Abstract
Mycobacterium tuberculosis, an etiological agent of pulmonary tuberculosis, causes significant morbidity and mortality worldwide. Pathogenic mycobacteria survive in the host by subverting host innate immunity. Dendritic cells (DCs) are professional antigen-presenting cells that are vital for eliciting immune responses to infectious agents, including pathogenic mycobacteria. DCs orchestrate distinct Th responses based on the signals they receive. In this perspective, deciphering the interactions of the proline-glutamic acid/proline-proline-glutamic acid (PE/PPE) family of proteins of M. tuberculosis with DCs assumes significant pathophysiological attributes. In this study, we demonstrate that Rv1917c (PPE34), a representative member of the proline-proline-glutamic-major polymorphic tandem repeat family, interacts with TLR2 and triggers functional maturation of human DCs. Signaling perturbations implicated a critical role for integrated cross-talk among PI3K-MAPK and NF-κB signaling cascades in Rv1917c-induced maturation of DCs. However, this maturation of DCs was associated with a secretion of high amounts of anti-inflammatory cytokine IL-10, whereas Th1-polarizing cytokine IL-12 was not induced. Consistent with these results, Rv1917c-matured DCs favored secretion of IL-4, IL-5, and IL-10 from CD4+ T cells and contributed to Th2-skewed cytokine balance ex vivo in healthy individuals and in patients with pulmonary tuberculosis. Interestingly, the Rv1917c-skewed Th2 immune response involved induced expression of cyclooxygenase-2 (COX-2) in DCs. Taken together, these results indicate that Rv1917c facilitates a shift in the ensuing immunity toward the Th2 phenotype and could aid in immune evasion by mycobacteria.
Keywords: Cyclooxygenase (COX) Pathway, Dendritic Cell, Innate Immunity, MAPKs, NF-kappaB Transcription Factor, Phosphatidylinositol 3-Kinase, Toll-like Receptors (TLR)
Introduction
Tuberculosis is a chronic infectious disease and a leading cause of death worldwide (1). A significant increase in mycobacterial infections with multidrug-resistant and extensively drug-resistant strains, variable vaccine efficacy of Mycobacterium bovis bacillus Calmette-Guérin, and emergence of tuberculosis infection in HIV-infected subjects have emphasized the need of deciphering pathogenesis of tuberculosis as well as to identify novel therapeutic targets to combat the disease (2, 3). Therefore, the functional characterization of mycobacterial antigens that are potent modulators of host immune responses to pathogens assumes critical importance. Protective immunity against intracellular pathogens such as M. tuberculosis depends principally on cell-mediated immunity executed by efficient anti-infectious functions of type 1 T helper (Th1) subset of CD4+ T cells (4–6). The polarization of Th1 responses is orchestrated by IL-12 secreted by antigen-presenting cells (APCs)7 such as dendritic cells (DCs). A hallmark of Th1-type CD4+ T cells is the production of IFN-γ that activates a plethora of innate cell-mediated immunity, including activation of NK cells, effector functions of cytotoxic T cells, and microbicidal properties of macrophages. In contrast, Th2 cells that secrete IL-4, IL-5, IL-9, and IL-13 are critical for antibody class switching in B cells as well for inducing a humoral response (7–10). Th2-driven immunity is significantly efficient in the eradication of extracellular pathogens but markedly deficient in clearing intracellular infections, including pathogenic mycobacteria. Importantly, IL-10 secreted by innate cells such as DCs and macrophages promotes induction of Th2 responses (11, 12). A large body of evidence suggests that M. tuberculosis induces the secretion of IL-10 that ultimately suppresses the secretion of IL-12 and IFN-γ from APCs and T cells, respectively, culminating in a skewed Th1/Th2 balance toward unprotective Th2 responses. This eventually leads to inhibition of the immunoprotective responses of the host with a concomitant increase in the vulnerability to chronic mycobacterial infections (8, 13–15).
Primary polarization of Th cells occurs during their priming in the secondary lymphoid tissues such as lymph nodes and spleen. DCs have the unique ability to capture mycobacterial antigens at the site of infection, mainly alveolar spaces in the lungs, and to migrate to lymph nodes where they prime naive T cells (16, 17). In this complex process, immature DCs present in lungs and other tissues are required to undergo maturation and activation. During this maturation process, DCs lose their phagocytic capacity and mature into a proficient APCs exhibiting higher surface expression of the antigen-presenting molecules MHC class I and II and costimulatory molecules such as CD40, CD80, and CD86 and secretion of a large range of immunomodulatory cytokines, all of which determine the type and strength of Th responses (18, 19).
Sequencing of M. tuberculosis genome identified PE and PPE gene clusters, exemplified by the presence of conserved Pro-Glu (PE) or Pro-Pro-Glu (PPE), near the N terminus of their gene products (20). The 69 members of the PPE family are further subdivided into different subgroups on the basis of differences in their C-terminal motif. There are four PPE subfamilies, one of which, characterized by the motif Gly-Xaa-Xaa-Ser-Val-Pro-Xaa-Xaa-Trp, constitutes the “PPE-SVP” subfamily, and the second group is “PPE-PPW,” which includes the highly conserved Gly-Phe-Xaa-Gly-Thr and Pro-Xaa-Xaa-Pro-Xaa-Xaa-Trp sequence motifs at the C terminus. The major polymorphic tandem repeat PPE subfamily contains multiple C-terminal repeats of the motif Asn-Xaa-Gly-Xaa-Gly-Asn-Xaa-Gly, whereas the last PPE subfamily consists of proteins with a low percentage of homology at the C terminus (21, 22). The uniqueness of the PPE genes is further illustrated by the fact that these genes are restricted to mycobacteria (20, 23). Many PPE proteins are also known to induce strong T and B cell responses and to associate with the cell wall. Following surface exposure, these PPE proteins could act as agonists to various surface receptors of APCs resulting in modulation of the host immune responses (24–28). In this regard, information related to the effects of PPE antigens on the maturation and functions of human DCs and the underlying signaling events remains scanty.
In this investigation, we demonstrate that cell wall-associated/secretory Rv1917c (PPE34) antigen acts as a TLR2 agonist and induces functional maturation of human DCs. Rv1917c-matured DCs preferentially secreted IL-10 and triggered IL-4, IL-5, and IL-10 production from CD4+ T cells thus polarizing Th2 responses in vitro. Importantly, skewed Th2 phenotype by Rv1917c could also be observed in T cells derived from tuberculosis patients. Interestingly, Rv1917c-skewed Th2 immune response involved induced expression of cyclooxygenase-2 in DCs. We further demonstrate the signaling integration of PI3K, MAPK, and NF-κB pathways during Rv1917c-triggered maturation of DCs. These results conjecture that polarization of crucial T cell responses in favor of Th2 immunity by Rv1917c could aid in immune evasion by mycobacteria in humans.
EXPERIMENTAL PROCEDURES
Antibodies and Reagents
Recombinant human IL-4, GM-CSF, and IFN-γ were purchased from ImmunoTools (Friesoythe, Germany). Fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies (mAbs) to HLA-DR, CD80, and CD1a and phycoerythrin-conjugated mAbs to CD86 and CD83 were from BD Biosciences, and phycoerythrin-conjugated mAb to CD40 was from BD Biosciences. Anti-Thr-202/Tyr-204 phospho-ERK1/2, anti-ERK1/2, anti-Thr-180/Tyr-182 phospho-p38 MAPK, p38 MAPK, anti-NF-κB p65 antibodies were purchased from Cell Signaling Technology. Anti-Ser-473 phospho-Akt antibody was from eBiosciences. Anti-β-actin, anti-COX-2, and anti-PGE2 antibodies were obtained from Sigma. Anti-TLR1, anti-TLR2, anti-TLR4, and anti-TLR6 were procured from Imgenex. Cell wall, cytosol, cell membrane, and culture filtrate protein fractions of virulent M. tuberculosis were obtained from the tuberculosis research materials and vaccine testing contract at Colorado State University.
