injury (18, 19). Contrary to its mitogenic effect, it has also been reported that IGF-1 signaling is important for maintenance of the differentiated SMC phenotype (20, 21). IGF-1 is a potent survival factor for vascular SMCs. Thus, we have previously reported that oxidized low density lipoprotein (oxLDL), a highly pro-atherogenic lipoprotein present in circulation and in atherosclerotic plaques (22, 23), down-regulates IGF-1 in vascular cells (16, 24), and overexpression of IGF-1 receptor (IGF-1R) prevents oxLDL-induced apoptosis of vascular cells (25). Intriguingly, IGF-1 and IGF-1R expression are reduced in areas of advanced human plaque staining positive for oxLDL (26, 27), indicating a potential association between reduced IGF-1 function and the acellular phenotype of advanced plaques. Collectively, our studies suggest that IGF-1 serves as a survival factor for vascular SMCs, preventing SMC loss from atherosclerotic plaques, and potentially contributing to plaque stabilization (25, 28). Indeed, we have recently shown that IGF-1 infusion in apolipoprotein E-deficient mice reduces atherosclerotic plaque burden (29).
In this study, we examined the potential action of TZDs on IGF-1R expression and signaling in vascular SMC. Our findings indicate that TZDs up-regulate IGF-1R levels via a PPARγ independent pathway, uncovering a novel mechanism that could contribute to the potential cardiovascular effects of TZDs.
EXPERIMENTAL PROCEDURES
Cell Culture
Cultured human aortic SMC (HASMC, Lonza) were grown in SmGM-2 medium (Lonza) supplemented with 5% fetal calf serum, antibiotics, 0.5 μg/ml of human recombinant epidermal growth factor, 5 μg/ml of insulin, and 1 μg/ml of human recombinant fibroblast growth factor. The cells were used for experiments between passages 4 to 10. All the experiments were done in a serum-free condition using a 1:1 mixture of Dulbecco's modified essential medium and F-12 nutrient solution (Invitrogen).
Reagents
Reagents were obtained as follows. Rosiglitazone and pioglitazone were from Cayman Chemical; the neutralizing monoclonal antibody against IGF-1R, αIR3, from EMD Biosciences; rabbit polyclonal anti-IGF-1R β-chain antibody and monoclonal anti-PPARγ antibody from Santa Cruz Biotechnology; Cell-death Detection ELISA was from Roche Applied Science. The adenovirus encoding human PPARγ-1 was a generous gift from Dr. Yuqing Chen (30). The tk-pPPREx3-Luc (31) vector for the reporter gene assay was a generous gift from Dr. Ronald M. Evans, Salk Institute.
Bicistronic Vectors
The bicistronic plasmid vectors were created in our laboratory (32). Briefly, the Renilla luciferase (RLuc) reporter gene from plasmid pRL-TK (Promega) was extracted by NheI/XbaI digestion and inserted into the Klenow-filled XhoI site of plasmid pCI (Promega) giving the pC-RL plasmid. Firefly luciferase (FLuc) reporter gene was then subcloned from the pGL3 plasmid (Promega) into the SmaI site of pC-RL. In the resulting plasmid, called pBiC, transcription of the first cistron corresponding to RLuc is under control of the cytomegalovirus promoter. The sequence coding for the entire 5′ UTR (943 bp) of the IGF-IR mRNA was amplified by PCR using plasmid pBSK-943 (32) as template and oligonucleotides 5′-AAAGAATTCAGTGTGTGGCGGCGGCGG-3′ and 5′-AAAGTCGACTCCTTTTATTTGGGACGA-3′, which contained an EcoRI site and a SalI site, respectively. The PCR product was then inserted in the pGEM-T easy vector (Promega), giving the pGEM-943 plasmid, and sequenced. The EcoRI/SalI fragment containing the sequence coding for the 5′ UTR was then introduced in the EcoRI and SalI sites of pBiC vector giving the pBiC-943 vector. The pBiC-EMCV vector was constructed by inserting encephalomyocarditis virus (EMCV)-derived internal ribosomal entry site (IRES) sequence between the two cistrons (32).
Lipoprotein Preparation
Native LDL (nLDL) was separated from human plasma of healthy donor (purchased from The Blood Center, New Orleans, LA) by sodium bromide stepwise density gradient centrifugation, and then dialyzed against PBS containing 0.25 mm EDTA to remove sodium bromide. OxLDL was prepared as previously described. Briefly, an aliquot of the nLDL fraction was passed through a 10DG desalting column (Bio-Rad) to remove EDTA, then the nLDL fraction (0.2 mg/ml, diluted in PBS) was incubated with 5 μm CuSO4 at 37 °C for 3 h. The reaction was stopped by adding EDTA (final concentration 0.25 mm). The oxLDL prepared under these conditions showed an increase in relative mobility on agarose gel electrophoresis, and the value for thiobarbituric acid-reactive substances in oxLDL was 37.2 ± 1.2 nmol of malondialdehyde/mg of protein. Thiobarbituric acid-reactive substance was not detectable in nLDL.
Western Blot Analysis
Western blot analysis was performed as described previously (28). In brief, cells were washed with PBS and lysed in RIPA buffer, containing 150 mm NaCl, 20 mm Tris-Cl, pH 7.2, 1 mm EDTA, 1% Nonidet P-40, 5 mm dithiothreitol, 0.1 mm phenylmethylsulfonyl fluoride, 1 mm sodium orthovanadate, 0.1 m okadaic acid, 0.1 μm aprotinin, 10 μg/ml of leupeptin, and 10 mm NaF. Lysates were subjected to 10% SDS-PAGE and Western blotting analysis. Immunopositive bands were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences). Blots were stripped and reprobed with monoclonal anti-β-actin antibody as a control for equal loading.
