Abstract
The distribution and function of neurons coexpressing the dopamine D1 and D2 receptors in the basal ganglia and mesolimbic system are unknown. We found a subset of medium spiny neurons coexpressing D1 and D2 receptors in varying densities throughout the basal ganglia, with the highest incidence in nucleus accumbens and globus pallidus and the lowest incidence in caudate putamen. These receptors formed D1-D2 receptor heteromers that were localized to cell bodies and presynaptic terminals. In rats, selective activation of D1-D2 heteromers increased grooming behavior and attenuated AMPA receptor GluR1 phosphorylation by calcium/calmodulin kinase IIα in nucleus accumbens, implying a role in reward pathways. D1-D2 heteromer sensitivity and functional activity was up-regulated in rat striatum by chronic amphetamine treatment and in globus pallidus from schizophrenia patients, indicating that the dopamine D1-D2 heteromer may contribute to psychopathologies of drug abuse, schizophrenia, or other disorders involving elevated dopamine transmission.
Keywords: Calcium/Calmodulin-dependent Protein Kinase (CaMK), G Protein-coupled Receptors (GPCR), Neurological Diseases, Neuropeptide, Neuroscience, AMPA Receptor, Amphetamine, Dopamine D1-D2 Receptor Heteromer, Nucleus Accumbens, Schizophrenia
Introduction
Dopaminergic signaling within basal ganglia occurs within at least two populations of GABAergic medium spiny neurons (MSNs),2 containing the neuropeptides dynorphin (DYN) and substance P, or containing enkephalin (ENK) (1, 2). There is controversy as to the colocalization of dopamine D1 and D2 receptors (D1R, D2R) within these two neuronal subtypes. It is generally agreed that whereas D1R is largely segregated to the DYN/substance P-expressing direct striatonigral pathway, D2R is predominantly localized to the ENK-expressing indirect striatopallidal pathway, which is supported by recent studies using fluorophore-tagged promoter elements of D1R and D2R in bacterial artificial chromosome transgenic mice to quantify the cells expressing the receptors within these pathways (3, 4). Although strict segregation of D1R and D2R has been suggested (5, 6) it was noted in these studies that although ∼60% of MSNs in nucleus accumbens (NAc) expressed D1R, ∼50% expressed D2R. This indicated a certain fraction of NAc MSNs expressed both receptors, a finding consistent with numerous studies showing colocalization of D1R and D2R in a proportion of striatal neurons (7–11).
In keeping with these findings, we have shown that D1R and D2R interacted in striatum to form a D1-D2 heteromeric complex that could be immunoprecipitated (9, 12). The D1-D2 heteromer was distinct from its constituent receptors in that it coupled to Gq/11 to activate phospholipase C and generate intracellular calcium release, representing a novel signaling pathway directly linking dopamine action to calcium (9, 13). However, despite growing evidence that neurons coexpressing D1R and D2R embody a significant fraction of MSNs in NAc, their regional distribution, phenotypic characterization, and functional role within basal ganglia are entirely unknown. Given the apparent relationship between dopamine receptor expression and DYN or ENK content, we hypothesized that neurons expressing the D1-D2 heteromer would contain both DYN and ENK, and, accordingly, these neurons would not show preferential localization to either the direct striatonigral or indirect striatopallidal pathways. Given the pervasive role of intracellular calcium signaling in all aspects of neuronal function, we hypothesized that activation of the D1-D2 heteromer would influence neuronal transmission and behavioral output, whereas the activity of the heteromer would respond to alterations in dopamine transmission, such as induced by drugs of abuse or in schizophrenia.
EXPERIMENTAL PROCEDURES
Animals
Seventy-five adult male Sprague-Dawley rats and 20 adult gene-deleted (D1R−/− or D5R−/−) or control mice were used. Animals were housed and tested in compliance with the guidelines described in the Guide to the Care and Use of Experimental Animals (Canadian Council on Animal Care).
Apparatus
The behavioral testing environment was a noncolony room containing 16 clear polycarbonate activity monitors (20 × 20 × 45 cm3). An array of six infrared photocells was attached outside the longer sides of the cages. The photocells were spaced 7.5-cm apart and 2-cm above the floor of the cage.
Drugs
SKF 83959 hydrobromide and SKF 83822 hydrobromide (Tocris Bioscience) were dissolved in physiological saline containing 5% dimethyl sulfoxide and administered subcutaneously. Raclopride (Sigma Aldrich) was dissolved in saline and administered intraperitoneally. For non-drug injections, an equivalent volume of saline was administered. All drug injections were administered at a volume of 1.0 ml/kg.
Immunohistochemistry
Fluorescence immunohistochemistry was performed as described previously (9) from rat brain caudate putamen (CP), NAc, ventral pallidum, entopeduncular nucleus, globus pallidus (GP), substantia nigra (SN), and ventral tegmental area (VTA) as described. Free-floating sections were incubated with primary antibodies (1:200) for 60 h at 4 °C (D1R (rabbit) from Sigma-Aldrich; D2R (rat), ENK (mouse), and synaptophysin (mouse, SYN) from Chemicon; DYN (guinea pig) from Neuromics; post-synaptic density 95 (mouse, PSD95) from Abcam). Specificity of the dopamine receptor antibodies for the D1R and D2R have been tested previously (9) and were validated in D1R or D2R gene-deleted mice. Additional controls were performed in the absence of the primary or secondary antibodies. Antibody dilutions were also used to identify the optimal working concentrations. To minimize background and prevent cross-excitation of the secondary antibody-linked fluorophores (Alexa Fluor, Invitrogen), only three primary antibodies were used on the tissue at any given time. Images were obtained using an Olympus Fluoview 1000 confocal microscope at 63× magnification. Lower magnification images (40×) were obtained for the purpose of cell counting. Cell counting was performed using 300 μm2 regions.
