Abstract
SENP1 (SUMO-specific protease 1) has been shown to be essential for the stability and activity of hypoxia-inducible factor 1 (HIF-1α) under hypoxia conditions. However, it is unknown how SENP1 activation and hypoxia signaling are coordinated in the cellular response to hypoxia. Here, we report the essential role of SENP1 in endothelial cells as a positive regulator of hypoxia-driven VEGF production and angiogenesis. SENP1 expression is increased in endothelial cells following exposure to hypoxia. Silencing of HIF-1α blocks SENP1 expression in cell response to hypoxia. Mutation of the hypoxia response element (HRE) on the Senp1 promoter abolishes its transactivation in response to hypoxia. Moreover, silencing of SENP1 expression decreases VEGF production and abrogates the angiogenic functions of endothelial cell. We also find that the elongated endothelial cells in embryonic brain section and vascular endothelial cells in embryonic renal glomeruli in Senp1−/− mice are markedly reduced than those in wild-type. Thus, these results show that hypoxia implies a positive feedback loop mediated by SENP1. This feedback loop is important in VEGF production, which is essential for angiogenesis in endothelial cells.
Keywords: Epithelial Cell, Gene Regulation, Hypoxia, Signal Transduction, Sumoylation, SENP1, VEGF
Introduction
SUMOylation has emerged as an important mechanism in the regulation of multiple cellular signaling pathways (1–3). SUMOylation is catalyzed by the activating (E1), conjugating (E2), and ligating (E3) enzymes. It also can be reversed by a family of sentrin/SUMO-specific5 proteases (SENPs) (1, 2, 4). There are six human SENPs, each with different subcellular locations and substrate specificities (1, 4). These SENPs can be divided into three subfamilies based on their sequence homology, substrate specificity, and cellular localization. The first subfamily consists of SENP1 and SENP2, which are able to deconjugate either SUMO-1 or SUMO-2/3-modified proteins. The second subfamily members, SENP3 and SENP5, and third subfamily members, SENP6 and SENP7, prefer SUMO-2/3 as substrates (1, 2, 4). The embryonic lethality shown in many SENP knock-out mice indicates that the functions of SENPs are not redundant and have specific substrate specificity (5–7).
SENP1 has been reported to play an important role in regulating cellular response to hypoxia (6). It is well known that the cellular response to hypoxia is mainly mediated by HIF-1α (8–11). HIF-1α is a basic helix-loop-helix transcription factor and can regulate the expression of many hypoxia-responsive genes, which control multiple cellular processes in response to hypoxia (8, 10, 12–14). At normoxia, HIF-1α is degraded but is stabilized and activated under hypoxic conditions (8, 12, 15–24). Several laboratories showed that hypoxia could induce HIF-1α SUMOylation (6, 25–29). These studies suggest that SUMOylation plays an important role in the regulation of HIF-1α under hypoxia condition, although the impact of SUMOylation on HIF-1α activity is controversial (6, 25, 27–28). We have shown that hypoxia-induced stabilization of HIF-1α is dependent on SENP1, which deconjugates hypoxia-induced SUMOylated HIF-1α and prevents the degradation mediated by the ubiquitination of SUMOylated HIF-1α (6). Mutation of the Senp1 gene in mouse results in embryonic anemia due to the defect in the hypoxia-induced HIF-1α signaling (6).
We observed that hypoxia-induced erythropoietin expression in Senp1−/− embryos was much lower than that in Senp1 wild-type, indicating that SENP1 is essential for hypoxia-induced HIF-1α signaling (6). However, it is unknown how SENP1 activation and hypoxia signaling are coordinated in the cellular response to hypoxia. In this study, we report that hypoxia can induce SENP1 transcription and identify a positive feedback loop in which SENP1 regulates hypoxia-induced HIF-1α signaling. We have also demonstrated that SENP1 plays an essential role in HIF-1α-driven VEGF production and angiogenesis in endothelial cells.
EXPERIMENTAL PROCEDURES
siRNA Stable-transfected HUVECs and Culture
We purchased primary human umbilical vein cells (HUVECs) from ATCC. The retrovirus containing SENP1 siRNA or nonspecific siRNA, which were described previously (6), infected HUVECs to generate si-SENP1-transfected HUVEC cells (si-SENP1-HUVECs) or nonspecific siRNA-transfected cells (si-NS-HUVECs) after puromycin selection. The si-SENP1-HUVEC cells were transfected with a SENP1 siRNA-resistant mutant (SENP1m with a mutation in the DNA sequence and with a wild-type in protein sequence) to generate si-SENP1+SENP1m-HUVEC cells. All HUVECs were grown in a complete endothelial cell medium (ScienCell). For starvation, HUVECs were cultured in basal medium without serum and endothelial cell growth supplement. Hypoxic treatment (1% O2) was performed in a specially designed hypoxia incubator (Thermo Electron, Forma Therapeutics) and generated by flushing a mixture of air and N2 plus 5% CO2.
