Abstract
Many GTPases regulate intracellular transport and signaling in eukaryotes. Guanine nucleotide exchange factors (GEFs) activate GTPases by catalyzing the exchange of their GDP for GTP. Here we present crystallographic and biochemical studies of a GEF reaction with four crystal structures of Arabidopsis thaliana ARA7, a plant homolog of Rab5 GTPase, in complex with its GEF, VPS9a, in the nucleotide-free and GDP-bound forms, as well as a complex with aminophosphonic acid-guanylate ester and ARA7·VPS9a(D185N) with GDP. Upon complex formation with ARA7, VPS9 wedges into the interswitch region of ARA7, inhibiting the coordination of Mg2+ and decreasing the stability of GDP binding. The aspartate finger of VPS9a recognizes GDP β-phosphate directly and pulls the P-loop lysine of ARA7 away from GDP β-phosphate toward switch II to further destabilize GDP for its release during the transition from the GDP-bound to nucleotide-free intermediates in the nucleotide exchange reaction.
Keywords: Enzyme Mechanisms, G Proteins, Membrane Trafficking, Nucleic Acid, X-ray Crystallography, Guanine Nucleotide Exchange Factor, Rab5, Vps9 Domain, Nucleotide-bound Complex, Nucleotide Exchange Mechanism
Introduction
Small GTPases work as a molecular switch, which is turned off by its intrinsic GTPase activity hydrolyzing GTP to GDP. To turn the switch on, the bound GDP should be removed to introduce a GTP. Normally, this exchange reaction is much slower than the rate of intrinsic GTPase activity because nucleotide binds to small GTPase tightly with the Mg2+ ion. GEF5 enhances the nucleotide exchange of its cognate GTPase by destabilizing the Mg2+ ion and GDP binding to the GTPase. Recognition of the GDP-bound GTPase by GEF is a critical but transient step prior to the formation of a nucleotide-free GTPase·GEF binary complex (1, 2). Because the nucleotide-free complex is stable in vitro, most GTPase·GEF complexes have been crystallized and structurally analyzed as the nucleotide-free form in the past, with a few exceptions containing nucleotides. One of them is a structure of eFF1A·eEF1Bα in complex with different di- and triphosphate nucleotides, in which β- and γ-phosphate electron densities of the nucleotides were ambiguous and only GMP could have been modeled (3). Another example is the Arf1·Sec7 complex, in which introducing either an abortive inhibitor or a mutation on the GEF allowed for GDP binding into the Arf1·Sec7 complex (4, 5). The complex structure between ROP4·GDP, a member of the plant Rho family, and its GEF PRONE8 indicated that GDP bound to the ROP4·PRONE8 complex loosely (6). A complex between Cdc42 and the DHR2 GEF domain of DOCK9 GEF has been crystallized in three different nucleotide forms: nucleotide-free, GDP-bound, and GTP/Mg2+-bound. These structures demonstrate that the nucleotide sensor found in the α10 helix of the DHR2 domain is responsible for the exclusion of GDP/Mg2+ and the introduction of GTP/Mg2+ (7). It should be noted that none of the nucleotides were directly recognized by the GEFs in the complex structures mentioned above. These exceptions have encouraged us to investigate the transient nucleotide recognition in the GEF-catalyzed reaction.
Rab small GTPases regulate vesicular transport in eukaryotes (8, 9), including plants (10). ARA7, an Arabidopsis homolog of mammalian Rab5, controls endosomal fusion (11) and is specifically activated by VPS9a, an Arabidopsis GEF for ARA7, which contains a Vps9 domain (12, 13). To understand the detailed mechanisms of the initial reaction pathway of the GEF catalyzed guanine nucleotide exchange, in particular the destabilization mechanisms responsible for the release of GDP for which no direct structural data are available (8), we have used protein crystallography and biochemical assays to identify the small GTPase·GEF intermediates in Rab small GTPases. The ARA7·VPS9a complex was crystallized in three different nucleotide states: the nucleotide-free form, the GDP-bound form, and the GDP analog, GDPNH2-bound form. In addition, we obtained the structure of the ARA7·GDP·VPS9a(D185N) mutant to compare with the GDPNH2-bound form. The four complex structures provide a mechanistic description of the intermediates of guanine nucleotide exchange in Rab5·Vps9, in which GEF interacts with the nucleotide directly and GDP is released by an interplay of the two conserved residues, the aspartate finger of VPS9a and the P-loop lysine of ARA7.
EXPERIMENTAL PROCEDURES
Proteins
Amino acid sequences of ARA7 and VPS9a were obtained from the Arabidopsis thaliana genome data base in the MIPS (Munich Information Center for Protein Sequences, available on the World Wide Web), with accession codes At4g19640.1 and At3g19770.1, respectively. ARA7 (residues 1–179) and VPS9a (residues 1–265) or mutants of VPS9a were produced as glutathione S-transferase (GST)-fused proteins using the pGEX4T1 expression vector (GE Healthcare) in Escherichia coli strains DH5α and Rosetta-gami(DE3)pLysS (Novagen), respectively. The GST domain was cleaved by thrombin. ARA7 was purified in the GDP-bound form in the absence of Mg2+, without the addition of GDP. After mixing ARA7·GDP and VPS9a, nucleotide-free ARA7·VPS9a was separated using an anionic exchanger followed by size exclusion chromatography in a solution containing 10 mm Tris-HCl, pH 7.4, 50 mm NaCl, and 1 mm EDTA. Both selenomethionine-labeled ARA7 and VPS9a were expressed in the E. coli strain B834(DE3)pLysS (Novagen), mixed, and purified as described above.
Pull-down Assay
Mutations in ARA7 and VPS9a were introduced by PCR-based mutagenesis. Protein extracts from yeast cells expressing WT or mutant ARA7 under the control of the constitutive TDH3 promoter were incubated with GST-VPS9a or GST, which were both prebound to the glutathione-Sepharose 4B resin (GE Healthcare). After incubation, the resins were washed three times, separated by SDS-PAGE, and analyzed by immunoblot using the anti-ARA7 antibody.
Yeast Two-hybrid Assay
The cDNAs encoding the wild type, the constitutively active (Q69L), the dominant negative (S24N), and the nucleotide-free (N123I) forms of ARA7 were subcloned into pAD-GAL4–2.1 (Stratagene). The ORF of VPS9a was subcloned into pBD-GAL4-GWRFC by the LR reaction (Invitrogen). The constructs were co-transformed into the AH109 strain (Clontech), and interactions were tested by spotting the same number of co-transformed yeast cells on His plates. At least three independent transformants were tested for each interaction. Empty vectors were co-transformed for the negative controls.