Confocal Microscopy FRET and Data Processing
FRET acceptor bleaching was performed sections from rat brain incubated for 24 h at 4 °C with primary antibodies to D1 and D2 receptors, and the species-specific secondary antibodies were conjugated to Alexa Fluor 488 or Alexa Fluor 350 dyes. Small regions of interest of the acceptor, D1-Alexa Fluor 488, were exposed to high intensity (90%) bleaching using an argon laser at 488 nm. Prebleach and postbleach images were sequentially acquired with an Olympus Fluoview FV 1000 laser scanning confocal microscope with a 60×/1.4 numeric aperture objective. The anti-D2-Alexa Fluor 350 was excited with a krypton laser at 405 nm, whereas the anti-D1-Alexa Fluor 488 was excited with an argon laser at 488 nm. The emissions were collected at appropriate emission bands 430/20 nm and 530/20 nm, respectively. Low laser intensities (<2%) were used to avoid acquisition bleaching. The percentage increase of the donor intensity post-bleaching (Dpost) was compared with the donor intensity pre-bleaching (Dpre) and represents the efficiency of FRET (designated as E), which was calculated according to the equation, E = 100 × ((Dpost − Dpre)/Dpre). Quantitative confocal microscopy FRET was performed as described previously (12).
Behavioral Experiments
Behavioral analysis was conducted daily for 7 days. On each day of testing, animals were administered SKF 83959 or SKF 83822 (both 0.4 mg/kg subcutaneously) and placed immediately inside the activity chamber. Their locomotor and grooming activity was then monitored for 60 min. The measurement of grooming behavior followed a previously described protocol (14). The grooming of the animals was scored for 30 s every 6–7 min, for a total of 4 min (2 min sampled from the first 30 min of testing and 2 min sampled from the last 30 min of testing). Immediately following behavioral testing on the final day (day 7), animals were decapitated, and brains were rapidly removed. NAc and CP tissues were dissected, flash frozen, and immediately stored at −80 °C until use in immunoblotting. To assess the effects of raclopride on grooming responses to SKF 83959, animals were administered injections of SKF 83959 once daily for 3 days, and grooming behavior was monitored for 60 min following drug administration. On the third day of testing, animals were injected with raclopride (0.5 mg/kg intraperitoneal) 10 min prior to SKF 83959.
Immunoblot
Tissues from the NAc and CP were retrieved from animals that underwent the 7-day treatment protocol and suspended in cell lysis buffer, and 15–30 μg of protein were incubated in sample buffer for 3 min at 95 °C. Samples were separated by SDS-PAGE on a 10% gel and electroblotted on a PVDF transfer membrane for 2.5 h. Membranes were blocked and incubated overnight at 4 °C with gentle shaking with primary antibody to calcium/calmodulin kinase IIα (CaMKII) (1:1000, rabbit, Cell Signaling), to phosphorylated CaMKII-Thr286 (1:5000, mouse, Affinity Bioreagents), to GluR1 (1:2000), phospho-GluR1-Ser845 (1:2000), and phospho-GluR1-Ser831 (1:2000, rabbit, Chemicon). Membranes were then washed in Tris-buffered saline with Tween 20 and incubated for 2 h at room temperature with secondary antibody (Bio-Rad). Antibody labeling of proteins were detected with enhanced chemiluminescence (Amersham Biosciences), and signal intensity was quantified using Zeiss AxioVision4 software.
Radioligand Binding
Binding experiments were performed on 50 μg of rat striatal protein or 25–35 μg of the P2 fraction human globus pallidus with 1–2 nm [3H]raclopride in the presence of agonist as described (13).
Human Tissue Samples
Post-mortem human tissues were generously donated by the Canadian Brain Tissue Bank (Toronto Western Hospital) and by Dr. W. W. Tourtellotte, J. S. Riehl, and D. Kamrava of the National Neurological Research Bank. Samples were from patients of similar ages. Three of the four schizophrenia patients had received antipsychotic treatment, whereas the remaining patient was neuroleptic-naive.
Incorporation of [35S]GTPγS into Dopamine Receptors
The incorporation of [35S]GTPγS was measured as described previously (15).
RESULTS
Specificity of D1R and D2R Antibodies and FRET Controls
Specificity of the dopamine receptor antibodies for the D1R and D2R were tested using the five dopamine receptors (D1–D5) expressed individually in HEK293 cells (9). Testing was also performed in striatal tissue of D1R or D2R gene-deleted mice where we showed no reactivity of the D1R or D2R antibody, respectively (Fig. 1A). When the primary D1R and D2R and associated secondary antibodies were combined, no cross-excitation of the secondary fluorophores was evident (Fig. 1B). Additional images depicting antibody specificity are shown in the NAc shell (Fig. 1C). Controls were also performed in the absence of the primary or secondary antibodies to exclude cross-reactivity.
FIGURE 1.
Control data depicting antibody specificity for D1 and D2 receptor coexpression and FRET analysis. A, confocal images revealed no reactivity of the D1-Alexa Fluor 488 antibody in D1R gene-deleted mouse CP. Similarly, the D2-Alexa Fluor 350 antibody did not exhibit reactivity in CP tissue of D2R gene-deleted mice. Note the high level of dendritic staining in this region. B, there was no cross-reactivity of the antibody-linked secondary fluorophores when coincubated with striatal tissue. Emission was detected only when excitation occurred at the appropriate wavelength, with no spectral bleed-through. C, D1R and D2R antibody specificity was further shown by labeling of cells coexpressing both receptors (white arrows) shown together with neurons expressing only the D1R within the same section of NAc shell. D and E, confocal FRET images of a neonatal striatal neuron or in NAc. In both the dendrites (D, inset) and cell bodies, the FRET signal occurred only when the donor (Alexa Fluor 350) and acceptor (Alexa Fluor 488) were present together (white arrows). When either the donor or acceptor was absent, no FRET signal was generated (blue arrows).
