Abstract
Trans-differentiation of quiescent hepatic stellate cells (Q-HSCs), which exhibit epithelial and adipocytic features, into myofibroblastic-HSC (MF-HSCs) is a key event in liver fibrosis. Culture models demonstrated that Hedgehog (Hh) pathway activation is required for transition of epithelioid/adipocytic Q-HSCs into MF-HSCs. Hh signaling inhibits adiposity and promotes epithelial-to-mesenchymal transitions (EMTs). Leptin (anti-adipogenic, pro-EMT factor) promotes HSC trans-differentiation and liver fibrosis, suggesting that the pathways may interact to modulate cell fate. This study aimed to determine whether leptin activates Hh signaling and whether this is required for the fibrogenic effects of leptin. Cultures of primary HSCs from lean and fa/fa rats with an inherited ObRb defect were examined. Inhibitors of PI3K/Akt, JAK/STAT, and Hh signaling were used to delineate how ObRb activation influenced Hh signaling and HSC trans-differentiation. Fibrogenesis was compared in wild type and db/db mice (impaired ObRb function) to assess the profibrotic role of leptin. The results demonstrate that leptin-ObR interactions activate Hh signaling with the latter necessary to promote trans-differentiation. Leptin-related increases in Hh signaling required ObR induction of PI3K/Akt, which was sufficient for leptin to repress the epithelioid/adipocytic program. Leptin-mediated induction of JAK/STAT was required for mesenchymal gene expression. Leptin-ObRb interactions were not necessary for HSC trans-differentiation to occur in vitro or in vivo but are important because liver fibrogenesis was attenuated in db/db mice. These findings reveal that leptin activates Hh signaling to alter gene expression programs that control cell fate and have important implications for liver fibrosis and other leptin-regulated processes involving EMTs, including development, obesity, and cancer metastasis.
Keywords: Adipocyte, Cell Differentiation, Fibroblast, Liver, Liver Injury, Epithelial-to-mesenchymal Transition, Fibrosis
Introduction
Stromal remodeling occurs routinely during embryogenesis and is a component of tissue reconstruction after injury in many adult organs, including liver (1). Myofibroblastic cells (MFs)2 are key mediators of stromal remodeling because they are major producers of both matrix proteins and factors that degrade matrix. Hence, MF populations generally expand during this phase of tissue repair (2). Various sources contribute MFs to wound-healing efforts, depending on the severity and duration of injury and the tissue involved. Transition of quiescent, adipocytic hepatic stellate cells (Q-HSCs) to MFs and the subsequent growth of these newly generated MFs are major mechanisms for enriching damaged livers with MFs (3). Therefore, it is important to characterize the signal transduction pathways that regulate these processes.
Several factors that increase during liver injury facilitate the formation of MFs from Q-HSCs and/or promote the survival and proliferation of the newly generated MFs, including transforming growth factor β (TGF-β), connective tissue growth factor, ligands for Toll-like receptors 2 and 9, certain proinflammatory cytokines, platelet-derived growth factor (PDGF), and leptin. Presumably, these factors are somewhat redundant, however, because knocking down any one of them in experimental animals has not been sufficient to abrogate either fibrogenesis or fibrinolysis entirely (2). Apoptotic liver cells themselves are also known to promote activation of HSCs to a MF phenotype. This occurs when HSCs phagocytose apoptotic liver cells (4). In addition, dying liver epithelial cells produce and release Hedgehog (Hh) ligands which operate as paracrine signals to activate Hh-dependent mechanisms that are required for Q-HSCs to become MFs (5). Hh ligands are also necessary for MFs to retain their fibroblastic phenotype, proliferate in response to mitogens, and survive during standard culture conditions (6).
Because Hh signaling mediates both the formation of MFs from Q-HSCs, and the growth of the newly generated MF populations, the Hh pathway may play a pivotal role in controlling HSC fate. If true, this hypothesis implies that the Hh pathway modulates signals that are initiated when other profibrogenic factors interact with their receptors on HSCs. To evaluate this possibility further, we examined how manipulating Hh signaling altered HSC responses to leptin.
We selected leptin for scrutiny because there is already an extensive body of literature detailing mechanisms by which leptin promotes myofibroblastic transition and proliferation of HSCs (7–16). That work demonstrated that activation of the long form of the leptin receptor, ObRb, and consequent activation of PI3K/Akt and JAK2/STAT3 signaling played critical roles in mediating the leptin effects. Q-HSCs are also adipocyte-like cells, and both leptin and Hh have been demonstrated to regulate the fate of adipocytes. In preadipocytes (which are fibroblastic cells), the Hh pathway is active. As the preadipocytes differentiate to become adipocytes, the Hh pathway is progressively silenced, and leptin gene expression is gradually induced (17). Leptin, in turn, prevents further adipocytic differentiation, thereby controlling the net mass of adipose tissue (18). Like leptin, Hh ligands are also potent inhibitors of adipogenesis. A recent genome-wide screen of factors that control adipose mass in Drosophila identified the Hh pathway as the major negative regulator of fat mass in flies (19). Similarly, transgenic mice with adipocyte-targeted disruption of SuFu, a major inhibitor of Hh signaling, exhibited excessive Hh signaling, aborted differentiation of adipocyte precursors, and failure to develop adipose depots (19). Evidence suggested that Hh acted upstream of peroxisome proliferator-activated receptor (PPAR) γ to block adipocytic differentiation by inhibiting the induction of this key adipogenic transcription factor and thereby, maintaining the typical fibroblastic preadipocyte phenotype.
Similar to mature adipocytes, Q-HSCs are lipid-laden and express PPARγ. PPARγ is down-regulated as Q-HSCs transition to become MFs, and agents that activate PPARγ both block the generation of MFs from Q-HSCs and cause MF-HSCs to revert back to their quiescent, adipocytic phenotype (20). Hh pathway activity increases as Q-HSCs repress PPARγ and transition to become MF-HSCs. Thus, PPARγ activity is low, and Hh pathway activity is high, in MF-HSCs. Treating MF-HSCs with Hh inhibitors permits PPARγ expression to reemerge and causes the cells to lose myofibroblastic characteristics (21). Thus, the Hh pathway acts upstream of PPARγ to inhibit PPARγ induction and maintain the fibroblastic phenotype of both preadipocytes and MF-HSCs.