Crystallography
Crystals of nucleotide-free ARA7·VPS9a were obtained from a mixture of 12 mg/ml nucleotide-free ARA7·VPS9a and an equal amount of a reservoir solution containing 3% PEG 4000, 50 mm imidazole malate, pH 5.5, 50 mm NaCl, and 30 mm β-mercaptoethanol equilibrated against the reservoir solution by hanging drop vapor diffusion at 16 °C. Molecular replacement, using the structures of Rab5 and Rabex-5, failed. Therefore, nucleotide-free selenomethionine ARA7·VPS9a was crystallized under the same conditions. As a cryoprotectant, 20% ethylene glycol was added to the reservoir solution. Crystals of ARA7·GDPNH2·VPS9a were obtained from a mixture of 2.5 mg/ml nucleotide-free ARA7·VPS9a with 43 mm guanosine-5′-(β,γ-imido)triphosphate (GppNHp) (Sigma) and an equal quantity of the reservoir solution containing 20% PEG 3350, 200 mm lithium citrate, pH 7.5, and 10 mm DTT, equilibrated against the reservoir solution by hanging drop vapor diffusion at 16 °C. ARA7·GDP·VPS9a and ARA7·GDP·VPS9a(D185N) were crystallized in the same manner as the ARA7·GDPNH2·VPS9a complex except that 1 mm GDP, instead of 43 mm GppNHp, was used. Crystals of nucleotide-bound ARA7·VPS9a were frozen without adding cryoprotectant. Diffraction data were collected at the Stanford Synchrotron Radiation Lightsource, SPring-8, and the Photon Factory. The crystallographic data and refinement statistics are summarized in supplemental Figs. 11 and 12. The diffraction data were processed using HKL2000 (14). The initial phase set of the nucleotide-free ARA7·VPS9a crystal data were calculated using SOLVE/RESOLVE (15), followed by iterative automatic and manual model refinement by REFMAC5 (16) in the CCP4 suite (17) with COOT (18) or O (19), respectively. Molecular replacement by MOLREP (20) in the CCP4 suite gave an initial model of the other crystal data using the coordinates of the nucleotide-free selenomethionine ARA7·VPS9a. The model was refined as described above, with the help of wARP (21). Structural figures were prepared by RASMOL (22), MOLSCRIPT (23), and RASTER3D (24). The stereochemical quality of the protein structures was checked by PROCHECK (25), and no main-chain torsion angels were located in the disallowed regions of the Ramachandran plot. The crystallographic data and refinement statistics are summarized in supplemental Figs. 11 and 12. The electron density maps around the nucleotides and in the surrounding residues are shown in supplemental Fig. 5.
GEF Activity
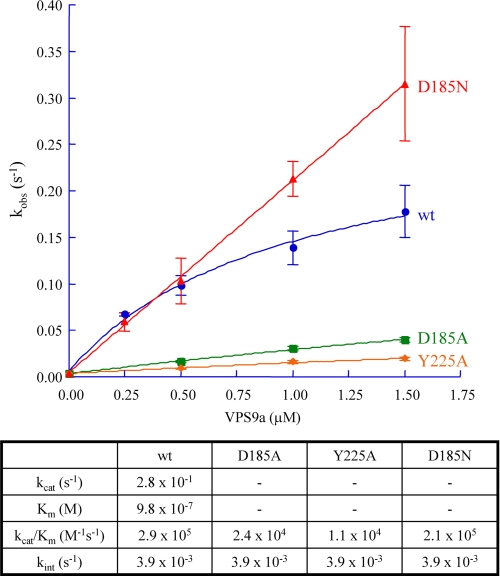
Single turnover guanine nucleotide exchange events on ARA7 GDP were initiated by adding 100 μm GppNHp to a solution containing 0.5 μm ARA7 GDP in 20 mm Tris-HCl, pH 8.0, 150 mm NaCl, and 0.5 mm MgCl2 at 25 °C, with 0.0–1.5 μm VPS9a and its three mutants, D185A, D185N, and Y225A. Nucleotide exchange of Ara7 from GDP to GppNHp was detected as a decrease in the intrinsic tryptophan fluorescence of ARA7, reflecting the state of its nucleotides, following the procedures used for Rab5 and Rab7 (26). Tryptophan fluorescence was excited at 290 nm and detected at 340 nm in a fluorescence spectrophotometer (F-2500, Hitachi). Data were collected for 700 s following the addition of GppNHp. The pseudo-first order rate constants, kobs, were obtained by fitting the decrease in intrinsic tryptophan fluorescence to a sum of single exponentials. Each kobs was measured three times, whereas the intrinsic nucleotide exchange rate of ARA7 without VPS9a, kint, was measured only once and was determined to be 3.9 × 10−3 s−1. The kobs value for the wild-type VPS9a was fit to a pseudo-Michaelis-Menten hyperbolic function to determine the apparent kcat and Km, whereas the values of kobs for the VPS9a mutants were obtained from the slopes corresponding to the GEF efficiencies, kcat/Km.
Surface Plasmon Resonance
For determination of binding affinities between GST-fused VPS9a and ARA7, plasmon resonance experiments were carried out using a BIAcoreTM 2000 system (BIAcore) using a sandwich assay with anti-GST antibody to immobilize GST or GST-fused proteins onto the sensor chip CM5 (BIAcore). All data collection was performed in the HBS-EP buffer, containing 10 mm HEPES (pH 7.4), 150 mm NaCl, 3 mm EDTA, and 0.005% surfactant P20 (pH 7.4) (BIAcore), and the HBS·Mg2+P buffer, containing 10 mm HEPES (pH 7.4), 150 mm NaCl, 1 mm MgCl2, and 0.005% surfactant P20 (pH 7.4) (BIAcore). Values of the dissociation constants (KD) were computed with BIAcoreTM software (BIAcore), and the final KD values were calculated as the average of three measurements.
Preparation of GDPNH2
GppNHp was dissolved in water to a concentration of 400 mm and then boiled for 10 min at 95 °C. The purity of GDPNH2 and GppNHp was checked by anionic exchange chromatography.
