Abstract
Resveratrol (RSV) is a naturally occurring polyphenol that has been found to exert antioxidant, anti-inflammatory, and neuroprotective properties. However, how RSV exerts its beneficial health effects remains largely unknown. Here, we show that RSV inhibits insulin- and leucine-stimulated mTOR signaling in C2C12 fibroblasts via a Sirt1-independent mechanism. Treating C2C12 cells with RSV dramatically inhibited insulin-stimulated Akt, S6 kinase, and 4E-BP1 phosphorylation but had little effect on tyrosine phosphorylation of the insulin receptor and activation of the p44/42 MAPK signaling pathway. RSV treatment also partially blocked mTOR and S6 kinase phosphorylation in TSC1/2-deficient mouse embryonic fibroblasts, suggesting the presence of an inhibitory site downstream of TSC1/2. Knocking out PDK1 or suppressing AMP-activated protein kinase had little effect on leucine-stimulated mTOR signaling. On the other hand, RSV significantly increased the association between mTOR and its inhibitor, DEPTOR. Furthermore, the inhibitory effect of RSV on leucine-stimulated mTOR signaling was greatly reduced in cells in which the expression levels of DEPTOR were suppressed by RNAi. Taken together, our studies reveal that RSV inhibits leucine-stimulated mTORC1 activation by promoting mTOR/DEPTOR interaction and thus uncover a novel mechanism by which RSV negatively regulates mTOR activity.
Keywords: Adipocyte, Diabetes, Insulin Resistance, Metabolism, Obesity
Introduction
The naturally occurring polyphenol resveratrol (RSV)3 has received great attention during the past few years due to its beneficial roles in longevity, cardioprotection, and immune regulation. How RSV exerts its biological function remains to be fully elucidated, but activation of the NAD+-dependent deacetylase Sirt1 has been suggested as an important mechanism for the life span extension and cancer prevention properties of RSV (1). Several targets of RSV such as AMP-activated protein kinase (AMPK), Akt, and NF-κB have also been identified (2–4). However, it remains controversial as to whether activation of these signaling pathways is Sirt1-dependent and whether additional targets and mechanisms are involved in RSV-initiated beneficial function in cells.
mTOR (mammalian target of rapamycin) is a member of the phosphatidylinositol 3-kinase (PI3K)-related protein kinase subfamily that plays a critical role in the regulation of various cellular events such as cell growth and proliferation (5, 6). mTOR exists in two distinct complexes, TORC1 and TORC2, which differ in subunit compositions and biological functions (7). The rapamycin-sensitive mTORC1, which consists of five components, including mTOR, Raptor (regulatory-associated protein of mTOR), mLST8 (mammalian lethal with Sec13 protein 8; also known as GβL), PRAS40 (proline-rich Akt substrate of 40 kDa), and DEPTOR (DEP domain-containing and mTOR-interactive protein) (8), regulates protein synthesis and cell growth by phosphorylating downstream target proteins such as p70 ribosomal S6K1 (S6 kinase 1) and the eukaryotic initiation factor 4E-BP1 (5, 9). The rapamycin-insensitive TORC2, which comprises six components, including mTOR, Rictor (rapamycin-insensitive companion of mTOR), mSIN1 (mammalian stress-activated protein kinase-interacting protein 1), Protor-1 (protein observed with Rictor-1), mLST8, and DEPTOR (8), functions as an Akt kinase that phosphorylates Ser473 of Akt in the “hydrophobic motif” that is essential for full activation of this kinase (8, 10).
mTOR is activated by diverse upstream signals such as amino acids, insulin, and insulin-like growth factor-1. Although it is well established that insulin activates mTORC1 through the PI3K/Akt/TSC1/2 signaling pathway (11), the mechanism by which amino acids stimulates mTORC1 activity appears to be PI3K/Akt/TSC1/2 pathway-independent (12, 13). There is some evidence suggesting that RSV negatively regulates insulin- or angiotensin II-activated mTOR signaling through the PI3K/Akt pathway (3, 14, 15), yet whether RSV regulates amino acid-stimulated mTOR activation and its underlying mechanism remains largely unknown.
In this study, we investigated whether and how RSV regulates leucine-stimulated mTOR/S6K signaling. We found that RSV inhibits mTOR signaling via a Sirt1-independent mechanism. In addition, we found that disruption of PDK1/Akt signaling and TSC1/2 expression has no significant effect on the inhibitory effect of RSV on leucine-stimulated mTOR activation. Furthermore, we found that RSV promotes the association between mTOR and DEPTOR, an inhibitor of mTOR, thus uncovering a novel mechanism by which RSV inhibits mTOR signaling.
MATERIALS AND METHODS
Plasmids and Reagents
The pAd-Track-Sirt1 plasmid was obtained from Addgene. The AMPK inhibitor Compound C and Akt inhibitor III were from Calbiochem. Sirtinol, insulin, leucine, glutathione agarose beads, lysozyme, and RSV were from Sigma. Protein A-Sepharose beads were from Amersham Biosciences. Antibodies to Sirt1 and tubulin were from Upstate Biotech and Sigma, respectively. All other antibodies were from Cell Signaling.