In cultured HSCs, the transition from Q-HSCs (which have high levels of PPARγ but little Hh activity) to MF-HSCs (which have low PPARγ but high Hh activity) is associated with down-regulation of BMP7, Id2, desmoplakin, and E-cadherin expression, and concomitant up-regulation of TGF-β, snail, vimentin, fibronectin, α-smooth muscle actin (α-SMA), matrix metalloproteinases, and type 1 collagen (Col1α1). Similar changes in gene expression are known to occur as epithelial (or endothelial) cells acquire mesenchymal characteristics, suggesting that the generation of MF-HSCs from adipocytic precursors involves a process that resembles epithelial/endothelial-to-mesenchymal transition (EMT). Blocking Hh pathway activation prevents/reverses this EMT-like process and inhibits HSCs from becoming/remaining myofibroblastic (21). Interestingly, recent studies of endocardial cushion remodeling in the developing heart, as well as certain types of adult epithelial tumors, demonstrate that leptin is also capable of promoting EMT (22–24). To our knowledge, however, the possibility that leptin might interact with Hh to modulate EMT during development, tumor metastasis, or the (trans)-differentiation of adipocytic cells has not been investigated.
Given the apparent overlap between the actions of leptin and Hh in several types of stromal cells, including HSCs, the present study evaluated the hypothesis that leptin activates the Hh pathway to promote HSC acquisition/maintenance of a myofibroblastic phenotype. These results confirm this hypothesis and thereby advance fundamental knowledge about the mechanisms underlying the leptin actions in HSCs. Moreover, the findings extend earlier evidence which suggested that Hh pathway activation is a conserved target of profibrogenic factors. This concept, in turn, has important implications for a broad spectrum of clinical disorders that involve deregulated stromal cells, including cirrhosis, metastatic cancers, certain congenital cardiac abnormalities, and obesity.
EXPERIMENTAL PROCEDURES
Animal Care
Adult, male Sprague-Dawley rats were obtained from Charles River Laboratories (Wilmington, MA). Zucker-Leprfa and Zucker-Lepr+ were obtained from Harlan Laboratories (Indianapolis, IN). Obese, diabetic db/db mice were obtained from Jackson Laboratories (Bar Harbor, ME). To induce liver injury and fibrosis, six db/db and five lean control mice were fed methionine choline-deficient (MCD) diets (MP Biomedicals, Solon, OH) for a total of 8 weeks. Control db/db and WT mice were permitted ad libitum consumption of water and standard rodent food. Upon completion of 8 weeks of treatment, mice were killed. Livers were harvested and either formalin-fixed or snap frozen. Animal experiments fulfilled National Institutes of Health and Duke University IACUC requirements for humane animal care.
Cell Isolation and Culture
Primary HSCs were isolated from Sprague-Dawley, Zucker-Leprfa and Zucker-Lepr+ rats, assessed for purity and viability, and seeded at a density of 3 × 102 cells/mm2 in DMEM supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin as described previously (6, 21). For studies that involved treatments with leptin, adenoviral vectors and/or various pharmacologic inhibitors, day 7 HSC cultures were cultured overnight in serum-depleted medium (0.1% FBS) before treatments were initiated.
Adenoviral Transduction of HSCs
Ad5GFP, which contains the GFP gene driven by the cytomegalovirus promoter, was used as a control virus. The Ad5dnAkt virus expresses the dominant negative forms of Akt. Pilot studies demonstrated that maximally efficient transduction occurred at a multiplicity of infection of 100. Subsequent experiments were carried out with this multiplicity of infection for 24 h, then virus-containing medium was aspirated and replaced with fresh medium (6).
Pharmacological Treatment of HSCs
Primary MF-HSCs were treated with cyclopamine (5 μm; Toronto Research Chemicals, Toronto, Canada) or a catalytically inactive analog tomatidine (5 μm) for 24 h to inhibit Hh signaling (21), LY294002 (25 μm; Cell Signaling Technology, Danvers, MA) for 30 min to inhibit PI3K signaling (6), and AG490 (50 μm; Tocris Biosciences, Ellisville, MO) for 30 min to inhibit JAK signaling (13) prior to treatment with leptin (100 ng/ml; R&D Systems, Minneapolis, MN) or vehicle control. This dose of leptin was selected based on evidence that it optimally induced transcriptional activation of the type 1 collagen gene (7) and stimulated downstream kinase activity and proliferation in cultured rodent and human HSCs (13–15).
mRNA Quantification by Real-time Reverse Transcription-PCR (RT-PCR)
Total RNA was extracted with TRIzol (Invitrogen), reverse-transcribed using Superscript reverse transcriptase (Invitrogen) as described (21). For primers, see Table 1.
TABLE 1.
RT-PCR Primers for Analysis
| Gene | GenBank | Direction | Sequence |
|---|---|---|---|
| αSMA | X06801 | Forward | GTGGATCACCAAGCAGGAGGAGT |
| Reverse | CATAGCACGATGGTCGATTG | ||
| Col1α1 | XM_213440 | Forward | CTGCATACACAATGGCCTAA |
| Reverse | GGGTCCCTCGACTCCTA | ||
| Fibronectin | NM_019143 | Forward | GTGGCTGCCTTCAACTTCTC |
| Reverse | GTGGGTTGCAAACCTTCAAT | ||
| PPARγ | NM_013124 | Forward | CCCTGGCAAAGCATTTGTAT |
| Reverse | ACTGGCACCCTTGAAAAATG | ||
| GFAP | NM_017009 | Forward | GGGAGTCGGCCAGTTACCAG |
| Reverse | CCCGCATCTCCACCGTCT | ||
| S100A4 | NM_012618 | Forward | ATACTCAGGCAACGAGGGTG |
| Reverse | CTTCCGGGGCTCCTTATC | ||
| BMP7 | XM_342591 | Forward | GTGGTCAACCCTCGGCACA |
| Reverse | GGCGTCTTGGAGCGATTCTG | ||
| Desmoplakin | XM_001058477 | Forward | GGAAGTCAGCCAAGCAAAAC |
| Reverse | GGCTCTCCTTTTCACACTGC | ||
| E-cadherin | NM_009864 | Forward | ACCTCTGGGCTGGACCGA |
| Reverse | CCTGATACGTGCTTGGGTTGAA | ||
| Shh | NM_017221 | Forward | ACAAGAAACTCCGAACGATT |
| Reverse | GCCCTCAGTCACTCGAAG | ||
| Gli2 | XM_222557 | Forward | ATAAGCGGAGCAAGGTCAAG |
| Reverse | CAGTGGCAGTTGGTCTCGTA | ||
| S9 | NM_029767 | Forward | GACTCCGGAACAAACGTGAGGT |
| Reverse | CTTCATCTTGCCCTCGTCCA |
Western Blotting
Western blotting was to demonstrate changes in relevant proteins; results were normalized to β-actin expression (21).