RESULTS
Interaction between VPS9a and ARA7
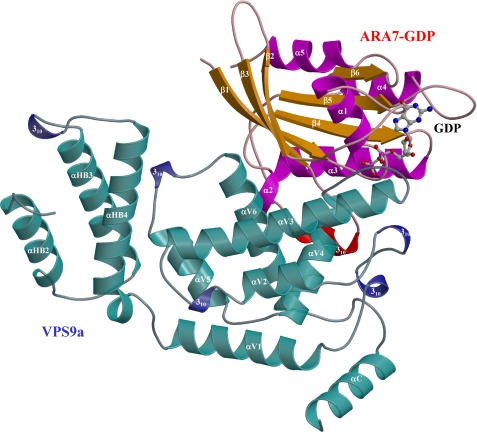
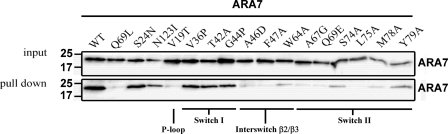
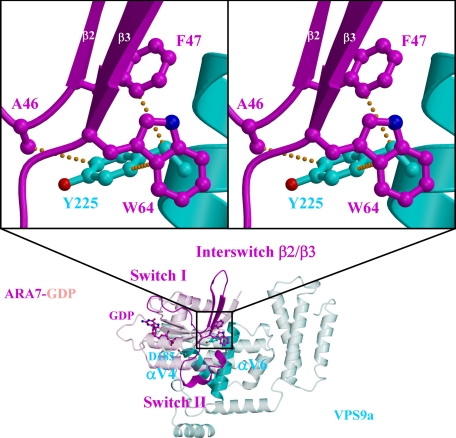
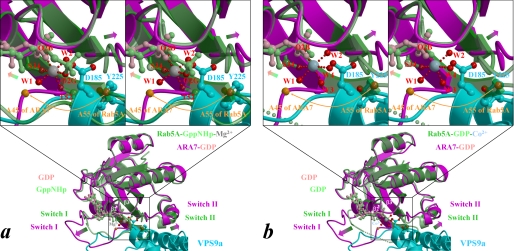
The four crystal structures showed remarkable similarities in the overall configurations of VPS9a and ARA7, suggesting that the complex remains largely intact during GDP release from ARA7 (Fig. 1 and supplemental Fig. 1, a–c). Small GTPases have two regions, switches I and II, which are structurally different in the GTP and GDP-bound forms (27). VPS9a uses two α-helices, αV4 and αV6, and three loops, αV1/αV2, αV3/αV4, and αV6/αC, to recognize the P-loop, interswitch region, and switch II of ARA7 (supplemental Fig. 2, a–d), in contrast to the other GEFs for Rab, MSS4 (28), and Sec2 (29), which recognize their cognate Rab GTPases mainly through the switch I region. Interactions seen in the ARA7·VPS9a crystals were confirmed by the GST pull-down experiments (Fig. 2). ARA7 mutants, V36P, T42A, and G44P, whose residues belong to switch I, interact with VPS9a as tightly as the GDP dominant mutant S24N, which was known to interact with VPS9a strongly both in vivo and in vitro. On the other hand, several other ARA7 mutants whose residues are conserved in the switch II and interswitch regions, A46D, F47D, W64A, A67G, Q69E, S74A, L75A, M78A, and Y79A, could not bind to VPS9a, which suggested that these residues in switch II and the interswitch region were critical in binding to VPS9a, and this mutational analysis of ARA7 was consistent with the observation of the specific interactions in the ARA7·VPS9a crystal structure. In particular, there were two sets of critical interactions holding ARA7 and VPS9a together; one was a hydrophobic interaction of VPS9a Tyr225 with the interswitch region of ARA7, and the other was an electrostatic interaction between the two conserved residues, VPS9a Asp185 and ARA7 Lys23 (supplemental Fig. 12, a–d). VPS9a Tyr225 makes three van der Waals contacts with ARA7 Ala46, Phe47, and Trp64 at the bottom of the interswitch region of ARA7 (Fig. 3). By comparing the structures of ARA7·GDP·VPS9a and Rab5A·GppNHp·Mg2+ (30) or the form B of Rab5A·GDP·Co2+ (31), we found that the hydrophobic contacts partially unzipped the antiparallel β-sheet (β2/β3) of ARA7 in the interswitch region to widen the nucleotide binding pocket, as observed in Arf1·Gea2 (32) (Fig. 4, a and b). Structural changes of ARA7 upon binding to VPS9a were largely confined to the switch regions: switch I in ARA7·GDP·VPS9a opened, and switch II partially rearranged. As a result, GDP also shifted along its long axis, by 0.5 Å, away from the interaction region with VPS9a (Fig. 4, a and b). Another striking effect of the binding of VPS9a to ARA7 was to remove Mg2+ from ARA7, which would lead to the destabilization of GDP binding in the ARA7·VPS9a complex. Indeed, none of the ARA7·VPS9a complex structures contained Mg2+. Moreover, Mg2+ could not be introduced into any of the crystals reported here by co-crystallization. Introducing Mg2+ ion in the nucleotide-free and GDP-bound ARA7·VPS9a structures at the position corresponding to that of Rab5A·GDP structure (the form B of 1TU4) (31) would produce severe steric hindrance between one of the coordinating water molecules, W4, and one of the oxygen atoms of VPS9a Asp185 (Fig. 4).
FIGURE 1.
Structure of ARA7·GDP·VPS9a. ARA7·GDP·VPS9a is colored based on the secondary structures labeled in supplemental Fig. 3, a and b. GDP is drawn as a stick model with small spheres to indicate carbon (gray), oxygen (red), and phosphorus (magenta) atoms.
FIGURE 2.
Interactions between VPS9a and mutants of ARA7. Some mutations in the ARA7 structure severely affected the interactions with VPS9a, as predicted from the three-dimensional structure. The quantity of the ARA7 protein contained in 10% of the yeast lysate used in each assay is shown as an ARA7 input.
FIGURE 3.
Hydrophobic contacts between ARA7 and VPS9a Tyr225. The side chain of Tyr225 on the αV6 of VPS9a (cyan) makes van der Waals contacts (yellow dots) with the side chains of Ala46, Phe47, and Trp64 in the interswitch region of ARA7 (magenta). The side chain of Asp185 on the αV4 of VPS9a is also shown.
FIGURE 4.
Expected structural changes of ARA7 upon binding to VPS9a. a and b, the form B of Rab5A·GppNHp·Mg2+ (Protein Data Bank entry 1R2Q; green) and Rab5A·GDP·Co2+ (chain B of Protein Data Bank entry 1TU4; green), respectively, are superimposed on the structure of ARA7·GDP (magenta) bound to VPS9a (cyan). A subset of the VPS9a structure, including Asp185 and Tyr225, whose side chains are drawn as ball-and-stick models, is displayed for clarity. Nucleotides in Rab5A and ARA7 are drawn as ball-and-stick models and colored in light green and pink, respectively. Structural differences between the switches and the phosphate-binding cassette are indicated by green-to-magenta arrows. The interswitch region of ARA7 is unzipped by hydrophobic contacts with VPS9a (Fig. 3), and the unzipping is represented by the Cα positions of the ARA7 Ala45 and Rab5A Ala55, indicated by orange spheres and an arrow. The side-chain oxygens of VPS9a and the oxygens coordinated to Co2+ (blue-gray sphere), or Mg2+ (gray sphere), of Rab5A are drawn as red spheres with red dots indicating coordination to the metal.