An example of quantitative confocal FRET analysis (12) in neuronal cell bodies can be found in supplemental Fig. S1 and was performed using eleven control images (supplemental Tables S1 and S2) in accordance with an algorithm (16) designed to remove both the donor and acceptor spectral bleed-through signals and to correct for variation in fluorophore expression level associated with FRET imaging. As shown in both neonatal striatal neurons and NAc core (Fig. 1, D and E), the FRET signal was absent unless both the donor and acceptor were present.
D1R and D2R Colocalize Selectively in DYN/ENK-positive Neurons
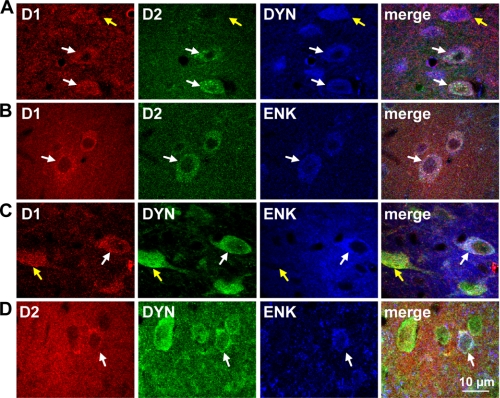
It was observed that the cell bodies of some neurons in NAc coexpressed D1R and D2R (Fig. 2, A and B), and these neurons also coexpressed DYN or ENK (white arrows). In contrast, MSNs that expressed solely the D1R (yellow arrows) or D2R expressed DYN or ENK, respectively. D2R-only expressing neurons, lacking ENK, were also evident and were likely indicative of a cholinergic neuronal population. Coexpression of DYN and ENK was also observed in NAc, and some of these neurons also expressed the D1R or the D2R (core, 62.6 ± 3.9%; shell, 79.0 ± 3.6%; n = 20 sections) (Fig. 2, C and D). However, although DYN/ENK coexpressing neurons could exist in the absence of the D1R or D2R, 100% of D1R and D2R coexpression occurred in the presence of DYN or ENK. Taken together, these findings indicated that D1/D2 coexpression was confined to a unique population of neurons expressing both neuropeptides. DYN/ENK neurons were also identified in CP (Fig. 3), some of which also expressed the D1R and D2R (8.0 ± 1.4%, n = 20 sections). D1/D2-DYN/ENK neurons were also found in varying quantities in ventral pallidum, GP, and entopeduncular nucleus (supplemental Fig. S2).
FIGURE 2.
Colocalization of the D1 and D2 receptor with dynorphin and enkephalin in rat nucleus accumbens. A and B, confocal images revealed D1R and D2R colocalization with DYN or with ENK (white arrows) in NAc. Neurons that expressed only D1R were for positive for DYN and neurons expressing only D2R were positive for ENK (yellow arrows). C and D, DYN+-ENK+-coexpressing neurons also expressed D1R or D2R (white arrows) in NAc. D1R was not expressed in DYN−/ENK+ neurons, nor was D2R expressed in DYN+/ENK− cells (yellow arrows).
FIGURE 3.
Colocalization of the D1 and D2 receptor with dynorphin and enkephalin in rat caudate putamen. A and B, confocal images showed that the D1R and D2R were colocalized with DYN or with ENK in CP (white arrows). C and D, neurons coexpressing DYN and ENK also expressed D1R or D2R (white arrows). DYN and ENK also exhibited coexpression in the absence of D1R (yellow arrows). E and F, neurons that expressed only D1R were positive for DYN, and neurons expressing only D2R were positive for ENK (yellow arrows).
To assess the proportion of D1R that also coexpressed the D2R in any given region, cell counting of D1R- and D2R-positive neurons was performed in 300 μm2 areas of multiple sections. The fraction of D1R-expressing neurons that contained D2R showed substantial inter-regional variation (Fig. 4A, left panel), ranging from ∼7–60%, although the density of D1/D2 coexpressing neurons did not appear to coincide with the number of D1R-expressing cells (Fig. 4A, right panel) nor with their anatomical localization to regions within the striatonigral or striatopallidal pathways. The highest density of D1R-expressing neurons was found in CP (109.8 ± 3.6 neurons/300 μm2, n = 25 sections), although this region also exhibited the lowest proportion of neurons coexpressing the D2R (6.8 ± 0.4%). Conversely, the lowest levels of D1R-expressing neurons were found in GP (11.6 ± 0.6 neurons/300 μm2, n = 25 sections), with the highest proportion of D2R coexpression (59.0 ± 3.1%). D1R expression was also observed in the other basal ganglia nuclei and mesolimbic structures, including both NAc subregions, and we showed that a significant proportion of these NAc neurons coexpressed the D2R (core: 24.6 ± 2.1%, shell: 33.8 ± 4.4%). In the NAc core, shell, and in CP, the number of D2R-expressing cells (core, 24.2 ± 2.6; shell, 29.1 ± 2.0; CP, 105.1 ± 4.4 neurons/300 μm2; n = 16–25 sections) was marginally lower than D1R-expressing cells with the proportion of D2R neurons coexpressing D1R being ∼27%, ∼36, and ∼7%, respectively.
FIGURE 4.
The D1 and D2 receptor colocalized in basal ganglia and formed D1-D2 receptor heteromers. A, quantification of the number of D1R-expressing neurons per 300 μm2 area (left panel) and the proportion of D1R-expressing neurons also expressing the D2R (right panel; n = 16–25 sections/region). B, fluorescence from fluorophore-tagged D1R and D2R in neurons coexpressing them showed equal amounts of the receptor in both NAc subregions but differing expression in CP (n = 43–62 neurons/region). AU, arbitrary units. **, p < 0.01, Student's t test. C, example of a representative neuron showing colocalization and interaction (FRET) of D1R and D2R in an NAc core cell body as assessed from individual microdomains (circles). Scale panel denotes FRET efficiency. D, interaction between D1R and D2R as determined by FRET. The FRET signal indicated equivalent FRET efficiencies in NAc core and shell and lower in CP and indicated the presence of D1-D2 heteromers. E, the proportion of D1/D2-coexpressing neurons that exhibited D1-D2 heteromer formation was 91% in NAc and only 24% in CP. Data in bar graphs expressed as mean ± S.E. NAcC, NAc core; NAcS, NAc shell; VP, ventral pallidum; EPN, entopeduncular nucleus.