Expression and Purification of SH3-interacting Domain of Rv1917c
The SH3-interacting domain of Rv1917c expands from amino acid 511 to 700 of wild-type protein. In this context, we cloned the SH3-interacting domain (190 amino acids, 25 kDa) as well as a larger fragment from amino acids 348 to 824 (476 amino acids, 55 kDa) encompassing the SH3-interacting domain of Rv1917c by PCR amplification from genomic DNA using domain-specific primers as follows: 5′-CGCGGATCCCCCGCGTCTCCGCTTGCGATCGGCTT-3′ (forward) and 5′-GCTCTAGAGGTGCCCGCGATTTCGATCGGGATGT-3′ (reverse) for SH3-interacting domain and 5′-GGAATTCCATATGAACACGGGCAGCTTCAATCTA-3′ (forward) and 5′-CGGGATCCGTTGTAGTCGCCGAGGTTTG-3′ (reverse) for larger fragment encompassing the SH3-interacting domain.
The amplified PCR products were ligated into the pJET vector (Fermentas), and the recombinant clones carrying the appropriate gene insert were confirmed by DNA sequencing. The gene insert was subcloned into pET series vectors for protein expression and purification. Escherichia coli BL21 cells carrying recombinant plasmids were induced with isopropyl β-d-thiogalactopyranoside, and His-tagged recombinant proteins were purified with Ni-nitrilotriacetic acid (Ni-NTA) columns (Bio-Rad).
Generation of Polyclonal Antibodies to Rv1917c
The polyclonal antibodies against Rv1917c were generated in rabbits by subcutaneous injection of 1 mg of purified SH3-interacting domain of Rv1917c emulsified with an equal volume of Freund's adjuvant (Sigma). The experiments were approved by the Institutional Ethics Committee for Animal Experimentation and Institutional Biosafety Committee, Indian Institute of Science, Bangalore. The first immunization was carried out with Freund's complete adjuvant followed by two booster immunizations with Freund's incomplete adjuvant at 21-day intervals. The antibody titers in the serum were determined by ELISA 2 weeks post final immunization.
Generation and Culture of Human Dendritic Cells
PBMCs were isolated from buffy coats of healthy donors purchased from Hôpital Hôtel Dieu, Etablissement Français du Sang, Paris, France, upon ethical approval for the use of such materials. Monocytes were positively isolated from PBMCs using CD14 magnetic beads (Miltenyi Biotec, France). The purity of the monocytes was >98%. Immature DCs were generated from monocytes by culturing them for 7 days in RPMI 1640 medium containing 10% FCS, 50 units/ml penicillin, 50 μg/ml streptomycin, IL-4 (500 IU/106 cells), and GM-CSF (1000 IU/106 cells). Immature 7-day-old DCs (0.5 × 106/ml) were cultured with cytokines alone or cytokines and Rv1917c for 48 h.
Tuberculosis Patients and Healthy Subjects
The study population included tuberculosis bacilli-infected patients (n = 11) or healthy controls (n = 4) reporting to the National Institute of Mental Health and Neurosciences, Bangalore, India. Active tuberculosis disease in patients was confirmed by the presence of acid-fast bacilli in sputum smear examinations and growth of bacilli in BACTEC cultures as well as by radiological abnormalities in chest x-ray. The healthy subjects were negative for active tuberculosis disease. The samples from HIV-positive subjects were excluded from the study. The study subjects had given written consent, and the study was approved by the institutional bioethics committee.
Analysis of the Expression of Surface Molecules by Flow Cytometry
Cell surface staining was performed with specifically labeled mAbs, and samples were processed for flow cytometry (LSR II, BD Biosciences). For each sample, 5000 events were recorded. Data were analyzed using BD FACS DIVA software (BD Biosciences).
Mixed Lymphocyte Reaction
Responder CD4+ T cells used in allogeneic MLR were isolated from PBMCs of healthy donors using CD4 magnetic beads (Miltenyi Biotec). DCs were treated with Rv1917c for 48 h followed by extensive washing and were co-cultured with 1 × 105 responder allogeneic CD4+ T cells at DC:T cell ratios of 1:10, 1:20, 1:40, and 1:80. On day 4 of co-culture, the cells were pulsed for 16–18 h with 0.5 μCi of [3H]thymidine. Radioactive incorporation was measured by standard liquid scintillation counting. The proliferation of cells was measured as counts/min (mean ± S.E. of quadruplicate values) after subtracting values of responder T cell cultures alone.
Analysis of Cytokines
Cytokines were quantified in cell-free culture supernatants using BD CBA human inflammation kit and human Th1/Th2 kits (BD Biosciences).
Treatment of DCs with Pharmacological Inhibitors of Signaling Pathways
All the pharmacological inhibitors were obtained from Calbiochem, reconstituted in sterile DMSO (Sigma), and used at the following concentrations: LY294002 (50 μm), SB203580 (20 μm), U0126 (10 μm), Bay 11-7082 (20 μm), and l-1-tosylamido-2-phenylethyl chloromethyl ketone (20 μm). DMSO at 0.1% concentration was used as the vehicle control. In all experiments with inhibitors, a tested concentration was used after careful titration experiments assessing the viability of the macrophages using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. In experiments with inhibitors, the cells were treated with a given inhibitor for 60 min followed by treatment with Rv1917c.
Immunoblotting Analysis
Cells were washed with ice-cold PBS, scraped off the culture dish, and collected by centrifugation. Cell pellets were lysed, and cell lysates were RIPA buffer constituting 50 mm Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm PMSF, 1 μg/ml each of aprotinin, leupeptin, pepstatin, 1 mm Na3VO4, 1 mm NaF. Protein concentration in each cell lysate was determined by Bradford's method of protein detection. Equal protein amounts from each cell lysate were subjected to SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Millipore) by the semidry Western blotting (Bio-Rad) method. Nonspecific binding was blocked with 5% nonfat dry milk powder in TBST (20 mm Tris-HCl (pH 7.4), 137 mm NaCl, and 0.1% Tween 20) for 60 min. The blots were incubated overnight at 4 °C with primary antibodies diluted in TBST with 5% BSA. After washing with TBST, blots were incubated with anti-rabbit or anti-mouse IgG secondary antibody conjugated to HRP (Jackson ImmunoResearch) diluted in TBST with 5% BSA for 2 h. After further washing in TBST, the immunoblots were developed with the ECL system (PerkinElmer Life Sciences) following the manufacturer's instructions.
Nuclear and Cytosolic Subcellular Fractionation
DCs were cultured in 35-mm dishes and treated as indicated. After treatment, cells were harvested by centrifugation at 13,000 rpm for 5 min, and cell pellets were washed with phosphate-buffered saline (PBS) and gently resuspended in ice-cold Buffer A (10 mm HEPES (pH 7.9), 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, and 0.5 mm PMSF). After incubation on ice for 15 min, cell membranes were disrupted with 10% Nonidet P-40, and the nuclear pellets were recovered by centrifugation 13,000 × g for 15 min at 4 °C, and the supernatant was used as cytosolic extract. Nuclear pellets were lysed with ice-cold Buffer C (20 mm HEPES (pH 7.9), 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, and 1 mm PMSF), and nuclear extracts were collected after centrifugation at 13,000 × g for 20 min at 4 °C.
Transient Transfections and Immunofluorescence
For immunofluorescence studies, HEK-293 cells or DCs were seeded in 35-mm dishes on coverslips. HEK-293 cells were transfected with indicated plasmid construct (Vector or TLR2 cDNA construct) using ESCORT III (Sigma). HEK-293 cells were treated with 2 μg/ml FITC-labeled Rv1917c for 1 h, and DCs were pretreated with either anti-TLR1, TLR2, TLR4, TLR6 blocking antibodies, or isotype control antibodies for 1 h followed by treatment with FITC-labeled Rv1917c. Cells were washed and fixed with cold methanol for 15 min at room temperature. Coverslips with cells were mounted on a slide with Fluoromount G. Immunofluorescent images were acquired by confocal microscopy (Carl Zeiss, Germany), and the images were analyzed for the integrated density of the fluorescence and for the area of the cells using Zeiss LSM image browser software.