Cell Culture
C2C12 cells (American Type Culture Collection) were cultured in DMEM (ATCC 30-2002) supplemented with 10% newborn calf serum and 1% penicillin/streptomycin. TSC1/2−/− and wild-type mouse embryonic fibroblasts (MEFs) (generous gifts of Dr. Kunliang Guan, University of California at San Diego) were grown in DMEM (ATCC 11960) supplemented with 10% newborn calf serum and 1% penicillin/streptomycin. Transfections were performed using Lipofectamine reagent (Invitrogen) according to the manufacturer's protocol. The Sirt1−/− cells were generated as described previously (16).
Generation of PDK1 Knock-out MEF Cells, AMPK-suppressed C2C12 Myoblasts, and DEPTOR-suppressed C2C12 Myoblasts
To generate PDK1-deficient cell lines, we isolated MEFs from a embryonic day 13.5 fetus that was homozygous for floxed PDK1 alleles (17). The cells were immortalized with a 3T3 protocol, and PDK1−/− MEFs were generated by retrovirus-mediated expression of the Cre gene. PDK1 MEFs were maintained in DMEM (Invitrogen 119995) supplemented with 10% FBS (Invitrogen 10437-028) and 1% penicillin and streptomycin (CellGro 30-0001-CI). To generate AMPK-suppressed C2C12 myoblasts, a plasmid encoding the AMPK short hairpin RNA construct cloned in the pSM2 vector (Open Biosystems RMM1766-96744125) was cotransfected with a plasmid encoding the puromycin resistance gene (pSV2-puro) into C2C12 cells. Stable cell lines containing the AMPK shRNA construct were selected using puromycin as described (18). Stable cell lines expressing the pSM2 vector alone were used as the control. To generate DEPTOR-suppressed C2C12 myoblasts, a plasmid encoding mouse DEPTOR short hairpin RNA constructs cloned into the pLKO.1 vector (Open Biosystems TRCN0000110157 and TRCN0000110159) (19) was transfected into C2C12 myoblasts together with a plasmid encoding the puromycin resistance gene (pSV2-puro). Stable cell lines were selected with puromycin as described in our previous study (18). The cells stably expressing the pLKO.1 vector were used as a control.
Purification of GST-S6K1 Protein
Overnight cultured BL21 cells harboring the pGEX-S6K1-(332–421) plasmid (a generous gift of Dr. Jie Chen, University of Illinois at Urbana-Champaign) were diluted 1:10 with fresh LB medium containing 50 μm ampicillin and cultured at 37 °C for 80 min. Cells were then cultured at 30 °C, and the production of the GST-S6K1-(332–421) fusion protein was induced by the addition of 1 mm isopropyl β-d-thiogalactopyranoside. Cells were cultured for 2.5 h, harvested, and lysed with lysis buffer containing 20 mg lysozyme. The GST-S6K1-(332–421) fusion protein was affinity-purified on glutathione-agarose.
mTOR Activity in Vitro Assays
The mTOR in vitro activity assays were performed according to the procedure described previously (19, 20). In brief, C2C12 cells grown on a 100-mm plate were lysed with 500 μl of ice-cold lysis buffer (40 mm HEPES (pH 7.4), 2 mm EDTA, 10 mm pyrophosphate, 10 mm glycerophosphate, 0.3% CHAPS, 1 mm sodium orthovanadate, 10 μg/ml leupeptin, 10 μg/ml aprotinin, 1 mm phenylmethanesulfonyl fluoride, and phosphatase inhibitor mixtures). Cell lysates were clarified by centrifugation, and cell lysates were incubated with 2 μl of anti-mTOR antibody overnight at 4 °C. This was followed by the addition of 30 μl of protein A beads and incubation with rotation for an additional 2 h. The beads were washed three times with low salt buffer (40 mm HEPES (pH 7.4), 150 mm NaCl, 2 mm EDTA, 10 mm pyrophosphate, 10 mm glycerophosphate, and 0.3% CHAPS) and then twice with buffer containing 25 mm HEPES (pH 7.4) and 20 mm KCl. The mTOR activity assays were initiated by the addition of 30 μl of kinase assay buffer containing 25 mm HEPES (pH 7.4), 20 mm KCl, 10 mm MgCl2, 50 mm ATP, 1 μCi of [γ-32P]ATP, and 500 ng of GST-S6K1-(332–421) fusion protein. The reaction was continued for 20 min at 37 °C, stopped by the addition of SDS loading buffer, and boiled at 95 °C for 5 min. The phosphorylation of GST-S6K1 was determined by autoradiography.
Statistical Analysis
Quantification of S6K phosphorylation and S6K protein levels was performed by analyzing Western blots using NIH Scion Image software. The ratio of S6K phosphorylation to the protein level of this kinase was used for statistical analysis in each experiment. Results are expressed as the mean ± S.E. Differences between groups were examined for statistical significance using analysis of variance (ANOVA).