D1R and D2R Form D1-D2 Receptor Heteromers in Neuronal Cell Bodies of Nucleus Accumbens and Caudate Putamen
We have shown by coimmunoprecipitation from striatum that D1R and D2R exist within an oligomeric complex (9, 12). To directly demonstrate the presence of the D1-D2 heteromer in situ, we used two confocal FRET methodologies that used specific antibodies tagged to endogenously expressed D1R or D2R. Preliminary experiments were conducted in NAc core using acceptor photobleaching, a method based on the fact that the specific photo-destruction of the acceptor (D1-Alexa Fluor 488) would lead to an increase in the donor (D2-Alexa Fluor 350) fluorescence if both D1R and D2R were in close proximity. When compared, prebleaching and postbleaching images of the same neuronal subregion showed that although the fluorescence of the acceptor (D1-Alexa Fluor 488) decreased to a low 6.6% of the original intensity, which represented a decrease of 93.3 ± 5.0%, the fluorescence of the donor (D2-Alexa Fluor 350) increased by 21.1 ± 3.0%. This indicated that the two receptors were in close proximity, allowing FRET to occur between the fluorophores linked to antibodies recognizing the D1R and D2R, with an apparent high FRET efficiency of 21.1 ± 3.0%.
To further quantify the parameters indicating the interaction between D1R and D2R within D1-D2 heteromers, notably the corrected FRET, the calculated FRET efficiency, and the distance separating both receptors, we used a second quantitative confocal FRET methodology. This methodology was applied to striatal sections in situ (Fig. 4, B–E). In neurons coexpressing both receptors, relative receptor expression levels were evaluated by assessing the fluorophore intensities of labeled D1R and D2R (Fig. 4B). There was equal expression of D1R and D2R in NAc core and shell neuronal cell bodies and reduced expression of D1R relative to D2R in CP cell bodies. In cell bodies of neurons in the NAc core (Fig. 4C), shell, and CP, high magnification revealed that receptor colocalization was not evenly dispersed but was more punctate, and FRET measurements were obtained from multiple microdomains, or regions of interest, within each neuron as indicated by the circles (Fig. 4C). The FRET efficiency and distance provided was an average number based on hundreds of individual regions of interest. Each region of interest was between 0.8–1 μm in diameter with a confocal tomographic section thickness of 2.27 μm. In both NAc subregions, D1R and D2R interactions displayed similarly high FRET efficiency (∼20%) (Fig. 4D), and the receptor antibody-linked fluorophores were calculated to be in close proximity with a relative distance of 5–7 nm (50–70 Å), indicative of D1-D2 heteromer formation. In contrast, FRET efficiency was significantly lower in CP (∼5%) with a relative distance of 8–9 nm (80–90 Å) between the receptors also indicative of D1-D2 heteromer formation but suggestive of weaker receptor interactions, fewer heteromers, and/or the presence of lower order oligomers. Additionally, we determined that, in the cell bodies of neurons coexpressing D1R and D2R in NAc, the majority exhibited heteromer formation (∼91%), whereas in CP, a smaller proportion of D1R- and D2R-coexpressing neurons (∼24%) formed the D1-D2 receptor complex (Fig. 4E).
Dopamine D1-D2 Receptor Heteromers in Neuropil of Nucleus Accumbens and Caudate Putamen Are Presynaptic
To discern the distribution of D1R and D2R in the neuronal projection areas, we assessed synaptic localization of the complex by coimmunostaining with the presynaptic marker SYN (or PSD95) (Fig. 5). In the NAc core and shell (Fig. 5, A and B), individual D1R or D2R was found at either pre- or postsynaptic sites as evidenced by colocalization with either SYN (Fig. 5, A and B, top rows) or PSD95 (Fig. 4, A and B, bottom rows) (yellow circles, D1R; purple color overlay, D2R), although the distribution of D2R expression was more diffuse. In contrast, colocalization of D1R and D2R was consistently visualized selectively with SYN on presynaptic terminals both opposing neuronal cell bodies and within the neuropil (white circles) and did not appear to exhibit anatomical overlap with PSD95 (white circles). These colocalized presynaptic D1R and D2R existed as D1-D2 heteromers as determined by quantitative FRET with FRET efficiencies (core, 0.17 ± 0.01; shell, 0.19 ± 0.01 region of interest/region; n = 45–55) (supplemental Fig. S3) being slightly lower than that observed in the NAc cell bodies (Fig. 4D). Likewise in CP, D1R and D2R coexpression was observed solely with SYN (Fig. 5C), and these receptors also formed D1-D2 heteromers. However, unlike that observed in CP cell bodies, the FRET efficiency within the neuropil of this region was relatively high (0.14 ± 0.01, n = 48) indicating that the synaptic D1-D2 heteromers may play a more predominant physiological role in this region.
FIGURE 5.
The D1 and D2 receptor are colocalized on presynaptic terminals in rat nucleus accumbens and caudate putamen but are extrasynaptic in the ventral tegmental area. A–C, the D1R and D2R colocalized with SYN but not PSD95 (white circles) in the NAc core, shell, and CP. D, colocalization of D1R and D2R in the neuropil of the VTA was scarce and was not observed with SYN or PSD95 (white circles). In all regions, individual D1R colocalized with SYN or PSD95 (yellow circles). Individual D2R also showed considerable, but diffuse, overlap with SYN or PSD95 (purple color, merge).
