Abstract
Na+-coupled ascorbic acid transporter-2 (SVCT2) activity is impaired at acid pH, but little is known about the molecular determinants that define the transporter pH sensitivity. SVCT2 contains six histidine residues in its primary sequence, three of which are exofacial in the transporter secondary structure model. We used site-directed mutagenesis and treatment with diethylpyrocarbonate to identify histidine residues responsible for SVCT2 pH sensitivity. We conclude that five histidine residues, His109, His203, His206, His269, and His413, are central regulators of SVCT2 function, participating to different degrees in modulating pH sensitivity, transporter kinetics, Na+ cooperativity, conformational stability, and subcellular localization. Our results are compatible with a model in which (i) a single exofacial histidine residue, His413, localized in the exofacial loop IV that connects transmembrane helices VII-VIII defines the pH sensitivity of SVCT2 through a mechanism involving a marked attenuation of the activation by Na+ and loss of Na+ cooperativity, which leads to a decreased Vmax without altering the transport Km; (ii) exofacial histidine residues His203, His206, and His413 may be involved in maintaining a functional interaction between exofacial loops II and IV and influence the general folding of the transporter; (iii) histidines 203, 206, 269, and 413 affect the transporter kinetics by modulating the apparent transport Km; and (iv) histidine 109, localized at the center of transmembrane helix I, might be fundamental for the interaction of SVCT2 with the transported substrate ascorbic acid. Thus, histidine residues are central regulators of SVCT2 function.
Keywords: Amino Acid, Antioxidant, Ascorbic Acid, Cell pH, Membrane Proteins, Protein Structure, Vitamin C, Histidine, SVCT2
Introduction
The Na+-coupled ascorbic acid cotransporters SVCT1 and SVCT2 transport ascorbic acid down the electrochemical sodium gradient (1–5). SVCT1 and SVCT2 have a 65% amino acid sequence identity and a similar hydropathy profile and are predicted to conform to a secondary structure model containing 12 transmembrane-spanning helices, with the hydrophilic amino and C-terminal domains located intracellularly (1–5). Most tissues express SVCT2, with SVCT1 showing a more restricted tissue distribution (3, 6). SVCT1 and SVCT2 show different kinetic properties; SVCT1 has an apparent ascorbic acid transport Km in the range of 50–200 μm, whereas the Km of SVCT2 is lower, in the range of 10–30 μm (1–3, 6–9). Both transporters are activated by Na+ in a cooperative manner, with a Hill coefficient (nH) near 2 (1–3, 6–9). We showed that both substrates, ascorbic acid and sodium, modify the kinetic properties of SVCT2 in a reciprocal manner (7). When extracellular sodium increases, the Km for ascorbic acid transport decreases more than 100 times without affecting the transport Vmax, converting a low affinity form of the transporter into a high affinity transporter (7). In turn, ascorbic acid modifies the sodium cooperativity of SVCT2 in a complex concentration-dependent manner, with maximal Na+ cooperativity observed at 100 μm ascorbic acid (7). Moreover, SVCT2 is completely dependent on the presence of calcium or magnesium ions for function, with the bivalent ions switching the transporter from an inactive into an active form by increasing the transport Vmax without affecting the Km or the sodium cooperativity. In contrast, SVCT1 is active in the complete absence of bivalent cations (7).
Little is currently known about the functional-structural determinants that define the activity of SVCT1 and SVCT2. The available information is restricted to the effect of protein phosphorylation on the functional activity and subcellular localization of SVCT2 (10), with evidence indicating that the C-terminal region is fundamental for the differential sorting and apical localization of SVCT1 in polarized cells (9–14) and that N-linked glycosylation sites are important for maintaining ascorbic transporter function (15).
Evidence has been published indicating that the functional activity of SVCT2 is impaired at acid pH (3, 6, 16). This finding may have physiological implications for the regulation of ascorbic acid transport and metabolism in organs and tissues that show restricted areas of lowered pH under physiological conditions, such as the gut, kidneys, and bladder (17–23). In a similar manner, vitamin C metabolism may be altered in muscle cells that are exposed to increased lactate concentrations and therefore to lowered pH after active exercise (24, 25). It has also been observed that increased brain activity is associated with the release of lactate from glial cells, which generates restricted areas of lowered pH that may affect the metabolism of vitamin C in cells characterized by expression of SVCT2 (26–28). Another important area is related to possible alterations in the metabolism of vitamin C in tumor cells, because of the lower pH associated with the tumor microenvironment during active tumor cell proliferation (29–31). In fact, there is currently a controversy about the role of vitamin C in cancer biology and its possible therapeutic applications, with contradictory data indicating positive (protective) as well as negative (deleterious) effects of vitamin C in cancer cell survival (32–34). It is noteworthy that cancer cells show increased capacity to acquire vitamin C, and breast cancer tissue shows a higher vitamin content than normal tissue (35, 36).
Although the characteristics of the pH effect on SVCT2 function point to the possible involvement of histidine residues, we lack information on the identity of the structural elements involved (3, 6, 16). SVCT2 contains six histidine residues in its primary sequence, three of which are exofacial in the transporter secondary structure model. Using site-directed mutagenesis, we replaced each histidine residue in SVCT2 with glutamine and used chemical modification with diethylpyrocarbonate (DEPC)2 to identify histidine residues responsible for SVCT2 pH sensitivity (7, 37). Each mutant was expressed in HEK-293 cells and examined for subcellular localization and function, including transport kinetics (Km and Vmax of ascorbic acid transport), Na+ cooperativity (nH and Na50+), sensitivity to acid pH, and effect of DEPC. The results of these experiments revealed that five histidine residues, His109, His203, His206, His269, and His413, from a total of six present in SVCT2, are central regulators of SVCT2 function, participating at different degrees in modulating pH sensitivity, transporter kinetics, Na+ cooperativity, conformational stability, and subcellular localization. We conclude that histidine residues are important regulators of SVCT2 function.
EXPERIMENTAL PROCEDURES
Cell Culture
Human melanoma cells (SK-MEL-131) were grown in RPMI 1640 containing 25 mm Hepes (pH 7.4). Human kidney embryonic cells (HEK-293) were grown in DMEM high glucose (pH 7.4). Both media were supplemented with 10% (v/v) fetal bovine serum and penicillin/streptomycin (100 units/ml) (7).
Transport Assays
Transport experiments were carried out as described previously using 2–3-day-old cell monolayers in 12-well tissue culture plates (7, 9). Unless otherwise indicated, ascorbic acid transport was measured at 37 °C in medium containing 100 μm radiolabeled ascorbic acid for the indicated times, using 12-well plates containing ∼200,000 cells/well. The transport medium contained 15 mm Hepes buffer (pH 7.6), 135 mm NaCl, 5 mm KCl, 1.8 mm CaCl2, 0.8 mm MgCl2, and 0.1 mm DTT. Monolayers were washed with transport medium, and uptake experiments were initiated by replacing the medium with prewarmed transport medium containing 0.1 μCi of l-[14C]ascorbic acid (PerkinElmer Life Sciences) and cold ascorbate at the concentrations indicated in the figure legends. Uptake was terminated by adding ice-cold stopping solution (transport medium containing 0.2 mm HgCl2). The monolayers were washed and lysed in 10 mm Tris/HCl (pH 8.0), 0.2% SDS, and the incorporated radioactivity was determined by scintillation spectrometry (7, 9). For 2-deoxy-d-glucose transport, the transport medium contained 0.2 μCi of [3H]-2-deoxy-d-glucose (PerkinElmer Life Sciences) and cold 2-deoxy-d-glucose for a final concentration of 1 mm. Data are presented as the average ± S.D. and correspond to a minimum of three assays performed independently in triplicate. Kinetic parameters were determined by non-linear fitting of the Michaelis-Menten equation, and data were plotted using the linear transformation of Lineweaver-Burk.
Expression of SVCT2 Histidine Mutants in HEK-293 Cells
The full-length SVCT2 cDNA cloned in pBluescript IIKS(+) was used for generating the different histidine mutants by means of a QuikChange site-directed mutagenesis kit (Stratagene), in accordance with the manufacturer's instructions. The mutant clones were subcloned into the expression vector pEGFP-N1 (Clontech) to generate a clone encoding the fusion protein SVCT2-GFP. Each clone was subjected to automated sequencing to verify its correctness and the introduction of the desired mutation. Ten mutants were constructed to test the role of the histidine residues in SVCT2 pH sensitivity: three exofacial single mutants, three endofacial single mutants, three exofacial double mutants, and a single exofacial triple mutant. HEK-293 cells were transfected with plasmids containing the cDNA encoding the GFP-tagged proteins using Lipofectamine (Invitrogen), in accordance with the manufacturer's instructions. The efficiency of transfection was estimated by direct visualization of the transfected cells under a fluorescent microscope, and transfected cells at greater than 95% transfection efficiency were analyzed for cellular localization of the expressed proteins by confocal microscopy using an Olympus DSU confocal microscope (7) and surface biotinylation and analyzed for function by measuring the transport of ascorbic acid. Unless otherwise indicated, in all transfection experiments, localization studies and transport assays were performed 48 after transfection. For confocal analysis, 30–40 successive optical slices along the z axis (each 0.1 μm thick) were obtained from each sample.