In Vitro Tube Formation of HUVECs
Each well of prechilled 24-well plates was coated with 100 μl/well of Matrigel (BD Biosciences) and incubated at 37 °C for 1 h. 4 × 104 HUVECs in 0.5-ml medium were seeded onto the solidified gel. After incubation for 16 h, the endothelial tubes were assessed with a photomicroscope.
Endothelial Sprouting Beads Assay
We performed HUVEC sprouting beads assay as described in Nehls et al. (30). Briefly, microcarrier beads coated with denatured collagen (Cytodex 3, Sigma) were seeded with HUVECs and embedded in fibrin gels in 96-well plates. For preparation of fibrin gels, bovine fibrinogen (Sigma) was dissolved in endothelial cell medium at a concentration of 2.5 mg/ml. Aprotinin was added at a concentration of 0.05 mg/ml, and the solution was passed through a 0.22-μm filter. Then, the fibrinogen solutions were transferred to 96-well plates together with HUVEC-coated beads at a density of 50 beads/well. Clotting was induced by the addition of thrombin (1.2 units/ml). After clotting was complete, gels were equilibrated with medium at 37 °C with or without VEGF supplement. Photographs were taken after 3 days of incubation.
Senp1 Promoter
The potential Senp1 promoter (−859 to +141) was amplified by PCR from the genomic DNA of NIH3T3 cells, subcloned into pGL3-Basic (Promega). The hypoxia response element (HRE) sites were mutated by using a site-directed mutagenesis kit (Stratagene) with the following primer pairs: HRE1 (−498), 5′-CCTGTGATCAGTCAAGAATGTCAAGCGTGACAAG-3′ (forward) and 5′-CTTGTCACGCTTGACATTCTTGACTGATCACAGG-3′ (reverse); HRE2 (−489), 5′-GTCAAGCGTGTCAAGAATGACAAGATGCACAC-3′ (forward) and 5′-GTGTGCATCTTGTCATTCTTGACACGCTTGAC-3′ (reverse).
ChIP
We performed ChIP by using the Upstate ChIP assay kit (Millipore) and followed the procedures suggested by the manufacturer. Briefly, the cells were first incubated with 1% formaldehyde at room temperature for 10 min and then pelleted and resuspended in 200 μl of lysis buffer (1% SDS, 10 mm EDTA, 50 mm Tris-HCl, pH 8.0). Cell lysates were sonicated with a Sonicator ultrasonic processor (Misonix, Inc.) until the DNA was cleaved into 500–1000 bp in size. The extracts were immunoprecipitated with mouse anti-human HIF-1α monoclonal antibody (BD Transduction Laboratories) and normal preimmune mouse IgG (Santa Cruz Biotechnology). PCR primers were used for amplification of the HRE segment of the SENP1 promoter (5′-GACGTTCCTTAGTGCTGGCGGGTAGGTTTGA-3′ (forward) and 5′-GGCACCAAGTTTGTGGAGCTGAGAACGGG-3′ (reverse)).
Real-time Quantitative PCR
Total RNA was isolated by the TRIzol kit (Invitrogen). RNA was treated with DNase (Promega, Madison, WI). Complementary DNA was synthesized using the cDNA synthesis kit (Takara) according to the manufacturer's instructions. Fluorescence real-time RT-PCR was performed with the double-stranded DNA dye SYBR Green PCR Core Reagents (PE Biosystems, Warrington, UK) using the ABI PRISM 7300 system (PerkinElmer Life Sciences). PCR was done in triplicate, and S.D. representing experimental errors were calculated. All data were analyzed by ABI PRISM SDS software (version 2.0, PerkinElmer Life Sciences). This software, which is coupled to the instrument, allows the determination of the threshold cycle (Ct) that represents the number of the cycle where the fluorescence intensity is significantly above the background fluorescence intensity. Pairs of PCR primers used to amplification of the target genes were as follows: SENP1, 5′-ATCAGGCAGTGAAACGTTGGAC-3′ (forward) and 5′-GCAGGCTTCATTGTTTATCCCA-3′ (reverse); β-actin, 5′-CATCCTCACCCTGAAGTACCC-3′ (forward) and 5′-AGCCTGGATAGCAACGTACATG-3′) (reverse).
Immunohistochemistry
Tissue samples were fixed in buffered formalin and embedded in paraffin. Anti-mouse CD31 antibody (BD Biosciences) was used for immunostaining mouse brain tissue, the VECTASTAIN ABC system (Vector Laboratories) was used for immunohistochemistry.
Statistical Analysis
The values were expressed as mean ± S.D. The paired t test was used for statistical analysis between two groups. The significant level was set at p < 0.05.