RESULTS
RSV Inhibits Insulin- and Leucine-stimulated mTOR/S6K Signaling in C2C12 Cells via a Sirt1-independent Mechanism
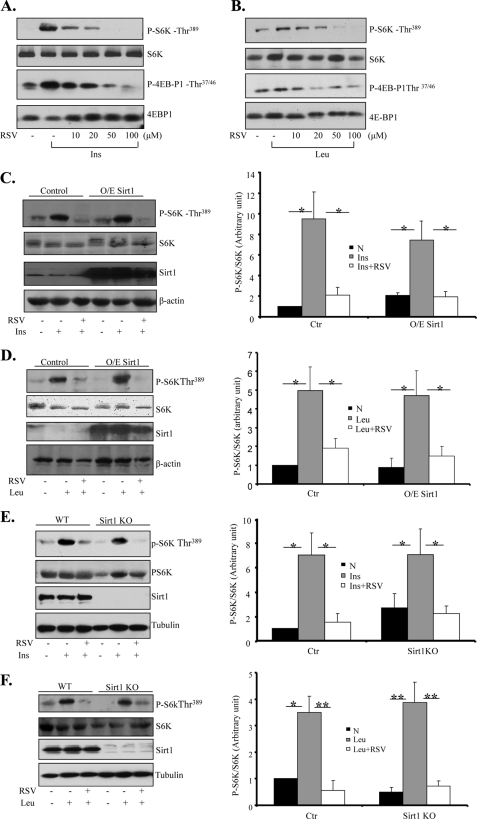
To determine the potential effect of RSV on mTOR activity, C2C12 myoblasts were treated with insulin or leucine in the presence or absence of RSV. Insulin and leucine treatments dramatically stimulated S6K and 4E-BP1 phosphorylation at Thr389 and Thr37/46, respectively (Fig. 1, A and B). The stimulatory effect of insulin and leucine on S6K or 4E-BP1 phosphorylation was significantly reduced by cotreating the cells with RSV in a dose-dependent (Fig. 1, A and B) and time-dependent (supplemental Fig. S1, A and B) manner. Treating the cells with RSV had little effect on the protein levels of Raptor, Rictor, or Sirt1 (supplemental Fig. S1C), suggesting that the reduced TORC1 signaling is not due to reduced expression levels of these mTOR components.
FIGURE 1.
RSV inhibits insulin- and leucine-stimulated mTOR activation via a Sirt1-independent mechanism. A, serum-starved C2C12 myoblasts were pretreated with or without RSV at the indicated concentrations for 20 min, followed with or without 10 nm insulin (Ins) for 10 min. B, serum-starved C2C12 cells were pretreated with or without 10 mm leucine for 60 min and then cotreated with RSV for 30 min. C, serum-starved C2C12 myoblasts transiently overexpressing (O/E) Sirt1 were pretreated with 50 μm RSV for 20 min, followed with or without 10 nm insulin for 10 min. D, serum-starved C2C12 cells transiently overexpressing Sirt1 were pretreated with 10 mm leucine for 60 min, followed with or without 50 μm RSV for 30 min. E, serum-starved Sirt1+/+ and Sirt1−/− MEFs were pretreated with 50 μm RSV for 20 min and then treated with or without 10 nm insulin for 10 min. KO, knock-out. F, serum-starved Sirt1+/+ and Sirt1−/− MEF cells were pretreated with 10 mm leucine for 60 min, followed with or without 50 μm RSV for 30 min. The expression and phosphorylation of proteins in cell lysates were determined by Western blotting using the indicated antibodies. Tubulin was used as a loading control (Ctr) for all experiments. All data (C–F) are representative of at least three independent experiments with similar results and were quantified using the NIH Scion Image program. Differences between groups were examined for statistical significance using ANOVA. *, p < 0.05; **, p < 0.01. N, no addition.
To elucidate the mechanism by which RSV inhibits mTOR signaling, we examined the potential involvement of Sirt1, which has been suggested to be a key mediator of RSV function (21, 22). Interestingly, overexpression of Sirt1 had no significant effect on RSV-induced suppression of insulin- or leucine-stimulated S6K phosphorylation in C2C12 myoblasts (Fig. 1, C and D). In addition, RSV greatly suppressed insulin- and leucine-stimulated S6K and 4E-BP1 phosphorylation in both wild-type and Sirt1−/− MEFs (Fig. 1, E and F). Consistent with the view that Sirt1 is dispensable for the inhibitory effect of RSV, inhibition of Sirt1 by sirtinol had no significant effect on the inhibitory role of RSV in insulin-stimulated S6K and 4E-BP1 phosphorylation in C2C12 myoblasts (supplemental Fig. S1D).
RSV Inhibits Leucine-stimulated mTORC1 Activation via a PI3K/Akt-independent Mechanism
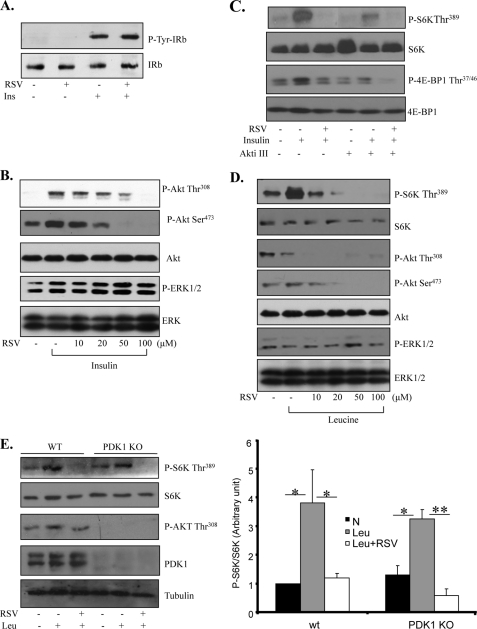
Activation of the PI3K/Akt pathway is essential for insulin-stimulated mTOR activation. To determine whether inhibition of the PI3K/Akt pathway provides a mechanism by which RSV suppresses insulin-stimulated mTOR activation, we cotreated C2C12 myoblasts with insulin and RSV. Treating the cells with RSV did not inhibit insulin-stimulated tyrosine phosphorylation of the insulin receptor (Fig. 2A), nor did it affect insulin-stimulated ERK1/2 phosphorylation (Fig. 2B). On the other hand, RSV treatment reduced insulin-stimulated Akt phosphorylation at Thr308 and Ser473 in a dose-dependent manner (Fig. 2B). Consistent with these findings, inhibition of Akt by Akt inhibitor III markedly blocked insulin-stimulated S6K and 4E-BP1 phosphorylation (Fig. 2C). Interestingly, an additive inhibitory effect on insulin-stimulated S6K and 4E-BP1 phosphorylation was observed when the cells were treated with both the Akt inhibitor and RSV (Fig. 2C), suggesting that RSV has an Akt-independent inhibitory effect on mTOR activity.