Colocalization Studies
To create the different organelle marker constructs, full-length cDNAs encoding protein-disulfide isomerase (NM 000918.3; endoplasmic reticulum marker), glutaredoxin-2a (Grx2a, NM 016066.3; mitochondrial marker), glucose transporter-1 (GLUT1, NM 006516.2; plasma membrane marker), and syntaxin-6 (Stx6, AJ002078.1; Golgi apparatus marker) were amplified by PCR with PfuUltra® II Fusion HS polymerase (Stratagene) from a cDNA prepared from mRNA isolated from HEK-293 cells. Each resulting PCR product was inserted into the EcoRI-SacII fragment of plasmid pDsRED-N1 (Clontech). Each clone was subjected to automated sequencing, analyzed by BLAST at the NCBI server, and transfected into HEK-293 cells, and its localization was tested using commercially available antibodies against the respective organellar markers. The sequence of each clone was 100% identical with the corresponding published sequences, and the localization analysis revealed the correct subcellular localization of each protein. For transient expression, HEK-293 cells were grown to 80–90% confluence in 24- and 6-well culture plates. Transfection was performed using Satisfection (Stratagene), following the manufacturer's instructions. For coexpression experiments, cells were grown in circular glass coverslips (Marienfeld GMbH & Co. KG), and equal molar amounts of each construct were transfected (1:1 ratio). After 48 h, cells were washed once in ice-cold PBS, fixed in 4% paraformaldehyde for 15 min, washed 3 times in PBS, and mounted using Vectashield hard set mounting medium (Vector Laboratories, Inc.). The fluorescence associated with each expressed protein (SVCT2 and the organellar markers) was detected using a spinning disc confocal microscope (Olympus DSU). Each sample was examined using successive optical slices along the cell z axis and was further processed for colocalization with CellR (Olympus Soft Imaging Solutions GmbH).
Surface Biotinylation of Plasma Membrane Proteins
HEK-293 cells, grown in 6-well plates, were transfected with plasmids encoding SVCT2-GFP or the histidine mutants. All of the biotinylation procedures were carried out at 4 °C. Twenty-four hours after transfection, cell surface proteins were labeled with biotin. For this, cells were washed twice with cold rinsing solution (phosphate-buffered saline with 1 mm MgCl2 and 0.1 mm CaCl2, pH 7.35) and incubated in rinsing solution containing 0.5 mg/ml EZ-Link Sulfo-NHS-Biotin (Pierce) for 30 min at 4 °C. Cells were washed twice with quenching solution (rinsing solution containing 100 mm glycine) (38). The cells were lysed in lysis buffer (radioimmune precipitation buffer, pH 7.4, containing protease inhibitors) and sonicated (39). One group of cells was processed in parallel without biotinylation, lysed as above, and saved for analysis (designated the total extract). Twenty-five percent of each cleared lysate from the biotinylated samples was saved for analysis (designated the total extract + biotin fraction). The remaining portion was incubated with avidin beads (Pierce) 1 h at room temperature with end-over-end rotation. The samples were then centrifuged at 12,000 rpm for 5 min and washed with lysis buffer, with salt wash buffer (0.1% Triton X-100, 500 mm NaCl, 5 mm EDTA, and 50 mm Tris, pH 7.5), and with no salt wash buffer (10 mm Tris, pH 7.5). Biotinylated proteins were eluted with SDS sample buffer (62.5 mm Tris-Cl, pH 6.8, 2% SDS, and 100 mm mercaptoethanol) at 95 °C for 5 min (40). The remainder of the procedure followed standard Western blotting protocols. SVCT2-GFP was detected using an anti-GFP antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). For Western blot, samples (50 μg of total extract, 50 μg of biotinylated total extract, and the biotin-avidin eluate) were separated using 10% SDS-PAGE, transferred to PVDF membranes (39), blocked with 2.5% nonfat skim milk in PBS, incubated with primary antibody (anti-GFP) in blocking buffer overnight at room temperature, washed with PBS, and incubated with anti-mouse-HRP antibody for 3 h at room temperature. The proteins were visualized using an enhanced chemiluminescence detection system (PerkinElmer Life Sciences). As a loading control, the PVDF membrane containing the untreated total extract was stripped by acid wash, and the blot was reprobed with a primary anti-GAPDH antibody, followed by incubation with a secondary antibody and analysis by chemiluminescence. Samples were strictly processed in parallel (biotinylation, avidin selection, separation by SDS-PAGE, and transfer to PVDF membrane, incubation with primary and secondary antibodies, and analysis by chemiluminescence).
pH Effect
To test the effect of pH on transport, cells were preincubated for 30 min at different pH values (pH 6.0–8.0), before measuring ascorbic acid transport in a 5-min assay at the same preincubation pH. For testing the reversibility of the pH effect, cells were incubated at pH 6.0 for a 30-min period, washed, changed to transport buffer at pH 7.4, and assayed for ascorbic acid transport in a 5-min assay at pH 7.4 at the times indicated in the figure legends. To test the effect of pH on the kinetics of ascorbic acid or 2-deoxy-d-glucose, cells were incubated for 30 min at different pH values before measuring transport using a 5-min transport assay and ascorbic acid concentrations from 2 to 200 μm or 1 mm 2-deoxy-d-glucose added to traces of 3H-radiolabeled 2-deoxy-d-glucose, and apparent transport Vmax and Km were obtained using non-linear analysis from the respective concentration-response curves. At pH 7.4, the apparent ascorbic acid transport Vmax in melanoma cells was 132 ± 4 pmol/106 cells × min, a value that was used as control (100%). The effect of pH on the cooperative Na+ activation was determined in a similar manner, except that transport of 100 μm ascorbic acid was measured in the presence of graded Na+ concentrations from 5 to 135 mm. Values of nH and Na50+ were obtained from the respective concentration-response curves using non-linear analysis.
DEPC Treatment
To test the effect of the modification with DEPC on transport, cells were incubated at 37 °C and pH 6.0 with graded DEPC concentrations from 0.001 to 10 mm or with fixed DEPC concentrations (300 or 30 μm), washed, and assayed for transport at the pH values indicated in the figure legends, at pH 7.4 or 6.0, or at pH 7.4 and 6.0 in the presence of graded Na+ concentrations (5–135 mm). Transport data were expressed as the means ± S.D. of experiments performed in triplicate.
Statistics
Transport data were processed for non-linear analysis with the program IgorPro (WaveMetrics).
RESULTS AND DISCUSSION
SVCT2 Function Is Impaired at Acid pH in Melanoma Cells
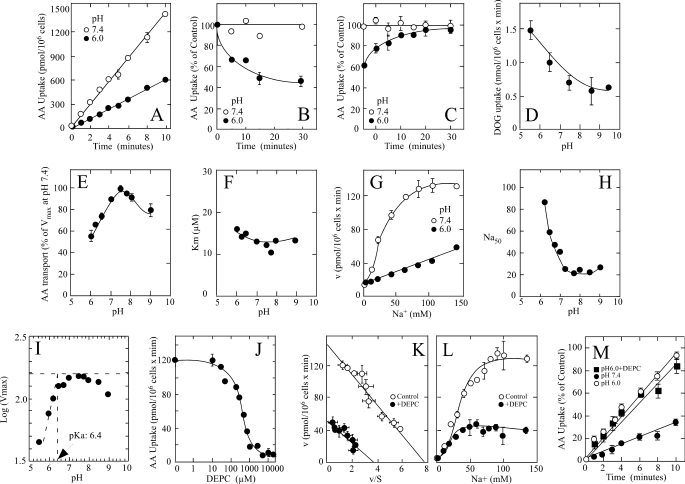
We have shown that the human melanoma cell line SK-MEL expresses the ascorbic acid transporter SVCT2 and characterized the transporter functional properties (7). Therefore, we used this cellular model to characterize SVCT2 pH sensitivity. Ascorbic acid transport by melanoma cells was acutely sensitive to changes in extracellular pH (Fig. 1A). Melanoma cells that were maintained at pH 6.0 during the overall course of the assay (30-min preincubation followed by a 10-min transport assay) transported ascorbic acid at a reduced rate of 60 pmol/min/million cells, compared with a transport rate of 135 pmol/min/million cells in melanoma cells that were maintained at pH 7.4 (Fig. 1A). The effect of pH on transport developed rapidly, with a half-time of less than 5 min (Fig. 1B), and was completely reversible; when cells incubated for 30 min at pH 6.0 were returned to pH 7.4 before the transport assay, they recovered their full capacity to transport ascorbic acid with a half-time of about 5 min (Fig. 1C). This result has important mechanistic implications because it refutes the possibility that exposure at pH 6.0 could inactivate the transporter in an irreversible manner and signals the reversible protonation-deprotonation of specific residues as a central event in the pH effect. The specificity of the pH effect on the transport of ascorbic acid was demonstrated in experiments in which we measured transport of 2-deoxy-d-glucose by melanoma cells at different pH, following the protocols devised for the transport of ascorbic acid. Under these conditions, 2-deoxy-d-glucose transport occurred at a higher velocity at pH 6.0 than at pH 7.4 (Fig. 1D), indicating that changes in extracellular pH affect the activity of different transporters in a highly specific manner. 2-Deoxy-d-glucose is a glucose analog that is transported in cells by facilitative glucose transporters of the GLUT family, of which four members, GLUT1, GLUT2, GLUT3, and GLUT4, are expressed by melanoma cells (35, 41).