Morphometry
To quantify liver fibrosis, 5-μm sections were stained with Picrosirius red (Sigma) with the proportion of Picrosirius red-stained tissue assessed for morphometric analysis as described previously (25).
Hepatic Hydroxyproline Quantification
Hydroxyproline content in whole liver specimens was quantified colorimetrically with results expressed relative to WT controls as described previously (25).
Statistical Analysis
Results are expressed as means ± S.E. Comparisons between groups were performed using the nonparametric Wilcoxon-Rank-Sums test using SAS version 9.1 software (SAS Institute, Cary, NC). p values are two-tailed; significance was accepted at the 5% level.
RESULTS
Leptin (Ob)/Ob Receptor Interactions Differentially Modulate Expression of Mesenchymal and Epithelial Genes in HSCs
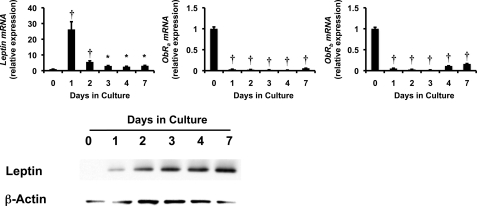
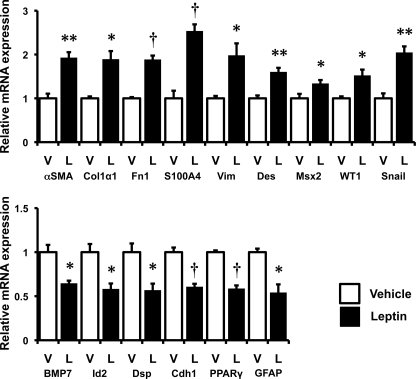
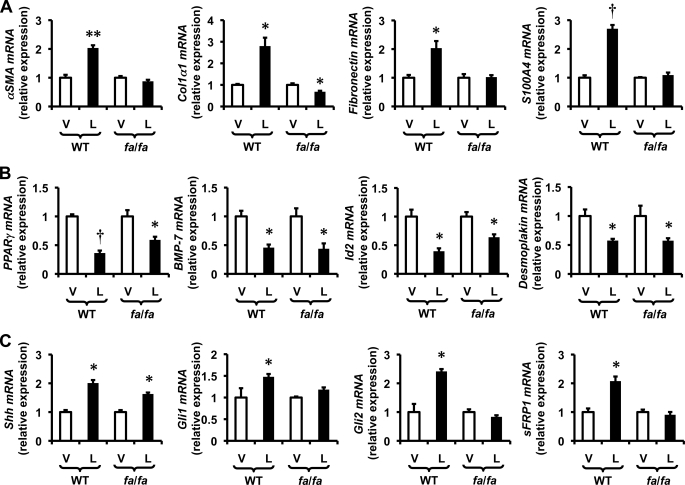
HSCs are known to express both leptin and leptin receptors. Quantitative RT-PCR (qRT-PCR) and Western blot analysis were used to characterize changes in the expression of these factors during culture-related activation of primary rat Q-HSCs into MF-HSCs. Although culture strongly repressed mRNA expression of both ObRa and ObRb, leptin mRNAs were rapidly induced, leading to progressive accumulation of leptin protein as HSCs became myofibroblastic (Fig. 1). These findings are consistent with findings that have been reported by other groups (8, 26, 27) and suggest that leptin signaling activity might increase during HSC trans-differentiation, despite associated repression of leptin receptor mRNAs. To further evaluate leptin receptor function in MF-HSCs, day 7 cultures of MF-HSCs were treated with exogenous leptin, and effects on HSC gene expression were assessed by qRT-PCR. Leptin treatment enhanced expression of various myofibroblast-related genes (e.g. α-SMA, Col1α1, fibronectin, vimentin, S100A4, and desmin) and increased mRNA expression of prototypical mesenchymal transcription factors (e.g. msx2, wt1, and snail), while repressing expression of markers of Q-HSCs (e.g. PPARγ and gfap) and epithelial cells (e.g. BMP7, Id2, desmoplakin, E-cadherin) (Fig. 2). Some, but not all, of the effects of leptin were lost when leptin was added to cultures of primary HSCs from fa/fa rats, which have an inherited defect in ObRb that reduces its function. ObRb-defective HSCs were unable to up-regulate expression of mesenchymal/myofibroblastic genes further when treated with exogenous leptin, but retained leptin-related repression of epithelial/quiescence markers (Fig. 3). Together, these results demonstrate that leptin must engage ObRb, the long form of its receptor, to increase HSC expression of mesenchymal myofibroblastic genes. However, distinct leptin receptors and/or residual functional components of the mutant ObRb transduce signals that permit leptin to repress expression of genes that mediate epithelial characteristics.
FIGURE 1.
Effects of stellate cell trans-differentiation on expression of leptin and its receptors. Primary HSCs were isolated from healthy adult male Sprague-Dawley rats, pooled, and cultured on plastic dishes in serum-containing medium. RNA was isolated at different time points and changes in gene expression were evaluated by qRT-PCR. top, leptin, ObRa, and ObRb. Results are representative of triplicate experiments. Results are mean ± S.E. (error bars) of triplicate experiments. *, p < 0.05; †, p < 0.005. bottom, protein harvested from these primary rat HSCs and analyzed by Western blotting.
FIGURE 2.
Effects of leptin on stellate cell gene expression. Primary rat HSCs were cultured in serum-containing medium and treated with leptin (L; 100 ng/ml) or vehicle (V). RNA was isolated, and changes in gene expression were evaluated by qRT-PCR. top, myofibroblastic/mesenchymal markers: α-SMA, Col1α1, fibronectin (Fn1), S100A4, vimentin (Vim), desmin (Des), Msx2, WT1, and snail. bottom, epithelial markers: BMP7, Id2, desmoplakin (Dsp), E-cadherin (Cdh1), and markers of quiescent HSCs: PPARγ and GFAP. Results are mean ± S.E. (error bars) of triplicate experiments. *, p < 0.05; **, p < 0.01; †, p < 0.005.
FIGURE 3.
Some effects of leptin are mediated via interaction with ObRb. Primary HSCs were isolated from obese fa/fa rats and their lean littermates, pooled, and cultured on plastic dishes in serum-containing medium. Culture-activated HSCs were treated with leptin as described in Fig. 2. RNA was isolated, and changes in gene expression were evaluated by qRT-PCR. A, myofibroblastic/mesenchymal markers: α-SMA, Col1α1, fibronectin (Fn1), and S100A4. B, PPARγ (quiescence marker) and epithelial markers: BMP7, Id2, and desmoplakin. C, Hedgehog pathway genes: Shh, Gli1, Gli2, and sFRP1. Results are mean ± S.E. (error bars) of triplicate experiments. *, p < 0.05; **, p < 0.01; †, p < 0.005.