Comparison with Rabex-5·Rab21 Complex Structure
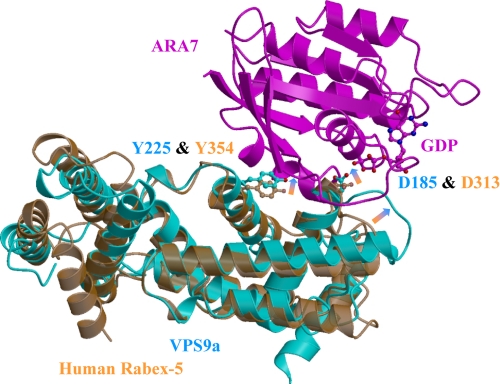
The structures of VPS9a, in each of the four complexes presented in this study, resembled that of the apo-form human Rabex-5 Vps9 domain described previously (33). The root mean square deviation between 184 corresponding Cα positions of VPS9a and Rabex-5 was 1.5 Å, despite the low sequence identity, 27% (supplemental Fig. 3a), between the two domains (Fig. 5). Superposition of the ARA7·VPS9a structure with the apo-form Rabex-5 structure (Fig. 5) revealed an intriguing possibility of side-chain movements of two key residues of VPS9a, Asp185 and Tyr225, corresponding to Asp313 and Tyr354 of Rabex-5. These movements introduce additional interactions with ARA7: three polar interactions with VPS9a Asp185 and three van der Waals contacts with VPS9a Tyr225 (Fig. 5 and supplemental Fig. 2, a–d). The N-terminal helical structure was conserved between the two molecules, despite the low sequence identity of the N-terminal regions, 19% for the first 97 residues (supplemental Fig. 3a), and the lack of αHB1 in VPS9a in the structure.
FIGURE 5.
Superposition of human Rabex-5 to VPS9a. Apo-form of human Rabex-5 (Protein Data Bank entry 1TXU; orange) is superposed to ARA7·GDP·VPS9a (magenta and cyan). The sequence identity between Vps9 of Rabex-5 and VPS9a is low, 27% (supplemental Fig. 3a). The root mean square deviation between 184 corresponding Cα positions of VPS9a and Rabex-5 was 1.5 Å. Possible structural shifts upon the complex formation are indicated by orange-to-cyan arrows.
Aspartate Finger of VPS9a and P-loop Lysine of ARA7
The most unique and direct ARA7 recognition was provided by VPS9a Asp185 near the β-phosphate of GDP and ARA7 Lys23. This corresponds to the invariant aspartate residue in Vps9-containing proteins, which has been described as crucial for GDP release from Rab5 and, accordingly, termed the “aspartate finger” (33), in analogy with the “glutamate finger” corresponding to the invariant glutamate in Sec7 proteins, such as Gea2 (32) or ARNO (34). On the other hand, ARA7 Lys23 is conserved in many GTPases as part of the diphosphate binding loop containing four consensus residues in an eight-residue sequence, GXXXXGK(T/S) (22). This conserved lysine residue contacts and neutralizes the β-phosphate of GDP in the complex (supplemental Fig. 4a). A side-chain oxygen of the VPS9a Asp185 was stabilized by a hydrogen bond to the main-chain NH of ARA7 Gly68 in switch II of ARA7. The carboxyl oxygen atoms of VPS9a Asp185 also formed electrostatic interactions with the side-chain NH3+ of ARA7 Lys23 (supplemental Fig. 4a). Interestingly, a side-chain oxygen of the glutamate finger, Gea2 Glu654, also forms a salt bridge with Arf1 Lys30, corresponding to ARA7 Lys23.
Crystal Structures of Two Complexes, ARA7·GDPNH2·VPS9a and ARA7·GDP·VPS9a(D185N)
Destabilization of GDP was structurally investigated using two crystal structure variants, ARA7·GDPNH2·VPS9a and ARA7·GDP·VPS9a(D185N). The crystal structure of ARA7·GDPNH2·VPS9a was accidentally obtained during crystallization trials of ARA7 in the GTP-bound form, in which an excess of GppNHp was added to the nucleotide-free ARA7·VPS9a solution. The structure of ARA7·GDPNH2·VPS9a did not exhibit significant overall structural changes compared with that of the nucleotide-free ARA7·VPS9a (supplemental Fig. 1, a and b). The electron density corresponding to the GDPNH2 molecule, a product of GppNHp hydrolysis, was clearly observable and excluded the possibility of residual GppNHp (supplemental Figs. 5 and 6b). The shape of the electron density itself did not provide evidence for the preferential binding of GDPNH2 over GDP at the current resolution of the complex structures, because the hydrolysis of GppNHp potentially produces either GDP or GDPNH2. However, an anion exchange chromatography analysis with a fresh GppNHp solution indicated the presence of GDPNH2, most likely as a hydrolysis product. Moreover, we established a protocol for the almost complete conversion of GppNHp to GDPNH2 by simply boiling the former in the absence of Mg2+ (supplemental Fig. 7, a and b). The addition of 5 mm GDPNH2 to the nucleotide-free ARA7·VPS9a complex solution resulted in reproducible crystal growth. In contrast, an addition of 5 mm GDP to the same solution caused precipitation of the proteins. These results suggested that our ARA7·GDPNH2·VPS9a had nothing but GDPNH2.
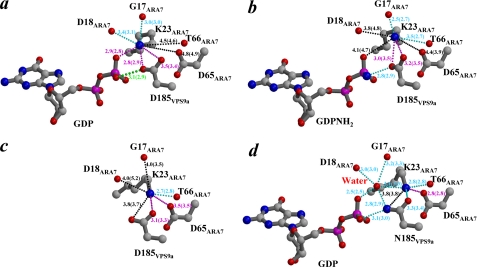
Compared with the complex structure of ARA·GDP·VPS9a, there was an interesting difference. The distance between the side chain of Asp185 in VPS9a and the β-phosphate of GDP in the ARA7· GDPNH2·VPS9a is shorter than that in the ARA·GDP·VPS9a (Fig. 6, a and b). This observation has prompted us to investigate the possibility of an inhibitory effect in the GEF reaction by GDPNH2. However, the GEF activity assay showed no effective inhibition of GEF activity in vitro for the addition of up to 50 μm GDPNH2 (data not shown). To further analyze the effect of inhibition of nucleotide exchange, the D185N mutant of VPS9a was designed based on the GDPNH2-bound ARA7·VPS9a complex structure. We anticipated that the interaction mode and affinity between VPS9a(D185N) and ARA7·GDP would be almost the same as those of ARA7·GDPNH2·VPS9a or would be stronger than those of ARA7·GDPNH2·VPS9a, except that the polarity of the hydrogen bond between the β-phosphate and the VPS9a(D185N) Asn185 would be opposite compared with the case between GDPNH2 and VPS9a Asp185 in ARA7·GDPNH2·VPS9a. Whereas the crystal structure of the ARA7·GDP·VPS9a(D185N) complex confirmed most of these, it also reveals the existence of a new water molecule between GDP β-oxygen and NH3+ of ARA7 Lys23 possibly due to the repulsion between the side-chain NH2 of VPS9a Asn185 and NH3+ of ARA7 Lys23 (Fig. 6d). Interestingly, the binding of GDP in the ARA7·GDP·VPS9a(D185N) mutant is weaker than in the wild-type ARA7·GDP·VPS9a, as judged from their higher temperature factors (supplemental Fig. 8, a and d).