RESULTS
Hypoxia Induces SENP1 Expression
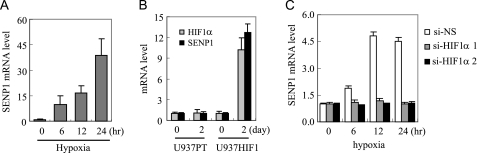
In a previous study, we found that SENP1 was essential for erythropoietin, a HIF-1α target gene, expression under hypoxia conditions (6). We hypothesized that hypoxia might induce SENP1 expression, which would further enhance HIF-1α activity. To test this possibility, we determined the expression of SENP1 in HUVEC cells in response to hypoxia by using real-time PCR. As shown in Fig. 1A, the expression of SENP1 transcripts was significantly increased in HUVEC cells after 6 h of treatment with hypoxia, suggesting that hypoxia is an important factor in the regulation of SENP1 expression. As HIF-1α mediates most of the cellular response to hypoxia, we reasoned that the hypoxia-induced SENP1 expression was mediated through the HIF-1α signaling pathway. U937HIF1 (a Tet-off inducible HIF-1α U937 cell line) and vector control U937PT cell lines (31) were used to test this hypothesis. At day 2 after Tet was withdrawn, the mRNA level of HIF-1α was significantly increased in U937HIF1, but not in U937PT. Similarly, SENP1 expression was also significantly up-regulated in U937HIF1, but not in U937PT cells, after Tet was withdrawn (Fig. 1B), suggesting that HIF-1α could control SENP1 transcription. We further confirmed the role of HIF-1α in hypoxia-induced SENP1 expression by siRNA approach. As shown in Fig. 1C, silencing of HIF-1α expression abolished the hypoxia-induced SENP1 expression in U937 cells. All these data suggest that SENP1 expression is regulated by the hypoxia-induced HIF-1α signaling pathway.
FIGURE 1.
SENP1 expression is increased by hypoxia. A, real-time PCR of SENP1 mRNA in HUVEC cells at indicated time after hypoxia (1% O2) treatment. B, real-time PCR of HIF-1α and SENP1 mRNA in U937PT and U937HIF1 cells (31) before or at day 2 after Tet withdrawal. C, real-time PCR of SENP1 mRNA in nonspecific siRNA (si-NS) or HIF-1α-specific siRNA (si-HIF-1α 1 and si-HIF-1α 2) stable-transfected U937 cells at the indicated time after hypoxia treatment. The data are presented in means ± S.D. of three independent experiments.
Two HREs in the Promoter Are Essential for Hypoxia-induced Senp1 Expression
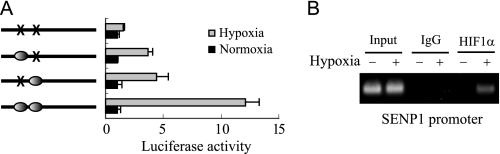
The above results indicate that SENP1 might be a target gene of HIF-1α. To test this, we first searched the hypoxia-responsive element (HRE) in the Senp1 promoter. The HRE core sequence (A/G)CGTG was found in around −498 bp and −489 bp in the mouse Senp1 promoter (32). We generated a series of truncated mouse Senp1 promoter-driven luciferase reporter genes. The results from a luciferase assay showed that Senp1 promoter region around the predicted HRE core sequence was required for hypoxia response (data not shown). Moreover, we confirmed the HRE sites located in Senp1 promoter by using a mutation approach. As shown in Fig. 2A, mutation of any one of the two HRE sites of mouse Senp1 promoter significantly decreased its response to hypoxia, and the two-site mutant lost all hypoxia-induced Senp1 transcription. Additionally, ChIP assay confirmed that HIF-1α was a transcription factor of SENP1 transcription. As shown in Fig. 2B, hypoxia- but not normoxia-treated cells showed increased in HIF-1α binding to the Senp1 promoter. These data strongly suggest that HIF-1α is a transcription factor that regulates SENP1 expression under hypoxia conditions.
FIGURE 2.
Two HRE sites in the Senp1 promoter are essential for response to hypoxia. A, the wild-type and potential HRE site mutant of the Senp1 promoter-driven luciferase activity was measured at 12 h after hypoxia treatment or under normoxia (set as 1). The data are presented in means ± S.D. of three independent experiments. B, ChIP assay of HIF-1α binding on the SENP1 promoter under normoxia or hypoxia conditions in HUVEC cells.