Reporter Gene Assay
For the PPARγ-dependent reporter gene assay, transient expression was achieved in human aortic SMC using 50:1 mixture of tk-PPREx3-Luc plasmid and pGL4.75-hRluc/CMV plasmid (Promega) by electroporation (Nucleofector reagent and equipment from Lonza, Basel, Switzerland). Transfected cells were cultured in SmGM-2 medium for 24 h, and then exposed to PPARγ ligands in serum-free DMEM/F-12 medium. PPRE-responsive luciferase expressions were determined and normalized to RLuc expressions using the Dual-luciferase Reporter Assay System (Promega). For the bicistronic reporter assay, after a vector DNA transfection by electroporation as described above, the cells were exposed to 0–10 μmol/liter of TZD for 24 h, followed by the dual-luciferase activity assay.
Real Time PCR Analysis of Gene Expression
Human aortic SMC were exposed to testing agents in serum-free DMEM/F-12 for 24 h and then lysed in Tripure reagent (Roche). Total RNA was extracted and precipitated by isopropyl alcohol, and was further purified using the RNeasy kit (Qiagen). The total RNA was subjected to a reverse transcriptase reaction using RT2 First Strand Kit (SuperArray Bioscience, Frederick, MD), subsequently followed by real time PCR analysis. To analyze IGF-1R gene expression, a specific primer set purchased from SuperArray Bioscience (catalogue number, PPH00350E) was used in RT2 PCR Master Mix, taking β-actin (catalogue number, PPH00073E) gene expression as an internal control. To analyze the expression of the bicistronic construct, we used primer sets specific for the RLuc and FLuc sequences: Rluc, 5′-AACGCGGCCTCTTCTTATTT-3′ and 5′-ATTTGCCTGATTTGCCCATA-3′; Fluc, 5′-TCGCCAGTCAAGTAACAAC-3′ and 5′-ACTTCGTCCACAAACACAA-3′.
DNA Fragmentation Analysis
Fragmented DNA in cytoplasmic fractions was detected and quantified by Cell Death Detection ELISA kits (Roche Molecular Biochemicals) according to the manufacturer's protocol. Briefly, cells were exposed to 60 μg/ml of nLDL or oxLDL for 24 h in serum-free medium, then lysed in 100 μl of lysis buffer, and centrifuged for 10 min at 200 × g. The resulted supernatants were placed into the streptavidin-coated microtest plates, together with biotinylated anti-histone antibody and peroxidase-conjugated anti-DNA antibody. After incubation and wash, the peroxidase activity retained in the immunocomplex was determined photometrically with 2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid) as substrate (absorbance at 405 nm with a reference wavelength at 490 nm).
Statistical Analysis
Data are presented as mean ± S.E. Statistical analysis was performed using one-way analysis of variance or Student's t test, with a value of p < 0.05 considered to be significant. All experiments were performed a minimum of three times.
RESULTS
IGF-1R and Insulin Receptor Expression in Human Aortic SMCs
Human aortic SMCs express IGF-1R, insulin receptor (InsR), and their hybrid receptor (heterotetramer consisting of one each of α- and β-subunits of IGF-1R and one each of α- and β-subunits of InsR (17)). Consistent with a previous report (33), human aortic SMCs responded to physiological doses of IGF-1 but not to insulin (supplemental Fig. S1). A high dose of insulin (76 ng/ml = 13 nm), which can stimulate IGF-1R and hybrid receptors, up-regulated Akt phosphorylation, although to a lesser extent than 10 ng/ml (1.3 nm) IGF-1 (supplemental Fig. S1). The growth medium for SMCs (SmGM2; Lonza) is supplemented with high dose insulin, which caused ligand-induced down-regulation of InsR and also of IGF-1R, as did insulin at a high dose (supplemental Fig. S2). Therefore, switching to serum-free medium caused a significant up-regulation of IGF-1R. Assessment of hybrid receptor expression by immunoprecipitation (supplemental Fig. S3) indicated that in the presence of a high dose of insulin (5 μg/ml) the ratio of IGF-1R to IGF-1R/InsR hybrids to InsR was 100:1.3:0.5, although in the serum-free condition it was 100:13.5:4.1. Overall, human aortic SMCs express predominantly IGF-1R and IGF-1R/InsR hybrids and a low level of InsR.
Thiazolidinediones Up-regulate IGF-1R Expression
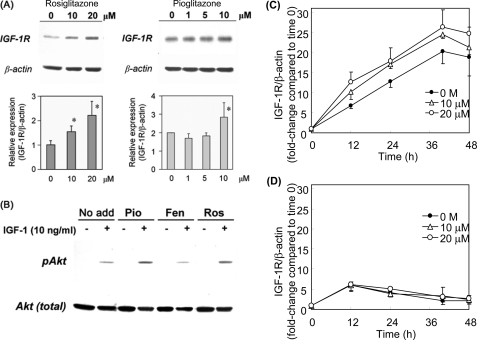
To determine potential effects of TZDs on IGF-1R expression, we exposed human aortic SMCs to TZDs (rosiglitazone or pioglitazone) for 24 h and examined IGF-1R expression by Western blot analysis. As shown in Fig. 1A, both TZDs dose dependently up-regulated IGF-1R levels. We further tested high doses of TZDs and found that 40 μm rosiglitazone up-regulated IGF-1R by ∼1.4-fold, whereas 80 μm rosiglitazone caused cell toxicity (supplemental Fig. S4). We then examined whether the increase in receptor protein levels was associated with up-regulation of the IGF-1R downstream signaling activity. After exposure to 10 μmol/liter of rosiglitazone and pioglitazone for 24 h, SMCs were incubated with 10 ng/ml of DES-IGF-1 for 10 min, and cell lysates were subjected to Western blot analysis for levels of Akt phosphorylation. DES-IGF-1 induced robust Akt phosphorylation (Fig. 1B) and preincubation with rosiglitazone and pioglitazone substantially increased the response to DES-IGF-1, whereas the same concentration of fenofibrate, a ligand for PPARα, had no effect (Fig. 1B). These results indicate that both TZDs up-regulate IGF-1R protein and its downstream signaling activity.