PI3K/Akt Signaling Globally Mediates Effects of Leptin-ObRb on HSC Trans-differentiation
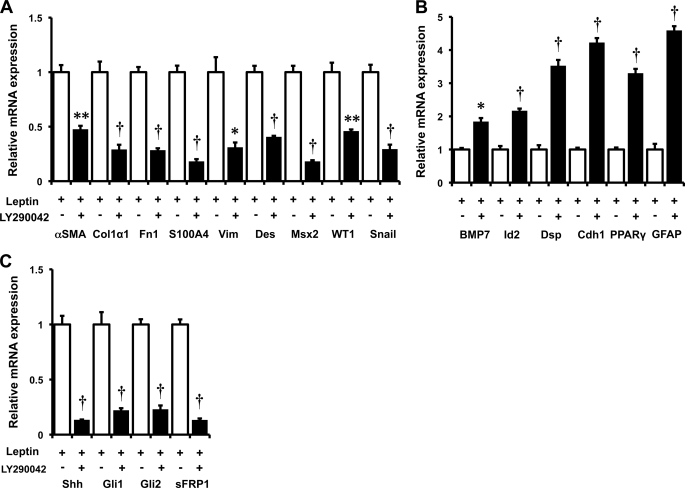
All leptin receptors couple with PI3K and result in activation of Akt, whereas only ObRb activates JAK-STAT signaling (8, 28). To determine the role of PI3K in leptin-mediated trans-differentiation of HSCs, primary rat HSCs were treated with leptin in the absence or presence of the PI3K inhibitor, LY294002. LY294002 reversed all of the effects of leptin, blocking its ability to increase mRNA expression of various mesenchymal/myofibroblastic genes, as well as leptin-related repression of different epithelial/quiescence-associated mRNAs (Fig. 4). LY294002 effects on mRNA expression were paralleled by comparable changes in the respective proteins (Fig. 5). Adenovirally mediated transfer of dominant negative Akt (dnAkt) similarly inhibited leptin-mediated changes in mRNA and protein expression, whereas infection with mock adenoviral vectors (Ad5GFP) did not alter leptin actions (Fig. 5 and supplemental Fig. 1). These data demonstrate that leptin-leptin receptor interactions that result in activation of PI3K/Akt signaling are required for leptin to induce expression of myofibroblastic/mesenchymal genes as well as to repress expression of epithelial and quiescence markers. Because the former, but not the latter, also require signaling that is uniquely triggered by ObRb, the PI3K/Akt pathway likely interacts with the JAK/STAT pathway to mediate the full effects of leptin on HSC trans-differentiation.
FIGURE 4.
Effect of PI3K inhibition on leptin signaling in primary HSCs. Primary rat HSCs were cultured in serum-containing medium and pretreated with the PI3K antagonist, LY294003 (25 μm) for 30 min prior to the addition of leptin (L; 100 ng/ml) or vehicle (V). RNA was isolated, and changes in gene expression were evaluated by qRT-PCR. A, myofibroblastic/mesenchymal markers: αSMA, Col1α1, fibronectin (Fn1), S100A4, vimentin (Vim), desmin (Des), Msx2, WT1, and snail. B, epithelial markers: BMP7, Id2, desmoplakin (Dsp), and E-cadherin (Cdh1) and markers of quiescent HSCs: PPARγ and GFAP. C, Hh pathway genes: Shh, Gli1, Gli2, and sFRP1. Results are mean ± S.E. (error bars) of triplicate experiments. *, p < 0.05; **, p < 0.01; †, p < 0.005.
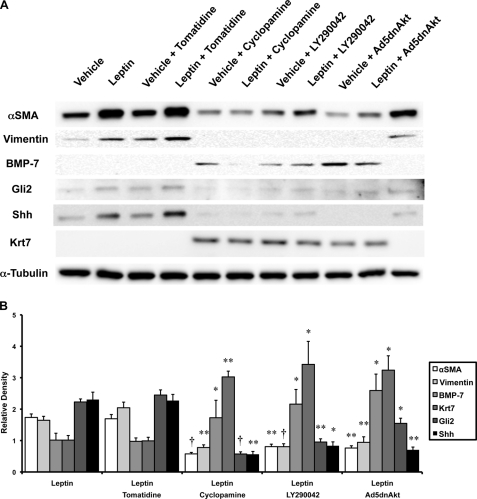
FIGURE 5.
Effects of PI3K and AKT inhibition on leptin signaling in HSCs. Primary rat HSCs were cultured in serum-containing medium as described in Fig. 2. HSCs were pretreated with LY294003 (25 μm) or vehicle (dimethyl sulfoxide) for 30 min (to inhibit PI3K), Ad5dnAkt (multiplicity of infection 100) or Ad5GFP (multiplicity of infection 100) for 24 h (to inhibit Akt), or cyclopamine (5 μm) or tomatidine (5 μm) for 24 h (to inhibit Hh pathway signaling) prior to the addition of leptin (100 ng/ml) or vehicle. To inhibit AKT, HSCs were treated with Ad5dnAkt prior to treatment with leptin(100 ng/ml) or vehicle. A, protein was harvested, and changes in gene expression were confirmed by Western blot analysis. Results are representative of triplicate experiments. B, densitometry was performed on all treatment groups. Leptin-treated groups are demonstrated as mean ± S.D. (error bars) of triplicate experiments. *, p < 0.05; **, p < 0.01; †, p < 0.005.
JAK/STAT Pathway Engagement Required for Some of the Downstream Consequences of ObRb Activation during HSC Trans-differentiation
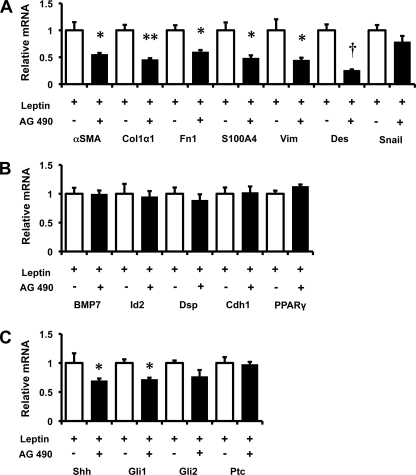
To clarify the role of JAK/STAT signaling in the ability of leptin to influence myofibroblastic trans-differentiation of HSCs, HSCs were treated with leptin in the absence or presence of AG490, an inhibitor of JAK signaling. Pretreatment with AG490 inhibited leptin-mediated induction of various myofibroblastic genes, including α-SMA, Col1α1, fibronectin, vimentin, S100A4, and desmin, without appreciably down-regulating levels of snail transcripts (Fig. 6A). These findings confirm that leptin-related induction of myofibroblastic genes requires JAK/STAT signaling (7) and suggest that the latter operates downstream of snail transcription to modulate myofibroblastic trans-differentiation. Interestingly, however, such ObRb-deficient HSCs remained capable of down-regulating various epithelial markers (BMP7, Id2, desmoplakin, E-cadherin) and PPARγ, a classical marker of Q-HSCs, when treated with leptin (Fig. 6B). The latter findings demonstrate that in HSCs, as in other cell types (29, 30), mechanisms that mediate acquisition of the myofibroblastic phenotype are somewhat distinct from those that promote loss of epithelial characteristics during EMT.