FIGURE 6.
Polar interactions around ARA7 Lys23. a–d, oxygens (red) or a nitrogen (blue), surrounding the side-chain NH3+ of ARA7 Lys23 in ARA7·GDP·VPS9a, ARA7·GDPNH2·VPS9a, nucleotide-free ARA7·VPS9a, and ARA7·GDP·VPS9a(D185N), respectively, are shown. The side-chain NH3+ of ARA7 Lys23 is surrounded by a β-position oxygen of GDP, side-chain COO− (ARA7 Asp65 and VPS9a Asp185), and main-chain carbonyl oxygens (Gly17, Asp18, and Thr66 of ARA7). Possible hydrogen bonds and electrostatic interactions are shown with cyan dots and magenta dots, respectively. Distances exceeding 3.5 Å are shown as black dots. In ARA7·GDP·VPS9a(D185N), a water molecule is located between the side-chain NH3+ of ARA7 Lys23 and the β-position oxygen of GDP. A possible hydrogen bond between the side-chain COO− of VPS9a Asp185 and the β-position oxygen of GDP is highlighted by green dots.
Following these crystallographic observations, we then investigated GEF activities and binding affinities of the VPS9a(D185N) mutant. Surprisingly, this mutant exhibited a higher GEF activity in the presence of Mg2+ (Fig. 7). We therefore prepared additional mutants, D185A and Y225A, of VPS9a and measured their GEF activities. As expected, the D185A and Y225A mutants exhibited much lower GEF activities, reduced by 12 and 25 times, respectively, compared with the wild-type VPS9a. In contrast, the GEF efficiency of VPS9a(D185N) was only 1.3 times lower than that of the wild-type VPS9a with an apparently higher maximum rate constant (Fig. 7).
FIGURE 7.
GEF activity of VPS9a. The pseudo-first order rate constants are plotted against the concentrations of the wild-type and three mutants of VPS9a. For the wild-type VPS9a, the maximum rate constant and the apparent dissociation constants are estimated. Values of the rate constants are to be compared with the intrinsic nucleotide exchange rate of ARA7 in the absence of VPS9a, kint, 3.9 × 10−3 (1/s). Values of the dissociation constants are summarized in the table below the plots.
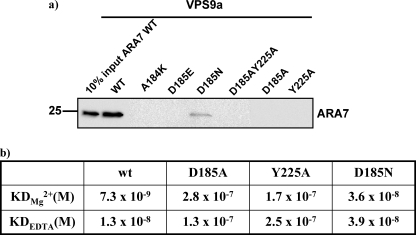
The mutational analysis was further extended with additional VPS9a mutants, A184K, D185E, and D185A/Y225A. A pull-down assay of these mutants showed that ARA7 could not interact with any other VPS9a mutants except for the VPS9a(D185N). Only this mutant showed rather weak binding to ARA7 (Fig. 8a). Furthermore, a surface plasmon resonance experiment was performed to measure the dissociation constants (KD) between VPS9a mutants and ARA7 using BIAcoreTM 2000 (BIAcore). The D185N mutant of VPS9a was shown to have much higher dissociation constants (KD) than that of wild-type VPS9a, increased by factors of 4.9 and 3 with Mg2+ and EDTA, respectively (Fig. 8b), although these KD values of the D185N mutant were smaller than those of other VPS9a mutants, D185A and Y225A. This result is consistent with the pull-down assay. These results corroborated well with the notion that the D185N mutant of VPS9a had a high GEF activity despite the weaker binding for ARA7 and that this high GEF activity of D185N mutant might have come from instability of GDP, which is observed in the crystal structure of ARA7·GDP·VPS9a(D185N).
FIGURE 8.
Interactions between ARA7 and mutants of VPS9a. a, mutations in the conserved Asp185 and Tyr225 of VPS9a strongly affect the interaction between ARA7 and VPS9a. Note that the D185N mutation of VPS9a does not completely abolish the interaction, which is consistent with the results shown in Fig. 4. The quantity of ARA7 protein contained in 10% of the yeast lysate used in each assay is shown as an ARA7 input. b, dissociation constants (KD) between ARA7 and VPS9a with 1 mm Mg2+ or 1 mm EDTA buffer.
DISCUSSION
Complex Formation
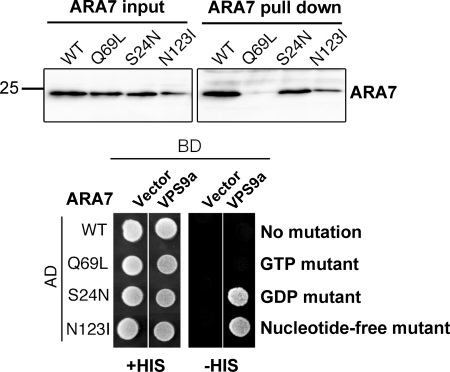
In the pull-down and yeast two-hybrid assays, VPS9a interacted with either the dominant negative (S24N) or the nucleotide-free (N123I) ARA7 but not with the constitutively active GTP-bound form (Q69L) (Fig. 9). The N-terminal catalytic domain (residues 1–265) showed essentially the same results as the full-length VPS9a (data not shown). Interestingly, there was a significant difference between the results of the yeast two-hybrid and pull-down experiments. VPS9a interacted with the wild-type ARA7 in the pull-down experiment but not in the yeast cells. Anion exchange column chromatography demonstrated that the purified wild-type ARA7 used in the pull-down experiment was completely in the GDP form (data not shown), which suggested that GTP in the wild-type ARA7 had been hydrolyzed to GDP by the intrinsic GTPase activity during purification. On the other hand, the wild-type ARA7 in the yeast two-hybrid experiment was expected to be present predominantly in the GTP form because the concentration of GTP is higher than that of GDP in the cell cytosol. Similar behaviors have been observed in other plant orthologs of Rab5, RHA1, and ARA6 (13). Thus, we conclude that the difference between the pull-down and yeast two-hybrid experiments for the interaction of VPS9a with the wild-type ARA7 reflects the two distinct nucleotide states.
FIGURE 9.