Immunoprecipitation Assay
Vector or TLR2 cDNA construct-transfected HEK-293 cells and DCs were washed briefly with ice-cold PBS, and cell lysates were prepared in 1× RIPA lysis buffer (50 mm Tris-HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA, 1 mm PMSF, 1 μg/ml each of aprotinin, leupeptin, pepstatin, 1 mm Na3VO4, 1 mm NaF). The cell lysate was incubated with Rv1917c immobilized on Ni-NTA beads at 4 °C overnight on a rotor. The beads were harvested, washed, and boiled in 5× Laemmli buffer for 5 min. The proteins were separated on 12% SDS-PAGE followed by transfer of proteins to polyvinylidene difluoride membranes (Millipore). The membranes were further probed with anti-TLR1, -TLR2, -TLR4, or -TLR6 antibodies as indicated.
Statistical Analysis
Levels of significance for comparison between samples were determined by the Student's t test distribution. The data in the graphs are expressed as the mean ± S.E. GraphPad Prism 3.0 software (GraphPad software) was used for all statistical analyses.
RESULTS
Rv1917c Induces Maturation of Human Dendritic Cells
The recent reports suggest that the PPE gene family can serve an important role in host-pathogen interactions by modulating innate immune responses during tuberculosis infection and hence can regulate the clinical course of tuberculosis (24–28). Because DCs are professional APCs and are important for eliciting both primary and secondary immune responses to pathogens, we explored whether Rv1917c, a typical member of PPE family, can regulate maturation of human DCs. Rv1917c open reading frame (ORF) encodes for protein with molecular mass equivalent to 143 kDa. Previous report had demonstrated the presence of Rv1917c in the cell wall but not in the culture filtrate fractions suggesting that Rv1917c could be a strongly hydrophobic protein and might not exhibit secretory properties (29). Furthermore, we assessed the localization of Rv1917c in various cellular fractions of virulent M. tuberculosis, and as demonstrated in supplemental Fig. S1, Rv1917c could be detected in the cell wall and cell membrane but not in the cytosol and culture filtrate fractions. Importantly, assessment of the purity of cell wall fractions was carried out by probing the cellular fractions with monoclonal antibodies reactive to cytosolic catalase-peroxidase KatG of M. tuberculosis. Accordingly, whereas the cytosolic fraction exhibited reactivity to antibodies against KatG, the cell wall fraction did not demonstrate any presence of KatG, thereby validating the purity of the cell wall preparations (data not shown).
In addition, preliminary in silico analysis of Rv1917c was performed to identify potential domains of importance, and a search against the domain data base demonstrated the presence of four eukaryotic SH3-interacting motifs PXXP in the region of protein expanding from amino acid 511 to 700. SH3 domains are ∼60-residue modules that often occur in signaling and cytoskeletal proteins and regulate a variety of cell fate decisions by virtue of their interaction with proline-rich interacting motifs PXXP in several eukaryotic as well as prokaryotic proteins (30–32). In this context, we cloned and expressed SH3-interacting domain (190 amino acids, 25 kDa) as well as a larger fragment encompassing the SH3-interacting domain of Rv1917c from amino acid 348–824 (476 amino acids, 55 kDa). Majority of the experiments were performed utilizing purified SH3-interacting domain, and results were also validated with a larger fragment of Rv1917c encompassing the SH3-interacting domain (supplemental Fig. S2).
Immature DCs were cultured with Rv1917c for 48 h, and expression of various cell surface markers was analyzed by flow cytometry. A protein concentration of 5 μg/ml was utilized in all these experiments after titration analysis. Rv1917c enhanced the expression of co-stimulatory molecules CD80, CD86, and CD40 (Fig. 1, A and B). Furthermore, Rv1917c treatment augmented the expression of DC terminal maturation marker CD83 (Fig. 1, A and B). A decrease in the expression of DC differentiation marker CD1a is a well defined marker for DC maturation (33, 34). When analyzed, Rv1917c decreased CD1a expression on DCs (Fig. 1B). In addition, expression of the antigen-presenting molecule, HLA-DR, was also enhanced, further validating DC maturation potential of Rv1917c.
FIGURE 1.
Recombinant Rv1917c induces maturation of human DCs. A and B, 7-day-old immature DCs (0.5 × 106 cells/ml) were cultured with GM-CSF and IL-4 alone (Control) or GM-CSF, IL-4, and 5 μg/ml of Rv1917c for 48 h followed by analysis for the expression of surface markers by flow cytometry. The percentage of cells expressing the indicated markers is shown in A, and mean fluorescence intensities (MFI) are shown in B. Data are presented as mean ± S.E. from five independent donors. *, p < 0.05 versus control.
Several lines of evidence indicate that the maturation of DCs induced by Rv1917c was not due to contaminating endotoxins or LPS. For all experiments, we used purified Rv1917c protein preparations that were passed through a polymyxin B-agarose column. Accordingly, we could not detect endotoxins in protein preparations as analyzed by the E-Toxate kit (Sigma). Furthermore, an unrelated His-tagged mycobacterial lipase protein produced and processed by the same procedure failed to trigger maturation of DCs (33). Importantly, proteinase K treatment abrogated the ability of Rv1917c to induce maturation of DCs as analyzed by cell surface expression of CD80, CD86, CD83, CD40, HLA-DR, and CD1a indicating that the maturation of DCs was induced by intact Rv1917c protein and not by contaminating endotoxins (supplemental Fig. S3).
Rv1917c Triggers the Secretion of Immunomodulatory Cytokines by DCs
DCs secrete a wide range of cytokines that regulate inflammation and adaptive T cell responses. As a “proof of concept,” we therefore analyzed whether the maturation process of DCs induced by Rv1917c is coupled with the secretion of immunomodulatory cytokines such as IL-6, IL-8, IL-10, IL-12, and TNF-α. As demonstrated in Fig. 2, A–D, Rv1917c-stimulated DCs secrete high levels of IL-6, IL-8, TNF-α, as well as immunoregulatory, suppressive cytokine IL-10. Interestingly, Rv1917c failed to trigger secretion of pro-inflammatory and Th1-promoting cytokine IL-12 (Fig. 2E).
FIGURE 2.
Rv1917c triggers secretion of immunoregulatory cytokines from human DCs. A–E, DCs (0.5 × 106 cells/ml) were cultured with GM-CSF and IL-4 alone (Control) or GM-CSF, IL-4, and 5 μg/ml of Rv1917c for 48 h, and secretion of IL-6 (A), IL-8 (B), TNF-α (C), IL-10 (D), and IL-12 (E) in cell-free culture supernatants was analyzed by cytokine bead array assay. Data are presented as mean ± S.E. from six independent donors. *, p < 0.05 versus control.
Rv1917c-stimulated DCs Induce CD4+ T Cell Proliferation in Vitro
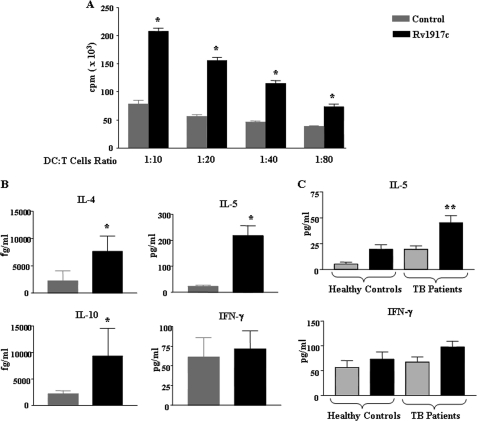
One of the functional attributes of DCs is their significant ability to stimulate CD4+ T cells at very low stimulator to responder ratios (18, 19, 35). The priming of T cell responses by DCs is regulated by several receptor-ligand interactions such as engagement of the peptide-MHC complex with T cell receptor of T cells (signal 1) and of co-stimulatory CD80/CD86, CD40, and adhesion molecules CD54 and CD58 on DCs with CD28, CD154, CD11a, and CD2, respectively, on T cells (signal 2) (18, 19, 35, 36). To ascertain that Rv1917c antigen-matured DCs were potent T cell stimulators, allogeneic T cells were co-cultured with them at various DC:T cell ratios, and the extent of T cell stimulation was assayed by measuring thymidine incorporation. As demonstrated in Fig. 3A, Rv1917c-stimulated DCs stimulated CD4+ T cells at ratios as low as 1:80, thus indicating that Rv1917c matured DCs were efficient T cell stimulators.