FIGURE 6.
Effects of JAK/STAT inhibition on HSC response to leptin. Primary rat HSCs were cultured in serum-containing medium as described in Fig. 2. HSCs were treated with the JAK inhibitor, AG490 (25 μm), for 30 min prior to treatment with leptin (100 ng/ml). RNA was isolated and changes in gene expression were evaluated by qRT-PCR. A, myofibroblastic/mesenchymal markers: α-SMA, Col1α1, fibronectin (Fn1), S100A4, vimentin (Vim), desmin (Des), and snail. B, epithelial markers: BMP7, Id2, desmoplakin (Dsp), E-cadherin (Cdh1) and the marker of quiescent HSCs, PPARγ. C, Hh pathway genes: Shh, Gli1, Gli2, and Ptc. Results are mean ± S.E. (error bars) of triplicate experiments. *, p < 0.05; **, p < 0.01; †, p < 0.005.
Activation of the Hh Pathway Is Conserved Requirement for Leptin-mediated Induction of HSC Trans-differentiation
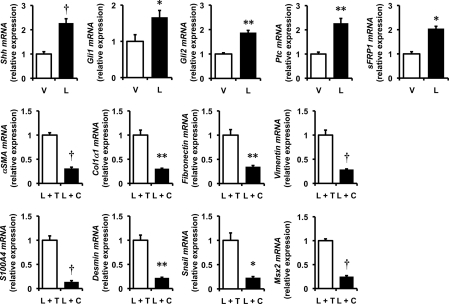
Like leptin signaling, activation of the Hh pathway also exerts global effects on HSC gene expression, increasing expression of myofibroblastic/mesenchymal genes while repressing expression of quiescence/epithelial markers (6, 21). To determine whether or not leptin interacts with the Hh pathway to alter the phenotype of HSCs, qRT-PCR was used to evaluate the effects of leptin on HSC expression of Hh ligands and target genes. Leptin increased expression of Sonic hedgehog (Shh) ligand, its receptor, patched (Ptc), the Hh-regulated transcription factors, Gli1 and Gli2, and the Gli-target gene, soluble frizzled-related peptide (sFRP)1, demonstrating that leptin increases production of Shh and activates the Hh pathway (Figs. 5 and 7). Moreover, adding cyclopamine (a specific Hh pathway inhibitor) to leptin completely blocked all of the actions of leptin, whereas the tomatidine (the inactive cyclopamine analog) had no effect. These results prove that leptin requires the Hh pathway to exert its effects on HSC trans-differentiation.
FIGURE 7.
Effects of leptin on the Hh pathway in HSCs. Primary rat HSCs were cultured in serum-containing medium. A, primary MF-HSCs were treated with leptin (L; 100 ng/ml) or vehicle (V). RNA was isolated, and changes in Hh pathway gene expression (Shh, Gli1, Gli2, Ptc, and sFRP1) were evaluated by qRT-PCR. The effects of Hh pathway inhibition were demonstrated by treating primary HSCs with cyclopamine (C) (5 μm) or its biologically inactive analog, tomatidine (T) (5 μm), for 2 h prior to treatment with leptin (L) (100 ng/ml). RNA was isolated and changes in myofibroblastic gene expression (α-SMA, Col1α1, fibronectin, vimentin, S100A4, desmin, snail, and Msx2) were evaluated by qRT-PCR. Results are mean ± S.E. (error bars) of triplicate experiments. *, p < 0.05; **, p < 0.01; †, p < 0.005.
Cross-talk among PI3K/Akt, JAK/STAT, and Hh Signaling Pathways Modulates Mesenchymal and Epithelial Gene Expression Profiles during HSC Trans-differentiation
Studies in fa/fa HSCs with defective ObRb showed that the long form of the leptin receptor was not necessary for leptin to increase Shh, but it was required for leptin to promote downstream Hh signaling because leptin failed to up-regulate expression of Gli1, Gli2, or sFRP1 in HSCs from fa/fa rats (Fig. 3). Previous work from our group and others indicated that activation of PI3K/Akt signaling stimulates expression of Shh in cells that are capable of producing Hh ligands (6). Therefore, we evaluated the effects of LY294002 and dnAkt on leptin-mediated induction of Shh mRNA and protein. Both LY294002 and dnAkt abrogated leptin-mediated induction of Shh (Figs. 4 and 5 and supplemental Fig. 1). The treatments also reversed all of the other actions of leptin on HSC gene expression (Figs. 4 and 5 and supplemental Fig. 1). Interestingly, inhibiting JAK/STAT signaling with AG490 partially inhibited leptin-mediated induction of Shh and abrogated up-regulation of Gli1, but did not significantly impact leptin-related induction of Gli2 or Ptc (Fig. 6C). These data suggest that the previously demonstrated effects of leptin on mesenchymal gene expression in HSCs (Figs. 2 and 3) involves ObRb-dependent effects on JAK/STAT signaling that modulate Shh induction of Gli1. The role of Gli2 in this process is less clear because JAK/STAT inhibition (Fig. 6C) did not reproduce the effects of mutant ObRb on Gli2 expression (Fig. 3). In any case, the aggregate findings suggest a model in which leptin-related changes in HSC gene expression are dependent upon PI3K/Akt-dependent signals that induce HSC expression of Shh ligand. Shh ligand-initiated signals, in turn, activate Hh signaling and culminate in autocrine induction of a myofibroblastic/mesenchymal phenotype once the JAK/STAT pathway has been activated, but do not require JAK/STAT pathway activation to repress HSC expression of quiescence/epithelial genes.