FIGURE 1.
pH sensitivity and effect of DEPC on SVCT2-mediated ascorbic acid (AA) transport in melanoma cells. A, time course of ascorbic acid transport at pH 6.0 (●) and 7.4 (○). B, time course of the effect of pH 6.0 (●) and 7.4 (○) on ascorbic acid transport. C, reversibility of the pH effect in cells incubated at pH 6.0 (●) or 7.4 (○) and assayed at pH 7.4. D, effect of pH on 2-deoxy-d-glucose (DOG) transport. E, effect of pH on the apparent ascorbic acid transport Vmax. F, effect of pH on the apparent ascorbic acid transport Km. G, effect of Na+ on ascorbic acid transport at pH 6.0 (●) and 7.4 (○). H, effect of pH on the Na50+. I, Dixon-Webb graph of the effect of pH on SVCT2 function. J, DEPC concentration-response curve for the effect of DEPC on ascorbic acid transport. K, ascorbic acid apparent transport Vmax and Km in cells treated with (●) or without (○) 300 μm DEPC. L, Na+ cooperativity in cells treated with (●) or without (○) 300 μm DEPC. M, effect of DEPC on the pH effect. Ascorbic acid transport at pH 6.0 (●) or 7.4 (■ and ○) in cells treated with (■) or without (○ and ●) 300 μm DEPC. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate. Ascorbic acid transport, DEPC modification, and pH effects were assayed as indicated under “Experimental Procedures.”
Acid pH Attenuates SVCT2 Na+ Activation and Induces Loss of Na+ Cooperativity in Melanoma Cells
We performed concentration-response experiments to determine whether extracellular pH affected the transporter kinetics. The saturation curves in the pH range 6.0–9.0 were consistently hyperbolic and revealed that the decreased ascorbic acid transport observed at pH below 7.0 and higher than 8.0 was due to a decreased transport Vmax (Fig. 1E), with no appreciable changes in the transport Km (Fig. 1F). The overall effect of acid pH was a 50% decrease in the transport Vmax.
Because SVCT2 is a cotransporter of Na+ and ascorbic acid, we explored whether extracellular pH affected the Na+ cooperativity. The effect of Na+ at pH 6.0 was characterized by a concentration-response curve that showed a linear dependence on Na+ concentration (Fig. 1G). The slope of the line revealed that maximal activation would not be reached at physiological Na+ concentrations, indicating that at pH 6.0, the transporter is only partially active due to a marked attenuation of the activation by Na+. In fact, the Na50+ increased in an exponential manner with decreasing pH, reaching a value greater than 90 mm Na+ at pH 6.3 (Fig. 1H).
DEPC Treatment Abolishes the pH Effect on SVCT2 in Melanoma Cells
The reversibility of the effect of extracellular pH on ascorbic acid transport suggests the protonation-deprotonation of lateral chains of amino acid residues exposed to the extracellular milieu as the pH decreases and then increases. Analysis of the pH-dependent changes in the transport Vmax by the Dixon-Webb equation (log of Vmax versus pH) revealed a pKa value of 6.4 for the lateral chains of amino acids protonated at acid pH (Fig. 1I). This is consistent with a possible role for histidine residues exposed to the extracellular milieu in mediating the pH effect. We analyzed this by testing the effect of chemical modification with DEPC, a reagent that under restricted conditions (pH 6.0) modifies histidine residues with elevated selectivity (42–45). Another useful property of DEPC is that the plasma membrane is impermeable to this reagent and therefore reacts only with amino acid residues accessible from the extracellular milieu (43, 46). The melanoma cells were incubated with graded DEPC concentrations for 30 min at pH 6.0, washed free of DEPC, and subjected to a standard ascorbic acid transport assay at pH 7.4. DEPC decreased, in a concentration-dependent manner, ascorbic acid transport by melanoma cells, with an IC50 around 300 μm and maximal inhibition at 3 mm (Fig. 1J). Further experiments performed using 300 μm DEPC revealed the treatment decreased the apparent transport Vmax, from 133 pmol/min/million cells in control cells to 50 pmol/min/million cells in DEPC-treated cells (Fig. 1K). However, the apparent ascorbic acid transport Km remained unaffected at a value of 25 ± 3 μm in DEPC-treated cells, compared with a value of 22 ± 4 μm in untreated control cells (Fig. 1K), and neither the Na+ cooperativity (nH = 2.0) nor the Na50 value (35 mm) was affected by DEPC treatment (Fig. 1L). Interestingly, ascorbic acid transport in DEPC-treated melanoma cells was insensitive to acid pH (Fig. 1M). Thus, although control cells transported ascorbic acid at a decreased rate of 3.5 pmol/min/million cells at pH 6.0, DEPC-treated cells had a transport rate of 8.8 pmol/min/million cells, which was similar to that of control cells at pH 7.4 (9.5 pmol/min/million cells) (Fig. 1M).
SVCT2 Function Is Impaired at Acid pH and DEPC Treatment Abolished the pH Effect in SVCT2-expressing HEK-293 Cells
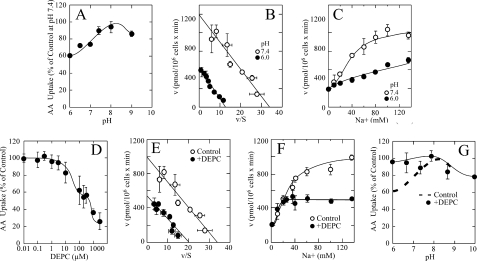
The previous results, supporting the proposal that histidine residues are involved in mediating the effect of extracellular pH on SVCT2 function, were obtained using human melanoma cells that endogenously express SVCT2. We further analyzed this by using HEK-293 cells overexpressing SVCT2 as a fusion protein with the green fluorescent protein (GFP). SVCT2-GFP maintains the kinetic and functional properties of native SVCT2 and is localized to the plasma membrane in HEK-293 cells (7). SVCT2-GFP expressed in HEK-293 cells showed a sensitivity to extracellular pH similar to that of SVCT2 from melanoma cells, with a 40–50% decrease in ascorbic acid transport at pH 6.0 compared with pH 7.4 (Fig. 2A). Concentration-response experiments revealed that the decreased transport at pH 6.0 was associated with changes in the transport Vmax, which decreased to a value of 400 pmol/min/million cells at pH 6.0 compared with 990 pmol/min/million cells at pH 7.4 (Fig. 2B), without appreciable changes in the ascorbic acid transport Km, which remained at 26 μm (Fig. 2B). Most importantly, the Na+ cooperativity was lost at pH 6.0, and the transport rate showed a linear dependence on increasing Na+, indicating a marked attenuation of the activation by Na+ (Fig. 2C).
FIGURE 2.
pH sensitivity and effect of DEPC on SVCT2-mediated ascorbic acid transport in transfected HEK-293 cells. A, effect of pH on ascorbic acid transport. B, effect of pH 6.0 (●) and 7.4 (○) on the apparent ascorbic acid transport Vmax and Km. C, effect of pH 6.0 (●) and 7.4 (○) on the Na+ cooperativity. D, concentration-response curve of the effect of DEPC on ascorbic acid transport. E, ascorbic acid apparent transport Vmax and Km in cells treated with (●) or without (○) 300 μm DEPC. F, Na+ cooperativity in cells treated with (●) or without (○) 300 μm DEPC. G, pH sensitivity in cells treated with (●) or without (dashed line) 300 μm DEPC. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate. Ascorbic acid transport, DEPC modification, and pH effects in transfected cells were assayed as indicated under “Experimental Procedures.”
DEPC decreased ascorbic acid transport in SVCT2-GFP-expressing HEK-293 cells in a concentration-dependent manner (Fig. 2D). The concentration-response curve was complex, suggesting the presence of at least two functional components of different affinity involved in the inhibitory effect of DEPC (Fig. 2D). At 300 μm, DEPC decreased the ascorbic acid transport Vmax to a value of 540 pmol/min/million cells (from 990 pmol/min/million cells in untreated control cells), without altering the transport Km, which remained at 24 μm (Fig. 2E), or the Na+ cooperativity (Fig. 2, E and F). Most importantly, DEPC treatment abolished the sensitivity of SVCT2 to acid pH (Fig. 2G). Thus, our data support a model in which exofacial histidine residues determine the pH sensitivity of SVCT2, a process that involves the loss of the Na+ cooperativity and attenuation of the activation by Na+, generating a partially inactive transporter with a decreased transport Vmax but without alteration in the transport Km.
SVCT2 Single and Double Histidine Mutants Are Targeted to the Plasma Membrane and Are Functional
We hypothesized that protonation of exofacial histidine residues in SVCT2, which are modifiable with DEPC, is responsible for the inhibitory effect of acid pH on transport. SVCT2 possess six histidine residues, His29, His109, His203, His206, His269, and His413, which can be segregated into two groups depending on their accessibility to the extracellular milieu (Fig. 3A). Three histidine residues, His29 in the intracellular amino-terminal moiety, His109 in the endofacial half of transmembrane helix I, and His269 in the endofacial loop II connecting transmembrane helices IV-V, are localized at the endofacial membrane face and therefore are not expected to be directly affected by changes in extracellular pH or accessible to chemical modification with DEPC. In contrast, histidine residues His203 and His206 in the exofacial loop II, connecting transmembrane helices III-IV, and His413 in the exofacial loop IV, connecting helices VII-VIII, are exofacial and are expected to be exposed to the extracellular milieu, to be sensitive to changes in extracellular pH, and to be accessible to chemical modification with DEPC.
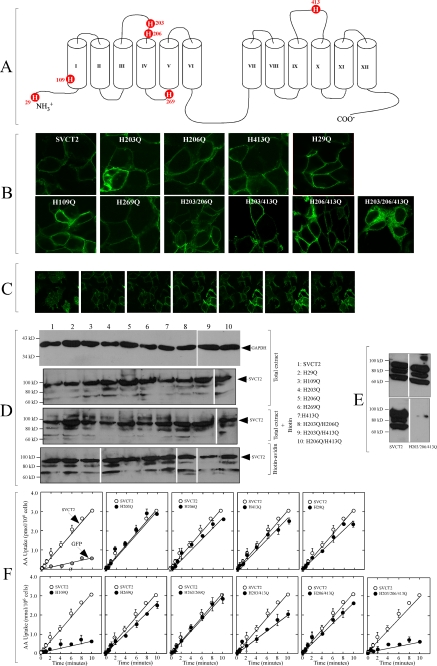
FIGURE 3.