FIGURE 2.
RSV inhibits leucine-stimulated mTOR activation in a PI3K/Akt-independent manner. A, serum-starved C2C12 myoblasts were pretreated with 50 μm RSV for 20 min and then stimulated with or without 10 nm insulin (Ins) for 10 min. The tyrosine phosphorylation and protein levels of the immunoprecipitated insulin receptor were determined by Western blotting using the antibodies indicated. B, serum-starved C2C12 cells were pretreated with or without RSV at the indicated concentrations for 20 min, followed with or without 10 nm insulin for 10 min. The insulin-stimulated phosphorylation of Akt and ERK1/2 and the protein levels of these kinases in cell lysates were determined by Western blotting using the antibodies indicated. C, serum-starved C2C12 cells were pretreated with or without the Akt inhibitor III (Akti III) for 60 min, followed with 50 μm RSV for 20 min. Cells were then stimulated with or without 10 nm insulin for 10 min and lysed. The insulin-stimulated S6K and 4E-BP1 phosphorylation in cell lysates was determined by Western blotting using the antibodies indicated. D, serum-starved C2C12 cells were pretreated with or without 10 mm leucine for 60 min and then cotreated using different concentrations of RSV for 30 min. The phosphorylation and protein levels of S6K, Akt, and ERK1/2 were determined by Western blotting with the indicated antibodies. E, serum-starved wild-type and PDK1-null MEFs were pretreated with or without leucine for 60 min and then cotreated with or without 50 μm RSV for 30 min. The phosphorylation and protein levels of the interesting signaling molecules were determined by Western blotting with the indicated antibodies and were quantified using the NIH Scion Image program. KO, knock-out. Unless indicated otherwise, all data are representative of at least three independent experiments with similar results. Differences between groups were examined for statistical significance using ANOVA. *, p < 0.05; **, p < 0.01. N, no addition; IRb, insulin receptor β-subunit.
To determine the mechanism by which RSV inhibits leucine-stimulated mTOR signaling, we examined whether leucine stimulates Akt phosphorylation in C2C12 cells. Although leucine significantly enhanced S6K phosphorylation, it had no stimulatory effect on Akt and ERK1/2 phosphorylation (Fig. 2D). In addition, leucine stimulated S6K phosphorylation in PDK1 knock-out MEFs (Fig. 2E). Taken together, these results suggest that, rather than suppressing the PI3K/PDK1/Akt signaling pathway, a distinct mechanism is involved in mediating the inhibitory effect of RSV on leucine-stimulated mTOR activation.
TSC1/2 and AMPK Are Not Involved in the RSV-mediated Regulation of mTOR Activity
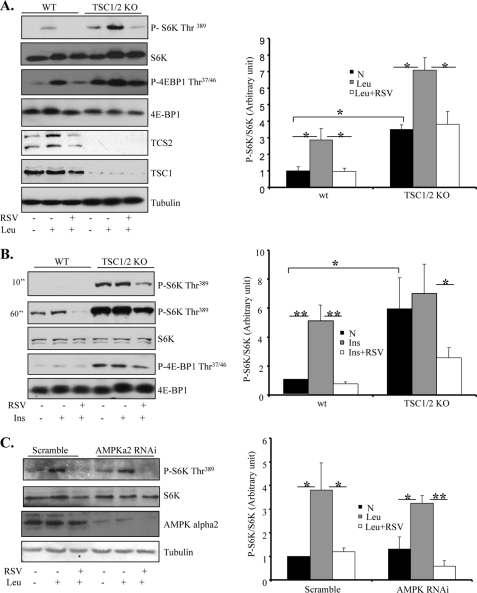
mTOR signaling is negatively regulated by the TSC1/2 complex-mediated pathway (23, 24). To investigate whether RSV inhibits mTOR signaling by acting on TSC1/2, we examined the effect of RSV on mTOR signaling in TSC1/2-null cells. As expected, the phosphorylation of S6K and 4E-BP was markedly increased in TSC1/2-deficient MEFs compared with wild-type MEFs (Fig. 3, A and B). Although insulin treatment had no significant effect on the TSC1/2 deficiency-induced increase in S6K and 4E-BP1 phosphorylation (Fig. 3B), the phosphorylation of S6K and 4E-BP1 was further stimulated by leucine in TSC-null cells (Fig. 3A). Interestingly, treating the cells with RSV greatly inhibited the leucine-stimulated phosphorylation of S6K and 4E-BP1 in TSC-null cells (Fig. 3A), suggesting that RSV inhibits mTOR signaling by activating at a site downstream of TSC1/2.