Leptin-ObRb Signaling Is Important, but Not Absolutely Required, for HSC Trans-differentiation
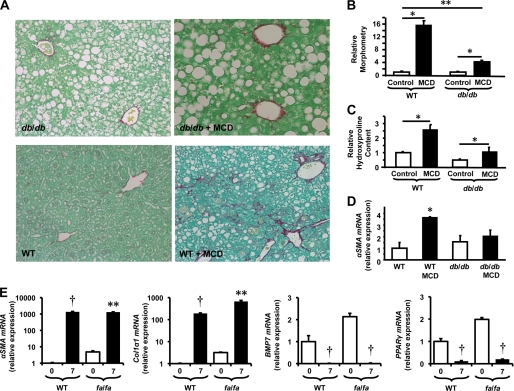
Although leptin is only one of the factors that stimulate HSCs to become myofibroblastic, ob/ob mice that are genetically deficient in leptin and fa/fa rats that have inherited deficiencies in ObRb have been reported to be protected from liver fibrosis induced by either carbon tetrachloride or thioacetamide (26). ObRb is also defective in db/db mice (31), but this strain is capable of developing considerable liver fibrosis during fatty liver damage (32). Therefore, we examined responses to fatty liver damage caused by feeding MCD diets to db/db mice and WT littermate controls to verify the relative significance of leptin signaling in liver fibrogenesis. Compared with MCD diet-fed WT mice, MCD diet-fed db/db mice had inhibited induction of the myofibroblast marker, α-SMA, and developed significantly less liver fibrosis as assessed both by Picrosirius red staining and by hepatic hydroxyproline assays (Fig. 8, A and B). These findings validate earlier evidence of impaired hepatic fibrogenesis in other rodent strains with defective leptin signaling (7, 10, 33) and together suggest that leptin interactions with ObRb play a dominant role in gating myofibroblastic responses that occur in HSCs after Hh pathway activation. To evaluate this concept more directly, culture-related changes in HSC gene expression were compared in parallel cultures of primary HSCs obtained from fa/fa rats and from the parental wild type strain (Fa/Fa). fa/fa HSCs with defective ObRb activated fully during culture, exhibiting normal activation of Hh signaling, induction of myofibroblastic/mesenchymal genes, and repression of epithelial/quiescence genes (Fig. 8E). These findings demonstrate that other factors present in the culture system were able to compensate for defective leptin-ObRb signaling while supporting the concept that leptin plays a major role in promoting HSC trans-differentiation in injured livers because liver fibrosis is reduced significantly in leptin-deficient (33) and leptin-resistant rodents (Fig. 8, A and B), despite the presence of other fibrogenic factors.
FIGURE 8.
Effects of defective ObRb function on fibrogenic repair of liver damage and stellate cell trans-differentiation. Obese diabetic db/db mice and wild type (WT) controls were treated with MCD or control diets for 8 weeks to induce liver injury and fibrosis. A, representative sections of Picrosirius red-stained livers from db/db and WT mice (magnification, × 1,000,510). B–D, morphometric analysis of Picrosirius red-stained sections (B), relative hydroxyproline content (C), and relative αSMA mRNA content from control and MCD diet-treated db/db and WT mice (D). D, primary HSCs from ObRb-defective fa/fa (Zucker-Leprfa) rats and lean (Zucker-Lepr+) rats cultured in serum-containing medium. Changes in gene expression were evaluated by qRT-PCR. For HSCs from each strain, mRNA expression in day 7 cultures were compared with that of freshly isolated cells (day 0). Mean ± S.E. (error bars) data from triplicate experiments are graphed. *, p < 0.05; **, p < 0.01; †, p < 0.005.
DISCUSSION
Evidence that leptin deficiency and leptin receptor defects significantly protect rodents from experimentally induced liver fibrosis (7, 10) stimulated extensive efforts to characterize the effects of leptin on HSCs, the major source of fibrous matrix in injured livers. That work showed that HSCs express several leptin receptor isoforms (ObRa, ObRb, and ObRe) (15) and generally demonstrated a requirement for leptin interaction with ObRb, the long form of its receptor, to activate the downstream kinases, PI3K/Akt and JAK2/STAT3 (8, 12, 13). One or both of the kinase-dependent signaling pathways, in turn, were proven to mediate subsequent induction of profibrogenic genes (8, 12, 13), phagocytic activity (14), and proliferation (11) while inhibiting apoptosis (8). HSCs were also shown to produce leptin, suggesting a possible autocrine role for leptin in HSC fibrogenesis and growth. Evidence that treating HSCs with siRNA to knock down expression of leptin significantly inhibited the myofibroblastic phenotype of cultured HSCs (34) supported this concept. Other reports, however, failed to demonstrate such leptin-dependent effects when HSCs were cultured in serum-enriched medium (27, 35), prompting speculation that leptin was neither necessary nor sufficient for HSC activation and leaving lingering questions about the ultimate significance of leptin-HSC interactions.
The present study provides novel evidence that leptin stimulates HSC production of Shh ligand, activates Hh signaling, and requires Hh pathway activity to induce/maintain the myofibroblastic transition in HSCs. The results demonstrate that leptin-mediated induction of Hh signaling requires interaction of leptin with its receptors and resultant activation of PI3K/Akt signaling. The present work suggests that the dependence on PI3K/Akt signaling occurs, at least in part, because Shh expression in HSCs requires PI3K/Akt activation. In this regard, HSCs are similar to certain gastric epithelial cells (36). In both cell types, various factors that activate PI3K/Akt stimulate Shh expression. For example, we reported previously that PDGF, an acknowledged mitogen for MF-HSCs, stimulates HSC production of Shh mRNA and protein via PI3K/Akt-dependent mechanisms. Those studies also showed that Hh pathway activity was required for PDGF-related mitogenicity (6). Interestingly, other researchers demonstrated that leptin up-regulates HSC expression of PDGF receptors and showed that this plays an important role in leptin-related HSC growth and fibrogenesis (37). Thus, the new data advance understanding about this process by demonstrating that HSC-derived Shh acts in an autocrine fashion to initiate signaling that culminates in the activation of Gli transcription factors with resultant induction of Hh-regulated target genes, including snail.