FIGURE 1.
Rosiglitazone and pioglitazone up-regulate IGF-1R levels and downstream signaling in human aortic SMCs. A, IGF-1R (β-chain) expression, dose response. Human aortic SMCs were incubated for 24 h with rosiglitazone or pioglitazone at the indicated doses, and IGF-1R protein levels were determined by Western blot analysis with β-actin levels as a loading control. *, p < 0.01 versus 0 μmol/liter (n = 4). B, rosiglitazone and pioglitazone up-regulate IGF-1-dependent phosphorylation of Akt. SMCs were incubated either in serum-free medium alone (No add), or with 10 μmol/liter of pioglitazone (Pio), 10 μmol/liter of fenofibrate (Fen), or 10 μmol/liter of rosiglitazone (Ros) for 24 h, and then stimulated with DES-IGF-1 (10 μmol/liter) for 10 min. Akt phosphorylation (Ser473) was assessed by Western blot analysis. C, time course of IGF-1R induction by rosiglitazone. SMCs were incubated with 0–20 μmol/liter of rosiglitazone for up to 48 h, and IGF-1R protein levels were determined by Western blot analysis. D, effect of cycloheximide. SMCs were preincubated with 10 μg/ml of cycloheximide for 1 h, and then co-incubated with cycloheximide and rosiglitazone for up to 48 h and IGF-1R protein levels measured. C and D are representative data from 3 independent experiments. Data are mean ± S.E.
To gain insights into potential mechanisms, we assessed the time course of IGF-1R induction by rosiglitazone (Fig. 1, C and D). In the serum-free control condition IGF-1R expression increased over 40 h, and then plateaued (Fig. 1C). We found that insulin in the complete medium (SmGM-2) suppressed IGF-1R expression, as indicated by the dose-dependent down-regulation of IGF-1R by increasing doses of insulin and up-regulation of IGF-1R when culturing cells in serum-free medium, compared with SmGM-2 medium containing insulin (supplemental Fig. S2). Rosiglitazone markedly enhanced IGF-1R expression (2-fold increase as early as 12 h) and this effect persisted up to 48 h (Fig. 1C). Rosiglitazone was also effective in up-regulating IGF-1R in the complete medium (supplemental Fig. S5). Cycloheximide, used to inhibit de novo synthesis of IGF-1R (Fig. 1D), did not inhibit the initial increase in IGF-1R expression seen over 12 h in response to serum deprivation (Fig. 1D). This initial increase resulted from conversion of pro-receptor to mature receptor as evidenced by disappearance of ∼250 kDa proreceptor dimers (data not shown). However, in the presence of cycloheximide, the IGF-1R levels declined after 12 h of incubation, indicating that the latter phase of up-regulation (>12 h) under serum-free conditions is due to increased synthesis of IGF-1R. More importantly, cycloheximide completely abolished rosiglitazone-induced IGF-1R expression at any time points compared with serum-free control; hence the TZDs up-regulation of IGF-1R is likely mediated by increased receptor synthesis.
PPARγ Activation by a Non-TZD Ligand Down-regulates IGF-1R Expression
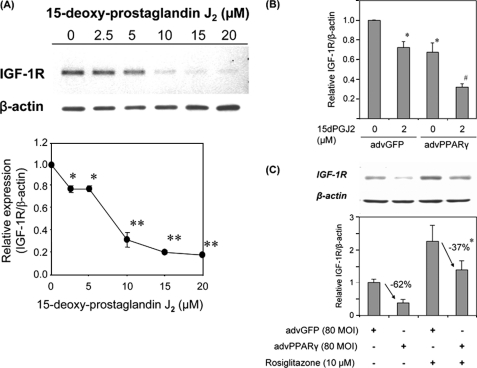
To determine potential mechanisms whereby TZDs up-regulate IGF-1R, we tested the non-TZD PPARγ ligand, 15-deoxy-Δ12,14-prostaglandin J2 (PGJ2) (31) for its effect on IGF-1R expression. As shown in Fig. 2A, PGJ2 dose dependently down-regulated IGF-1R. We tested low doses of PGJ2 in a separate experiment and found that 1 μm PGJ2 moderately down-regulated IGF-1R (∼25% decrease, supplemental Fig. S6). We further tested the effect of PGJ2 action in combination with PPARγ overexpression by adenoviral vector. An adenovirus encoding human PPARγ-1 markedly increased PPARγ protein levels compared with the control adenovirus (encoding green fluorescent protein; advGFP, data not shown). Intriguingly, IGF-1R protein levels were decreased in cells infected with advPPARγ (Fig. 2B). PGJ2 (2 μmol/liter) decreased IGF-1R protein levels by ∼30% (Fig. 2B) in advGFP-infected human aortic SMCs; however, in SMCs infected with advPPARγ there was a synergistic decrease in IGF-1R in response to PGJ2 (more than 50% decrease compared with advPPARγ-infected cells without PGJ2 co-incubation). This result strongly suggests that activation of PPARγ by PGJ2 leads to down-regulation of IGF-1R.
FIGURE 2.