D1R and D2R colocalization with SYN, but not PSD95, was additionally observed in ventral pallidum and GP (supplemental Fig. S4). However, in VTA (Fig. 5D), SN compacta and SN reticulata (supplemental Fig. S4), unambiguous instances of D1R and D2R colocalization in the neuropil were scarce, and colocalization of both receptors with SYN or PSD95 was not observed. Conversely, individual D1R or D2R were colocalized with either marker in this region, demonstrating both the pre- and postsynaptic localization of these receptors in MSNs. This scarcity of synaptic D1R and D2R colocalization in SN and VTA was not due to a lack of DYN-ENK projections to these regions as projections from striatum to SN/VTA that co-contain substance P and ENK (17) or D1R and D2R (18) have been documented. A more plausible explanation is that dopamine receptor trafficking to the dendrites exhibits region-dependent specificity. That is, although D1R and D2R may be coexpressed in cell bodies of neurons within a given brain region, it cannot be inferred that the two receptors would concurrently coexist in their distal dendrites. This suggests that the functional properties of cell body versus dendritic D1-D2 heteromers may differ, with the physiological properties of synaptic D1-D2 heteromers not being required in the SN or VTA. Nonetheless, despite the absence of synaptic D1-D2 heteromers in some regions, our neuro-anatomical evidence suggests that there exists a significant sub-circuitry of D1-D2/DYN-ENK MSNs that interconnect the basal ganglia nuclei and likely also form intraregional synaptic connections between MSNs, in addition to the classical D1R striatonigral and D2R striatopallidal projections.
D1-D2 Heteromer Activation Induces Grooming and Inhibits AMPA Receptor GluR1 Phosphorylation at Ser831
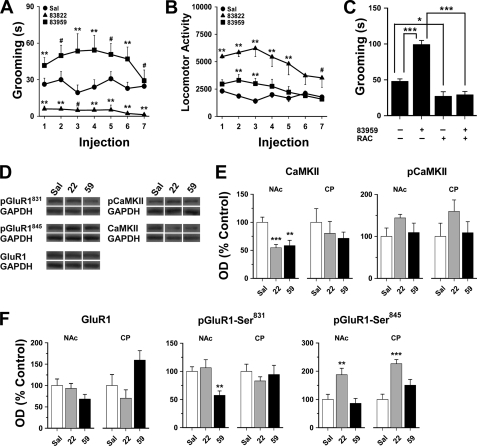
Given the presence of the D1-D2 heteromer in basal ganglia, the effects of selective D1-D2 heteromer activation on locomotion and grooming were examined by treatment with SKF 83959, a drug we have previously shown to selectively activate the D1-D2 heteromer-induced calcium signal without activating D1R or D2R homo-oligomers (13). Activation of the D1-D2 heteromer by SKF 83959 significantly increased the amount of time rats spent grooming, whereas SKF 83822, a drug that activates only D1R homo-oligomers, attenuated grooming (mean across injections, 26.0 ± 2.2 s versus 4.5 ± 0.8 s; drug effect, F(2,21) = 35.0, p < 0.0001, Fig. 6A). These drug-induced changes in grooming remained relatively consistent across the drug injection regimen as evidenced by the lack of an injection effect (F(6,126) = 0.9, p = 0.47) or a drug x injection interaction (F(12,126) = 0.7, p = 0.78). Antagonism of either receptor within the heteromer has been shown to attenuate D1-D2 heteromer-induced signaling (9, 12, 13), and an acute injection of the D2R antagonist raclopride greatly attenuated, but did not eliminate, grooming in both SKF 83959-treated rats and controls (Fig. 6C). As SKF 83959 does not activate D2R homo-oligomers, inhibition of SKF 83959-induced grooming by raclopride indicates that this behavior is in large part induced by the D1-D2 heteromer. However, it is important to note that the D5 receptor may have also contributed to SKF 83959-induced grooming as the drug additionally exhibits high affinity (19) and acts as an agonist at this receptor (12). Furthermore, although grooming induced by SKF 83959 would not involve D2R homo-oligomer activation, the antagonist raclopride does invariably target D2Rs. As raclopride alone significantly reduced grooming, a role for D2R homo-oligomers in mediating this behavior cannot be equivocally excluded. SKF 83959 or SKF 83822 both stimulated locomotion (Fig. 6B), though the magnitude of the responses differed (drug effect, F(2,21) = 12.6, p < 0.0001) as did the time course (supplemental Fig. S5).
FIGURE 6.
Repeated D1-D2 receptor heteromer activation induced grooming in rats and attenuated AMPA GluR1 receptor phosphorylation in nucleus accumbens. A, SKF 83959 significantly increased the amount of time spent grooming compared with saline (Sal) controls, whereas SKF 83822 attenuated it (both drugs administered at 0.4 mg/kg subcutaneously for 7 days). B, SKF 83959 stimulated locomotion to a lesser degree than SKF 83822 (n = 8 rats/group). **, p < 0.05 compared with every other group; *, p < 0.05 compared with controls; #, p < 0.05 compared with the other drug treatment group; repeated measures analysis of variance followed by Student's t test for post hoc comparisons. C, antagonism of the D2 receptor by acute administration of raclopride (RAC) (0.5 mg/kg intraperitoneal) attenuated grooming following three injections of SKF 83959 or saline (n = 6 rats/group). *, p < 0.05; ***, p < 0.001, as indicated. D, representative blots depicting the effects of seven daily injections of SKF 83959 or SKF 83822 on total and phosphorylated CaMKII and GluR1 levels in NAc (n = 8 rats/group). GAPDH was used as a loading control. E, SKF 83959 or SKF 83822 diminished the total expression and had no effect on phosphorylated levels of CaMKII in NAc with no drug effects in CP. F, in NAc, SKF 83959 significantly reduced CaMKII-mediated phosphorylation of GluR1 at Ser831. SKF 83822 elevated protein kinase A-mediated GluR1 phosphorylation at Ser845 in both NAc and CP. **, p < 0.01; ***, p < 0.001. Data were expressed as mean ± S.E. data in bar graphs analyzed by analysis of variance followed by post-hoc comparisons by Student's t test.