Subcellular expression and functional activity of SVCT2 histidine mutants expressed in HEK-293 cells. A, predicted location of histidine residues in SVCT2 two-dimensional 12-helix model. Histidine residues His203, His206, and His413 are exofacial, and histidine residues His29, His109, and His269 were considered to be endofacially localized. B, confocal microscopy of SVCT2-GFP and the different mutants in transfected HEK-293 cells. An optical slice from the middle section of cells is shown. C, confocal analysis of SVCT2-GFP subcellular localization in HEK-293 cells. Seven equally interspersed optical slices, selected to encompass the complete height of the cells, from top to bottom, are shown. D, surface biotinylation of plasma membrane proteins in transfected HEK-293 cells. Proteins from untreated (Total extract), biotinylated (Total extract + Biotin), and biotin-avidin-treated cells (Biotin-avidin) were separated by SDS-PAGE, transferred to a PVDF membrane, incubated with anti-GFP and a secondary HRP-coupled antibody, and revealed by chemiluminescence. Samples were strictly processed in parallel (biotinylation, avidin selection, separation by SDS-PAGE and transfer to PVDF membrane, incubation with primary and secondary antibodies, and analysis by chemiluminescence). The film containing the final image was cut in pieces and reordered for clarity of presentation. Numbers to the left correspond to the migration of molecular size markers. The arrowheads to the right indicate the migration of the main SVCT2-GFP band at about 90 kDa. E, surface biotinylation of the triple mutant H203Q/H206Q/H413Q. Experimental details were as described in D. F, time course ascorbic acid (AA) transport assay 48 h after transfection in HEK-293 cells expressing SVCT2-GFP or the different mutants. Cells expressing SVCT2-GFP or GFP alone were used as the positive and negative controls, respectively. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate. Other experimental details were as indicated under “Experimental Procedures.”
We replaced each histidine residue of SVCT2 with a glutamine residue by site-directed mutagenesis, generating three endofacial single mutants (H29Q, H109Q, and H269Q), three exofacial single mutants (H203Q, H206Q, and H413Q), three exofacial double mutants (H203Q/H206Q, H203Q/H413Q, and H206Q/H413Q), and the triple exofacial mutant H203Q/H206Q/H413Q. Each mutant was expressed in HEK-293 cells and examined for subcellular localization and function, including transport kinetics (Km and Vmax of ascorbic acid transport), Na+ cooperativity (nH and Na50+), sensitivity to acid pH, and effect of DEPC.
Confocal analysis revealed that the fluorescence associated with the expression of nine of the 10 mutants was associated with the plasma membrane (the exception is the triple mutant H203Q/H206Q/H413Q), with similar fluorescence levels observed for the mutants and SVCT2 (Fig. 3B). We obtained multiple optical slices from each sample to scan the complete cell z axis, which confirmed that the fluorescence associated with the mutants was specifically localized at the plasma membrane level (Fig. 3C) (data not shown). Cotransfection experiments with fluorescent protein markers for different organelles revealed that the fluorescence associated with expression of SVCT2 and the different mutants colocalized with the plasma membrane marker GLUT1 but not with the endoplasmic reticulum marker PDI (49), the Golgi apparatus marker Stx6 (50), or the mitochondrial marker Grx2a (51) (supplemental Fig. 1S) (data not shown). No plasma membrane-associated fluorescence was observed for the triple mutant H203Q/H206Q/H413Q, with the fluorescence associated with intracellular punctuate and reticulate-like structures distributed around the cell nucleus (Fig. 3B). Colocalization experiments with markers for cellular organelles confirmed retention in the endoplasmic reticulum and the Golgi apparatus (supplemental Fig. 1S).
Biotinylation of surface proteins confirmed the plasma membrane localization of SVCT2 and the nine mutants (Fig. 3D). Although there were differences in the relative intensity of the protein bands corresponding to the different mutants, we observed similar variations in intensity when analyzing different samples of a given mutant that we ascribed to experimental variations inherent to the biotinylation protocol. Also, the presence of more than one band of variable intensity has been previously documented for SVCT2 in different cellular models (5, 47, 48). These experiments confirmed that the triple mutant H203Q/H206Q/H413Q was not accessible to surface biotinylation (Fig. 4E).
FIGURE 4.
Altered kinetic properties of SVCT2 histidine mutants (A–H) expressed in HEK-293 cells. Ascorbic acid transport was measured 48 h after transfection. Shown is the Eadie-Hofstee transformation of the ascorbic acid concentration-response curves (2–200 μm) for the effect of the mutations on the transport rate. Apparent transport Km and Vmax were obtained using non-linear analysis from the Michaelis-Menten equation. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate. ○, SVCT2; ●, mutant SVCT2.
With the exception of the single endofacial mutant H109Q and the triple mutant H203Q/H206Q/H413Q, eight of the plasma-membrane associated mutants were active and transported ascorbic acid at a rate similar to SVCT2-expressing cells (Fig. 4F). For H109Q and H203Q/H206Q/H413Q, the ascorbic acid transport rate was about 15% of that of SVCT2-expressing cells and similar to cells transfected with GFP (Fig. 4F). Because abundant plasma membrane localization of the mutant protein H109Q was demonstrated by confocal microscopy and surface biotinylation (Fig. 4, B and D), these results strongly support the concept that H109Q is an inactive mutant that lacks the capacity to transport ascorbic acid. One possible explanation for these results is that replacement of H109Q produces a large rearrangement that disrupts the normal conformation of SVCT2 in such a manner that an inactive transporter results. However, the correct sorting of the mutant protein to the plasma membrane argues against this possibility. An alternate and more interesting possibility is that His109 is an important structural element of the substrate-binding site in SVCT2, and therefore its replacement will have a major effect on the capacity of SVCT2 to bind and transport ascorbic acid. This proposal is consistent with data indicating that His51 in SVCT1, the equivalent of His109 in SVCT2, is fundamental in defining the specificity of SVCT1 for ascorbic acid; replacement of His51 in SVCT1 with a glutamine residue rendered a transporter unable to transport ascorbic acid (52). Similar findings were reported for His31 in the bacterial YgfO xanthine permease from the nucleobase-ascorbate transporter family (53).
SVCT2 Histidine Mutants Show Altered Ascorbic Acid Transport Km
Concentration-response studies revealed differences in the kinetic properties of exofacial histidine mutants. The exofacial mutants H203Q and H206Q (exofacial loop II) had kinetic constants similar to SVCT2 of around 20 μm (Fig. 4, A and B, and Table 1), whereas the exofacial mutant H413Q (exofacial loop IV) showed a 3-fold increase in the apparent transport Km but maintained a similar Vmax compared with SVCT2 (Fig. 4C and Table 1). The double mutant H203Q/H206Q showed a 3-fold increase in the ascorbic acid transport Km (Fig. 4D and Table 1), whereas the double mutants H203Q/H413Q and H206Q/H413Q had apparent ascorbic acid transport Km values similar to that of SVCT2 (Fig. 4, E and F, and Table 1), indicating that the simultaneous introduction of a mutation in the exofacial loop II neutralized the effect of a mutation in the exofacial loop IV. These loops are at a distance of about 200 amino acid residues in SVCT2 primary structure from each other. An interesting possibility is that exofacial loops II and IV are in close contact in the three-dimensional structure of the transporter and are able to interact with each other; this interaction would be disrupted in the single and the double exofacial histidine mutants, altering the transport Km without affecting the general structural features of the transporter. Validation of this proposal will require a three-dimensional model of SVCT2.
TABLE 1.
Functional properties of SVCT2 histidine mutants
Values are from one experiment of two performed in triplicate. PM, plasma membrane.
| Mutant | Localization | Vmax | Km | nH | Na50+ |
|---|---|---|---|---|---|
| pmol/106 cells/min | μm | ||||
| SVCT2 | PM | 994 ± 34 | 24 ± 13 | 1.7 ± 0.1 | 29 ± 2 |
| H203Q | PM | 865 ± 34 | 28 ± 13 | 2.2 ± 0.2 | 33 ± 2 |
| H206Q | PM | 914 ± 34 | 28 ± 13 | 2.3 ± 0.3 | 22 ± 2 |
| H413Q | PM | 982 ± 11 | 68 ± 2 | 1.7 ± 0.2 | 20 ± 2 |
| H29Q | PM | 994 ± 34 | 24 ± 13 | 1.7 ± 0.1 | 34 ± 2 |
| H109Q | PM | ||||
| H269Q | PM | 1307 ± 24 | 95 ± 4 | 1.7 ± 0.1 | 20 ± 2 |
| H203Q/H206Q | PM | 986 ± 14 | 67 ± 3 | 1.6 ± 0.4 | 28 ± 2 |
| H203Q/H413Q | PM | 632 ± 21 | 28 ± 2 | 1.4 ± 0.1 | 26 ± 1 |
| H206Q/H413Q | PM | 994 ± 34 | 24 ± 13 | 1.7 ± 0.1 | 29 ± 2 |
| H203Q/H206Q/H413Q | Intracellular | 98.2 ± 2.4 | 23 ± 2 | 2.2 ± 0.2 | 34 ± 2 |
With regard to the endofacial histidine mutants, the mutant H29Q maintained the kinetic properties of SVCT2 (Fig. 4G and Table 1). In contrast, the mutant H269Q had an altered transport Km, which increased ∼5-fold, compared with SVCT2 control, to 95 μm without showing appreciable changes in the transport Vmax (Fig. 4H and Table 1). His269 is in the interface between the beginning of transmembrane helix V and the C-terminal end of the endofacial loop II connecting transmembrane helices IV-V. Presently, we have insufficient data to propose an explanation for the role of histidine 269 in SVCT2 function.