PPARγ expression and PGJ2 coordinately down-regulate IGF-1R levels. A, PGJ2 dose response. SMCs were exposed to 0–20 μmol/liter of PGJ2 for 24 h and IGF-1R levels were determined by Western blot analysis. *, p < 0.05 and **, p < 0.01 versus 0 μmol/liter (n = 4). B, SMCs were infected with advGFP or advPPARγ (40 multiplicity of infection (MOI), respectively) and exposed to the indicated doses of PGJ2 for 24 h. IGF-1R protein levels were assessed by Western blot analysis. *, p < 0.01 versus advGFP, PGJ2 untreated, n = 4; #, p < 0.01 versus advPPARγ, PGJ2 untreated, n = 4. C, rosiglitazone attenuates advPPARγ-induced down-regulation of IGF-1R. SMCs were infected with advGFP or advPPARγ and exposed to 10 μmol/liter of rosiglitazone for 24 h. IGF-1R levels were assessed by Western blot analysis, and the extent of decrease in IGF-1R caused by advPPARγ was analyzed and compared between no rosiglitazone and 10 μmol/liter of rosiglitazone conditions. *, p < 0.01, n = 4. Data are mean ± S.E.
Rosiglitazone Attenuates IGF-1R Down-regulation Induced by PPARγ Overexpression
We also examined the effect of a TZD, rosiglitazone, in combination with advPPARγ on IGF-1R expression. As shown in Fig. 2C, PPARγ overexpression down-regulated IGF-1R by 62% (advGFP versus advPPARγ, p < 0.01, n = 4) and this decrease was significantly blunted by rosiglitazone (p < 0.05, n = 4). These data suggest that the mechanism whereby TZDs up-regulate IGF-1R is distinct from their action as PPARγ agonists; it appears that TZDs antagonize PPARγ-dependent down-regulation of IGF-1R.
Rosiglitazone Has No Effect on IGF-1R mRNA Levels, but Increases IGF-1R Translation
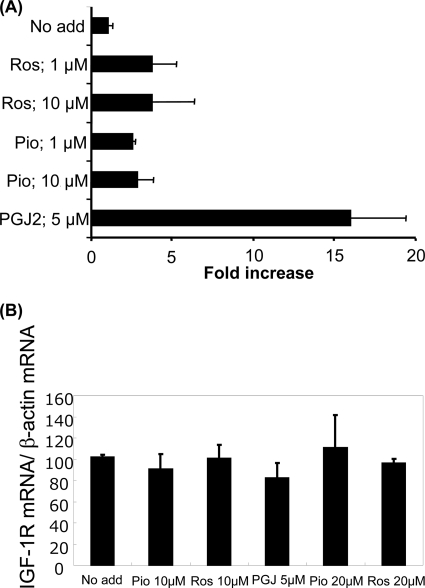
PPARγ is a nuclear receptor, which regulates transcription of target genes upon ligand binding. Because TZDs and PGJ2 showed sharply contrasting effects on IGF-1R expression, we tested their ability to induce PPARγ-dependent reporter gene expression (Fig. 3A). Rosiglitazone, pioglitazone, and PGJ2 enhanced luciferase gene expression driven by the PPARγ-responsive promoter in human aortic SMCs (Fig. 3A). Thus TZDs as well as PGJ2 have authentic activity as PPARγ ligands in human aortic SMCs.
FIGURE 3.
TZDs enhance PPARγ-mediated luciferase expression but do not alter IGF-1R mRNA levels. A, SMCs were transfected with tk-pPPREx3-Luc and pGL4.75-hRluc/CMV vectors and exposed to TZDs (rosiglitazone, Ros; pioglitazone, Pio) and PGJ2 for assessment of reporter gene expression. The FLuc activity normalized to RLuc activity is expressed as a relative value to the control (No add). A representative experiment from 2 independent experiments is shown. B, IGF-1R mRNA levels were determined by quantitative real time PCR in aortic human SMCs. The cells were treated with TZDs and PGJ2 at the indicated doses for 24 h. The experiment was repeated 3 times. Data are mean ± S.E.
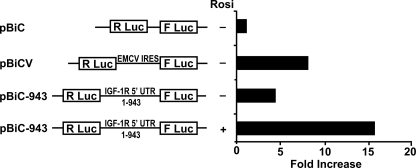
To determine potential transcriptional regulation of IGF-1R gene expression by PPARγ ligands, we measured IGF-1R mRNA levels by real time PCR. Neither TZDs nor PGJ2 altered IGF-1R mRNA levels at 24 h (Fig. 3B). We also tested the effect of TZDs and PGJ2 on IGF-1R gene promoter activity using a −2350/+640 bp IGF-1R gene promoter luciferase construct (34). Neither 20 μmol/liter of rosiglitazone nor 5 μmol/liter of PGJ2 altered IGF-1R promoter activity in human aortic SMCs (data not shown). These data suggested that TZD up-regulation of IGF-1R was mediated not by transcriptional but by translational or post-translational mechanisms. We have previously reported that the IGF-1R mRNA has an IRES in its 5′-untranslated region, which directs 5′-m7G cap independent translation initiation (32). We therefore examined potential IRES-dependent regulation of IGF-1R translation by TZDs using a bicistronic vector strategy as previously described (32). Briefly, in the basic bicistronic construct (pBiC), translation of the first cistron (RLuc) is 5′-m7G cap-dependent, whereas the second cistron (FLuc) requires translation by internal ribosomal entry. These constructs were transfected into human aortic SMC, which were then treated with rosiglitazone in serum-free condition for 24 h. We used pBiC with an EMCV-derived IRES sequence inserted between the two cistrons as a positive control (pBiCV). The insertion of EMCV-IRES resulted in significant expression of FLuc in transfected SMCs (Fig. 4). SMCs transfected with pBiC-943, which has 943 base pairs of 5′ UTR of the IGF-1R mRNA sequence inserted in the intercistronic region, also had significant levels of FLuc expression, consistent with our previous report (32). Rosiglitazone markedly enhanced FLuc expression in pBiC-943-transfected cells, suggesting that TZDs enhance IRES-mediated IGF-1R translation. We also assessed the effect of rosiglitazone in complete medium (SmGM-2; supplemental Fig. S7). Interestingly, in complete medium the expression of FLuc in pBiC-943-transfected cells was substantially lower than in the serum-free condition (supplemental Fig. S6). Rosiglitazone also enhanced FLuc expression in complete medium, although the extent of the increase was less than that in serum-free medium (supplemental Fig. S7). To exclude the possibility that rosiglitazone-induced promoter activity within the IGF-1R 5′ UTR, we analyzed expression of the two cistrons by real time PCR. Fluc/RLuc mRNA expression ratio was 1.36 ± 0.10 (n = 3) in pBiC plasmid-transfected cells. Rosiglitazone did not increase the ratio in pBiC943 plasmid-transfected cells (1.40 ± 0.29 in control culture condition versus 1.07 ± 0.32 in 10 μmol/liter of rosiglitazone, n = 3), indicating that no promoter activity was induced within the IGF-1R 5′ UTR by rosiglitazone.