Nucleotide-dependent interactions between ARA7 and VPS9a. Pull-down and yeast two-hybrid assays indicated that VPS9a preferred the dominant negative (S24N) or the nucleotide-free (N123I) forms of ARA7 to the constitutively active (Q69L) form. The quantity of the ARA7 protein contained in 10% of the yeast lysate used in each pull-down assay is shown (ARA7 input). AD and BD were fused to the transcription activating domain and DNA binding domain, respectively, in the yeast two-hybrid assay.
Protonation of GDP β-Phosphate
We present here four complex structures of plant Rab5 and its GEF, which indicate several unexpected features. The first is the observation of an unusually close contact, 2.9–3.1 Å, between a β-phosphate oxygen of GDP and a side-chain oxygen of VPS9a Asp185 (Fig. 6a). This distance is too short for two negatively charged oxygen atoms and suggests that it is likely that one of these two oxygen atoms is protonated to form a hydrogen bond. Protonation of nucleotides can be modulated by the surrounding electronic environment. The pKa for GDP is 6.7 (35), and GDP alone in solution is largely deprotonated at pH 7.5, the conditions under which the ARA7·GDP·VPS9a complexes were crystallized. However, the pKa for GDP in ARA7·VPS9a should be higher due to the interaction with the side-chain COO− of VPS9a Asp185. An example of such a pKa increase has been reported, for example, for the G12D mutant of Ras with an increase of 1.4 pH units in the γ-phosphate pKa in Ras(G12D)·GppNHp·Mg2+ relative to Ras(G12)·GppNHp·Mg2+, resulting in the protonation of GppNHp (36). Such a pKa increase caused by the negative charges is also consistent with the pKa increase of the nucleotides caused by the removal of Mg2+, as suggested by quantum mechanical calculations (35, 36), or with the arginine finger of RasGAP, predicted by computer simulations (37). These observations support the notion that a similar pKa increase of GDP would be caused by the presence of VPS9a Asp185 and the absence of Mg2+ in the vicinity and result in the protonation of the GDP β-phosphate in ARA7·GDP·VPS9a, leading to the short distance between the β-phosphate oxygen of GDP and the side-chain oxygen of VPS9a Asp185.
GDP Analog GDPNH2 and VPS9a(D185N) Mutation
Second, a comparison of the two ternary complex structures, ARA7·GDPNH2·VPS9a and ARA7·GDP·VPS9a(D185N) gives a further insight into the stability of GDP in the complexes. We have shown that the distance between the β-phosphate and VPS9a Asp185 in ARA7·GDPNH2·VPS9a was shorter than that in ARA7·GDP·VPS9a (Fig. 6, a and b) and that VPS9a(D185N) has higher GEF activity due to the instability of GDP (Fig. 6d). We at first anticipated that VPS9a(D185N) mutation would have the same effect as the GDP analog, GDPNH2, which can provide the NH2 base to the β-phosphate oxygen and block the nucleotide exchange completely with stronger affinity between β-phosphate of GDP and the substituted Asn185 residue of VPS9a. However, the structure of the D185N mutant of VPS9a turned out to be counter to our expectations; as mentioned above, the introduction of the new water molecule between GDP β-oxygen and NH3+ of ARA7 Lys23 destabilized GDP, and accordingly, this D185N mutant obtained a higher GEF activity. Based on these observations, design and search for new chemical compounds mimicking the nucleotide analogues, which would block the GEF reaction efficiently, are being pursued.
Initial Pathway of the GEF Reaction
Third, these four complex structures shed light on the initial GEF exchange reaction pathway. The Rab5 GEF destabilizes GDP by modifying the local environment of the β-phosphate with one of the carboxyl oxygen atoms of the aspartate finger, VPS9a Asp185. This process is coupled with the movement of the side chain of ARA7 Lys23. The electrostatic interaction between GDP β-oxygen and NH3+ of ARA7 Lys23 is observed in the ARA7·GDP·VPS9a structure (Fig. 6a). On the other hand, it is absent in the other two complex structures; the distance is much longer in the ARA7·GDPNH2·VPS9a structure, and the water molecule is inserted in the ARA7·GDP·VPS9a(D185N) structure (Fig. 6, b and d). Taken together, VPS9a appears to use three steps to increase the pKa of the β-phosphate in GDP-bound ARA7, leading to the putative protonation of the β-phosphate of GDP: 1) removing Mg2+ from GDP, 2) placing the side-chain COO− of VPS9a aspartate finger in the vicinity of the β-phosphate of GDP, and 3) destabilizing the electrostatic interaction between the side-chain NH3+ of ARA7 Lys23 and GDP by moving the P-loop lysine from GDP toward switch II Glu65. As a net result, deprotonation of the GDP β-phosphate oxygen is induced, which would destabilize the GDP and enhance its release due to repulsion between the two oxygen anions of GDP and VPS9a Asp185 (supplemental Fig. 9, a–f). In the following, we will discuss these three steps with reference to the other small GTPase·GEF complex structures.
Removal of Mg2+ Ion
As one of the first steps of the GEF reaction, the Mg2+ ion is removed from the nucleotide binding site of small GTPase·GEF complexes. The exclusion of the Mg2+ ion leads to a decrease in the nucleotide affinity of the nucleotide binding site. This is accomplished in three distinct ways by various conserved residues: switch II alanine of Ras in Ras·SOS (38) and Rac in Rac·Tiam1 (39), valine of nucleotide sensor in DOCK9 (7), glutamate finger of Sec7 (32, 34), and aspartate finger of VPS9a (this work)(33). Interestingly, Ala27 of Arf·Gea2 (32), which corresponds to the switch II alanine in Ras and Rac, is flipped outward, and instead the glutamate finger of Sec7 or Gea2 is supplied into the Mg2+ binding site and pushes the Mg2+ ion out. DOCK9 uses the side chain of the valine residue of the nucleotide sensor to occlude the nucleotide coordinated Mg2+ directly from the Cdc42·GDP·Mg2+, leading to destabilization of GDP. In contrast, the introduction of GTP·Mg2+ into the nucleotide-free Cdc42·DOCK9 complex pushes out the valine side chain, resulting in the release of activated Cdc42 from DOCK9. In this structure, the side chain of Cdc42 switch II Ala59 does not interfere with the Mg2+ binding, unlike that of Ras Ala59 and Rop Ala62 (6).
GDP Destabilization by Aspartate Finger
The exclusion of Mg2+ alone does not seem to be enough to induce the dissociation of GDP from ARA7 small GTPase, although it has been reported that the loss of Mg2+ weakens nucleotide affinity by 500–1000 times (40). The trans-type GEFs, such as Vps9 and Sec7 domains with the acidic finger, do not have extra motifs, such as the PRONE WW motif stabilizing the nucleotide-free form or the nucleotide sensor of DOCK proteins responsible for the removal of GDP and Mg2+. Instead, they accomplish the task of removing Mg2+ and GDP by inserting the acidic finger into the nucleotide binding site first to push Mg2+ out of the small GTPase·GEF complex and then to make contacts with both GDP β-phosphate and the P-loop lysine. Through the latter two contacts, VPS9a influences directly the P-loop lysine to move away from GDP toward the acidic residue of switch II (see below). After the GDP release, the acidic finger keeps the contact with the P-loop lysine to stabilize the nucleotide-free complex. This direct GDP recognition by VPS9a, as opposed to indirect GDP recognition by switch II acidic residues, makes the ARA7·VPS9 GEF mechanism distinct from those of the other small GTPases·GEFs, such as Rop4·PRONE8 and Cdc42·DOCK9 complexes.