Effects of repeated D1-D2 heteromer activation by SKF 83959 on CaMKII levels and activity revealed a significant reduction in total CaMKII expression in NAc, but not CP, and no changes in the phosphorylation state (Fig. 6E). Consistent with this reduction in CaMKII expression, SKF 83959 also inhibited total CaMKII-mediated phosphorylation at Ser831 of GluR1 (20) in NAc, and a trend toward reduced total GluR1 expression in this region was also observed (p < 0.06) (Fig. 6F). Together, these findings may suggest an inhibitory influence of repeated D1-D2 heteromer activation on AMPA GluR1-containing receptor transmission in NAc and are consistent with the relatively higher density of D1-D2 heteromers here compared with CP. As expected, D1R-mediated activation of protein kinase A by SKF 83822 significantly increased striatal phosphorylation at Ser845 of GluR1 (20).
Sensitization of D1-D2 Heteromer by Amphetamine Treatment in Rat Striatum or in Schizophrenia Brain Globus Pallidus
Because phosphorylation of AMPA GluR1 in NAc by CaMKII has been shown to be integral in the pathophysiology underlying drug seeking (21), alterations in striatal heteromeric function in response to (AMPH) was examined. An index of total D2R agonist-detected high affinity state (D2high), obtained using quinpirole competition of [3H]raclopride binding, showed an increase following repeated AMPH treatment, an effect that was maintained 21 days after drug withdrawal (Fig. 7A). The contribution of D2R within the D1-D2 heteromer to this increase in the D2high state was evaluated by SKF 83959 displacement of [3H]raclopride at heteromeric D2R (Fig. 7B). Although raclopride can bind to both D2R and D3R, we have previously shown that SKF 83959 occupied the pertussis toxin-resistant D2R within the D1-D2 heteromer with high affinity as an agonist, but had low affinity for the Gi-coupled D2R homo-oligomers (13). Similarly, SKF 83959 has low affinity for the D3 receptor (19). Thus, it was shown that AMPH significantly increased the proportion of the D1-D2 heteromer in the agonist-detected high affinity state in striatum and increased the affinity of SKF 83959 for this complex by ∼10-fold (Fig. 7B). These effects were maintained following drug withdrawal. In D1R−/− mice, the striatal D1-D2 heteromer high affinity state was absent, as there was no detectable high affinity displacement of [3H]raclopride by SKF 83959. In contrast, the high affinity site was present in D5R−/− mice and a significant increase in the D2high state was observed in response to repeated AMPH treatment (supplemental Fig. S6). Consistent with the observed increase in the agonist-detected high affinity state of the D1-D2 heteromer, AMPH increased the sensitivity of striatal G protein activation by dopamine, although this effect was absent after drug withdrawal (Fig. 7C). The contribution of D1-D2 heteromer activation, evaluated by SKF 83959-induced GTPγS binding, also showed a heightened sensitivity following AMPH administration, indicating the high affinity state of the D1-D2 heteromer was functionally supersensitive in response to repeated increases in dopamine transmission. Furthermore, AMPH treatment induced a significant increase in FRET efficiency between D1R and D2R in neuronal cell bodies in NAc, but not in CP (Fig. 7D), demonstrating that AMPH not only enhanced the activity and sensitivity of heteromers but may additionally increase D1-D2 heteromer density in this region.
FIGURE 7.
Modulation of D1-D2 receptor heteromer activity in rat striatum by repeated amphetamine treatment and withdrawal. A, representative competition binding curves of [3H]raclopride binding by quinpirole in rat striatum are shown after AMPH (2.5 mg/kg, intraperitoneally) for 5 days or withdrawal for 21 days. The affinity of the D2 receptor for quinpirole was increased by ∼10-fold. The proportion of D2 receptors in the D2high in striatum was increased by AMPH compared with control (VEH), an effect that was maintained following drug withdrawal (WD) (bar graphs, n = 5–7 rats/group). B, representative competition binding curves of [3H]raclopride binding by SKF 83959 in rat striatum are shown after AMPH for 5 days or withdrawal for 21 days. The affinity of the D2 receptor within the D1-D2 heteromer for SKF 83959 was increased by ∼10-fold. The proportion of heteromeric D2high in striatum was elevated by AMPH compared with control (VEH), and this increase in D2high was still present after drug withdrawal (bar graphs) (n = 5–7 rats/group). C, percent stimulation (above baseline) of [35S]GTPγS binding to striatal membranes by dopamine or SKF 83959. AMPH enhanced dopamine-induced [35S]GTPγS incorporation and sensitized [35S]GTPγS incorporation by ∼100-fold in rat striatum after 5 days of treatment. This effect was absent after 21 days of withdrawal. D1-D2 heteromer activity was also markedly enhanced as indicated by rat striatal SKF 83959-induced [35S]GTPγS incorporation with a 100-fold increase in sensitivity. D, increased interaction (FRET) between D1 and D2 receptors in NAc, but not CP, following AMPH treatment (n = 15–22 neurons/region). Data are expressed as percent control. *, p < 0.05; **, p < 0.01; Student's t test.
As schizophrenia has been linked to increased dopamine transmission (22–24), it was assessed whether the fraction of high affinity D2R within the D1-D2 heteromer was altered in schizophrenia brains through SKF 83959 displacement of [3H]raclopride in human GP (Fig. 8). There was an ∼10-fold increase in the affinity of D2R within the D1-D2 heteromer for SKF 83959 in schizophrenia brain GP, together with a concomitant increase in the levels of heteromeric D2high compared with that in normal individuals (D2high, schizophrenia, 49.1 ± 4.0%; control, 28.5 ± 3.5%, p < 0.01, n = 4).
FIGURE 8.