FIGURE 4.
Rosiglitazone enhances IRES-dependent translation within the IGF-R 5′ UTR. The indicated bicistronic reporter plasmids were transfected into human aortic SMCs, and tested for IRES-dependent translation initiation by determining FLuc activity, which is dependent on translation of the second cistron. FLuc activity was normalized to RLuc activity, which is dependent on the first cistron, (5′ cap-dependent translation). The ratio of FLuc/RLuc activity was calculated basally (pBic), in the presence of a positive control (pBicV) and in the presence of pBic-943 (which contains the IGF-1R 5′ UTR) with or without 10 μmol/liter rosiglitazone (Rosi). Shown is a representative experiment repeated 3 times.
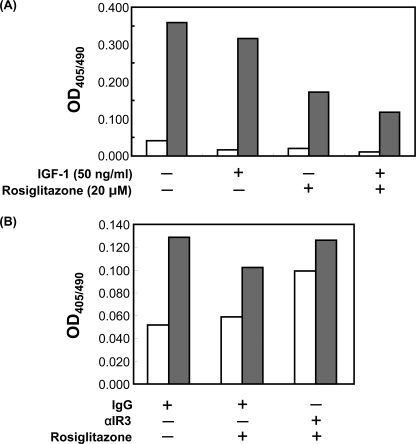
TZDs Enhance the Anti-apoptotic Effects of IGF-1
We have shown that oxLDL-induced apoptotic cell death of human aortic SMCs was effectively prevented by enhancement of IGF-1 signaling by overexpressing IGF-1R (28). Consistent with our previous study, DES-IGF-1 (50 ng/ml) partially prevented apoptotic genomic DNA fragmentation induced by oxLDL in human aortic SMCs (Fig. 5A). Intriguingly, rosiglitazone also prevented oxLDL-induced DNA fragmentation and this effect was more pronounced than that of DES-IGF-1. The combination of rosiglitazone and DES-IGF-1 additively lowered DNA fragmentation (Fig. 5A). To examine whether the anti-apoptotic effect of rosiglitazone was mediated by IGF-1R, we pre- and co-incubated cells with neutralizing monoclonal antibody against IGF-1R (αIR3) and measured oxLDL-induced DNA fragmentation (Fig. 5B). 20 μmol/liter of rosiglitazone attenuated oxLDL-induced genomic DNA fragmentation by 44%, however, αIR3 pre- and co-incubation completely reversed this effect of rosiglitazone. αIR3 also increased DNA fragmentation in cells incubated in native LDL consistent with inhibition of autocrine IGF-1, which is normally released from cultured SMC (35). These results suggested that the ability of TZDs to inhibit oxLDL-induced apoptosis is, at least in part, mediated by the IGF-1R.
FIGURE 5.
Rosiglitazone attenuates oxidized LDL-induced DNA fragmentation in human aortic SMCs. A, SMCs were co-incubated (24 h) with 50 ng/ml of DES-IGF-1 and 20 μmol/liter of rosiglitazone in serum-free medium containing 60 μg/ml of native LDL (open) or oxLDL (gray). Genomic DNA fragmentation was assessed by Cell Death Detection ELISA (Roche). Shown is representative data from 5 independent experiments. B, SMCs were co-incubated (24 h) with 20 μmol/liter of rosiglitazone and 5 μg/ml of αIR3 (neutralizing antibody against IGF-1R) in serum-free medium containing 60 μg/ml of native LDL (open) or oxidized LDL (gray). Genomic DNA fragmentation was assessed by Cell Death Detection ELISA (Roche). Data are from a representative experiment repeated 3 times.
DISCUSSION
In this study, we have made two important findings. First, TZDs, namely rosiglitazone and pioglitazone, up-regulated IGF-1 receptor levels in human aortic SMC via a PPARγ-independent translational mechanism. Second, TZD induction of IGF-1R expression led to enhanced downstream signaling and conferred a cell survival effect as demonstrated by the ability of rosiglitazone to rescue SMC from apoptotic cell death induced by the pro-atherogenic molecule, oxLDL. The discovery that TZDs up-regulate IGF-1R in SMC may be important for the understanding of the clinical effects of these widely used anti-diabetic drugs.