FIGURE 3.
RSV-promoted Inhibition of mTOR activation is TSC1/2- and AMPK-independent. A, serum-starved TSC1/2+/+ and TSC1/2−/− MEF cells were pretreated with 10 mm leucine for 60 min and then cotreated with or without 50 μm RSV for 30 min. B, serum-starved TSC1/2+/+ and TSC1/2−/− MEFs were pretreated with 50 μm RSV for 20 min, followed with or without 10 nm insulin (Ins) for 10 min. KO, knock-out. C, serum-starved AMPKα2-suppressed C2C12 cells were pretreated with or without leucine for 60 min and then cotreated with or without 50 μm RSV for 30 min. For all experiments, the phosphorylation and protein levels of S6K, 4E-BP1, and AMPK and the protein levels of TSC1 and TSC2 in cell lysates were determined by Western blotting with the indicated antibodies. All data are representative of at least three independent experiments with similar results, and A–C were quantified using the NIH Scion Image program. Differences between groups were examined for statistical significance using ANOVA. *, p < 0.05; **, p < 0.01. N, no addition.
Several recent studies suggest that the activation of the AMPK signaling pathway may play a role in the biological action of RSV (25–28). To determine the potential involvement of the AMPK signaling pathway in RSV-induced suppression of mTOR signaling, we examined S6K and 4E-BP1 phosphorylation in C2C12 cells in which the expression levels of AMPKα2 were suppressed by RNAi. Suppressing AMPKα2, which markedly inhibited AMPK activity (supplemental Fig. S1E), had no significant effect on leucine-stimulated mTOR signaling in C2C12 cells (Fig. 3C). In addition, the inhibitory effect of RSV on leucine-stimulated S6K phosphorylation remained intact in AMPKα2-suppressed cells (Fig. 3C), suggesting that RSV inhibits mTOR signaling via an AMPK-independent mechanism.
RSV Inhibits mTOR Activity via Promoting the Association between mTOR and Its Inhibitor, DEPTOR
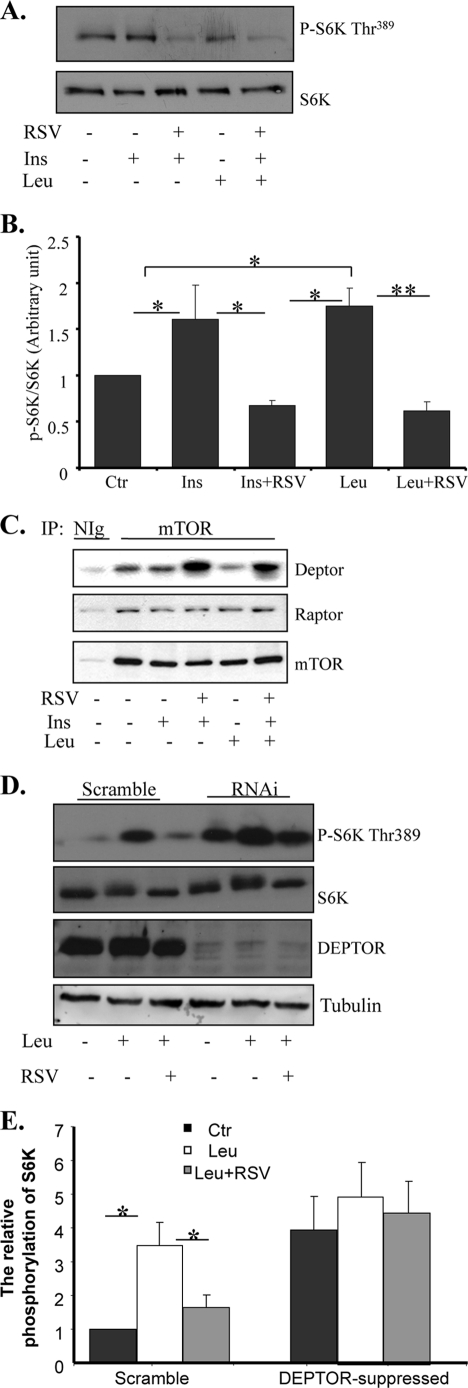
To determine whether RSV has a direct effect on mTOR, we examined mTOR activity by in vitro kinase assays using purified GST-S6K1-(332–421) fusion protein as a substrate. We found that mTOR isolated from C2C12 myoblasts cotreated with leucine and RSV displayed a lower in vitro kinase activity compared with the protein isolated from cells treated with leucine alone (Fig. 4, A and B), suggesting that RSV has a direct effect on the activity of mTOR. Because TORC1 activity is regulated via association with its interacting protein such as the positive regulator Raptor (29, 30) and the negative regulator DEPTOR (19), we examined the effect of RSV on the association between mTOR and these interactive proteins. We found that RSV treatment had no significant effect on the association between mTOR and Raptor (Fig. 4C). On the other hand, treating the cells with RSV greatly increased the association between mTOR and DEPTOR (Fig. 4C), a recently identified inhibitor of mTOR (19).