Increased agonist affinity of D1-D2 receptor heteromer in schizophrenia globus pallidus. A, representative competition binding curves of [3H]raclopride displaced with SKF 83959 in GP of individuals with schizophrenia showing increased affinity of the drug for the D2 receptor within the D1-D2 heteromer. B, the proportion of striatal D2 receptors in the high affinity state (D2high) within the D1-D2 heteromer was significantly higher in schizophrenia brain GP than in control tissue (schizophrenia (SCHIZ), D2high 49.1 ± 4.0%; control (CONT), D2high 28.5 ± 3.5%; n = 4–5/group). **, p < 0.01; Student's t test.
DISCUSSION
In the present study, we established that D1R and D2R were colocalized in a unique population of neurons that coexpressed DYN and ENK, which were prevalent throughout the mesolimbic and basal ganglia circuits. We further showed that the incidence of these D1/D2-coexpressing neurons showed substantial inter-regional variation, though the level of coexpression did not coincide with their anatomical localization to regions within the striatonigral or striatopallidal pathways. In addition, the proportion of D1/D2 coexpression did not correlate with the total number of D1R-expressing cells present, as CP, the region with the highest D1R expression, also exhibited the lowest proportion of D2R coexpression. Although these findings suggest that the functional contribution of D1/D2-coexpressing neurons may differ between regions, with a nominal contribution in CP, nonetheless, the marked presence of D1/D2-coexpressing neurons in many regions, particularly in NAc and GP, argues for a pervasive physiological role in the basal ganglia and mesolimbic circuits. That we additionally demonstrated a region-specific distribution of D1/D2 receptor coexpression at presynaptic (but not postsynaptic) terminals indicates that receptor coexpression may have a unique physiological function at a local level as well as distal effects through their efferent projections. Together, these findings suggest that these D1-D2/DYN-ENK neurons may play a critical role in regulating thalamic neurotransmission at numerous levels along both the striatonigral and striatopallidal pathways.
One physiological characteristic that was unique to striatal D1R- and D2R-coexpressing neurons was the occurrence of D1-D2 receptor heteromers, both within the cell bodies of these MSNs as well as at their presynaptic terminals. In NAc cell bodies, energy transfer between fluorophore-tagged D1R and D2R was quite high, an indication of stronger receptor-receptor interaction, high densities of heteromers, or higher order oligomers. In contrast, D1R and D2R interactions in CP cell bodies, present only in a small subset of D1/D2-coexpressing neurons, were quite weak. At presynaptic sites, however, D1R and D2R interaction in NAc and CP were more comparable, with FRET efficiency in CP neuropils being much higher than that observed in CP cell bodies. These findings, in association with the demonstration that SN and VTA appear to lack synaptic D1/D2 coexpression (and hence no D1-D2 heteromer) raises the possibility that functional properties of cell body versus dendritic D1-D2 heteromers may differ. We have shown recently that activation of D1-D2 heteromers increases brain-derived neurotrophic factor synthesis in NAc (12); however, it is also possible that the heteromer may be involved, both at a local level as well as through efferent projections, in the modulation of GABAergic synapses as has been shown for MSNs in culture (25–27), perhaps through the regulation of neurotransmitter uptake and/or release. A role for the D1-D2 heteromer in influencing neurotransmission is supported by the present findings, which showed that activation of the heteromer discretely modified behavioral activity and inhibited CaMKII-mediated AMPA GluR1 phosphorylation in NAc, leading to a potential reduction of AMPA receptor neurotransmission in MSNs in this region.
That the D1-D2 heteromer could effectively inhibit AMPA receptor phosphorylation strongly suggested a link between the D1-D2 heteromer and the reward pathways of brain (21, 28–30), a hypothesis supported by increased striatal D1-D2 heteromers in the high affinity state and its enhanced activity following AMPH administration. The increased sensitivity and activity of the D1-D2 heteromer following repeated increases in dopamine transmission indicated that it was functionally up-regulated and this sensitization persisted after agonist withdrawal.
There was a marked increase in the proportion of D1-D2 heteromers in the agonist-detected high affinity state in schizophrenia GP, in keeping with the presynaptic striatal hyperdopaminergia shown to occur in this clinical disorder (22). At the same time, it has also been proposed that abnormal regulation of calcium signaling may constitute the central dysfunction that is responsible for generating the psychopathology of schizophrenia, and many abnormalities related to calcium signaling have been described in schizophrenia brains (31). For the first time, these data bring together the most predominant mechanistic hypotheses of the molecular basis of schizophrenia. Thus, our findings of a D1-D2 heteromer mediating a Gq-linked calcium signal, whose sensitivity is up-regulated in schizophrenia, is the strongest evidence thus far for the unification of the dopamine hypothesis of schizophrenia with the biochemical abnormalities documented with the disorder.
The similarity of the findings between schizophrenia and chronic AMPH administration is particularly compelling, given that AMPH treatment with behavioral sensitization has been used as an animal model for schizophrenia (32), but no common biochemical finding linking the two has previously been documented. We postulate that, under conditions of increased dopamine transmission, the D1-D2 receptor heteromer may act to site-specifically reduce MSN neuronal activity to maintain homeostatic balance between the direct and indirect pathways; however, when dopamine transmission is persistently increased by repeated AMPH administration or in schizophrenia, the heteromer was sensitized and the high affinity state up-regulated. This finding may well provide the first neuropharmacological correlate between increased dopamine neurotransmission and a defined molecular entity, the D1-D2 heteromer, the sensitized state of which may be a marker linking hyperdopaminergia to its functional consequence. Future research will determine whether the D1-D2 receptor heteromer may represent a therapeutic target in psychopathologies involving increased dopamine neurotransmission, including both schizophrenia and drug addiction.
Supplementary Material
Acknowledgment
We thank Dr. H. C. Guan for assistance in performing the GTPγS assays.
This work was supported by a grant from the National Institute on Drug Abuse DA007223 (to S. R. G., B. F. O., and P. S.) and an Ontario Mental Health Foundation postdoctoral fellowship (to M. L. P.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6.