FIGURE 3.
Rv1917c-treated DCs stimulate CD4+ T cells to produce Th2 cytokines. A and B, DCs were cultured with GM-CSF and IL-4 alone (Control) or GM-CSF, IL-4, and 5 μg/ml of Rv1917c for 48 h. The Rv1917c-matured DCs were co-cultured with allogeneic CD4+ T cells at different DC to T cell ratios. After 4 days of co-culture, the cells were pulsed overnight with 0.5 μCi of [3H]thymidine to quantify T cell proliferation (A). Radioactive incorporation was expressed as counts/min (mean ± S.E. of quadruplet values). The data are representative for three independent donors. B, cell-free supernatants from DC:T cell co-cultures were analyzed for the cytokines IL-4, IL-5, IL-10, and IFN-γ. C, PBMCs obtained from tuberculosis patients (TB patients) (n = 11) and healthy control subjects (n = 4) were cultured with or without 5 μg/ml Rv1917c, and cell-free supernatants collected on day 4 were tested for concentrations of secreted IL-5 and IFN-γ. Data are represented as mean ± S.E. *, p < 0.05 versus control; **, p < 0.05 versus Rv1917c (Healthy Controls).
In addition to providing co-stimulatory signaling to CD4+ T cells, activated DCs secrete a wide range of immunomodulatory cytokines that regulate the polarization of CD4+ T cells toward different subtypes such as Th1, Th2, or Th17. Whereas IL-12 signals for differentiation of Th1 cells, IL-4 and IL-10 promote Th2 differentiation (18, 19, 35). Therefore, in the context of tuberculosis, DCs can bias CD4+ T cell priming toward a protective Th1 or immunosuppressive Th2 responses. Consistent with the pattern of cytokines secreted (Fig. 2E), Rv1917c-matured DCs promoted an augmented secretion of IL-4, IL-5, and IL-10 by CD4+ T cells (Fig. 3B). Interestingly, Rv1917c-stimulated T cells produced negligible levels of IFN-γ (Fig. 3B). These results clearly suggest that Rv1917c-matured DCs induce Th2-biased response as against the reported Th1 response induced by diverse mycobacterial antigens (33, 37).
Rv1917c Skews Th1/Th2 Balance toward Th2 ex Vivo
Because Rv1917c-matured DCs have the potential to shift the balance of immunity in favor of Th2, we hypothesized that patients with pulmonary tuberculosis have a high frequency of T cells specific to Rv1917c and these T cells exhibit a strong Th2-biased phenotype. In consensus with our hypothesis, Rv1917c elicited robust IL-5 secretion from T cells of patients with pulmonary tuberculosis compared with healthy individuals (Fig. 3C). Interestingly, Rv1917c did not induce secretion of IFN-γ from T cells of tuberculosis patients. Furthermore, the median ratio of Rv1917c-induced IFN-γ/IL-5 in tuberculosis patients as well as healthy individuals advocated the Th2 polarizing nature of Rv1917c (data not shown).
Rv1917c Interacts with TLR2
Pattern recognition receptors (PRRs), a class of innate immune response-expressed proteins, have the ability to recognize pathogen-associated molecular patterns in mycobacteria or cell wall antigens of mycobacteria. Among other PRRs, Toll-like receptors (TLRs) occupy a central place in host defense against invading pathogens, including mycobacteria, by activating intracellular signaling cascades, activation of innate cells, and secretion of inflammatory cytokines (37–42).
Earlier reports suggest that mycobacteria harbor antigens with the potential to interact with TLR1, TLR2, TLR4, and TLR6 that could have differential effects on ensuing host immune responses (37, 41, 43–49). In view of these observations, we analyzed the possible interaction of Rv1917c with different TLRs. A series of pulldown assays were performed to explore possible interaction of Rv1917c to various TLRs, and results signify that Rv1917c physically interacts with TLR2 (Fig. 4A). To further ascertain the specific binding of Rv1917c with TLR2, we blocked TLR1, TLR2, TLR4, and TLR6 by respective blocking antibodies and then analyzed binding of FITC-labeled Rv1917c to the cell surface of DCs. As demonstrated in Fig. 4B, blockade of TLR2, but not that of TLR1, TLR4, and TLR6, abrogated binding of Rv1917c to the DC surface. For further validation, HEK-293 cells, a human embryonic kidney cell line that lacks TLR2, were transfected with TLR2 expression construct, and the binding of Rv1917c to HEK-293 cells was analyzed. Results presented in Fig. 4C demonstrate that Rv1917c interacts preferentially with TLR2-transfected HEK-293 cells in comparison with vector-transfected cells. Similarly, Rv1917c could pull down TLR2 from TLR2-transfected HEK-293 cells thus further confirming the ligand potential of Rv1917c for TLR2 (Fig. 4D). These results clearly ascertain Rv1917c as a specific TLR2 agonist that could have a probable role in cell fate decisions.
FIGURE 4.
Rv1917c specifically recognizes TLR2 on cell surface. A, cell lysates from DCs were incubated with Rv1917c immobilized on Ni-NTA beads, and bead-bound proteins were analyzed for TLR1, TLR2, TLR4, and TLR6 by immunoblotting. B, DCs were pretreated with either blocking antibodies against TLR1, TLR2, TLR4, TLR6, or isotypic control antibodies followed by incubation with FITC-labeled Rv1917c, and interaction of Rv1917c with DCs was evaluated by confocal microscopy. C, preferential interaction of FITC-labeled Rv1917c with TLR2 cDNA-transfected HEK-293 cells. D, cell lysates from HEK-293 cells transfected with either TLR2 or vector were incubated with Rv1917c immobilized on Ni-NTA beads, and immunoprecipitation of TLR2 was evaluated. BF, Bright Field. Data are representative of two independent experiments.
Integrated Cross-talk of PI3K, MAPK, and NF-κB Signaling Pathways during Rv1917c-induced Maturation of Human DCs
The maturation of DCs often involves diverse sets of signaling events, and many species of mycobacteria are known to trigger the activation of PI3K/Akt and MAPK, including ERK1/2 and p38 MAPK (33, 50–57). Among the transcription factors, the active heterodimer p50/p65 forms of nuclear factor-κB (NF-κB) has been suggested to play a central role in immunological processes by inducing expression of a variety of genes involved in inflammatory responses (50, 51, 54, 57, 58). In this regard, Rv1917c triggered the activation of Akt as well as MAPKs, ERK1/2, and p38 MAPK (Fig. 5A). Interestingly, pharmacological inhibition data suggest that Rv1917c-mediated activation of ERK, but not p38 MAPK, required the involvement of PI3K (Fig. 5B). Accordingly, Rv1917c induced significant nuclear translocation of NF-κB from cytosol within 60 min of stimulation (Fig. 5C). The pharmacological inhibition of PI3K (LY294002), ERK1/2 (U0126), and p38 MAPK (SB203580) abrogated Rv1917c-articulated nuclear translocation of NF-κB (Fig. 5D). These results clearly suggest a significant role of PI3K- and MAPK-dependent NF-κB activation during Rv1917c-induced DC maturation.
FIGURE 5.
DC maturation triggered by Rv1917c involves PI3K, ERK1/2, and p38 MAPK-dependent activation of NF-κB. A, DCs were treated with Rv1917c for indicated time points, and phosphorylation of ERK1/2 (pERK1/2), p38 MAPK (pp38), and AKT (pAKT) was analyzed by immunoblotting. B, DCs were pretreated with LY294002 followed by stimulation with Rv1917c for indicated time points, and activation of ERK1/2 and p38 MAPK was analyzed. C, Rv1917c and LPS trigger nuclear translocation of p65 NF-κB. D, DCs were pretreated with ERK1/2 inhibitor (U0126, 10 μm), p38 MAPK inhibitor (SB203580, 20 μm), and PI3K inhibitor (LY294002, 50 μm) followed by treatment with Rv1917c and nuclear translocation of p65 NF-κB was analyzed. Med, Medium. Data are representative of three independent experiments.