TZDs are synthetic ligands for a nuclear receptor, PPARγ, and thus their actions are primarily mediated by PPARγ activation, leading to enhanced expression of target genes (36). Our data, however, indicate that TZD up-regulation of IGF-1R is unlikely to result from direct regulation of IGF-1R gene expression by PPARγ. Paradoxically, PPARγ overexpression and an endogenous ligand for PPARγ, PGJ2, coordinately down-regulated IGF-1R, indicating that activation of PPARγ signaling primarily suppressed IGF-1R expression. These contradictory observations suggested that the effect of TZDs was PPARγ-independent; however, they could not completely exclude potential involvement of PPARγ. For instance, TZDs can recruit cofactors different from those recruited by vacant PPARγ or PGJ2-liganded PPARγ, leading to a positive regulation for the target gene (37, 38). We thus examined IGF-1R mRNA levels, seeking possible up-regulation by TZDs and down-regulation by PGJ2. However, IGF-1R mRNA levels were not influenced by either TZDs or PGJ2, and reporter gene assays testing the activity of the IGF-1R gene promoter region (−2340/+640 bp) supported these results and showed no regulation by TZDs and PGJ2. Thus we concluded that there is no direct regulation of IGF-1R mRNA expression by TZDs and PGJ2.
Time course analysis of IGF-1R expression in the presence of cycloheximide suggested that TZDs up-regulated IGF-1R via a translational mechanism. There have been some reports that TZDs may have translational effects; rosiglitazone administration in rats caused cardiac hypertrophy associated with increased activity of the mammalian target of rapamycin pathway (39). This pathway is known to stimulate translation initiation via a 5′-m7G cap-dependent mechanism. On the other hand, TZDs are reported to inhibit the mammalian target of rapamycin pathway indirectly by activating AMP-activated protein kinase in cancer cells (40). Therefore, the effect of TZDs on the mammalian target of rapamycin pathway is cell-line specific. It has also been reported that pioglitazone as well as docosahexaenoic acid and eicosapentaenoic acid, which are endogenous ligands for PPARγ, enhanced adiponectin translation in cultured adipocytes (41). The bicistronic reporter gene assay clearly showed that TZD increased translation from the second cistron (FLuc) and real time PCR analysis indicated no cryptic promoter activity, which leads to transcription from the second cistron. Overall, our data indicates that rosiglitazone up-regulates IGF-1R expression by a translational mechanism, which is mediated by IRES-dependent translation initiation. Intriguingly, our data showed that serum deprivation caused IGF-1R up-regulation (Fig. 1C) associated with higher IRES activity (supplemental Fig. S6). These data suggest that IGF-1R up-regulation by serum deprivation is, at least in part, mediated by IRES-dependent translation. Furthermore, the up-regulation of IGF-1R induced by serum deprivation is likely related to removal of insulin, which is present at high concentrations in SmGM-2 medium (supplemental Fig. S2).
An increasing number of genes have been identified as potentially regulated by IRES-dependent translation (reviewed in Refs. 42 and 43), highlighting the importance of this mechanism for cellular protein translation. However, potential processes regulating IRES-dependent translation initiation are largely unclear. The structures of IRES elements thus far identified are widely diverse; i.e. there is little primary sequence or secondary structure similarities shared by them (42, 43). Moreover, it is known that the activity of one particular IRES element is cell-line and/or cellular condition specific (42, 43). It is thus difficult to identify potential proteins, which associate with IRES elements in mRNAs thereby regulating IRES-dependent translation initiation.
The IRES activity in the 5′ untranslated region of the IGF-1R mRNA was first described by our group in COS-1 cells and rat fibroblasts (32), and it has since been described in other cell types (44, 45). Thus far, polypyrimidine tract-binding protein (32), HuR (45), and heterogeneous nuclear RNP C1/C2 (44) have been reported to associate with the 5′ portion of the IGF-1R mRNA thereby regulating IRES-dependent translation initiation. To our knowledge, this is the first report describing potential IRES-mediated translation initiation of IGF-1R in vascular SMCs. We have performed preliminary experiments assessing protein expression levels for the polypyrimidine tract-binding protein, heterogeneous nuclear RNA C1/C2, and HuR; however, we detected no change in expression of these proteins in response to TZDs.3 It is thus possible that some unidentified regulator(s) of the IGF-1R IRES in vascular SMCs is regulated by TZDs, or that TZDs regulate the association of the above mentioned RNA-binding proteins or other unidentified RNA-binding proteins to the IGF-1R mRNA in vascular SMCs.
In view of their widespread clinical utilization for the treatment of diabetes, there has been much debate about whether TZDs are beneficial, detrimental, or have no effect on cardiovascular mortality and morbidity. Many studies have shown that TZDs exert beneficial effects in animal models of atherosclerosis (1–4). However, outcomes from human trials are inconclusive (5–8) and thus further studies are required (and are ongoing). In this study, we showed that TZDs not only up-regulate IGF-1R levels, but also enhance its downstream signaling, leading to enhanced cell survival in the presence of the pro-atherogenic and pro-apoptotic molecule, oxLDL. In this respect, it is important to note that there is increasing evidence that IGF-1 may have atheroprotective effects (17). It is, thus, reasonable to speculate that these effects of TZDs contribute to their potential vascular actions (36). Furthermore, one can speculate that the ability of TZDs to improve glycemic control could be, in part, related to their ability to up-regulate IGF-1R. Of note, TZDs did not regulate IGF-1 expression in vascular SMC.3 As discussed above, activity of IRES elements is cell-line and cellular condition dependent, thus it is hard to predict whether TZDs up-regulate IGF-1R in other cell types and tissues. However, our study provides a strong rationale to investigate potential TZD effects on IGF-1R expression and signaling in other cell types/tissues; e.g. examination of potential cell-growth promoting effects of TZDs via IGF-1R up-regulation in tumor cells.