FIGURE 4.
RSV enhances the association between mTOR and DEPTOR. A, serum-starved C2C12 cells were pretreated with or without leucine for 60 min and then cotreated with or without 50 μm RSV for 20 min, followed with or without insulin (Ins) stimulation for 10 min. Cells were lysed, and mTOR proteins were immunoprecipitated from the cell lysates and used for mTOR kinase activity assays. B, the quantification of S6K phosphorylation in A was performed using the NIH Scion Image program and normalized with S6K protein levels. Ctr, control. C, serum-starved C2C12 cells were pretreated with or without leucine for 60 min and then cotreated with 50 μm RSV for 30 min. The immunoprecipitated (IP) mTOR and the co-immunoprecipitated DEPTOR were determined by Western blotting using the specific antibodies indicated. Unless indicated otherwise, all data are representative of at least three independent experiments with similar results. Nlg, normal immunoglobulin. D, serum-starved DEPTOR-suppressed and scrambled C2C12 cells were pretreated with or without leucine for 60 min and then cotreated with or without 50 μm RSV for 30 min. The phosphorylation of S6K and the protein levels of S6K, DEPTOR, and tubulin were determined by Western blotting with specific antibodies. E, quantification of S6K phosphorylation in D was performed using the NIH Scion Image program and normalized with S6K protein levels. Differences between groups were examined for statistical significance using ANOVA. *, p < 0.05; **, p < 0.01.
To provide further evidence that DEPTOR is involved in RSV-induced inhibition of mTOR signaling, we examined the effect of RSV on leucine-stimulated S6K phosphorylation in C2C12 cells in which the expression levels of DEPTOR were suppressed by RNAi. Consistent with a previous finding (19), mTOR signaling was significantly enhanced in DEPTOR-suppressed cells, as demonstrated by increased S6K phosphorylation (Fig. 4, D and E). Although leucine treatment further stimulated mTOR activation in DEPTOR-suppressed cells, the inhibitory effect of RSV on mTOR activity was greatly reduced in DEPTOR-suppressed cells (Fig. 4, D and E), suggesting that DEPTOR mediates the inhibitory effect of RSV on leucine-stimulated mTOR activation.
DISCUSSION
RSV has been shown to exert numerous beneficial effects such as maintenance of glucose homeostasis, improvement of mitochondrial function, and extension of life span (31–35). The mechanisms underlying the beneficial health roles of RSV remain elusive, but there is evidence suggesting that activation of Sirt1 could play a role (31–33). However, several recent studies showed that RSV regulates cell growth, glucose homeostasis, and protection of the cardiovascular system via a Sirt1-independent mechanism (3, 28, 36, 37). In agreement with these latter findings, we found that RSV inhibits insulin- and leucine-stimulated mTOR signaling in Sirt1 knock-out MEFs (Fig. 1, E and F). In addition, inhibition of Sirt1 by sirtinol had no effect on insulin-stimulated mTOR signaling activation (supplemental Fig. S1D). Taken together, these results indicate that activation of Sirt1 is dispensable for the suppression effect of RSV on insulin- or leucine-stimulated mTOR signaling events.
Although we found that RSV inhibits both insulin- and leucine-stimulated mTOR signaling, our results suggest that the mechanisms of inhibition are distinct. It is well established that insulin stimulates mTOR signaling by activating the PI3K/PDK1/Akt signaling pathway (11, 38). Suppressing this signaling pathway could thus provide a mechanism underlying the RSV-mediated inhibition of mTOR signaling. Consistent with this view, we found that RSV treatment inhibited insulin-stimulated Akt phosphorylation at Thr308 in C2C12 cells (Fig. 2B). This result is also consistent with a recent finding that RSV suppresses PI3K signaling by binding to the ATP-binding site of PI3K (37, 39). However, inhibition of Akt did not completely block the inhibitory effect of RSV on mTOR activity (Fig. 2C), suggesting the presence of a distinct mechanism underlying the inhibitory effect of RSV on mTOR. We found that leucine activated mTOR signaling in C2C12 cells but had little effect on Akt activation (Fig. 2D). In addition, suppression of PDK1/Akt activity had little effect on leucine-stimulated S6K and 4E-BP1 phosphorylation (Figs. 2E). These results suggest that inhibition of the PI3K/PDK1/Akt signaling pathway is dispensable for the negative regulation RSV-promoted of leucine-stimulated mTOR signaling. There is some evidence indicating that RSV could exert its biological effect by activation of AMPK (40, 41). Because activation of AMPK enhances TSC1/2 activity that down-regulates mTOR signaling (42, 43), it is possible that RSV inhibits leucine-stimulated mTOR signaling by activation of AMPK. However, we found that disruption of TSC1/2 expression or suppression of the expression levels of AMPK had little effect on leucine-stimulated mTOR signaling in C2C12 cells or MEFs (Fig. 3), suggesting that RSV negatively regulates leucine-stimulated mTOR signaling by a mechanism independent of the AMPK signaling pathway. Consistent with this view, we found that RSV treatment markedly increased the association between mTOR and DEPTOR (Fig. 4B). Because the binding of DEPTOR to mTOR has been shown to inhibit mTOR activity (19, 44), this finding suggests that the enhanced interaction with DEPTOR could be a mechanism by which RSV inhibits insulin- and leucine-induced mTORC1 signaling. Consistent with this, suppressing DEPTOR expression levels by RNAi greatly diminished the inhibitory effect of RSV on leucine-stimulated mTORC1 signaling (Fig. 4C). Our study also showed that RSV treatment inhibited insulin-stimulated Akt phosphorylation at Ser473 (Fig. 2B), which is consistent with the finding that the binding of DEPTOR to mTOR suppresses both mTORC1 and mTORC2 activities (19).