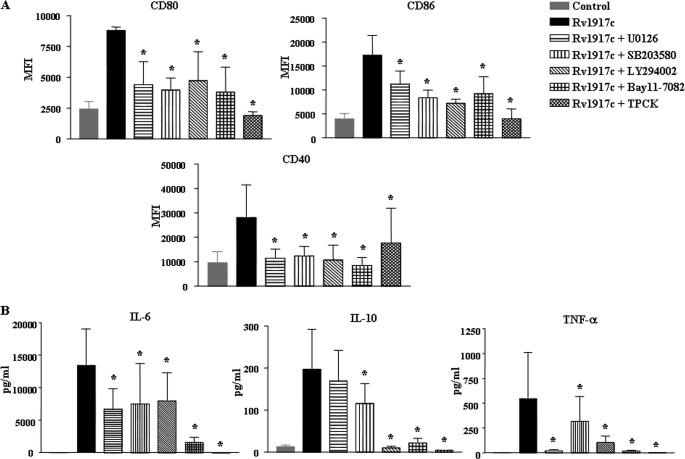
Furthermore, U0126 (ERK1/2 inhibitor), SB203580 (p38 MAPK inhibitor), LY294002 (PI3K inhibitor), Bay 11-082 or l-1-tosylamido-2-phenylethyl chloromethyl ketone (NF-κB inhibitor) abrogated Rv1917c-induced expression of DC maturation markers CD80, CD86, CD40, CD83, and HLA-DR, whereas rescued Rv1917c triggered decrease in CD1a expression (Fig. 6A and supplemental Fig. S4). In addition, inhibition of PI3K, ERK1/2, p38 MAPK, and NF-κB abolished Rv1917c-induced secretion of cytokines IL-6 and IL-10 as well as TNF-α from DCs (Fig. 6B). Altogether, these observations clearly implicate a role for signaling integration between members of PI3K, MAPK, and NF-κB pathways in Rv1917c-triggered maturation of human DCs.
FIGURE 6.
Involvement of PI3K, ERK1/2, p38 MAPK, and NF-κB pathways in Rv1917c-induced maturation of DCs. A, DCs were treated with pharmacological inhibitors of ERK1/2 (U0126, 10 μm), p38 MAPK (SB203580, 20 μm), PI3K (LY294002, 50 μm), NF-κB (Bay 11-7082, 20 μm; l-1-tosylamido-2-phenylethyl chloromethyl ketone, 20 μm), or DMSO (vehicle control) for 1 h prior to treatment with Rv1917c for 48 h, and the expression of CD80, CD86, and CD40 was analyzed by flow cytometry. B, DCs were treated as in A, and the level of secreted IL-6, IL-10, and TNF-α was analyzed. Data are presented as mean ± S.E. from three independent donors. *, p < 0.05 versus Rv1917c. MFI, mean fluorescence intensities.
Critical Role for COX-2 in Rv1917c-triggered Th2 Response
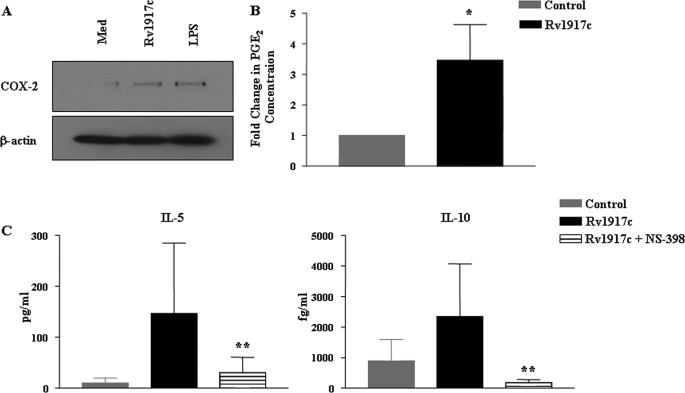
Increasing evidence supports the multifaceted effect of prostanoids like PGE2 on T cell differentiation. PGE2 has been shown to decrease IL-12 and increase IL-10 production by DCs and macrophages. PGE2 modulates a wide range of T cell effector functions, including promotion of the development of Th2 responses and inhibition of the production of Th1 cytokines IL-2 and IFN-γ (45, 56, 59). PGE2 is a biosynthetic product of an enzymatic reaction catalyzed by cycloxygenase-2 (COX-2). Thus PGE2 levels in cellular systems are tightly regulated by COX-2 activity (60, 61). In this context and consistent with the production of IL-10 by Rv1917c-stimulated DCs and promotion of Th2 responses by Rv1917c-educated DCs (Fig. 2 and Fig. 3, B and C), Rv1917c augmented expression and secretion of COX-2 and PGE2, respectively, in human DCs (Fig. 7, A and B). Furthermore, pharmacological inhibitor NS-398-mediated inhibition of COX-2 activity led to a decrease in Rv1917c-triggered secretion of immunosuppressive cytokine IL-10 in human DCs (data not shown). Interestingly, pretreatment of DCs with NS-398 has blocked Rv1917c-triggered secretion of Th2 cytokines IL-5 and IL-10 from T cells (Fig. 7C). These observations thus demonstrate that induced expression of COX-2/PGE2 represents a critical factor in Rv1917c-triggered Th2-biased immune responses.
FIGURE 7.
COX-2 activity is critical for Rv1917c-induced differentiation of CD4+ T cells into Th2 subtype. A and B, Rv1917c or LPS induces COX-2 expression (A) and PGE2 secretion (B) in human DCs. C, DCs pretreated with NS-398 followed by stimulation with Rv1917c for 48 h were co-cultured with allogeneic CD4+ T cells for 4 days, and cell-free supernatants were analyzed for level of T cell cytokines IL-5 and IL-10. Data in A represent one of the three independent experiments, and data in B and C (mean ± S.E.) are from three independent donors. *, p < 0.05 versus control; **, p < 0.05 versus Rv1917c.
DISCUSSION
A protective anti-mycobacterial immune response includes activation of Th1 CD4+ T cells resulting in release of microbicidal IFN-γ (5). However, patients with active tuberculosis exhibit biased Th2 immune responses during later stages of the infection conveying severity of the disease (62). DCs are known to prime and modulate T cell-mediated immunity toward mycobacterial antigens. Mycobacterial infection or its antigens could interfere in DC maturation and subsequent effector functions thus representing a novel immune evasion mechanism by mycobacteria (63–65). Although mycobacteria reside within phagolysosomes, diverse cell wall-associated antigens such as lipoarabinomannan, phosphatidylmyoinositol mannosides, PE/PPE antigens actively traffic out and access both intracellular compartments as well as extracellular environment (66–69). These findings strengthen the need to delineate the interaction of these antigens with human DCs to effectuate deeper insights into disease pathogenesis as well as to identify and develop credible vaccine candidates.
Many of the cell wall-associated or secreted antigens of pathogenic mycobacteria are conjectured to execute subversion of immune responses. For example, ESAT-6 inhibits effector functions of APCs by modulating TLR2-mediated signaling leading to inhibition of the activities of NF-κB and interferon regulatory factors (70). In addition, mannose-capped lipoarabinomannan induces expansion of regulatory T cells, whereas 10-kDa culture filtrate protein (CFP-10) down-regulates Th1 responses against mycobacterial antigens by modulating maturation of DCs (64). In this regard, restriction of the distinctive PE/PPE family of antigens to mycobacterial species as well as their unique expression profiles upon infection suggest their important role in adaptation or persistence of the pathogenic mycobacteria. Furthermore, multiple PPE antigens such as Rv2430c, Rv2608, and Rv3347c were found to be recognized by antibodies in the sera of infected patients suggesting elicitation of strong B cell responses to these antigens in patients with tuberculosis (28, 71, 72). Rv1196 was demonstrated to interact with TLR2 on the macrophage cell surface and induce IL-10 secretion (45). Furthermore, a transposon insertion mutant of Rv3018c exhibited attenuated growth in macrophages, suggesting a role in survival of the bacterium (73). However, there is no report deciphering the immunological consequences of interaction of PPE antigens with human DCs.