SENP1 Regulates VEGF Production in Endothelial Cells
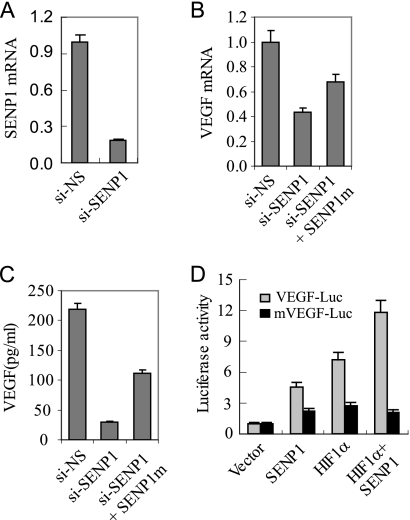
We previously reported that SENP1 was an essential regulator for the stability and activity of HIF-1α during hypoxia (6). Moreover, in the present study, we found that hypoxia-induced HIF-1α signaling could induce SENP1 expression (Figs. 1 and 2). Based on those observations, we proposed that SENP1 might regulate hypoxia-induced HIF-1α signaling pathway through a positive feedback loop. If it is true, SENP1 would be essential for the expression of hypoxia-induced HIF-1α target genes. Indeed, we have previously reported that SENP1 is essential for the expression of erythropoietin in the Senp1−/− embryos. Here, we asked whether the activity of SENP1 was important in the regulation of VEGF expression in endothelial cells, as VEGF plays an essential role in control the angiogenic activity of endothelial cells. To address this, we generated SENP1-silenced HUVECs by SENP1-specific siRNA. The mRNA level of SENP1 in si-SENP1-HUVEC was down by 80% in comparison with that in si-NS-HUVEC (Fig. 3A). We then compared the mRNA expression of VEGF in si-SENP1 with si-NS-HUVEC cells under hypoxia. As shown in Fig. 3B, VEGF message RNA was significantly decreased in si-SENP1-HUVEC cells in comparison with that in si-NS control cells under hypoxia conditions. We also measured the secreted VEGF protein in the cultural medium of these HUVEC cells by ELISA. VEGF protein secreted by si-SENP1-HUVEC was also much less than that of si-NS-HUVEC cells (Fig. 3C), indicating that SENP1 was essential for hypoxia-induced VEGF expression in endothelial cells. To determine the specificity of siRNA against SENP1, we transfected SENP1 siRNA-resistant mutant plasmid into si-SENP1-HUVEC cells and found VEGF expression was partially recovered in the transfected cells (Fig. 3, B and C). To further determine whether VEGF expression regulated by SENP1 in endothelial cells is mediated through HIF-1α, we employed a VEGF promoter-driven luciferase reporter assay. As shown in Fig. 3D, either overexpression of SENP1 or HIF-1α could induce VEGF transcription. Overexpression of both SENP1 and HIF-1α synergistically enhanced the activity of VEGF reporter. Importantly, mutation of HRE site in the VEGF promoter completely abolished the action of SENP1 on the VEGF transcription, indicating that HIF-1α was essential for SENP1 regulation in VEGF expression. These data suggest that SENP1 plays an important role in hypoxia-driven VEGF production in endothelial cells.
FIGURE 3.
SENP1 is essential for VEGF production in endothelial cells. A, real-time PCR of SENP1 mRNA in si-NS- or si-SENP1-HUVEC cells. The data are presented as means ± S.D. of three independent experiments. B, real-time PCR of VEGF mRNA in si-NS-, si-SENP1-, or si-SENP1+SENP1m-HUVEC cells. The data are presented as means ± S.D. of three independent experiments. C, ELISA assay of VEGF production in si-NS-, si-SENP1-, or si-SENP1+SENP1m-HUVEC cells. The data are presented as means ± S.D. of three independent experiments. D, the VEGF promoter (VEGF-Luc) or HRE-mutated VEGF promoter (mVEGF-Luc) reporter gene, HIF-1α, and SENP1 expression plasmids were co-transfected into 293 cells. The cells were treated with hypoxia (1% O2) for 12 h before luciferase assay. The data are presented as means ± S.D. of three independent experiments. Luc, luciferase.
SENP1 Is Essential for Angiogenic Activity of Endothelial Cells
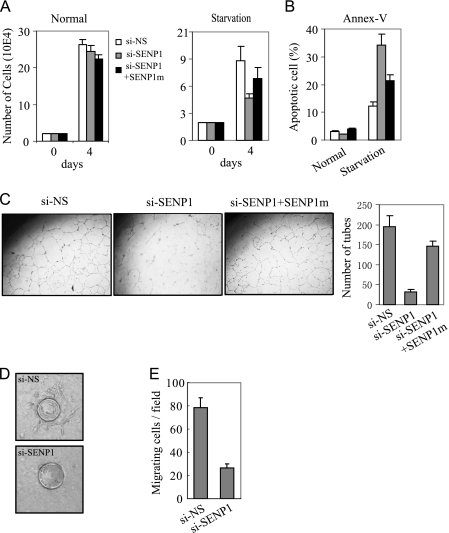
The above observation raised the possibility that SENP1 might have a regulatory role in the angiogenic function of endothelial cells. To address this, we performed a series of analysis on the angiogenic activity of si-NS-HUVEC and si-SENP1-HUVEC cells. Under complete culture conditions, there was no significant difference in cell proliferation between the si-SENP1- and si-NS-HUVEC cells (Fig. 4A). However, silencing SENP1 significantly decreased HUVEC cell survival (Fig. 4A) and increased apoptosis in basal medium without addition of serum and growth factors (starvation) (Fig. 4B). Both activities could be recovered by transfection of SENP1 siRNA-resistant mutant plasmid. Importantly, silencing of SENP1 expression markedly reduced the angiogenic tube formation of HUVEC cells under basal medium culture condition (Fig. 4C). Similarly, SENP1 siRNA-resistant mutant could markedly rescue the defect of the tube formation in si-SENP1-HUVEC cells (Fig. 4C). We also performed an in vitro endothelial sprouting assay to confirm the defect of angiogenic function in si-SENP1-HUVEC cells. The si-NS-HUVEC cells were coated on beads and sprouted for 3 days when cultured in basal medium, and showed multiple vessel structures protruding from each beads. However, silencing SENP1 blocked sprouting of HUVEC cells (Fig. 4D).