Interaction between P-loop Lysine and Switch II Acidic Residues
As the third step, it is necessary to move the P-loop lysine away from GDP. To do this, an acidic residue, either the first aspartate or the last glutamate in the conserved DTAGQE motif of switch II, attracts the P-loop lysine by an electrostatic interaction. During GDP release from ARA7·GDP·VPS9, the P-loop lysine swings from the GDP β-phosphate toward switch II Asp65, and as a result, it changes the interaction partner from the β-phosphate of GDP to the switch II aspartate. Usually, the latter interaction between the P-loop lysine and the switch II acidic residue is formed in the nucleotide-free complexes, as shown in Ras·SOS (38), Rac·Tiam1 (39), or Arf·Gea2 (32), and it is proposed that this coordination is important for keeping the stable nucleotide-free complex stable (40). In the Ran·RCC1 complex, the conserved P-loop lysine is detached from the position near GDP β-phosphate and is shifted toward the two acidic residues, Glu70 and Asp65 (Glu62 and Asp57 in Ras) (41). This glutamate residue is crucial for the Ran·RCC1 and Ras·Cdc25 reactions and is conserved in most Ras-like small GTPases.
Interestingly, the comparison of the GDP-bound (6) and nucleotide-free (42) complex structures of the Rops·PRONE8 shows that there is no overall structural change between these two forms and that the electrostatic interaction between ROP4 P-loop Lys19 and the β-phosphate oxygen of GDP is already formed in the GDP-bound state (3.7 Å in AC chains and 3.3 Å in BD chains in the ROP4·GDP·PRONE8, and 2.6 Å and 3.3 Å in the nucleotide-free ROP7·PRONE8, respectively). These observations might suggest that the structural rearrangements of the nucleotide binding site necessary for the GDP release were already accomplished in the GDP-bound state of the ROP4·GDP·PRONE8 complex. Also, the structure of the nucleotide-free ROP7·PRONE8 complex shows that the interaction between the ROP7 P-loop Lys19 and the switch II Glu65 is important but not sufficient for the stabilization of the nucleotide-free complex requiring assistance of the WW motif.
In our case, ARA7 Glu70, which corresponds to the conserved glutamate of switch II of Rop4/7, Ras, or Ran, is further away from the GDP, and the distance between the NH3+ nitrogen of Lys23 and the COO− oxygen of Glu70 is 12 Å. Thus, Glu70 of ARA7 switch II cannot play the same role as Glu65 of Rop4/7. Instead, Asp65 of ARA7 switch II is situated near the P-loop lysine and plays the same role as Rop4 Glu65 to attract the lysine NH3+ to stabilize the nucleotide-free form of ARA7·VPS9a. This is similar to the case of the Cdc42·GDP·DOCK9 structure, where the side chain of Cdc42 P-loop Lys16 is stabilized by the interaction with Cdc42 Asp57, not Glu62, of switch II (7).
Possibility of ARA7·GTP·VPS9a Complex
Finally, we consider if it would be possible for the ARA7·VPS9a complex to accommodate GTP without disintegrating into ARA7 and VPS9a. Superimposing the ARA7·VPS9a complex onto Rab5A·GppNHp·Mg2+ indicated that the phosphorus atom of the γ-phosphate would suffer severe steric hindrance with the OD2 of VPS9a Asp185 at a distance of 0.5 Å (Fig. 4a). The side-chain COO− of VPS9a Asp185 cannot drift far from the phosphorus position in the complex of ARA7·GDP·VPS9a because the COO− of VPS9a Asp185 interacts rather tightly with the ARA7 Lys23 side chain and the main-chain NH group of the ARA7 Gly68. However, a structural comparison with the crystal structure of the nucleotide-free Rab21·Rabex-5 complex (43) sheds light on the possibility of GTP binding. Surprisingly, a superposition of the two Vps9a domains, VPS9a and Rabex-5, reveals a very large rotation by 18° and displacement by 0.4 Å between the two GTPases, ARA7 and Rab21 (supplemental Fig. 10a). This seems to create a space large enough for binding of GTP in Rab21·Rabex-5 without obvious steric clashes. Hence, we constructed a model in which GTP is introduced to the corresponding position in the nucleotide-free Rab21·Rabex-5 structure (supplemental Fig. 10b). This model shows the recognition of the GTP γ-phosphate by the aspartate finger through hydrogen bonds, which are shifted from but similar to those observed for the β-phosphate of GDP in the ARA7·GDP·VPS9a structure.
Conclusion
It is now emerging that there are two mechanisms for destabilization of GDP in the nucleotide exchange reaction: either by a direct involvement of GEF with its acidic finger or by an indirect process where GEF assists the switch II glutamate of GTPase to approach GDP. However, there remain many critical questions, such as structural evidence for directionality of the GEF reaction (i.e. from GDP to GTP or vice versa) and whether GEF recognizes Mg2+-bound GTPase in the GDP form as the first step in the forward reaction. The more recent structure of a complex between Mg2+-bound Rho GTPase and DOCK complex structure (7) provides an example of such Mg2+ recognition, although it is GTP and not GDP that is recognized. These nucleotide-bound and nucleotide-free Cdc42·DOCK9 structures represent snap shots corresponding to steps much further down the GEF reaction. Further crystallographic studies on GDP-bound complexes with magnesium or other divalent metal ions would lead to a much better understanding of the initial reaction pathway of the GEF reaction of Rab5·VPS9a.
Supplementary Material
Acknowledgments
We thank the Photon Factory staff for encouragement, helpful discussions, and assistance in data collection. We thank the staff at the Stanford Synchrotron Radiation Lightsource and SPring-8 for assistance during data collection. We thank T. Demura of RIKEN for providing the plasmid pBD-GAL4-GWRFC.
This work was supported by a Protein 3000 Project grant and a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2 and Figs. 1–10.
The atomic coordinates and structure factors (codes 2EFC, 2EFD, 2EFE, and 2EFH) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- GEF
- guanine nucleotide exchange factor
- GDPNH2
- aminophosphonic acid-guanylate ester
- GppNHp
- guanosine-5′-(β,γ-imido)triphosphate.