In this context, we explored the effect of Rv1917c on activation and maturation of human DCs and its possible outcome in terms of the clinical course of tuberculosis. Rv1917c, a representative member of major polymorphic tandem repeat subfamily of PPE antigens, is surface-exposed, predicted to contain many putative O-glycosylation sites, and display high antigenic index (29). Interestingly, Rv1917c has been demonstrated to exhibit tandem repeat polymorphism among clinical isolates of M. tuberculosis (29, 74). Furthermore, DNA microarray expression data suggested selective enhancement in the expression of Rv1917c in M. tuberculosis bacilli during infection of mouse macrophages (75). Rv1917c exhibited strong humoral reactivity with cerebrospinal fluid of tuberculous meningitis patients attributing association of Rv1917c antigen in pathophysiological conditions of tuberculosis.8 Interestingly, as described previously, bioinformatics analysis using Motif Scan revealed the presence of four SH3-interacting motifs in Rv1917c expanding from amino acid 511 to 700. SH3-interactive domains across many proteins, including those that function as critical players in signal transduction events, often act as rate-limiting factors in effectuating the overall immune responses. Importantly, a prominent high incidence of SH3-interacting motifs in mycobacterial proteins such as Rv1917c suggest a possible interaction of these proteins with SH3 domain-containing proteins of various immune cells (31). In addition to mycobacterial cell wall localization, homology domain modeling ascertained possible cross-talk of Rv1917c with the molecules of host signaling pathways. In the perspective of this study, the presence of SH3-interacting domain clearly ascribes an immunomodulatory role for Rv1917c, a surface-exposed prototype member of PPE family proteins of M. tuberculosis. In the view of these observations, Rv1917c represents as an ideal PPE antigen prototype to study mycobacteria-specific innate immune responses in humans.
In this regard, we explored the implication of the SH3-interacting domain of Rv1917c in modulation of human DC maturation and in turn regulation of host immune responses. Our observations suggest that SH3-interacting domain of Rv1917c induces maturation of human DCs as assessed by analysis of phenotypic markers and cytokine secretion. Interestingly, although Rv1917c augmented in DCs the secretion of IL-10, it did not induce IL-12, a Th1-polarizing cytokine. In addition, Rv1917c-matured DCs were functionally competent as assessed by proliferation of CD4+ T cells. However, quantitative analysis of secreted cytokines revealed the potential of Rv1917c to skew the immune response in favor of Th2 immunity.
O-Glycosylation usually strongly influences the immune response; and to rule out the role of O-glycosylation in Rv1917c-induced maturation of human DCs, the SH3-interacting domain as well as a larger fragment encompassing the SH3-interacting domain of Rv1917c were expressed and purified using E. coli BL21 expression system. In addition, although the NetOGlyc program predicted a number of putative O-glycosylation sites, it failed to detect the presence of signal peptide for O-glycosylation in full-length as well as SH3-interacting domain or a larger fragment encompassing the SH3-interacting domain of Rv1917c. These results are in agreement with findings of a recent report, wherein staining with the glycan/protein double labeling kit (Roche Applied Science) and detection with the concanavalin A lectin failed to demonstrate glycosylation of Rv1917c (29). These results clearly suggest that Rv1917c might not be O-glycosylated during in vivo conditions.
Activation of host innate immunity is initiated by evolutionally conserved PRRs like TLRs that recognize pathogen-associated molecular patterns (42). Among TLRs, TLR2 acquires a unique place in terms of mycobacterial pathogenesis as it appears to be one of the major PRRs interacting with this organism (37, 38, 70). Interestingly, TLR2 is known to heterodimerize and activate the immune response in conjunction with TLR1 or TLR6 or Dectin1 (43, 46, 49, 76). However, the original concept suggesting role for TLR2/TLR1 and TLR2/TLR6 heteromers in recognition by various antigens has recently been confronted by a report suggesting that certain TLR2 agonists can stimulate cells from TLR1- or TLR6-deficient mice. These observations suggest the existence of multiple separate recognition pathways, including TLR2/TLR1, TLR2/TLR6, and TLR2 alone, which could function independent of each other (77). During infection, the activation of TLR2 in DCs has a pivotal function in their functional maturation that ultimately controls the strength of innate and acquired immune responses. Although NF-κB activation is a critical factor, activation of ERK1/2, p38 MAPK, and PI3K pathways assumes a rate-limiting step in DC maturation that regulates the strength of immune response and leads to generation of pathogen-tailored immune responses (50, 57, 58). Importantly, recent reports have suggested that activation of differential intracellular signaling pathways upon TLR ligation can lead to alternative T cell-mediated responses. It has been demonstrated that ERK/p38 MAPK activation ratio controls the Th1/Th2 switch and a high ERK/p38 MAPK activation ratio can promote pro-Th2 maturation via increased IL-10 release and diminished IL-12 synthesis (55, 78). In this context, TLR2-specific interaction of Rv1917c as opposed to TLR1 or TLR6 suggests that Rv1917c could utilize the TLR2 alone cellular recognition pathway. In addition, signaling perturbation experiments implicated an integrated cross-talk among the members of PI3K, ERK1/2, p38 MAPK, and NF-κB pathways during Rv1917c-induced DC maturation. These Rv1917c-triggered cellular events resulted in a switch of Th1/Th2 balance in favor of the Th2 type of cellular immunity.
PGE2 is one of the important key regulators of Th1/Th2 polarization (59). PGE2, a biosynthetic product of enzymatic activity of COX-2, binds to EP2 or EP4 receptor on the cell surface of immune cells such as DCs or T cells and interferes with a wide range of effector functions (60, 61). Furthermore, in vitro studies suggest that PGE2 paves a way for the development of Th2 response by inhibiting the production of the Th1 cytokines IL-2 and IFN-γ as well as augmenting IL-4 and IL-5 production (59–61). A variety of pathogenic microorganisms, including mycobacteria, activate COX-2 expression that could lead to suppression of Th1 polarization in favor of Th2 immunity (51, 56). Additionally, COX-2−/− mice infected with Helicobacter pylori were shown to have up-regulated IFN-γ and IL-12 expression compared with wild-type mice (61). Accordingly, Rv1917c strongly stimulated COX-2 expression and augmented the secretion of PGE2 from human DCs. Inhibition of COX-2 activity in DCs prominently suppressed Rv1917c-induced Th2 responses. These results clearly implicate a crucial role of the COX-2/PGE2 axis in the development of Th2 immunity in response to Rv1917c. Overall, this investigation identifies Rv1917c as a Th2-responsive mycobacterial antigen that can play a decisive role in controlling the clinical course of tuberculosis. Importantly, we would like to speculate that our analysis would render the designing and development of peptidomimics that specifically inhibit interaction between Rv1917c and pattern recognition receptors as well as host signaling proteins of DCs or macrophages. Because expansion of regulatory T cells and Th2 cells is a hallmark of chronic tuberculosis infection, the molecular adjuvants such as CCR4 antagonists that block these two suppressive populations might benefit tuberculosis vaccination strategies (79, 80). These insights, in our opinion, will broaden the understanding of the tuberculosis disease pathogenesis and might pave a way for their inclusion in drug development efforts against tuberculosis.
Supplementary Material
Acknowledgments
We acknowledge the kind help of Minakshi Sen DBT-Confocal Facility, for assistance in confocal microscopy studies. We are thankful to Dr. Douglas Golenbock, University of Massachusetts Medical School, Worcester, MA, for the kind gift of reagents. We acknowledge Dr. Shashidhar Buggi, Rajiv Gandhi Institute of Chest Diseases, Bangalore, India, for the kind help during the course of this study. Infrastructure support was from the Indian Council of Medical Research (Center for Advanced Study in Molecular Medicine), Department of Science and Technology.