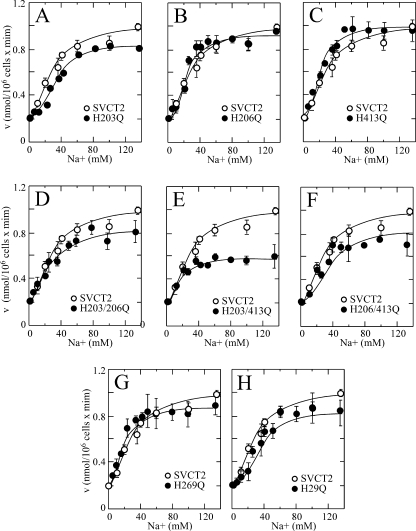
SVCT2 Histidine Mutants Show Unaltered Na+ Cooperativity
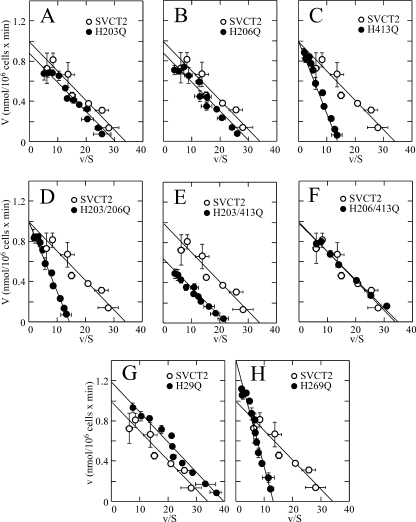
The histidine mutants conserved the capacity to be activated by Na+ in a cooperative manner, with minimal variations in the nH and the Na50+ that remained at values around 2.0 and 30 mm, respectively. This result applied to the single exofacial mutants (Fig. 5, A–C, and Table 1), the double exofacial mutants (Fig. 5, D–F, and Table 1), and the single endofacial mutants (Fig. 5, G and H, and Table 1). These results imply that replacing the histidine residues of SVCT2 with glutamine is associated with small, localized conformational changes that alter in a very specific manner the molecular interactions in the microenvironment surrounding each specific histidine residue. It is expected that massive conformational changes, if they were to occur in the histidine mutants, would have a major effect on the Na+ cooperativity, a fact that was not observed in the case of the histidine mutants. We have previously demonstrated the existence of a delicate functional balance between the Na+ and ascorbic acid binding sites in SVCT2 that defines, in a reciprocal manner, the extent of the Na+ cooperative effect and the ascorbic acid kinetic constants (7). Our present results advance our understanding of these functional interactions by indicating that they can be uncoupled by mutations in specific histidine residues.
FIGURE 5.
Unaltered cooperative Na+ activation of SVCT2 histidine mutants (A–H) expressed in HEK-293. Transport of 100 μm ascorbic acid was measured 48 h after transfection using a 5-min transport assay. Concentration-response curves for the effect of Na+ on ascorbic acid transport rate were constructed using graded Na+ concentrations from 5 to 135 mm. ○, SVCT2; ●, mutant SVCT2. Error bars, S.D.
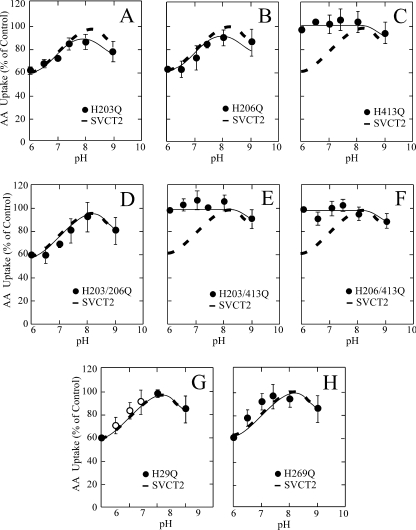
Site-directed Mutagenesis Reveals His413 Involvement in SVCT2 pH Sensitivity
Because the histidine mutants conserve, to a great extent, the functional properties of SVCT2, they were examined for their sensitivity to acid pH in order to identify possible histidine residues involved. We first examined the pH sensitivity of the single exofacial mutants. The mutants H203Q and H206Q (exofacial loop II) had a pH sensitivity similar to SVCT2, which is characterized by a 40% decrease in the ascorbic acid transport rate at pH 6.0 compared with pH 7.4 (Fig. 6, A and B). In contrast, the mutant H413Q (exofacial loop IV) lost the sensitivity to acid pH, with the result that ascorbic acid transport at pH 6.0 occurred at a rate similar to that at pH 7.4 (Fig. 6C). These results were confirmed when analyzing the pH sensitivity of the double exofacial histidine mutants (Fig. 6, D–F). The double mutant H203Q/H206Q was still sensitive to acid pH, with a 40% decrease in ascorbic acid transport at pH 6.0 compared with pH 7.4 (Fig. 6D), therefore eliminating the participation of histidine residues localized in the exofacial loop II in the acid pH effect on SVCT2 function. Conversely, the double mutants H203Q/H413Q and H206Q/H413Q were completely insensitive to the effect of acid pH on transport, transporting ascorbic acid at a similar rate at pH 6.0 and 7.4 (Fig. 6, E and F). Thus, the lack of an effect at pH 6.0 is due to the mutation H413Q in both double mutants and not to the mutation H203Q or H206Q. As expected, the endofacial histidine residues His29 and His269 had no bearing on the effect of extracellular pH on SVCT2 (Fig. 6, G and H). In both cases, transport at pH 6.0 was about 40% of that observed at pH 7.4.
FIGURE 6.
Altered pH sensitivity of SVCT2 histidine mutants expressed in HEK-293 cells. 48 h after transfection, the cells were incubated for 30 min at different pH values (6.0–9.0) and then subjected to a 5-min transport assay at the same pH. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate. AA, ascorbic acid.
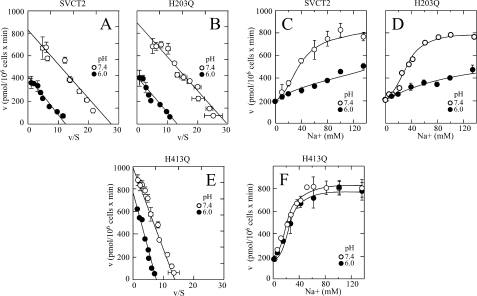
When analyzed in concert, our results indicate that His413 is the structural element that defines SVCT2 sensitivity to acid pH. Importantly, there was no relationship between the changes in the value of the ascorbic acid transport Km and changes in the sensitivity to acid pH. Thus, the single mutants H413Q and H269Q and the double mutant H203Q/H206Q showed similar increases in their ascorbic acid transport Km, but only the mutant H413Q was insensitive to acid pH (Figs. 4 (C, D, and H) and 6C). Indeed, when comparing the respective concentration-response curves for the effect of ascorbic acid on transport, we found that at 100 μm ascorbic acid, the concentration used to test the effect of acid pH on transport, the mutants transported ascorbic acid at a rate essentially equivalent with that of SVCT2 (data not shown). To further analyze this, we selected the exofacial mutants H203Q and H413Q to study the effect of pH 6.0 on four parameters of SVCT2 function that are differentially affected by acid pH: the apparent Km and Vmax of ascorbic acid transport and the nH and Na50+ for the cooperative activation by Na+. As expected, the mutant H203Q showed a response to acid pH identical to SVCT2 control, characterized by a 50% decrease in the apparent transport Vmax (Fig. 7, A and B), no measurable change in the transport Km (Fig. 7, A and B), and loss of the cooperative activation by Na+ (Fig. 7, C and D). On the other hand, the mutant H413Q had an ascorbic acid transport Km of 70 μm at pH 7.4, which is about 4-fold higher than that of SVCT2 (Fig. 7E), and although there was a decrease in the transport Vmax at pH 6.0, the transport Km remained constant at about 75 μm (Fig. 7E). Moreover, the mutant H413Q was cooperatively activated by Na+ in a similar manner at pH 6.0 and 7.4, with an nH and Na50+ of 2.0 and 34 mm, respectively (Fig. 7F), indicating a complete loss of acid pH sensitivity.
FIGURE 7.
Effect of pH on the kinetics and Na+ activation of SVCT2 and mutants H203Q and H413Q expressed in HEK-293 cells. Ascorbic acid transport was measured 48 h after transfection. A–C, effect of pH 6.0 (●) and 7.4 (○) on ascorbic acid transporter kinetics. Shown is the Eadie-Hofstee transformation of the ascorbic acid concentration-response curves for the effect of the mutations on SVCT2 (A), mutant H203Q (B), and mutant H413Q (C) kinetics. D–F, effect of pH 6.0 (●) and 7.4 (○) on the cooperative Na+ activation of SVCT2 (D), mutant H203Q (E), and mutant H413Q (F). Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate.