FIGURE 4.
SENP1 is essential for angiogenic activity of endothelial cells. A and B, the 2 × 104 si-NS-, si-SENP1-, or si-SENP1+SENP1m-HUVEC cells were cultured in basal medium with (Normal) or without serum and growth factors (Starvation). The cell number was counted at day 4 and is shown in means ± S.D. of three independent experiments (A). The apoptotic cells were detected by annexin V (Annex-V) staining and showed in means ± S.D. of three independent experiments (B). si-NS-, si-SENP1-, or si-SENP1+SENP1m-HUVEC cells were cultured on Matrigel-coated plates in basal medium without serum and analyzed for tubule formation (C, left and middle panels). Right panel, the tubes were accounted and presented as the number of tubes per field in means ± S.D. of three independent experiments. D, si-NS- or si-SENP1-HUVEC cells were mixed with gelatin-coated Cytodex beads and cultured in a collagen matrix with basal medium. The sprouting was shown after 3 days of incubation. E, trans-well migration assay of si-NS- or si-SENP1-HUVEC cells. The data are presented in means ± S.D. of three independent experiments.
Migration is another critical aspect of endothelial cells during angiogenesis. To determine whether SENP1 regulates endothelial cell migration, we measured the migration ability of si-NS- and si-SENP1-HUVEC cells through a Matrigel-coated Boyden chamber. We tested for chemotaxis by placing VEGF in the lower chamber. The motility of si-SENP1-HUVEC was greatly reduced in comparison with si-NS-HUVEC cells (Fig. 4E), suggesting that SENP1 was required for endothelial cell migration. All these data indicate that SENP1 is essential for angiogenic activity of endothelial cells.
Endothelial Cell Defects in Senp1−/− Embryos
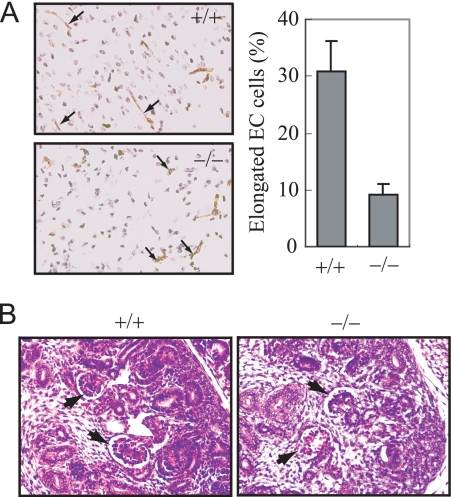
To further determine the role of SENP1 in endothelial cells, we examined morphologic changes of endothelial cells in Senp1−/− embryo sections. As shown in Fig. 5A, there were much fewer elongated endothelial cells in embryonic brain section of Senp1−/− than those in wild-type littermates at day 15, indicating SENP1 played an essential role in embryonic endothelial cell angiogenesis. We also observed much fewer vascular endothelial cells in embryonic renal glomeruli in Senp1−/− than those in wild-type littermates (Fig. 5B), suggesting that SENP1 was essential for the proliferation of endothelial cells during development.
FIGURE 5.
Endothelial cell defects in Senp1−/− embryos. A, the brain sections from Senp1+/+ and Senp1−/− embryos at day 15 were stained with anti-CD31 to label endothelial cells (left panel). Right panel, elongated endothelial cell (EC) cells were counted and presented as the percentage of total CD31-positive cells in means ± S.D. of 9 sections from three pairs of littermates. B, H&E-stained sections of kidney from E15 Senp1+/+ (+/+) and Senp1−/− (−/−) embryo. Arrows indicate glomeruli.
VEGF Mediates SENP1 Action in Angiogenesis of Endothelial Cells
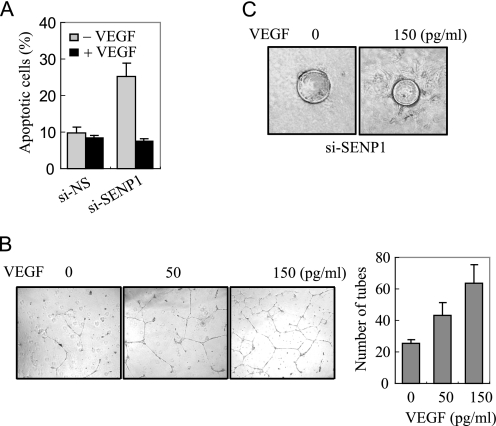
The above observations show that SENP1 is essential for VEGF production and angiogenic activity of endothelial cells. We thought that down-regulation of VEGF in si-SENP1-HUVEC might be responsible for the defect in angiogenic function; therefore, addition of VEGF should rescue the angiogenic ability of si-SENP1-HUVEC. To test this possibility, we carried out a VEGF rescue experiment in si-SENP1-HUVEC cells. As shown in Fig. 6A, apoptosis of si-SENP1-HUVEC induced by serum starvation was inhibited by addition of VEGF in its cultural medium. An in vitro tube formation assay also showed that addition of VEGF in the medium could significantly recover the defect in si-SENP1-HUVEC cells in a dose-dependent manner (Fig. 6B). We also carried out a spouting assay and confirmed that addition of VEGF in medium could promote si-SENP1-HUVEC sprouting from coated beads (Fig. 6C). These data suggest that VEGF production regulated by SENP1 is essential for angiogenic function in endothelial cells.