This work was supported in part by Indian Institute of Science, Departments of Biotechnology and Science and Technology, Council for Scientific and Industrial Research, India (to K. N. B.), INSERM, CNRS, and Université Pierre et Marie Curie and Université Paris Descartes, France (to S. V. K. and J. B.), and Coopération INSERM-ICMR-AO 2009/2010 (to K. N. B. and J. B.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4.
K. N. Balaji, unpublished data.
- APC
- antigen-presenting cells
- DC
- dendritic cells
- PE
- proline-glutamic acid
- PPE
- proline-proline-glutamic acid
- SH
- Src homology
- Ni-NTA
- nickel-nitrilotriacetic acid
- PBMC
- peripheral blood mononuclear cell
- PRR
- pattern recognition receptor
- TLR
- Toll-like receptor.
REFERENCES
- 1.Organization, W. H. (2007) WHO Report 2007 WHO/HTM/TB/2007.376, Geneva, Switzerland [Google Scholar]
- 2.Andersen P., Doherty T. M. (2005) Nat. Rev. Microbiol. 3, 656–662 [DOI] [PubMed] [Google Scholar]
- 3.Brandt L., Feino Cunha J., Weinreich Olsen A., Chilima B., Hirsch P., Appelberg R., Andersen P. (2002) Infect. Immun. 70, 672–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng C. G., Bean A. G., Hooi H., Briscoe H., Britton W. J. (1999) Infect. Immun. 67, 3242–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flynn J. L., Chan J. (2001) Annu. Rev. Immunol. 19, 93–129 [DOI] [PubMed] [Google Scholar]
- 6.McShane H., Behboudi S., Goonetilleke N., Brookes R., Hill A. V. (2002) Infect. Immun. 70, 1623–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diebold S. S. (2008) Immunol. Cell Biol. 86, 389–397 [DOI] [PubMed] [Google Scholar]
- 8.Józefowski S., Sobota A., Kwiatkowska K. (2008) BioEssays 30, 943–954 [DOI] [PubMed] [Google Scholar]
- 9.O'Shea J. J., Paul W. E. (2010) Science 327, 1098–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu J., Yamane H., Paul W. E. (2010) Annu. Rev. Immunol. 28, 445–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daly L. M., Johnson P. A., Donnelly G., Nicolson C., Robertson J., Mills K. H. (2005) Vaccine 23, 963–974 [DOI] [PubMed] [Google Scholar]
- 12.Liu L., Rich B. E., Inobe J., Chen W., Weiner H. L. (1998) Int. Immunol. 10, 1017–1026 [DOI] [PubMed] [Google Scholar]
- 13.Bermudez L. E., Champsi J. (1993) Infect. Immun. 61, 3093–3097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore K. W., de Waal Malefyt R., Coffman R. L., O'Garra A. (2001) Annu. Rev. Immunol. 19, 683–765 [DOI] [PubMed] [Google Scholar]
- 15.Murray P. J., Young R. A. (1999) Infect. Immun. 67, 3087–3095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Heer H. J., Hammad H., Kool M., Lambrecht B. N. (2005) Semin. Immunol. 17, 295–303 [DOI] [PubMed] [Google Scholar]
- 17.Marino S., Kirschner D. E. (2004) J. Theor. Biol. 227, 463–486 [DOI] [PubMed] [Google Scholar]
- 18.Reis e Sousa C. (2001) Immunity 14, 495–498 [DOI] [PubMed] [Google Scholar]
- 19.Reis e Sousa C. (2006) Nat. Rev. Immunol. 6, 476–483 [DOI] [PubMed] [Google Scholar]
- 20.Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S., Barry C. E., 3rd, Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., Connor R., Davies R., Devlin K., Feltwell T., Gentles S., Hamlin N., Holroyd S., Hornsby T., Jagels K., Krogh A., McLean J., Moule S., Murphy L., Oliver K., Osborne J., Quail M. A., Rajandream M. A., Rogers J., Rutter S., Seeger K., Skelton J., Squares R., Squares S., Sulston J. E., Taylor K., Whitehead S., Barrell B. G. (1998) Nature 393, 537–544 [DOI] [PubMed] [Google Scholar]
- 21.Adindla S., Guruprasad L. (2003) J. Biosci. 28, 169–179 [DOI] [PubMed] [Google Scholar]
- 22.Gordon S. V., Eiglmeier K., Garnier T., Brosch R., Parkhill J., Barrell B., Cole S. T., Hewinson R. G. (2001) Tuberculosis 81, 157–163 [DOI] [PubMed] [Google Scholar]
- 23.Voskuil M. I., Schnappinger D., Rutherford R., Liu Y., Schoolnik G. K. (2004) Tuberculosis 84, 256–262 [DOI] [PubMed] [Google Scholar]
- 24.Chaitra M. G., Shaila M. S., Nayak R. (2008) Microbes Infect. 10, 858–867 [DOI] [PubMed] [Google Scholar]
- 25.Chaitra M. G., Shaila M. S., Nayak R. (2008) J. Med. Microbiol. 57, 1079–1086 [DOI] [PubMed] [Google Scholar]
- 26.Choudhary R. K., Mukhopadhyay S., Chakhaiyar P., Sharma N., Murthy K. J., Katoch V. M., Hasnain S. E. (2003) Infect. Immun. 71, 6338–6343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mishra K. C., de Chastellier C., Narayana Y., Bifani P., Brown A. K., Besra G. S., Katoch V. M., Joshi B., Balaji K. N., Kremer L. (2008) Infect. Immun. 76, 127–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tundup S., Pathak N., Ramanadham M., Mukhopadhyay S., Murthy K. J., Ehtesham N. Z., Hasnain S. E. (2008) PLoS One 3, e3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sampson S. L., Lukey P., Warren R. M., van Helden P. D., Richardson M., Everett M. J. (2001) Tuberculosis 81, 305–317 [DOI] [PubMed] [Google Scholar]
- 30.Manninen A., Hiipakka M., Vihinen M., Lu W., Mayer B. J., Saksela K. (1998) Virology 250, 273–282 [DOI] [PubMed] [Google Scholar]
- 31.Ravi Chandra B., Gowthaman R., Raj Akhouri R., Gupta D., Sharma A. (2004) Protein Eng. Des. Sel. 17, 175–182 [DOI] [PubMed] [Google Scholar]
- 32.Shelton H., Harris M. (2008) Virol. J. 5, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bansal K., Elluru S. R., Narayana Y., Chaturvedi R., Patil S. A., Kaveri S. V., Bayry J., Balaji K. N. (2010) J. Immunol. 184, 3495–3504 [DOI] [PubMed] [Google Scholar]
- 34.Coventry B. J., Lee P. L., Gibbs D., Hart D. N. (2002) Br. J. Cancer 86, 546–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Münz C., Steinman R. M., Fujii S. (2005) J. Exp. Med. 202, 203–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aimanianda V., Haensler J., Lacroix-Desmazes S., Kaveri S. V., Bayry J. (2009) Trends Pharmacol. Sci. 30, 287–295 [DOI] [PubMed] [Google Scholar]
- 37.Pecora N. D., Gehring A. J., Canaday D. H., Boom W. H., Harding C. V. (2006) J. Immunol. 177, 422–429 [DOI] [PubMed] [Google Scholar]
- 38.Almeida P. E., Silva A. R., Maya-Monteiro C. M., Töröcsik D., D'Avila H., Dezsö B., Magalhães K. G., Castro-Faria-Neto H. C., Nagy L., Bozza P. T. (2009) J. Immunol. 183, 1337–1345 [DOI] [PubMed] [Google Scholar]