DEPC Treatment Abolishes the pH Effect on SVCT2-mediated Transport and Involves His203 and His413
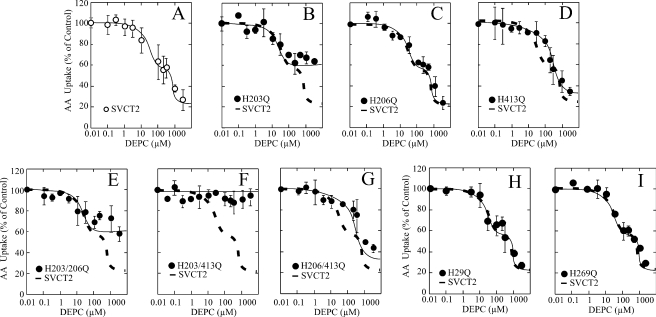
The mutagenesis experiments revealed that exofacial His413 is responsible for the effect of acid pH on SVCT2 function. We pursued this further by performing chemical modification with DEPC of the different mutants and analyzing the effect on ascorbic acid transport. DEPC decreased, in a concentration-dependent manner, ascorbic acid transport in HEK-293 cells expressing SVCT2-GFP (Fig. 8A). Analysis of the concentration-response curve revealed the presence of two functional components with different sensitivity to DEPC, with each component accounting for about 50% of the DEPC effect on transport. The highest affinity component was sensitive to DEPC concentrations from 1 to about 30 μm, and the lower affinity component was sensitive to DEPC concentrations from 100 μm to 3 mm (Fig. 8A). Maximal transport inhibition was observed at 3 mm DEPC, a concentration at which the transport rate was less than 20% of untreated controls (Fig. 8A). This clearly differs from our data showing a single histidine residue involved in the effect of acid pH.
FIGURE 8.
Effect of DEPC on ascorbic acid transport by SVCT2 (A) and mutants H203Q and H413Q (B–I) expressed in HEK-293 cells. Ascorbic acid (AA) transport was measured 48 h after transfection. The cells were incubated for 30 min at pH 6.0 with graded concentrations of DEPC, washed, and submitted to a 5-min transport assay using 100 μm ascorbic acid. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate.
The single exofacial mutants showed a varied response to DEPC. Thus, the exofacial mutant H203Q was only partially sensitive to DEPC, with a maximal decrease in ascorbic acid transport of about 40% observed at 100 μm DEPC and no further decrease observed at DEPC concentrations from 100 μm to 3 mm, indicating the selective absence of the low affinity component sensitive to high DEPC concentrations (Fig. 8B). On the other hand, the mutant H206Q was similar to SVCT2 with regard to the effect of DEPC, with no changes observed in the shape of the concentration-response curve and the extent of the inhibition (Fig. 8C). In contrast to H203Q, the mutant H413Q was insensitive to DEPC concentrations from 1 to 30 μm, indicating the loss of the high affinity component that was sensitive to low DEPC concentrations, but conserved the low affinity component sensitive to high DEPC concentrations (Fig. 8D). A maximal transport decrease of about 70% was observed at 3 mm DEPC, which revealed that the extent of inhibition was higher than expected, considering that each component accounted for about 50% of the DEPC inhibitory effect on transport in SVCT2, a functional difference that could have mechanistic implications.
These results were confirmed when analyzing the DEPC sensitivity of the three double exofacial mutants (Fig. 8, E–G). The double mutant H203Q/H206Q showed a sensitivity to DEPC identical with mutant H203Q, with loss of the low affinity component, conservation of the high affinity component sensitive to low DEPC concentrations, and maximal transport inhibition of about 40% at 30 μm DEPC (Fig. 8E). On the other hand, the double mutant H203Q/H413Q was completely insensitive to DEPC; concentrations from 0.1 μm to 3 mm DEPC had no effect on ascorbic acid transport (Fig. 8F). Most importantly, the double mutant H206Q/H413Q showed a sensitivity to DEPC identical with that of mutant H413Q, with loss of the high affinity component, conservation of the low affinity component sensitive to high DEPC concentrations, and maximal transport inhibition of about 70% at 3 mm DEPC (Fig. 8G).
The endofacial mutants H29Q and H269Q showed a response to DEPC similar to SVCT2, with the concentration-response curve containing two functional components with different sensitivity to DEPC and maximal inhibition at 3 mm DEPC (Fig. 8, H and I). This is expected from their endofacial localization that makes them inaccessible to DEPC.
Our end point for assaying the effect of DEPC on SVCT2 is the transport of ascorbic acid; therefore, our results cannot be used to identify which amino acid residues are chemically modified by DEPC. Even with this limitation, we can argue that endofacial histidines His29 and His269 are not modifiable with DEPC because the plasma membrane is impermeable to this reagent. This leaves the exofacial histidine residues His203, His206, and His413 as possible modification targets because, consistent with the SVCT2 secondary model, these residues should be accessible from the extracellular milieu. It is therefore reasonable to assume that they are subjected to chemical modification during DEPC treatment. From our present data, we conclude that exofacial histidine residues His203 and His413, located on two different exofacial loops in SVCT2, are the structural elements that define the two functional components with differential sensitivity to the inhibitory effect of DEPC on ascorbic acid transport.
The Effect of DEPC on SVCT2 Function Is Defined by the Selective Targeting of His413 at Low DEPC Concentrations
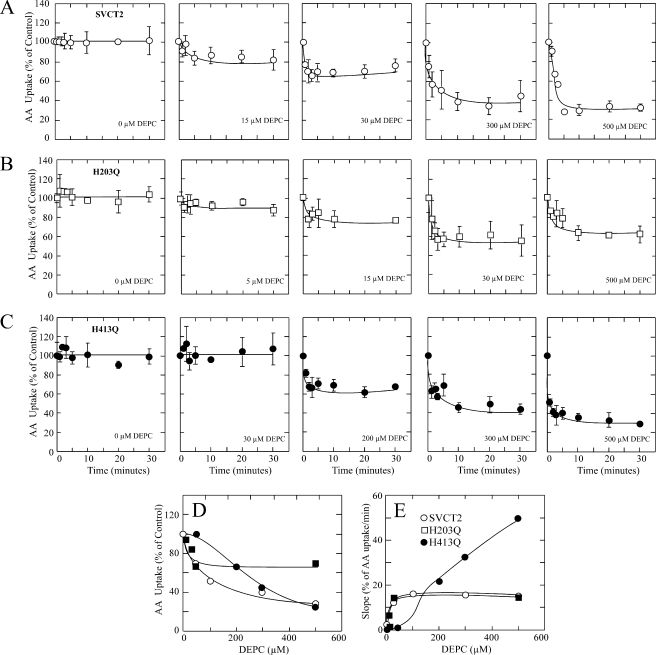
The effect of DEPC on SVCT2-mediated ascorbic acid transport developed rapidly; maximal transport inhibition was observed within the first 5 min of treatment at all DEPC concentrations tested and remained at that level for the 30-min duration of the experiment (Fig. 9A). Transport inhibition was observed at 15 μm DEPC, the extent of inhibition increased with increasing DEPC concentrations, and maximal transport inhibition of 80% was observed at 500 μm DEPC (Fig. 9, A and D). For mutant H203Q, maximal transport inhibition was also observed within 5 min of treatment at all DEPC concentrations tested and remained at that level for the 30-min duration of the experiment (Fig. 9B). The extent to which transport was inhibited increased with increasing DEPC concentrations; maximum transport inhibition of 40% was observed at 30 μm DEPC and remained at that level at 500 μm DEPC (Fig. 9, B and D), results that are consistent with the fact that His413 is the histidine residue responsible for the effect of DEPC in this particular mutant. For mutant H413Q, the DEPC effect on transport was almost instantaneous, with maximal transport inhibition observed within the first minute of treatment, and remained at that level for the 30-min duration of the experiment (Fig. 9C). There was no inhibition at DEPC concentrations of up to 30 μm, but clear transport inhibition was observed at 100 μm and higher DEPC concentrations, with maximal inhibition observed at 500 μm DEPC (Fig. 9, C and E). These results are consistent with the fact that His203 is the histidine residue responsible for the effect of DEPC in this particular mutant. However, ascorbic acid transport was inhibited by 70% in mutant His413 at 500 μm DEPC, which is similar to the inhibition observed for SVCT2 under similar conditions (Fig. 9, A, C, and D) and is about twice the extent of inhibition expected for this mutant (Fig. 8). The anomalous behavior of mutant H413Q was also evident when examining the effect of DEPC on the rate of transport inactivation (Fig. 9E). For SVCT2, the rate of ascorbic acid transport inactivation increased rapidly at low DEPC concentrations, reaching a maximal value at 30 μm DEPC that remained constant at DEPC concentrations of up to 500 μm (Fig. 9E). The behavior of the mutant H203Q matched exactly that of SVCT2, with the rate of transport inactivation increasing rapidly at low DEPC concentrations, reaching a maximal value at 30 μm DEPC that remained constant at DEPC concentrations of up to 500 μm (Fig. 9E). In contrast, the rate of transport inactivation for the mutant H413Q remained near zero at DEPC concentrations of 30 μm or less but increased rapidly with a linear dependence on concentration at higher concentrations of DEPC, with the result that the rate of inactivation at 500 μm DEPC was 3-fold higher than that of SVCT2 and the mutant H203Q (Fig. 9E).
FIGURE 9.
Time course of the effect of DEPC on ascorbic acid (AA) transport by SVCT2 and histidine mutants H203Q and H413Q. 48 h after transfection, cells expressing SVCT2 (A), mutant H203Q (B), or mutant H413Q (C) were incubated with graded concentrations of DEPC and submitted to a 5-min transport assay using 100 μm ascorbic acid. D, concentration-response curve for the effect of DEPC on the rate of ascorbic acid transport after a 10-min modification experiment with DEPC. E, concentration-response curve for the effect of DEPC on the initial rate of decrease of the ascorbic acid transport rate. For each curve, the slope of the initial decrease in transport rate was obtained by linearization of the first 3 min of the time course curve of DEPC treatment. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate.