REFERENCES
- 1.Sprang S. R., Coleman D. E. (1998) Cell 95, 155–158 [DOI] [PubMed] [Google Scholar]
- 2.Cherfils J., Chardin P. (1999) Trends Biochem. Sci. 24, 306–311 [DOI] [PubMed] [Google Scholar]
- 3.Andersen G. R., Valente L., Pedersen L., Kinzy T. G., Nyborg J. (2001) Nat. Struct. Biol. 8, 531–534 [DOI] [PubMed] [Google Scholar]
- 4.Renault L., Guibert B., Cherfils J. (2003) Nature 426, 525–530 [DOI] [PubMed] [Google Scholar]
- 5.Mossessova E., Corpina R. A., Goldberg J. (2003) Mol. Cell 12, 1403–1411 [DOI] [PubMed] [Google Scholar]
- 6.Thomas C., Fricke I., Scrima A., Berken A., Wittinghofer A. (2007) Mol. Cell 25, 141–149 [DOI] [PubMed] [Google Scholar]
- 7.Yang J., Zhang Z., Roe S. M., Marshall C. J., Barford D. (2009) Science 325, 1398–1402 [DOI] [PubMed] [Google Scholar]
- 8.Barr F., Lambright D. G. (2010) Curr. Opin. Cell Biol. 22, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zerial M., McBride H. (2001) Nat. Rev. Mol. Cell Biol. 2, 107–117 [DOI] [PubMed] [Google Scholar]
- 10.Ueda T., Nakano A. (2002) Curr. Opin. Plant. Biol. 5, 513–517 [DOI] [PubMed] [Google Scholar]
- 11.Ueda T., Yamaguchi M., Uchimiya H., Nakano A. (2001) EMBO J. 20, 4730–4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horiuchi H., Lippé R., McBride H. M., Rubino M., Woodman P., Stenmark H., Rybin V., Wilm M., Ashman K., Mann M., Zerial M. (1997) Cell 90, 1149–1159 [DOI] [PubMed] [Google Scholar]
- 13.Goh T., Uchida W., Arakawa S., Ito E., Dainobu T., Ebine K., Takeuchi M., Sato K., Ueda T., Nakano A. (2007) Plant Cell 19, 3504–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Otwinowski Z., Minor W. (1997) Methods Enzymol. 276, 307–326 [DOI] [PubMed] [Google Scholar]
- 15.Terwilliger T. C. (2003) Methods Enzymol. 374, 22–37 [DOI] [PubMed] [Google Scholar]
- 16.Murshudov G. N., Vagin A. A., Dodson E. J. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 240–255 [DOI] [PubMed] [Google Scholar]
- 17.Collaborative Computational Project 4 (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 760–76315299374 [Google Scholar]
- 18.Emsley P., Cowtan K. (2004) Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 [DOI] [PubMed] [Google Scholar]
- 19.Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. (1991) Acta Crystallogr. A 47, 110–119 [DOI] [PubMed] [Google Scholar]
- 20.Vagin A., Teplyakov A. (1997) J. Appl. Crystallogr. 30, 1022–1025 [Google Scholar]
- 21.Perrakis A., Sixma T. K., Wilson K. S., Lamzin V. S. (1997) Acta Crystallogr. D Biol. Crystallogr. 53, 448–455 [DOI] [PubMed] [Google Scholar]
- 22.Sayle R. A., Milner-White E. J. (1995) Trends Biochem. Sci. 20, 374–376 [DOI] [PubMed] [Google Scholar]
- 23.Kraulis P. J. (1991) J. Appl. Crystallogr. 24, 946–950 [Google Scholar]
- 24.Merritt E. A., Murphy M. E. (1994) Acta Crystallogr. D Biol. Crystallogr. 50, 869–873 [DOI] [PubMed] [Google Scholar]
- 25.Laskowski R. A., MacArthur M. W., Moss D. S., Thornton J. M. (1993) J. Appl. Crystallogr. 26, 283–291 [Google Scholar]
- 26.Simon I., Zerial M., Goody R. S. (1996) J. Biol. Chem. 271, 20470–20478 [DOI] [PubMed] [Google Scholar]
- 27.Milburn M. V., Tong L., deVos A. M., Brünger A., Yamaizumi Z., Nishimura S., Kim S. H. (1990) Science 247, 939–945 [DOI] [PubMed] [Google Scholar]
- 28.Itzen A., Pylypenko O., Goody R. S., Alexandrov K., Rak A. (2006) EMBO J. 25, 1445–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dong G., Medkova M., Novick P., Reinisch K. M. (2007) Mol. Cell 25, 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu G., Liu J., Terzyan S., Zhai P., Li G., Zhang X. C. (2003) J. Biol. Chem. 278, 2452–2460 [DOI] [PubMed] [Google Scholar]
- 31.Zhu G., Zhai P., Liu J., Terzyan S., Li G., Zhang X. C. (2004) Nat. Struct. Mol. Biol. 11, 975–983 [DOI] [PubMed] [Google Scholar]
- 32.Goldberg J. (1998) Cell 95, 237–248 [DOI] [PubMed] [Google Scholar]
- 33.Delprato A., Merithew E., Lambright D. G. (2004) Cell 118, 607–617 [DOI] [PubMed] [Google Scholar]
- 34.Béraud-Dufour S., Robineau S., Chardin P., Paris S., Chabre M., Cherfils J., Antonny B. (1998) EMBO J. 17, 3651–3659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith R. M., Martell A. E., Chen Y. (1991) Pure Appl. Chem. 63, 1015–1080 [Google Scholar]
- 36.Franken S. M., Scheidig A. J., Krengel U., Rensland H., Lautwein A., Geyer M., Scheffzek K., Goody R. S., Kalbitzer H. R., Pai E. F. (1993) Biochemistry 32, 8411–8420 [DOI] [PubMed] [Google Scholar]
- 37.Resat H., Straatsma T. P., Dixon D. A., Miller J. H. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 6033–6038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boriack-Sjodin P. A., Margarit S. M., Bar-Sagi D., Kuriyan J. (1998) Nature 394, 337–343 [DOI] [PubMed] [Google Scholar]
- 39.Worthylake D. K., Rossman K. L., Sondek J. (2000) Nature 408, 682–688 [DOI] [PubMed] [Google Scholar]
- 40.Vetter I. R., Wittinghofer A. (2001) Science 294, 1299–1304 [DOI] [PubMed] [Google Scholar]
- 41.Renault L., Kuhlmann J., Henkel A., Wittinghofer A. (2001) Cell 105, 245–255 [DOI] [PubMed] [Google Scholar]
- 42.Thomas C., Fricke I., Weyand M., Berken A. (2009) Biol. Chem. 390, 427–435 [DOI] [PubMed] [Google Scholar]
- 43.Delprato A., Lambright D. G. (2007) Nat. Struct. Mol. Biol. 14, 406–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.