How RSV promotes the binding of DEPTOR to mTOR remains unknown. One possibility may be that RSV directly interacts with mTOR or DEPTOR, leading to enhanced interaction between these two proteins. Alternatively, RSV may indirectly promote the interaction between mTOR and DEPTOR by binding to an auxiliary protein. Similar to the latter model, rapamycin has been shown to impair the association between mTOR and Raptor through binding to FKBP12 (43). It is also possible that RSV may activate a signaling pathway that leads to a chemical modification of either mTOR or DEPTOR, thus promoting the interaction between these proteins. Further studies will be needed to test these possibilities.
In summary, we have provided evidence showing that RSV inhibits leucine-stimulated mTOR signaling via an Akt-independent mechanism. In addition, we have demonstrated that RSV enhances the interaction between mTOR and its negative regulator, DEPTOR, thus uncovering a novel mechanism by which RSV negatively regulates leucine-stimulated mTOR signaling in cells.
Supplementary Material
Acknowledgments
We thank Debbie Hu for assistance with all of the cell culture work. We also thank Drs. Kunliang Guan and Jie Chen for the TSC−/− MEFs and the pGEX-S6K1-(332–421) plasmid, respectively.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 DK76902 (to F. L.) and RO1 DK80344 (to L. Q. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
- RSV
- resveratrol
- AMPK
- AMP-activated protein kinase
- PI3K
- phosphatidylinositol 3-kinase
- S6K
- S6 kinase
- MEF
- mouse embryonic fibroblast
- ANOVA
- analysis of variance.
REFERENCES
- 1.Valenzano M. M., Mistrangelo E., Lijoi D., Fortunato T., Lantieri P. B., Risso D., Costantini S., Ragni N. (2006) Eur. J. Obstet. Gynecol. Reprod. Biol. 124, 246–249 [DOI] [PubMed] [Google Scholar]
- 2.Kaeberlein M., Rabinovitch P. S. (2006) Nature 444, 280–281 [DOI] [PubMed] [Google Scholar]
- 3.Zhang J. (2006) Biochem. J. 397, 519–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kundu J. K., Surh Y. J. (2004) Mutat. Res. 555, 65–80 [DOI] [PubMed] [Google Scholar]
- 5.Wullschleger S., Loewith R., Hall M. N. (2006) Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 6.Yang Q., Guan K. L. (2007) Cell Res. 17, 666–681 [DOI] [PubMed] [Google Scholar]
- 7.Loewith R., Jacinto E., Wullschleger S., Lorberg A., Crespo J. L., Bonenfant D., Oppliger W., Jenoe P., Hall M. N. (2002) Mol. Cell 10, 457–468 [DOI] [PubMed] [Google Scholar]
- 8.Laplante M., Sabatini D. M. (2009) J. Cell Sci. 122, 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guertin D. A., Sabatini D. M. (2007) Cancer Cell 12, 9–22 [DOI] [PubMed] [Google Scholar]
- 10.Dong L. Q., Liu F. (2005) Am. J. Physiol. Endocrinol. Metab. 289, E187–E196 [DOI] [PubMed] [Google Scholar]
- 11.Scott P. H., Brunn G. J., Kohn A. D., Roth R. A., Lawrence J. C., Jr. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7772–7777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith E. M., Finn S. G., Tee A. R., Browne G. J., Proud C. G. (2005) J. Biol. Chem. 280, 18717–18727 [DOI] [PubMed] [Google Scholar]
- 13.Roccio M., Bos J. L., Zwartkruis F. J. (2006) Oncogene 25, 657–664 [DOI] [PubMed] [Google Scholar]
- 14.Kueck A., Opipari A. W., Jr., Griffith K. A., Tan L., Choi M., Huang J., Wahl H., Liu J. R. (2007) Gynecol. Oncol. 107, 450–457 [DOI] [PubMed] [Google Scholar]
- 15.Haider U. G., Sorescu D., Griendling K. K., Vollmar A. M., Dirsch V. M. (2002) Mol. Pharmacol. 62, 772–777 [DOI] [PubMed] [Google Scholar]
- 16.Wang R. H., Sengupta K., Li C., Kim H. S., Cao L., Xiao C., Kim S., Xu X., Zheng Y., Chilton B., Jia R., Zheng Z. M., Appella E., Wang X. W., Ried T., Deng C. X. (2008) Cancer Cell 14, 312–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue H., Ogawa W., Asakawa A., Okamoto Y., Nishizawa A., Matsumoto M., Teshigawara K., Matsuki Y., Watanabe E., Hiramatsu R., Notohara K., Katayose K., Okamura H., Kahn C. R., Noda T., Takeda K., Akira S., Inui A., Kasuga M. (2006) Cell Metab. 3, 267–275 [DOI] [PubMed] [Google Scholar]