In summary, our data indicate that the TZDs rosiglitazone and pioglitazone up-regulate IGF-1R expression and signaling in vascular SMC leading to improved cell survival in the presence of oxLDL. The effect of TZDs is likely mediated via enhancement of IRES-dependent translation of the IGF-1R mRNA. To our knowledge, this is the first report of regulation of vascular SMC IGF-1R via IRES-dependent translation. Furthermore, we are unaware of any previous report of a pharmaceutical compound regulating translation via enhancement of internal ribosomal entry. In view of the widespread clinical use of TZDs in the treatment of diabetes and in view of the widespread distribution of IGF-1R in humans leading to well known pleiotropic effects of IGF-1 on development, proliferation, anabolism, and cell differentiation, our findings may have important implications for understanding clinical effects of TZD compounds.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants R01HL070241 and R01HL080682 from the NHLBI.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S7 and supplemental “Methods.”
Y. Higashi, unpublished data.
- TZDs
- thiazolidinediones
- PPARγ
- peroxisome proliferator-activated receptor γ
- SMC
- smooth muscle cell
- IGF-1
- insulin-like growth factor-1
- IGF-1R
- insulin-like growth factor-1 receptor
- oxLDL
- oxidized low-density lipoprotein
- RLuc
- Renilla luciferase
- FLuc
- Firefly luciferase
- nLDL
- native LDL
- PGJ2
- 15-deoxy-Δ12,14-prostaglandin J2
- advGFP
- adenovirus encoding green fluorescent protein
- IRES
- internal ribosomal entry site
- pBiC
- basic bicistronic construct
- EMCV
- encephalomyocarditis virus
- pBiCV
- bicistronic construct with EMCV-derived IRES sequence inserted in between the two cistrons
- pBiC-943
- bicistronic construct with 943 bp of IGF receptor 5′ UTR inserted between the two cistrons
- InsR
- insulin receptor.
REFERENCES
- 1.Semple R. K., Chatterjee V. K., O'Rahilly S. (2006) J. Clin. Invest. 116, 581–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barish G. D. (2006) J. Nutr. 136, 690–694 [DOI] [PubMed] [Google Scholar]
- 3.Schiffrin E. L. (2005) Am. J. Physiol. Heart Circ. Physiol. 288, H1037–1043 [DOI] [PubMed] [Google Scholar]
- 4.Hsueh W. A., Bruemmer D. (2004) Hypertension 43, 297–305 [DOI] [PubMed] [Google Scholar]
- 5.Home P. D., Pocock S. J., Beck-Nielsen H., Curtis P. S., Gomis R., Hanefeld M., Jones N. P., Komajda M., McMurray J. J. (2009) Lancet 373, 2125–213519501900 [Google Scholar]
- 6.Lonn E. M., Gerstein H. C., Sheridan P., Smith S., Diaz R., Mohan V., Bosch J., Yusuf S., Dagenais G. R. (2009) J. Am. Coll. Cardiol. 53, 2028–2035 [DOI] [PubMed] [Google Scholar]
- 7.Nissen S. E., Nicholls S. J., Wolski K., Nesto R., Kupfer S., Perez A., Jure H., De Larochellière R., Staniloae C. S., Mavromatis K., Saw J., Hu B., Lincoff A. M., Tuzcu E. M. (2008) JAMA 299, 1561–1573 [DOI] [PubMed] [Google Scholar]
- 8.Nissen S. E., Wolski K. (2007) N. Engl. J. Med. 356, 2457–2471 [DOI] [PubMed] [Google Scholar]
- 9.Li A. C., Brown K. K., Silvestre M. J., Willson T. M., Palinski W., Glass C. K. (2000) J. Clin. Invest. 106, 523–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minamikawa J., Tanaka S., Yamauchi M., Inoue D., Koshiyama H. (1998) J. Clin. Endocrinol. Metab. 83, 1818–1820 [DOI] [PubMed] [Google Scholar]
- 11.Chen Z., Ishibashi S., Perrey S., Osuga Ji, Gotoda T., Kitamine T., Tamura Y., Okazaki H., Yahagi N., Iizuka Y., Shionoiri F., Ohashi K., Harada K., Shimano H., Nagai R., Yamada N. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 372–377 [DOI] [PubMed] [Google Scholar]
- 12.Collins A. R., Meehan W. P., Kintscher U., Jackson S., Wakino S., Noh G., Palinski W., Hsueh W. A., Law R. E. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 365–371 [DOI] [PubMed] [Google Scholar]
- 13.Marx N., Schönbeck U., Lazar M. A., Libby P., Plutzky J. (1998) Circ. Res. 83, 1097–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Law R. E., Goetze S., Xi X. P., Jackson S., Kawano Y., Demer L., Fishbein M. C., Meehan W. P., Hsueh W. A. (2000) Circulation 101, 1311–1318 [DOI] [PubMed] [Google Scholar]
- 15.Goetze S., Xi X. P., Kawano H., Gotlibowski T., Fleck E., Hsueh W. A., Law R. E. (1999) J. Cardiovasc. Pharmacol. 33, 798–806 [DOI] [PubMed] [Google Scholar]
- 16.Delafontaine P., Song Y. H., Li Y. (2004) Arterioscler. Thromb. Vasc. Biol. 24, 435–444 [DOI] [PubMed] [Google Scholar]