FIGURE 6.
VEGF mediates SENP1 action in the angiogenesis of endothelial cells. A, apoptosis assay of si-NS- or si-SENP1-HUVEC cells cultured in basal medium with or without VEGF. B, si-SENP1-HUVEC cells were cultured on Matrigel-coated plates in basal medium with 0, 50, or 150 (pg/ml) of VEGF and analyzed for tubule formation. Right panel, the tubes were counted and presented as the number of tubes per field in means ± S.D. of three independent experiments. C, si-NS- or si-SENP1-HUVEC cells were mixed with gelatin-coated Cytodex beads and cultured in a collagen matrix with basal medium with 0 or 150 pg/ml VEGF. The sprouting was shown after 3 days of incubation.
DISCUSSION
Hypoxia-induced cellular processes such as erythropoiesis, angiogenesis, and anaerobic metabolism are mediated by HIF-1α through regulating the expression of hypoxia-responsive genes (8–10, 12–14, 33). In this study, we indentified a novel hypoxia-responsive gene, SENP1, a member of a SUMO-specific protease family. We showed that SENP1 expression could be induced by hypoxia. Moreover, induction of SENP1 expression is mediated by hypoxia-inducible factor HIF-1α. To support this conclusion, we have gathered the following evidence. First, SENP1 expression was increased following the induction of HIF-1α expression in the inducible HIF-1α expression cells. Second, silencing of HIF-1α expression can block hypoxia-induced SENP1 expression. Third, two HRE sites were indentified in the Senp1 promoter, and mutation of the two caused the loss of trans-activation in response to hypoxia. Fourth, we also determined that hypoxia enhanced HIF-1α binding to the SENP1 promoter. Collectively, these results illustrated that HIF-1α is a transcription factor mediating the hypoxia-induced Senp1 expression. Although several studies have reported that SUMOylation can be induced by hypoxia (25–28), to our knowledge, this is the first study to directly show a SUMOylation enzyme as a hypoxia-responsive target gene.
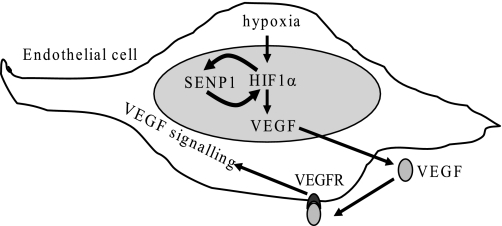
We have previously reported that SENP1 enhances HIF-1α stability and activity under hypoxia conditions (6). This study shows that hypoxia promotes SENP1 expression through HIF-1α transactivation. Therefore, we propose a positive feedback loop model to depict SENP1 action in the hypoxia-induced HIF-1α signaling pathway. In this model, SENP1, as one of the HIF-1α target genes, can be induced by hypoxia. Then induction of SENP1 expression further enhance hypoxia-induced HIF-1α activity through deconjugating SUMOylated HIF-1α, which is induced by hypoxia (Fig. 7). We have shown a similar regulatory mechanism involved by SENP1 in androgen signaling (34, 35). SENP1 was indentified as an androgen-responsive target gene, and SENP1 was shown to promote the activity of androgen receptor and the biological consequence resulted from the activated androgen receptor (34, 35). These results suggest the positive feedback loop model might be a general mechanism in cellular signaling regulated by SENP1.
FIGURE 7.
Model depicting the role of SENP1 in the regulation of hypoxia-induced HIF-1α signaling and the VEGF autocrine loop in endothelial cells by positive feedback.
The biological significance of SENP1 regulation in HIF-1α signaling has been clearly demonstrated in the regulation of VEGF production and angiogenesis in endothelial cells. Silencing of SENP1 expression in endothelial cells significantly decreased VEGF transcripts and protein production. VEGF, a key regulator of angiogenesis, is one of the well known targets of hypoxia-induced HIF-1α signaling (10, 15, 36–39). VEGF is not only essential for normal physiological angiogenesis but also critical in tumor angiogenesis (36, 40–48). As a consequence of down-regulation of VEGF, multiply angiogenic activities of endothelial cells were shown to decrease in SENP1-silenced endothelial cells. In Senp1−/− embryos, we have also observed the deficiency of endothelial cells. The elongated endothelial cells in brain tissue of Senp1−/− were decreased significantly. Vascular endothelial cells were much fewer in Senp1−/− embryonic renal glomeruli than those in wild-type. These results support the concept that SENP1 plays an important role in the regulation of angiogenic activity of endothelial cells.