Snail is a transcription factor that is known to play a major role in EMT, and many situations that promote EMT induce expression of snail mRNAs (38). Our results show that leptin functions as a pro-EMT factor in adult HSCs, as it does in the developing heart and certain types of cancer (22–24). Leptin increases expression of snail mRNA in HSCs, and this response is blocked by inhibitors of PI3K and Akt (which also prevent induction of Shh ligand), as well as cyclopamine (which directly inhibits Smoothened, the signaling competent Hh co-receptor). Therefore, leptin promotes HSC EMT by activating the Hh pathway, which transduces pro-EMT signals that promote acquisition of a mesenchymal, migratory phenotype in many other cell types (39, 40). Our new findings complement and extend earlier evidence which showed that the ability of leptin to promote the mesenchymal transition in HSCs requires functional ObRb receptors and is blocked by JAK/STAT inhibitors (8, 12, 13). Our results show, however, that the latter do not prevent leptin from up-regulating expression of snail mRNA. Further research is required to clarify whether this reflects post-transcriptional actions of leptin that modulate snail function and/or redundant leptin-mediated induction of other pro-EMT factors, such as slug or twist. In any case, the current aggregate data provide novel evidence that Hh-JAK/STAT interactions modulate mesen-chymal gene expression in HSCs. They also reveal that leptin remains capable of down-regulating HSC quiescence/epithelial markers when ObRb signaling is deficient and show that JAK/STAT inhibitors fail to attenuate these antiepithelial actions of leptin. However, as noted for leptin-mediated induction of mesenchymal genes, leptin-mediated repression of the quiescent/epithelial phenotype also depends upon Hh pathway activation. Together, these findings suggest that any leptin-ObR interactions that activate PI3K/Akt (and ultimately increase production of Shh ligand that triggers Hh signaling) are sufficient to repress PPARγ and the epithelial program, whereas full induction of the mesenchymal program requires additional engagement of the JAK/STAT pathway, a unique property of the ObRb-type receptor.
Although functional ObRbs are critical for leptin to activate Hh signaling and support myofibroblastic trans-differentiation of HSCs, other factors that regulate trans-differentiation and growth of MF-HSCs, such as PDGF-BB and TGF-β1, also activate and depend upon Hh signaling for their fibrogenic actions (6, 41). Indeed, as shown previously (26), in the present study primary HSCs from fa/fa rats with defective leptin receptors were fully capable of transitioning to become MFs when cultured in serum-enriched medium that contained other factors that activate Akt. These data are also consistent with earlier reports that demonstrated that leptin was not necessary to activate HSCs that were cultured in serum-enriched medium (35). Moreover, they support the concept that the Hh pathway is a conserved target of several important profibrogenic factors, and its activation triggers various responses (increased proliferative activity, reduced apoptosis, induction of mesenchymal genes, and repression of epithelial characteristics) that promote growth of hepatic MF populations and fibrogenesis (5, 6, 21, 42).
Parallel studies in db/db mice with an inherited ObRb defect were informative in establishing both the relative importance of ObRb for leptin-mediated fibrosis, as well as the significance of factors other than leptin for regulating fibrogenic repair of damaged livers. Unlike the fa/fa mutation which causes an amino acid substitution that reduces the efficiency of leptin binding to all leptin receptor isoforms (43, 44), the db/db mutation specifically targets the cytoplasmic tail of ObRb and abrogates its ability to activate JAK/STAT signaling (45, 46). Hence, leptin remains capable of activating PI3K/AKT signaling in db/db HSCs. As others reported in thioacetamide-treated db/db mice (10), db/db mice that were fed MCD diets to induce chronic fatty liver damage exhibited much less robust up-regulation of myofibroblast markers and significantly less liver fibrosis than WT mice with functional ObRbs, but still accumulated appreciable fibrous scar. Therefore, leptin-ObRb interactions play a major role in gating the growth of liver MF populations in the context of liver injury, as they do in cultured HSCs. However, in intact animals, as in cultured HSCs, these fibroproliferative actions of leptin can be compensated, at least in part, by other factors that activate similar fibrogenic programs. These findings advance fundamental understanding of the mechanisms by which leptin promotes liver fibrosis and have potentially broad implications for other processes that are regulated by leptin, particularly adipogenesis and EMT events that occur during development and carcinogenesis.
Supplementary Material

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
- MF
- myofibroblastic cell
- α-SMA
- α-smooth muscle actin
- EMT
- epithelial-to-mesenchymal transition
- Hh
- Hedgehog
- HSC
- hepatic stellate cell
- MCD
- methionine choline-deficient
- MF-HSC
- myofibroblastic HSC
- PPARγ
- peroxisome proliferator-activated receptor γ
- Ptc
- patched
- Q-HSC
- quiescent HSC
- qRT-PCR
- quantitative RT-PCR
- sFRP1
- soluble frizzled-related peptide 1
- Shh
- Sonic hedgehog.
REFERENCES
- 1.Wynn T. A. (2008) J. Pathol. 214, 199–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman S. L. (2008) Gastroenterology 134, 1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman S. L. (2008) Physiol. Rev. 88, 125–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canbay A., Taimr P., Torok N., Higuchi H., Friedman S., Gores G. J. (2003) Lab. Invest. 83, 655–663 [DOI] [PubMed] [Google Scholar]
- 5.Jung Y., Witek R. P., Syn W. K., Choi S. S., Omenetti A., Premont R., Guy C. D., Diehl A. M. (2010) Gut 59, 655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L., Wang Y., Mao H., Fleig S., Omenetti A., Brown K. D., Sicklick J. K., Li Y. X., Diehl A. M. (2008) J. Hepatol. 48, 98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saxena N. K., Ikeda K., Rockey D. C., Friedman S. L., Anania F. A. (2002) Hepatology 35, 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saxena N. K., Titus M. A., Ding X., Floyd J., Srinivasan S., Sitaraman S. V., Anania F. A. (2004) FASEB J. 18, 1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aleffi S., Petrai I., Bertolani C., Parola M., Colombatto S., Novo E., Vizzutti F., Anania F. A., Milani S., Rombouts K., Laffi G., Pinzani M., Marra F. (2005) Hepatology 42, 1339–1348 [DOI] [PubMed] [Google Scholar]