- 39.Harding C. V., Boom W. H. (2010) Nat. Rev. Microbiol. 8, 296–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kincaid E. Z., Wolf A. J., Desvignes L., Mahapatra S., Crick D. C., Brennan P. J., Pavelka M. S., Jr., Ernst J. D. (2007) J. Immunol. 179, 3187–3195 [DOI] [PubMed] [Google Scholar]
- 41.Means T. K., Wang S., Lien E., Yoshimura A., Golenbock D. T., Fenton M. J. (1999) J. Immunol. 163, 3920–3927 [PubMed] [Google Scholar]
- 42.Trinchieri G., Sher A. (2007) Nat. Rev. Immunol. 7, 179–190 [DOI] [PubMed] [Google Scholar]
- 43.Farhat K., Riekenberg S., Heine H., Debarry J., Lang R., Mages J., Buwitt-Beckmann U., Röschmann K., Jung G., Wiesmüller K. H., Ulmer A. J. (2008) J. Leukocyte Biol. 83, 692–701 [DOI] [PubMed] [Google Scholar]
- 44.Ferwerda G., Kullberg B. J., de Jong D. J., Girardin S. E., Langenberg D. M., van Crevel R., Ottenhoff T. H., Van der Meer J. W., Netea M. G. (2007) J. Leukocyte Biol. 82, 1011–1018 [DOI] [PubMed] [Google Scholar]
- 45.Nair S., Ramaswamy P. A., Ghosh S., Joshi D. C., Pathak N., Siddiqui I., Sharma P., Hasnain S. E., Mande S. C., Mukhopadhyay S. (2009) J. Immunol. 183, 6269–6281 [DOI] [PubMed] [Google Scholar]
- 46.Nakao Y., Funami K., Kikkawa S., Taniguchi M., Nishiguchi M., Fukumori Y., Seya T., Matsumoto M. (2005) J. Immunol. 174, 1566–1573 [DOI] [PubMed] [Google Scholar]
- 47.Nigou J., Vasselon T., Ray A., Constant P., Gilleron M., Besra G. S., Sutcliffe I., Tiraby G., Puzo G. (2008) J. Immunol. 180, 6696–6702 [DOI] [PubMed] [Google Scholar]
- 48.Ryffel B., Fremond C., Jacobs M., Parida S., Botha T., Schnyder B., Quesniaux V. (2005) Tuberculosis 85, 395–405 [DOI] [PubMed] [Google Scholar]
- 49.Tapping R. I., Tobias P. S. (2003) J. Endotoxin Res. 9, 264–268 [DOI] [PubMed] [Google Scholar]
- 50.Ardeshna K. M., Pizzey A. R., Devereux S., Khwaja A. (2000) Blood 96, 1039–1046 [PubMed] [Google Scholar]
- 51.Bansal K., Narayana Y., Patil S. A., Balaji K. N. (2009) J. Leukocyte Biol. 85, 804–816 [DOI] [PubMed] [Google Scholar]
- 52.Boislève F., Kerdine-Römer S., Pallardy M. (2005) Toxicology 206, 233–244 [DOI] [PubMed] [Google Scholar]
- 53.Dowling D., Hamilton C. M., O'Neill S. M. (2008) Cytokine 41, 254–262 [DOI] [PubMed] [Google Scholar]
- 54.Kapoor N., Narayana Y., Patil S. A., Balaji K. N. (2010) J. Immunol. 184, 3117–3126 [DOI] [PubMed] [Google Scholar]
- 55.Nakahara T., Moroi Y., Uchi H., Furue M. (2006) J. Dermatol. Sci. 42, 1–11 [DOI] [PubMed] [Google Scholar]
- 56.Pathak S. K., Bhattacharyya A., Pathak S., Basak C., Mandal D., Kundu M., Basu J. (2004) J. Biol. Chem. 279, 55127–55136 [DOI] [PubMed] [Google Scholar]
- 57.Rescigno M., Martino M., Sutherland C. L., Gold M. R., Ricciardi-Castagnoli P. (1998) J. Exp. Med. 188, 2175–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Valentinis B., Bianchi A., Zhou D., Cipponi A., Catalanotti F., Russo V., Traversari C. (2005) J. Biol. Chem. 280, 14264–14271 [DOI] [PubMed] [Google Scholar]
- 59.Tilley S. L., Coffman T. M., Koller B. H. (2001) J. Clin. Invest. 108, 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown J. R., DuBois R. N. (2005) J. Clin. Oncol. 23, 2840–2855 [DOI] [PubMed] [Google Scholar]
- 61.Meyer F., Ramanujam K. S., Gobert A. P., James S. P., Wilson K. T. (2003) J. Immunol. 171, 3913–3917 [DOI] [PubMed] [Google Scholar]
- 62.Balikó Z., Szereday L., Szekeres-Bartho J. (1998) FEMS Immunol. Med. Microbiol. 22, 199–204 [DOI] [PubMed] [Google Scholar]
- 63.Anis M. M., Fulton S. A., Reba S. M., Liu Y., Harding C. V., Boom W. H. (2008) Infect. Immun. 76, 671–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Natarajan K., Latchumanan V. K., Singh B., Singh S., Sharma P. (2003) J. Infect. Dis. 187, 914–928 [DOI] [PubMed] [Google Scholar]
- 65.Wolf A. J., Linas B., Trevejo-Nuñez G. J., Kincaid E., Tamura T., Takatsu K., Ernst J. D. (2007) J. Immunol. 179, 2509–2519 [DOI] [PubMed] [Google Scholar]
- 66.Balaji K. N., Goyal G., Narayana Y., Srinivas M., Chaturvedi R., Mohammad S. (2007) Microbes Infect. 9, 271–281 [DOI] [PubMed] [Google Scholar]
- 67.Bansal K., Kapoor N., Narayana Y., Puzo G., Gilleron M., Balaji K. N. (2009) PLoS One 4, e4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beatty W. L., Russell D. G. (2000) Infect. Immun. 68, 6997–7002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhoades E., Hsu F., Torrelles J. B., Turk J., Chatterjee D., Russell D. G. (2003) Mol. Microbiol. 48, 875–888 [DOI] [PubMed] [Google Scholar]
- 70.Pathak S. K., Basu S., Basu K. K., Banerjee A., Pathak S., Bhattacharyya A., Kaisho T., Kundu M., Basu J. (2007) Nat. Immunol. 8, 610–618 [DOI] [PubMed] [Google Scholar]
- 71.Chakhaiyar P., Nagalakshmi Y., Aruna B., Murthy K. J., Katoch V. M., Hasnain S. E. (2004) J. Infect. Dis. 190, 1237–1244 [DOI] [PubMed] [Google Scholar]
- 72.Singh K. K., Dong Y., Patibandla S. A., McMurray D. N., Arora V. K., Laal S. (2005) Infect. Immun. 73, 5004–5014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Camacho L. R., Ensergueix D., Perez E., Gicquel B., Guilhot C. (1999) Mol. Microbiol. 34, 257–267 [DOI] [PubMed] [Google Scholar]
- 74.O'Brien R., Flynn O., Costello E., O'Grady D., Rogers M. (2000) J. Clin. Microbiol. 38, 1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schnappinger D., Ehrt S., Voskuil M. I., Liu Y., Mangan J. A., Monahan I. M., Dolganov G., Efron B., Butcher P. D., Nathan C., Schoolnik G. K. (2003) J. Exp. Med. 198, 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yadav M., Schorey J. S. (2006) Blood 108, 3168–3175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Buwitt-Beckmann U., Heine H., Wiesmüller K. H., Jung G., Brock R., Akira S., Ulmer A. J. (2006) J. Biol. Chem. 281, 9049–9057 [DOI] [PubMed] [Google Scholar]
- 78.Caparrós E., Munoz P., Sierra-Filardi E., Serrano-Gómez D., Puig-Kröger A., Rodríguez-Fernández J. L., Mellado M., Sancho J., Zubiaur M., Corbí A. L. (2006) Blood 107, 3950–3958 [DOI] [PubMed] [Google Scholar]
- 79.Bayry J., Tchilian E. Z., Davies M. N., Forbes E. K., Draper S. J., Kaveri S. V., Hill A. V., Kazatchkine M. D., Beverley P. C., Flower D. R., Tough D. F. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 10221–10226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davies M. N., Bayry J., Tchilian E. Z., Vani J., Shaila M. S., Forbes E. K., Draper S. J., Beverley P. C., Tough D. F., Flower D. R. (2009) PLoS One 4, e8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.