In brief, the two central results are as follows: (i) the rate of transport inactivation is similar in SVCT2 and the mutant H203Q in which only His413 is present, and (ii) there was a marked increase in the inactivation rate of the mutant H413Q in which only His203 is present. These results can be rationalized if we assume that the decreased ascorbic acid transport observed after DEPC treatment is a direct result of the chemical modification of histidine residues His203 and His413 in SVCT2. We propose that the rate-limiting step of the overall effect of DEPC is determined by the rate of modification of His413, that the first DEPC-modified residue in SVCT2 is His413, and that His203 is not accessible for modification without the previous modification of His413. However, His203 is fully accessible to modification with DEPC in the mutant H413Q, as indicated by the increased rate of transport inactivation. This implies the occurrence of a localized conformational change due to the replacement of His413 with glutamine in the exofacial loop IV connecting transmembrane helices VII-VIII and that this change is transmitted and sensed by the microenvironment around His203 in the exofacial loop II connecting transmembrane helices III-IV. This proposal is supported by our results indicating that the mutant H413Q has an increased transport Km, a change that is reverted in the double mutant H203Q/H413Q, suggesting a functional-structural interaction between the exofacial loops II (His203) and IV (His413). Although the three-dimensional structure of SVCT2 is not known, the length of the exofacial loops II (29 amino acid residues) and IV (27 amino acid residues) makes it feasible that they may be close enough in SVCT2 to interact with each other.
His413 Is Responsible for SVCT2 Acid pH Sensitivity
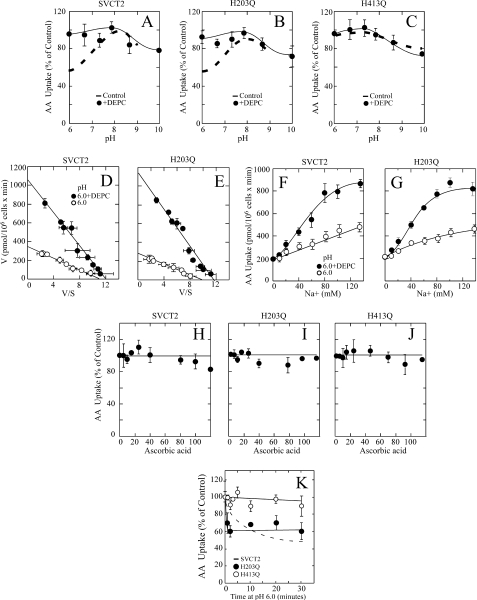
We explored whether modification of SVCT2 with 30 μm DEPC, to specifically target the high affinity component (His413), would alter SVCT2 pH sensitivity in a manner that would confirm the central role of His413. For SVCT2, 30 μm DEPC eliminated the acid pH effect on transport, as indicated by a similar ascorbic acid transport rate at pH 6.0 and 7.4 (Fig. 10A). Similarly, 30 μm DEPC eliminated the acid pH effect on transport for the mutant H203Q, as indicated by a similar ascorbic acid transport rate at pH 6.0 and 7.4 (Fig. 10B). No effect on transport was observed when the mutant H413Q, insensitive to the effect of acid pH by the lack of His413, was subjected to DEPC treatment (Fig. 10C).
FIGURE 10.
SVCT2 and mutants H203Q and H413Q; pH sensitivity and DEPC and ascorbic acid effect. Forty-eight hours after transfection, the cells were assayed for ascorbic acid transport. A–C, effect of pH on ascorbic acid transport in HEK-293 cells expressing SVCT2 (A), mutant H203Q (B), or mutant H413Q (C) and treated with (●) or without (dashed line) 30 μm DEPC. D–E, ascorbic acid transport kinetics at pH 6.0 in HEK-293 cells expressing SVCT2 (D) or mutant H203Q (E) and treated with (●) or without (○) 30 μm DEPC. F and G, Na+ cooperativity of ascorbic acid transport at pH 6.0 in cells expressing SVCT2 (F) or mutant H203Q (G) and treated with (●) or without (○) 30 μm DEPC. H–J, effect of ascorbic acid on the effect of DEPC on the transporter pH sensitivity. Transfected HEK-293 cells expressing SVCT2 (H), mutant H203Q (I), or mutant H413Q (J) were incubated for 30 min with 30 μm DEPC in the presence of increasing ascorbic acid concentrations, washed, and assayed for ascorbic acid transport at pH 6.0. K, time course of the effect of acid pH. SVCT2 (dashed line), mutant H203Q (●), and mutant H413Q (○) were incubated at pH 6.0 for the indicated times and assayed for ascorbic acid transport at pH 6.0. Transport data correspond to the means ± S.D. (error bars) of one experiment of two performed in triplicate.
We conclude that targeting His413 using modification with DEPC affects SVCT2 function similarly to its replacement with glutamine in the mutant H413Q. Consistent with this interpretation, further studies demonstrated that, when treated with 30 μm DEPC, both SVCT2 and the mutant H203Q acquired the functional properties observed for mutant H413Q (Fig. 10, D–G). At pH 6.0, DEPC-treated SVCT2 presented an augmented transport Km of 80 μm (Fig. 10D) and was cooperatively activated by Na+, with an nH of 2.0 and an Na50+ of 35 mm (Fig. 10F). Almost identical results were observed for the DEPC-treated mutant H203Q, which presented an augmented transport Km of 85 μm (Fig. 10E) and was cooperatively activated by Na+, with an nH of 2.0 and an Na50+ of 35 mm (Fig. 10G).
Is His413 directly involved in the binding of Na+ to SVCT2? Similar to SVCT2 and the mutant H203Q treated with 30 μm DEPC, the mutant H413Q had a transport Km of 70 μm, which may be interpreted as suggesting that His413 is involved in the binding of ascorbic acid. However, ascorbic acid failed to protect the functional activity of SVCT2 and the mutants H203Q and H413Q from the effect of DEPC (Fig. 10, H–J). Moreover, the absence of His413 (through replacement by site-directed mutagenesis or by selective targeting with DEPC) relieved the effect of acid pH on SVCT2 but failed to affect the Na+ activation profile (cooperativity and Na50+). Therefore, we conclude that His413 is not directly involved in the binding of Na+ to SVCT2.
Importantly, although protonation of histidines should be a fast event, the pH effect is very slow, occurring in a range of 10–20 min. One possibility to explain these results is that exposure to acidic extracellular pH might be associated with a secondary change in intracellular pH (54), a change that would be expected to be slow and therefore may be consistent with the slow time course of the pH effect on transport. Consistent with this proposal, the activity of the cation channel TRPM2 is modulated by changes in intracellular and extracellular pH, with participation of specific exofacial and endofacial histidine residues (55). However, our mutagenesis and DEPC modification studies identified exofacial His413 as the amino acid residue responsible for the pH effect, discarding the participation of endofacial histidine residues His29 and His269. However, we cannot discard the occurrence of a slow conformational rearrangement due to changes in intracellular pH as the basis for the slow effect of extracellular pH on transport.
Alternatively, our results imply that His413 may participate in maintaining the conformational integrity of SVCT2, with acid pH inducing a slow local conformational rearrangement that disrupts a functional (structural?) interaction between exofacial loops II and IV in SVCT2. This conformational rearrangement could explain the slow effect of pH on transport, which is in contrast with the much faster effect of DEPC modification. We tested this hypothesis by analyzing the time course of the pH effect on the mutants H203Q and H413Q. As expected, acid pH failed to affect the transport activity of the mutant H413Q but induced a rapid time-dependent decrease in the transport activity of the mutant H203Q, in a manner similar to the effect of DEPC modification. These results are consistent with the concept that the absence of His203 in the mutant H203Q disrupts the interaction between exofacial loops II and IV, augmenting the exposure of His413 to the extracellular milieu and accelerating the conformational rearrangement involved in the pH effect.
A final question is central to this discussion. What is the role of the substrate on the pH effect? Although there is no available information on the exact chemical identity of the transported substrate (protonated/anion), it is clear that at pH 6.0, ascorbic acid is still unprotonated (pKa = 4.2). Because presently we do not know the molecular architecture of the substrate binding site on the transporter, the interesting possibility that the ascorbate pKa may change upon binding is a matter that will require elucidation of the three-dimensional structure of SVCT2.
Conclusions
Five histidine residues, from a total of six present in SVCT2, are central regulators of SVCT2 function, modulating pH sensitivity (His413), transporter kinetics (His203, His206, His269, and His413), conformational stability and subcellular localization (His203, His206, and His413), and substrate interactions (His109). Our results indicate that acid pH impairs SVCT2 function through a mechanism involving a marked attenuation of the activation by Na+ and loss of Na+ cooperativity. At physiological Na+ concentrations, the transporter is only partially active, with a decreased Vmax and conservation of the transport Km. The Na+ concentration-response curve suggests that maximal activation of the transporter may be achieved at ∼300 mm Na+ at pH 6.0, which may have physiological implications for the transport of ascorbic acid in the kidney and at the proximal tubules and may also have pathophysiological implications during hypoxia-reoxygenation events.
Supplementary Material
This work was supported in part by Anillo de Investigación en Ciencia y Tecnología, Programa Bicentenario de Ciencia y Tecnología, Chile, Grant PBCT-ACT28; Fondecyt (Fondo Nacional de Desarrollo Científico y Tecnológico, Chile) Grants 1090501, 1060198, and 11080144; doctoral fellowships from the Comisión Nacional de Investigación Científica y Tecnológica, Chile; and equipment grants by the Mecesup program from the Chilean Education Ministry.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1S.
- DEPC
- diethylpirocarbonate.