This work was supported in part by Grant 30772462 from the State Key Laboratory of Oncogenes & Related Genes, National Natural Science Foundation of China (to J. C.), National Basic Research Program of China (973 Program) Grants 2009CB918403 and 2010CB912104 (to J. C.), the Shanghai Pujiang Program (to J. C.), and National Institutes of Health Grant CA 239520 (to E. T. H. Y.).
- SUMO
- small ubiquitin-like modifier
- HIF-1α
- hypoxia-inducible factor 1 α
- HRE
- hypoxia-responsive element
- HUVEC
- human umbilical vein endothelial cell.
REFERENCES
- 1.Yeh E. T. (2009) J. Biol. Chem. 284, 8223–8227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hay R. T. (2005) Mol. Cell 18, 1–12 [DOI] [PubMed] [Google Scholar]
- 3.Geiss-Friedlander R., Melchior F. (2007) Nat. Rev. Mol. Cell Biol. 8, 947–956 [DOI] [PubMed] [Google Scholar]
- 4.Mukhopadhyay D., Dasso M. (2007) Trends Biochem. Sci. 32, 286–295 [DOI] [PubMed] [Google Scholar]
- 5.Kang X., Qi Y., Zuo Y., Wang Q., Zou Y., Schwartz R. J., Cheng J., Yeh E. T. (2010) Mol. Cell 38, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng J., Kang X., Zhang S., Yeh E. T. (2007) Cell 131, 584–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiu S. Y., Asai N., Costantini F., Hsu W. (2008) PLoS. Biol. 6, e310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang G. L., Jiang B. H., Rue E. A., Semenza G. L. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmeliet P., Dor Y., Herbert J. M., Fukumura D., Brusselmans K., Dewerchin M., Neeman M., Bono F., Abramovitch R., Maxwell P., Koch C. J., Ratcliffe P., Moons L., Jain R. K., Collen D., Keshert E. (1998) Nature 394, 485–490 [DOI] [PubMed] [Google Scholar]
- 10.Ryan H. E., Lo J., Johnson R. S. (1998) EMBO J. 17, 3005–3015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kotch L. E., Iyer N. V., Laughner E., Semenza G. L. (1999) Dev. Biol. 209, 254–267 [DOI] [PubMed] [Google Scholar]
- 12.Huang L. E., Gu J., Schau M., Bunn H. F. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 7987–7992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iyer N. V., Kotch L. E., Agani F., Leung S. W., Laughner E., Wenger R. H., Gassmann M., Gearhart J. D., Lawler A. M., Yu A. Y., Semenza G. L. (1998) Genes Dev. 12, 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Curr. Opin. Genet. Dev. 11, 293–299 [DOI] [PubMed] [Google Scholar]
- 15.Pouysségur J., Dayan F., Mazure N. M. (2006) Nature 441, 437–443 [DOI] [PubMed] [Google Scholar]
- 16.Maxwell P. H., Wiesener M. S., Chang G. W., Clifford S. C., Vaux E. C., Cockman M. E., Wykoff C. C., Pugh C. W., Maher E. R., Ratcliffe P. J. (1999) Nature 399, 271–275 [DOI] [PubMed] [Google Scholar]
- 17.Jaakkola P., Mole D. R., Tian Y. M., Wilson M. I., Gielbert J., Gaskell S. J., Kriegsheim Av, Hebestreit H. F., Mukherji M., Schofield C. J., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) Science 292, 468–472 [DOI] [PubMed] [Google Scholar]
- 18.Ohh M., Park C. W., Ivan M., Hoffman M. A., Kim T. Y., Huang L. E., Pavletich N., Chau V., Kaelin W. G. (2000) Nat. Cell Biol. 2, 423–427 [DOI] [PubMed] [Google Scholar]
- 19.Masson N., Willam C., Maxwell P. H., Pugh C. W., Ratcliffe P. J. (2001) EMBO J. 20, 5197–5206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hon W. C., Wilson M. I., Harlos K., Claridge T. D., Schofield C. J., Pugh C. W., Maxwell P. H., Ratcliffe P. J., Stuart D. I., Jones E. Y. (2002) Nature 417, 975–978 [DOI] [PubMed] [Google Scholar]
- 21.Min J. H., Yang H., Ivan M., Gertler F., Kaelin W. G., Jr., Pavletich N. P. (2002) Science 296, 1886–1889 [DOI] [PubMed] [Google Scholar]
- 22.Mack F. A., Rathmell W. K., Arsham A. M., Gnarra J., Keith B., Simon M. C. (2003) Cancer Cell 3, 75–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama K., Frew I. J., Hagensen M., Skals M., Habelhah H., Bhoumik A., Kadoya T., Erdjument-Bromage H., Tempst P., Frappell P. B., Bowtell D. D., Ronai Z. (2004) Cell 117, 941–952 [DOI] [PubMed] [Google Scholar]