- 18.Lim M. A., Kikani C. K., Wick M. J., Dong L. Q. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14006–14011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson T. R., Laplante M., Thoreen C. C., Sancak Y., Kang S. A., Kuehl W. M., Gray N. S., Sabatini D. M. (2009) Cell 137, 873–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sancak Y., Thoreen C. C., Peterson T. R., Lindquist R. A., Kang S. A., Spooner E., Carr S. A., Sabatini D. M. (2007) Mol. Cell 25, 903–915 [DOI] [PubMed] [Google Scholar]
- 21.Kaeberlein M., McDonagh T., Heltweg B., Hixon J., Westman E. A., Caldwell S. D., Napper A., Curtis R., DiStefano P. S., Fields S., Bedalov A., Kennedy B. K. (2005) J. Biol. Chem. 280, 17038–17045 [DOI] [PubMed] [Google Scholar]
- 22.Borra M. T., Smith B. C., Denu J. M. (2005) J. Biol. Chem. 280, 17187–17195 [DOI] [PubMed] [Google Scholar]
- 23.Zhang H., Cicchetti G., Onda H., Koon H. B., Asrican K., Bajraszewski N., Vazquez F., Carpenter C. L., Kwiatkowski D. J. (2003) J. Clin. Invest. 112, 1223–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potter C. J., Huang H., Xu T. (2001) Cell 105, 357–368 [DOI] [PubMed] [Google Scholar]
- 25.Lin J. N., Lin V. C., Rau K. M., Shieh P. C., Kuo D. H., Shieh J. C., Chen W. J., Tsai S. C., Way T. D. (2010) J. Agric. Food Chem. 58, 1584–1592 [DOI] [PubMed] [Google Scholar]
- 26.Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J. E., Janle E. M., Lobo J., Ferruzzi M. G., Davies P., Marambaud P. (2010) J. Biol. Chem. 285, 9100–9113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puissant A., Robert G., Fenouille N., Luciano F., Cassuto J. P., Raynaud S., Auberger P. (2010) Cancer Res. 70, 1042–1052 [DOI] [PubMed] [Google Scholar]
- 28.Chan A. Y., Dolinsky V. W., Soltys C. L., Viollet B., Baksh S., Light P. E., Dyck J. R. (2008) J. Biol. Chem. 283, 24194–24201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. (2002) Cell 110, 177–189 [DOI] [PubMed] [Google Scholar]
- 30.Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 31.Baur J. A. (2010) Mech. Ageing Dev. 131, 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagouge M., Argmann C., Gerhart-Hines Z., Meziane H., Lerin C., Daussin F., Messadeq N., Milne J., Lambert P., Elliott P., Geny B., Laakso M., Puigserver P., Auwerx J. (2006) Cell 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- 33.Chen H., Yang Z., Gibbs D., Yang X., Hau V., Zhao P., Ma X., Zeng J., Luo L., Pearson E., Constantine R., Kaminoh Y., Harmon J., Tong Z., Stratton C. A., Cameron D. J., Tang S., Zhang K. (2008) Vision Res. 48, 690–694 [DOI] [PubMed] [Google Scholar]
- 34.Acharya S. H., Philip S., Viswanath A. K., Boroujerdi M., Waugh N. R., Pearson D. W. (2008) Diabet. Med. 25, 360–364 [DOI] [PubMed] [Google Scholar]
- 35.Baur J. A., Pearson K. J., Price N. L., Jamieson H. A., Lerin C., Kalra A., Prabhu V. V., Allard J. S., Lopez-Lluch G., Lewis K., Pistell P. J., Poosala S., Becker K. G., Boss O., Gwinn D., Wang M., Ramaswamy S., Fishbein K. W., Spencer R. G., Lakatta E. G., Le Couteur D., Shaw R. J., Navas P., Puigserver P., Ingram D. K., de Cabo R., Sinclair D. A. (2006) Nature 444, 337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Um J. H., Park S. J., Kang H., Yang S., Foretz M., McBurney M. W., Kim M. K., Viollet B., Chung J. H. (2010) Diabetes 59, 554–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang H., Shang X., Wu H., Gautam S. C., Al-Holou S., Li C., Kuo J., Zhang L., Chopp M. (2010) J. Exp. Ther. Oncol. 8, 25–33 [PMC free article] [PubMed] [Google Scholar]
- 38.Taha C., Liu Z., Jin J., Al-Hasani H., Sonenberg N., Klip A. (1999) J. Biol. Chem. 274, 33085–33091 [DOI] [PubMed] [Google Scholar]
- 39.Fröjdö S., Cozzone D., Vidal H., Pirola L. (2007) Biochem. J. 406, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dasgupta B., Milbrandt J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 7217–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gledhill J. R., Montgomery M. G., Leslie A. G., Walker J. E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 13632–13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang C., Mao X., Wang L., Liu M., Wetzel M. D., Guan K. L., Dong L. Q., Liu F. (2007) J. Biol. Chem. 282, 7991–7996 [DOI] [PubMed] [Google Scholar]
- 43.Memmott R. M., Dennis P. A. (2009) Cell. Signal. 21, 656–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Proud C. G. (2009) J. Mol. Cell Biol. 1, 61–63 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.