REFERENCES
- 1.Daruwala R., Song J., Koh W. S., Rumsey S. C., Levine M. (1999) FEBS Lett. 460, 480–484 [DOI] [PubMed] [Google Scholar]
- 2.Rajan D. P., Huang W., Dutta B., Devoe L. D., Leibach F. H., Ganapathy V., Prasad P. D. (1999) Biochem. Biophys. Res. Commun. 262, 762–768 [DOI] [PubMed] [Google Scholar]
- 3.Tsukaguchi H., Tokui T., Mackenzie B., Berger U. V., Chen X. Z., Wang Y., Brubaker R. F., Hediger M. A. (1999) Nature 399, 70–75 [DOI] [PubMed] [Google Scholar]
- 4.Wang H., Dutta B., Huang W., Devoe L. D., Leibach F. H., Ganapathy V., Prasad P. D. (1999) Biochim. Biophys. Acta 1461, 1–9 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y., Mackenzie B., Tsukaguchi H., Weremowicz S., Morton C. C., Hediger M. A. (2000) Biochem. Biophys. Res. Commun. 267, 488–494 [DOI] [PubMed] [Google Scholar]
- 6.Savini I., Rossi A., Pierro C., Avigliano L., Catani M. V. (2008) Amino Acids 34, 347–355 [DOI] [PubMed] [Google Scholar]
- 7.Godoy A., Ormazabal V., Moraga-Cid G., Zúñiga F. A., Sotomayor P., Barra V., Vasquez O., Montecinos V., Mardones L., Guzmán C., Villagrán M., Aguayo L. G., Oñate S. A., Reyes A. M., Cárcamo J. G., Rivas C. I., Vera J. C. (2007) J. Biol. Chem. 282, 615–624 [DOI] [PubMed] [Google Scholar]
- 8.Mackenzie B., Illing A. C., Hediger M. A. (2008) Am. J. Physiol. Cell Physiol. 294, C451–C459 [DOI] [PubMed] [Google Scholar]
- 9.Maulén N. P., Henríquez E. A., Kempe S., Cárcamo J. G., Schmid-Kotsas A., Bachem M., Grünert A., Bustamante M. E., Nualart F., Vera J. C. (2003) J. Biol. Chem. 278, 9035–9041 [DOI] [PubMed] [Google Scholar]
- 10.Liang W. J., Johnson D., Ma L. S., Jarvis S. M., Wei-Jun L. (2002) Am. J. Physiol. Cell Physiol. 283, C1696–C1704 [DOI] [PubMed] [Google Scholar]
- 11.Boyer J. C., Campbell C. E., Sigurdson W. J., Kuo S. M. (2005) Biochem. Biophys. Res. Commun. 334, 150–156 [DOI] [PubMed] [Google Scholar]
- 12.Subramanian V. S., Marchant J. S., Boulware M. J., Said H. M. (2004) J. Biol. Chem. 279, 27719–27728 [DOI] [PubMed] [Google Scholar]
- 13.Wu X., Zeng L. H., Taniguchi T., Xie Q. M. (2007) Cell Death Differ. 14, 1792–1801 [DOI] [PubMed] [Google Scholar]
- 14.Varma S., Sobey K., Campbell C. E., Kuo S. M. (2009) Biochemistry 48, 2969–2980 [DOI] [PubMed] [Google Scholar]
- 15.Subramanian V. S., Marchant J. S., Reidling J. C., Said H. M. (2008) Biochem. Biophys. Res. Commun. 374, 123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang W. J., Johnson D., Jarvis S. M. (2001) Mol. Membr. Biol. 18, 87–95 [DOI] [PubMed] [Google Scholar]
- 17.Nugent S. G., Kumar D., Rampton D. S., Evans D. F. (2001) Gut 48, 571–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown D., Breton S., Ausiello D. A., Marshansky V. (2009) Traffic 10, 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown D., Paunescu T. G., Breton S., Marshansky V. (2009) J. Exp. Biol. 212, 1762–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renkema K. Y., Velic A., Dijkman H. B., Verkaart S., van der Kemp A. W., Nowik M., Timmermans K., Doucet A., Wagner C. A., Bindels R. J., Hoenderop J. G. (2009) J. Am. Soc. Nephrol. 20, 1705–1713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosen S., Oliver J. A., Steinmetz P. R. (1974) J. Membr. Biol. 15, 193–205 [DOI] [PubMed] [Google Scholar]
- 22.Steinmetz P. R. (1974) Physiol. Rev. 54, 890–956 [DOI] [PubMed] [Google Scholar]
- 23.McKinney T. D., Burg M. B. (1977) J. Clin. Invest. 60, 766–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitts R. H. (1994) Physiol. Rev. 74, 49–94 [DOI] [PubMed] [Google Scholar]
- 25.Allen D. G., Lamb G. D., Westerblad H. (2008) Physiol. Rev. 88, 287–332 [DOI] [PubMed] [Google Scholar]
- 26.Pellerin L., Bouzier-Sore A. K., Aubert A., Serres S., Merle M., Costalat R., Magistretti P. J. (2007) Glia 55, 1251–1262 [DOI] [PubMed] [Google Scholar]
- 27.Brown A. M., Ransom B. R. (2007) Glia 55, 1263–1271 [DOI] [PubMed] [Google Scholar]
- 28.Castro M. A., Beltrán F. A., Brauchi S., Concha I. I. (2009) J. Neurochem. 110, 423–440 [DOI] [PubMed] [Google Scholar]
- 29.Swietach P., Vaughan-Jones R. D., Harris A. L. (2007) Cancer Metastasis Rev. 26, 299–310 [DOI] [PubMed] [Google Scholar]
- 30.Swietach P., Patiar S., Supuran C. T., Harris A. L., Vaughan-Jones R. D. (2009) J. Biol. Chem. 284, 20299–20310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright M. E., Michaud D. S., Pietinen P., Taylor P. R., Virtamo J., Albanes D. (2005) Cancer Causes Control 16, 1117–1123 [DOI] [PubMed] [Google Scholar]
- 32.Verrax J., Calderon P. B. (2009) Free Radic. Biol. Med. 47, 32–40 [DOI] [PubMed] [Google Scholar]
- 33.Chen Q., Espey M. G., Sun A. Y., Pooput C., Kirk K. L., Krishna M. C., Khosh D. B., Drisko J., Levine M. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 11105–11109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heaney M. L., Gardner J. R., Karasavvas N., Golde D. W., Scheinberg D. A., Smith E. A., O'Connor O. A. (2008) Cancer Res. 68, 8031–8038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spielholz C., Golde D. W., Houghton A. N., Nualart F., Vera J. C. (1997) Cancer Res. 57, 2529–2537 [PubMed] [Google Scholar]
- 36.Honegger C. G., Torhorst J., Langemann H., Kabiersch A., Krenger W. (1988) Int. J. Cancer 41, 690–694 [DOI] [PubMed] [Google Scholar]
- 37.Rivas C. I., Zúñiga F. A., Salas-Burgos A., Mardones L., Ormazabal V., Vera J. C. (2008) J. Physiol. Biochem. 64, 357–375 [DOI] [PubMed] [Google Scholar]
- 38.Wu H. Y., Hsu F. C., Gleichman A. J., Baconguis I., Coulter D. A., Lynch D. R. (2007) J. Biol. Chem. 282, 20075–20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ziane R., Huang H., Moghadaszadeh B., Beggs A. H., Levesque G., Chahine M. (2010) Biochemistry 49, 166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Penuela S., Bhalla R., Nag K., Laird D. W. (2009) Mol. Biol. Cell 20, 4313–4323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parente P., Coli A., Massi G., Mangoni A., Fabrizi M. M., Bigotti G. (2008) J. Exp. Clin. Cancer Res. 27, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolf B., Lesnaw J. A., Reichmann M. E. (1970) Eur. J. Biochem. 13, 519–525 [DOI] [PubMed] [Google Scholar]
- 43.Miles E. W. (1977) Methods Enzymol. 47, 431–442 [DOI] [PubMed] [Google Scholar]
- 44.Németh-Cahalan K. L., Hall J. E. (2000) J. Biol. Chem. 275, 6777–6782 [DOI] [PubMed] [Google Scholar]
- 45.Shoshan-Barmatz V., Weil S. (1994) Biochem. J. 299, 177–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jiang M., Dun W., Tseng G. N. (1999) Am. J. Physiol. 277, H1283–H1292 [DOI] [PubMed] [Google Scholar]
- 47.Liang W. J., Johnson D., Ma L. S., Jarvis S. M., Wei-Jun L. (2002) Am. J. Physiol. Cell Physiol. 283, C1696–C1704 [DOI] [PubMed] [Google Scholar]
- 48.Wu X., Itoh N., Taniguchi T., Nakanishi T., Tatsu Y., Yumoto N., Tanaka K. (2003) Arch. Biochem. Biophys. 420, 114–120 [DOI] [PubMed] [Google Scholar]
- 49.Hatahet F., Ruddock L. W. (2007) FEBS J. 274, 5223–5234 [DOI] [PubMed] [Google Scholar]
- 50.Corse E., Machamer C. E. (2000) J. Virol. 74, 4319–4326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lichty B. D., McBride H., Hanson S., Bell J. C. (2006) J. Gen. Virol. 87, 3379–3384 [DOI] [PubMed] [Google Scholar]
- 52.Varma S., Campbell C. E., Kuo S. M. (2008) Biochemistry 47, 2952–2960 [DOI] [PubMed] [Google Scholar]
- 53.Karena E., Frillingos S. (2009) J. Biol. Chem. 284, 24257–24268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee A. H., Tannock I. F. (1998) Cancer Res. 58, 1901–1908 [PubMed] [Google Scholar]
- 55.Du J., Xie J., Yue L. (2009) J. Gen. Physiol. 134, 471–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.