- 24.Schofield C. J., Ratcliffe P. J. (2004) Nat. Rev. Mol. Cell Biol. 5, 343–354 [DOI] [PubMed] [Google Scholar]
- 25.Bae S. H., Jeong J. W., Park J. A., Kim S. H., Bae M. K., Choi S. J., Kim K. W. (2004) Biochem. Biophys. Res. Commun. 324, 394–400 [DOI] [PubMed] [Google Scholar]
- 26.Shao R., Zhang F. P., Tian F., Anders Friberg P., Wang X., Sjöland H., Billig H. (2004) FEBS Lett. 569, 293–300 [DOI] [PubMed] [Google Scholar]
- 27.Berta M. A., Mazure N., Hattab M., Pouysségur J., Brahimi-Horn M. C. (2007) Biochem. Biophys. Res. Commun. 360, 646–652 [DOI] [PubMed] [Google Scholar]
- 28.Carbia-Nagashima A., Gerez J., Perez-Castro C., Paez-Pereda M., Silberstein S., Stalla G. K., Holsboer F., Arzt E. (2007) Cell 131, 309–323 [DOI] [PubMed] [Google Scholar]
- 29.Comerford K. M., Leonard M. O., Karhausen J., Carey R., Colgan S. P., Taylor C. T. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nehls V., Drenckhahn D. (1995) Microvasc Res 50, 311–322 [DOI] [PubMed] [Google Scholar]
- 31.Song L. P., Zhang J., Wu S. F., Huang Y., Zhao Q., Cao J. P., Wu Y. L., Wang L. S., Chen G. Q. (2008) Oncogene 27, 519–527 [DOI] [PubMed] [Google Scholar]
- 32.Wenger R. H., Stiehl D. P., Camenisch G. (2005) Sci. STKE 2005, re12. [DOI] [PubMed] [Google Scholar]
- 33.Kallio P. J., Okamoto K., O'Brien S., Carrero P., Makino Y., Tanaka H., Poellinger L. (1998) EMBO J. 17, 6573–6586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bawa-Khalfe T., Cheng J., Wang Z., Yeh E. T. (2007) J. Biol. Chem. 282, 37341–37349 [DOI] [PubMed] [Google Scholar]
- 35.Cheng J., Wang D., Wang Z., Yeh E. T. (2004) Mol. Cell. Biol. 24, 6021–6028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrara N., Gerber H. P., LeCouter J. (2003) Nat. Med. 9, 669–676 [DOI] [PubMed] [Google Scholar]
- 37.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. (2003) J. Cell Biol. 161, 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pugh C. W., Ratcliffe P. J. (2003) Nat. Med. 9, 677–684 [DOI] [PubMed] [Google Scholar]
- 39.Tang N., Wang L., Esko J., Giordano F. J., Huang Y., Gerber H. P., Ferrara N., Johnson R. S. (2004) Cancer Cell 6, 485–495 [DOI] [PubMed] [Google Scholar]
- 40.Fukumura D., Xavier R., Sugiura T., Chen Y., Park E. C., Lu N., Selig M., Nielsen G., Taksir T., Jain R. K., Seed B. (1998) Cell 94, 715–725 [DOI] [PubMed] [Google Scholar]
- 41.Greenberg J. I., Shields D. J., Barillas S. G., Acevedo L. M., Murphy E., Huang J., Scheppke L., Stockmann C., Johnson R. S., Angle N., Cheresh D. A. (2008) Nature 456, 809–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grunewald M., Avraham I., Dor Y., Bachar-Lustig E., Itin A., Jung S., Yung S., Chimenti S., Landsman L., Abramovitch R., Keshet E. (2006) Cell 124, 175–189 [DOI] [PubMed] [Google Scholar]
- 43.Ferrara N., Hillan K. J., Gerber H. P., Novotny W. (2004) Nat. Rev. Drug Discov. 3, 391–400 [DOI] [PubMed] [Google Scholar]
- 44.Gerber H. P., Hillan K. J., Ryan A. M., Kowalski J., Keller G. A., Rangell L., Wright B. D., Radtke F., Aguet M., Ferrara N. (1999) Development 126, 1149–1159 [DOI] [PubMed] [Google Scholar]
- 45.Holash J., Maisonpierre P. C., Compton D., Boland P., Alexander C. R., Zagzag D., Yancopoulos G. D., Wiegand S. J. (1999) Science 284, 1994–1998 [DOI] [PubMed] [Google Scholar]
- 46.Raleigh J. A., Calkins-Adams D. P., Rinker L. H., Ballenger C. A., Weissler M. C., Fowler W. C., Jr., Novotny D. B., Varia M. A. (1998) Cancer Res. 58, 3765–3768 [PubMed] [Google Scholar]
- 47.Ferrara N., Chen H., Davis-Smyth T., Gerber H. P., Nguyen T. N., Peers D., Chisholm V., Hillan K. J., Schwall R. H. (1998) Nat. Med. 4, 336–340 [DOI] [PubMed] [Google Scholar]
- 48.Kitamoto Y., Tokunaga H., Tomita K. (1997) J. Clin. Invest. 99, 2351–2357 [DOI] [PMC free article] [PubMed] [Google Scholar]