- 10.Qamar A., Sheikh S. Z., Masud A., Jhandier M. N., Inayat I. B., Hakim W., Mehal W. Z. (2006) Dig. Dis. Sci. 51, 1697–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Si H. F., Li J., Lü X. W., Jin Y. (2007) Exp. Biol. Med. 232, 427–436 [PubMed] [Google Scholar]
- 12.Niu L., Wang X., Li J., Huang Y., Yang Z., Chen F., Ni H., Jin Y., Lu X., Cao Q. (2007) Liver Int. 27, 1265–1272 [DOI] [PubMed] [Google Scholar]
- 13.De Minicis S., Seki E., Oesterreicher C., Schnabl B., Schwabe R. F., Brenner D. A. (2008) Hepatology 48, 2016–2026 [DOI] [PubMed] [Google Scholar]
- 14.Jiang J. X., Mikami K., Shah V. H., Torok N. J. (2008) Hepatology 48, 1497–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang Y., Zheng S., Chen A. (2009) Endocrinology 150, 3011–3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Handy J. A., Saxena N. K., Fu P., Lin S., Mells J. E., Gupta N. A., Anania F. A. (2010) J. Cell. Biochem. 110, 1195–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gregoire F. M. (2001) Exp. Biol. Med. 226, 997–1002 [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y. T., Wang Z. W., Higa M., Newgard C. B., Unger R. H. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 2391–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pospisilik J. A., Schramek D., Schnidar H., Cronin S. J., Nehme N. T., Zhang X., Knauf C., Cani P. D., Aumayr K., Todoric J., Bayer M., Haschemi A., Puviindran V., Tar K., Orthofer M., Neely G. G., Dietzl G., Manoukian A., Funovics M., Prager G., Wagner O., Ferrandon D., Aberger F., Hui C. C., Esterbauer H., Penninger J. M. (2010) Cell 140, 148–160 [DOI] [PubMed] [Google Scholar]
- 20.She H., Xiong S., Hazra S., Tsukamoto H. (2005) J. Biol. Chem. 280, 4959–4967 [DOI] [PubMed] [Google Scholar]
- 21.Choi S. S., Omenetti A., Witek R. P., Moylan C. A., Syn W. K., Jung Y., Yang L., Sudan D. L., Sicklick J. K., Michelotti G. A., Rojkind M., Diehl A. M. (2009) Am. J. Physiol. Gastrointest. Liver Physiol 297, G1093–G106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nath A. K., Brown R. M., Michaud M., Sierra-Honigmann M. R., Snyder M., Madri J. A. (2008) J. Cell Biol. 181, 367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fava G., Alpini G., Rychlicki C., Saccomanno S., DeMorrow S., Trozzi L., Candelaresi C., Venter J., Di Sario A., Marzioni M., Bearzi I., Glaser S., Alvaro D., Marucci L., Francis H., Svegliati-Baroni G., Benedetti A. (2008) Cancer Res. 68, 6752–6761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma D., Saxena N. K., Vertino P. M., Anania F. A. (2006) Endocr. Relat. Cancer 13, 629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Choi S. S., Sicklick J. K., Ma Q., Yang L., Huang J., Qi Y., Chen W., Li Y. X., Goldschmidt-Clermont P. J., Diehl A. M. (2006) Hepatology 44, 1267–1277 [DOI] [PubMed] [Google Scholar]
- 26.Ikejima K., Takei Y., Honda H., Hirose M., Yoshikawa M., Zhang Y. J., Lang T., Fukuda T., Yamashina S., Kitamura T., Sato N. (2002) Gastroenterology 122, 1399–1410 [DOI] [PubMed] [Google Scholar]
- 27.Ikejima K., Lang T., Zhang Y. J., Yamashina S., Honda H., Yoshikawa M., Hirose M., Enomoto N., Kitamura T., Takei Y., Sato N. (2004) Comp. Hepatol. 3, Suppl. 1, S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hegyi K., Fülöp K., Kovács K., Tóth S., Falus A. (2004) Cell Biol. Int. 28, 159–169 [DOI] [PubMed] [Google Scholar]
- 29.Zeisberg M., Neilson E. G. (2009) J. Clin. Invest. 119, 1429–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalluri R., Weinberg R. A. (2009) J. Clin. Invest. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua S., Jr., Liu S. M., Li Q., Yang L., Thassanapaff V. T., Fisher P. (2002) Diabetologia 45, 976–990 [DOI] [PubMed] [Google Scholar]
- 32.Sahai A., Malladi P., Pan X., Paul R., Melin-Aldana H., Green R. M., Whitington P. F. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 287, G1035–G1043 [DOI] [PubMed] [Google Scholar]
- 33.Honda H., Ikejima K., Hirose M., Yoshikawa M., Lang T., Enomoto N., Kitamura T., Takei Y., Sato N. (2002) Hepatology 36, 12–21 [DOI] [PubMed] [Google Scholar]
- 34.Xue X., Lin J., Song Y., Sun X., Zhou H. (2005) J. Huazhong Univ. Sci. Technolog. Med. Sci. 25, 655–657 [DOI] [PubMed] [Google Scholar]
- 35.Wang J., Leclercq I., Brymora J. M., Xu N., Ramezani-Moghadam M., London R. M., Brigstock D., George J. (2009) Gastroenterology 137, 713–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El-Zaatari M., Saqui-Salces M., Waghray M., Todisco A., Merchant J. L. (2009) Curr. Opin. Endocrinol. Diabetes Obes. 16, 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lang T., Ikejima K., Yoshikawa M., Enomoto N., Iijima K., Kitamura T., Takei Y., Sato N. (2004) Biochem. Biophys. Res. Commun. 323, 1091–1095 [DOI] [PubMed] [Google Scholar]
- 38.Li X., Deng W., Nail C. D., Bailey S. K., Kraus M. H., Ruppert J. M., Lobo-Ruppert S. M. (2006) Oncogene 25, 609–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalluri R., Neilson E. G. (2003) J. Clin. Invest. 112, 1776–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamouille S., Derynck R. (2007) J. Cell Biol. 178, 437–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jung Y., Brown K. D., Witek R. P., Omenetti A., Yang L., Vandongen M., Milton R. J., Hines I. N., Rippe R. A., Spahr L., Rubbia-Brandt L., Diehl A. M. (2008) Gastroenterology 134, 1532–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi S. S., Witek R. P., Yang L., Omenetti A., Syn W. K., Moylan C. A., Jung Y., Karaca G. F., Teaberry V. S., Pereira T. A., Wang J., Ren X. R., Diehl A. M. (2010) Hepatology 52, 278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takaya K., Ogawa Y., Isse N., Okazaki T., Satoh N., Masuzaki H., Mori K., Tamura N., Hosoda K., Nakao K. (1996) Biochem. Biophys. Res. Commun. 225, 75–83 [DOI] [PubMed] [Google Scholar]
- 44.Yamashita T., Murakami T., Otani S., Kuwajima M., Shima K. (1998) Biochem. Biophys. Res. Commun. 246, 752–759 [DOI] [PubMed] [Google Scholar]
- 45.Lee G. H., Proenca R., Montez J. M., Carroll K. M., Darvishzadeh J. G., Lee J. I., Friedman J. M. (1996) Nature 379, 632–635 [DOI] [PubMed] [Google Scholar]
- 46.Chen H., Charlat O., Tartaglia L. A., Woolf E. A., Weng X., Ellis S. J., Lakey N. D., Culpepper J., Moore K. J., Breitbart R. E., Duyk G. M., Tepper R. I., Morgenstern J. P. (1996) Cell 84, 491–495 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.