- 17.Higashi Y., Sukhanov S., Anwar A., Shai S. Y., Delafontaine P. (2010) Trends Endocrinol. Metab. 21, 245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khorsandi M. J., Fagin J. A., Giannella-Neto D., Forrester J. S., Cercek B. (1992) J. Clin. Invest. 90, 1926–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nichols T. C., du Laney T., Zheng B., Bellinger D. A., Nickols G. A., Engleman W., Clemmons D. R. (1999) Circ. Res. 85, 1040–1045 [DOI] [PubMed] [Google Scholar]
- 20.Hayashi K., Saga H., Chimori Y., Kimura K., Yamanaka Y., Sobue K. (1998) J. Biol. Chem. 273, 28860–28867 [DOI] [PubMed] [Google Scholar]
- 21.Martin K. A., Merenick B. L., Ding M., Fetalvero K. M., Rzucidlo E. M., Kozul C. D., Brown D. J., Chiu H. Y., Shyu M., Drapeau B. L., Wagner R. J., Powell R. J. (2007) J. Biol. Chem. 282, 36112–36120 [DOI] [PubMed] [Google Scholar]
- 22.Itabe H., Yamamoto H., Imanaka T., Shimamura K., Uchiyama H., Kimura J., Sanaka T., Hata Y., Takano T. (1996) J. Lipid Res. 37, 45–53 [PubMed] [Google Scholar]
- 23.Itabe H., Takeshima E., Iwasaki H., Kimura J., Yoshida Y., Imanaka T., Takano T. (1994) J. Biol. Chem. 269, 15274–15279 [PubMed] [Google Scholar]
- 24.Scheidegger K. J., James R. W., Delafontaine P. (2000) J. Biol. Chem. 275, 26864–26869 [DOI] [PubMed] [Google Scholar]
- 25.Li Y., Higashi Y., Itabe H., Song Y. H., Du J., Delafontaine P. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 2178–2184 [DOI] [PubMed] [Google Scholar]
- 26.Okura Y., Brink M., Itabe H., Scheidegger K. J., Kalangos A., Delafontaine P. (2000) Circulation 102, 2680–2686 [DOI] [PubMed] [Google Scholar]
- 27.Okura Y., Brink M., Zahid A. A., Anwar A., Delafontaine P. (2001) J. Mol. Cell. Cardiol. 33, 1777–1789 [DOI] [PubMed] [Google Scholar]
- 28.Higashi Y., Peng T., Du J., Sukhanov S., Li Y., Itabe H., Parthasarathy S., Delafontaine P. (2005) J. Lipid Res. 46, 1266–1277 [DOI] [PubMed] [Google Scholar]
- 29.Sukhanov S., Higashi Y., Shai S. Y., Vaughn C., Mohler J., Li Y., Song Y. H., Titterington J., Delafontaine P. (2007) Arterioscler. Thromb. Vasc. Biol. 27, 2684–2690 [DOI] [PubMed] [Google Scholar]
- 30.Fu M., Zhang J., Lin Yg Y., Zhu X., Willson T. M., Chen Y. E. (2002) Biochem. Biophys. Res. Commun. 294, 597–601 [DOI] [PubMed] [Google Scholar]
- 31.Forman B. M., Tontonoz P., Chen J., Brun R. P., Spiegelman B. M., Evans R. M. (1995) Cell 83, 803–812 [DOI] [PubMed] [Google Scholar]
- 32.Giraud S., Greco A., Brink M., Diaz J. J., Delafontaine P. (2001) J. Biol. Chem. 276, 5668–5675 [DOI] [PubMed] [Google Scholar]
- 33.Chisalita S. I., Johansson G. S., Liefvendahl E., Bäck K., Arnqvist H. J. (2009) J. Mol. Endocrinol. 43, 231–239 [DOI] [PubMed] [Google Scholar]
- 34.Scheidegger K. J., Du J., Delafontaine P. (1999) J. Biol. Chem. 274, 3522–3530 [DOI] [PubMed] [Google Scholar]
- 35.Scheidegger K. J., Cenni B., Picard D., Delafontaine P. (2000) J. Biol. Chem. 275, 38921–38928 [DOI] [PubMed] [Google Scholar]
- 36.Hamblin M., Chang L., Fan Y., Zhang J., Chen Y. E. (2009) Antioxid. Redox Signal. 11, 1415–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chui P. C., Guan H. P., Lehrke M., Lazar M. A. (2005) J. Clin. Invest. 115, 2244–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guan H. P., Ishizuka T., Chui P. C., Lehrke M., Lazar M. A. (2005) Genes Dev. 19, 453–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Festuccia W. T., Laplante M., Brûlé S., Houde V. P., Achouba A., Lachance D., Pedrosa M. L., Silva M. E., Guerra-Sá R., Couet J., Arsenault M., Marette A., Deshaies Y. (2009) J. Mol. Cell. Cardiol. 47, 85–95 [DOI] [PubMed] [Google Scholar]
- 40.He G., Sung Y. M., Digiovanni J., Fischer S. M. (2006) Cancer Res. 66, 1873–1878 [DOI] [PubMed] [Google Scholar]
- 41.Banga A., Unal R., Tripathi P., Pokrovskaya I., Owens R. J., Kern P. A., Ranganathan G. (2009) Am. J. Physiol. Endocrinol. Metab. 296, E480–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baird S. D., Turcotte M., Korneluk R. G., Holcik M. (2006) RNA 12, 1755–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitzgerald K. D., Semler B. L. (2009) Biochim. Biophys. Acta 1789, 518–528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng Z., Jackson N. L., Choi H., King P. H., Emanuel P. D., Blume S. W. (2008) J. Cell. Physiol. 217, 172–183 [DOI] [PubMed] [Google Scholar]
- 45.Meng Z., King P. H., Nabors L. B., Jackson N. L., Chen C. Y., Emanuel P. D., Blume S. W. (2005) Nucleic Acids Res. 33, 2962–2979 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.