- MSN
- medium spiny neuron
- NAc
- nucleus accumbens
- GP
- globus pallidus
- SN
- substantia nigra
- D1R
- D1 receptor
- VTA
- ventral tegmental area
- CaMKII
- calcium/calmodulin kinase IIα
- DYN
- dynorphin
- ENK
- enkephalin
- SYN
- synaptophysin
- PSD95
- post-synaptic density 95
- AMPH
- amphetamine.
REFERENCES
- 1.Reiner A., Medina L., Haber S. N. (1999) Neuroscience 88, 775–793 [DOI] [PubMed] [Google Scholar]
- 2.Steiner H., Gerfen C. R. (1998) Exp. Brain Res. 123, 60–76 [DOI] [PubMed] [Google Scholar]
- 3.Bertran-Gonzalez J., Bosch C., Maroteaux M., Matamales M., Hervé D., Valjent E., Girault J. A. (2008) J. Neurosci. 28, 5671–5685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee K. W., Kim Y., Kim A. M., Helmin K., Nairn A. C., Greengard P. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 3399–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. N., Monsma F. J., Jr., Sibley D. R. (1990) Science 250, 1429–1432 [DOI] [PubMed] [Google Scholar]
- 6.Gertler T. S., Chan C. S., Surmeier D. J. (2008) J. Neurosci. 28, 10814–10824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aubert I., Ghorayeb I., Normand E., Bloch B. (2000) J. Comp. Neurol. 418, 22–32 [PubMed] [Google Scholar]
- 8.Le Moine C., Bloch B. (1995) J. Comp. Neurol. 355, 418–426 [DOI] [PubMed] [Google Scholar]
- 9.Lee S. P., So C. H., Rashid A. J., Varghese G., Cheng R., Lança A. J., O'Dowd B. F., George S. R. (2004) J. Biol. Chem. 279, 35671–35678 [DOI] [PubMed] [Google Scholar]
- 10.Surmeier D. J., Song W. J., Yan Z. (1996) J. Neurosci. 16, 6579–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aizman O., Brismar H., Uhlén P., Zettergren E., Levey A. I., Forssberg H., Greengard P., Aperia A. (2000) Nat. Neurosci. 3, 226–230 [DOI] [PubMed] [Google Scholar]
- 12.Hasbi A., Fan T., Alijaniaram M., Nguyen T., Perreault M. L., O'Dowd B. F., George S. R. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 21377–21382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashid A. J., So C. H., Kong M. M., Furtak T., El-Ghundi M., Cheng R., O'Dowd B. F., George S. R. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Culver K. E., Rosenfeld J. M., Szechtman H. (2000) Psychopharmacology 151, 202–210 [DOI] [PubMed] [Google Scholar]
- 15.Seeman P., Guan H. C. (2008) Synapse 62, 819–828 [DOI] [PubMed] [Google Scholar]
- 16.Chen Y., Elangovan M., Periasamy A. (2005) in Molecular Imaging: FRET Microscopy and Spectroscopy (Periasamy A., Day R. N. eds) pp. 126–145, Oxford University Press, New York [Google Scholar]
- 17.Wang H. B., Laverghetta A. V., Foehring R., Deng Y. P., Sun Z., Yamamoto K., Lei W. L., Jiao Y., Reiner A. (2006) J. Chem. Neuroanat. 31, 178–199 [DOI] [PubMed] [Google Scholar]
- 18.Deng Y. P., Lei W. L., Reiner A. (2006) J. Chem. Neuroanat. 32, 101–116 [DOI] [PubMed] [Google Scholar]
- 19.Neumeyer J. L., Kula N. S., Bergman J., Baldessarini R. J. (2003) Eur. J. Pharmacol. 474, 137–140 [DOI] [PubMed] [Google Scholar]
- 20.Wang J. Q., Liu X., Zhang G., Parelkar N. K., Arora A., Haines M., Fibuch E. E., Mao L. (2006) J. Neurosci. Res. 84, 1621–1629 [DOI] [PubMed] [Google Scholar]
- 21.Anderson S. M., Famous K. R., Sadri-Vakili G., Kumaresan V., Schmidt H. D., Bass C. E., Terwilliger E. F., Cha J. H., Pierce R. C. (2008) Nat. Neurosci. 11, 344–353 [DOI] [PubMed] [Google Scholar]
- 22.Howes O. D., Kapur S. (2009) Schizophr. Bull. 35, 549–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kegeles L. S., Slifstein M., Frankle W. G., Xu X., Hackett E., Bae S. A., Gonzales R., Kim J. H., Alvarez B., Gil R., Laruelle M., Abi-Dargham A. (2008) Neuropsychopharmacology 33, 3111–3125 [DOI] [PubMed] [Google Scholar]
- 24.Seeman P., Guan H. C. (2009) Synapse 63, 935–939 [DOI] [PubMed] [Google Scholar]
- 25.Geldwert D., Norris J. M., Feldman I. G., Schulman J. J., Joyce M. P., Rayport S. (2006) BMC Neurosci. 7, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guzmán J. N., Hernández A., Galarraga E., Tapia D., Laville A., Vergara R., Aceves J., Bargas J. (2003) J. Neurosci. 23, 8931–8940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuno T., Schmauss C., Rayport S. (2007) BMC Neurosci. 8, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Conrad K. L., Tseng K. Y., Uejima J. L., Reimers J. M., Heng L. J., Shaham Y., Marinelli M., Wolf M. E. (2008) Nature 454, 118–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crombag H. S., Sutton J. M., Takamiya K., Lee H. K., Holland P. C., Gallagher M., Huganir R. L. (2008) Behav. Brain. Res. 191, 178–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ping A., Xi J., Prasad B. M., Wang M. H., Kruzich P. J. (2008) Brain Res. 1215, 173–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lidow M. S. (2003) Brain Res. Brain Res. Rev. 43, 70–84 [DOI] [PubMed] [Google Scholar]
- 32.Featherstone R. E., Kapur S., Fletcher P. J. (2007) Prog. Neuropsychopharmacol Biol. Psychiatry 31, 1556–1571 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.