Abstract
The bile acid receptor farnesoid X receptor (FXR) is expressed in adipose tissue, but its function remains poorly defined. Peroxisome proliferator-activated receptor-γ (PPARγ) is a master regulator of adipocyte differentiation and function. The aim of this study was to analyze the role of FXR in adipocyte function and to assess whether it modulates PPARγ action. Therefore, we tested the responsiveness of FXR-deficient mice (FXR−/−) and cells to the PPARγ activator rosiglitazone. Our results show that genetically obese FXR−/−/ob/ob mice displayed a resistance to rosiglitazone treatment. In vitro, rosiglitazone treatment did not induce normal adipocyte differentiation and lipid droplet formation in FXR−/− mouse embryonic fibroblasts (MEFs) and preadipocytes. Moreover, FXR−/− MEFs displayed both an increased lipolysis and a decreased de novo lipogenesis, resulting in reduced intracellular triglyceride content, even upon PPARγ activation. Retroviral-mediated FXR re-expression in FXR−/− MEFs restored the induction of adipogenic marker genes during rosiglitazone-forced adipocyte differentiation. The expression of Wnt/β-catenin pathway and target genes was increased in FXR−/− adipose tissue and MEFs. Moreover, the expression of several endogenous inhibitors of this pathway was decreased early during the adipocyte differentiation of FXR−/− MEFs. These findings demonstrate that FXR regulates adipocyte differentiation and function by regulating two counteracting pathways of adipocyte differentiation, the PPARγ and Wnt/β-catenin pathways.
Keywords: Adipocyte, Adipose Tissue, Adipose Tissue Metabolism, Lipid Droplet, Metabolic Syndrome, Metabolism, Metabolic Regulation, PPAR, Wnt Pathway, FXR
Introduction
The nuclear receptor farnesoid X receptor (FXR)3 is a transcription factor that belongs to the nuclear receptor superfamily that is endogenously activated by bile acids (1). FXR was initially found to regulate bile acid metabolism and to protect the liver from the deleterious effect of excessive bile acid accumulation (2–4). The phenotype of FXR-deficient (FXR−/−) mice further established a role for FXR in lipid metabolism (5). Recently, FXR was shown to be implicated in the control of hepatic glucose metabolism and peripheral insulin sensitivity (6–9). FXR modulates the fasting-refeeding transition in mice (8), and genetic murine models of diabetes display an increased FXR expression (10). Although FXR deficiency was associated with peripheral insulin resistance (6, 7), activation of FXR by bile acids (6) or specific synthetic agonists (7, 9) conversely improves glucose homeostasis in rodent models of diabetes. Finally, FXR appears to be involved in the regulation of adaptive thermogenesis in response to fasting or cold exposure (11).
The primary role of adipose tissue is to store energy in form of triglycerides (TG) in the adipocytes, which can be liberated as fatty acids upon energy requirement. Adipose tissue is also an endocrine organ secreting hormones involved in metabolic homeostasis (12). Preadipocyte differentiation into mature adipocytes is a finely tuned process that is regulated by a complex network of transcription factors. In this cascade, the peroxisome proliferator-activated receptor-γ (PPARγ) (13) and CAAT/enhancer-binding protein (C/EBP) α (14) act as key regulators. Early regulators of preadipocyte differentiation are other members of the CAAT/enhancer-binding protein family, C/EBPβ and C/EBPδ (15), which induce the expression of PPARγ and C/EBPα (16). Among the extracellular signaling pathways that regulate adipogenesis is the Wnt pathway (17). The non-canonical and canonical Wnt signaling pathways, being respectively β-catenin-independent and -dependent, are negative regulators of adipogenesis (17). In the absence of Wnt proteins, β-catenin is localized in the cytoplasm in a protein complex containing Axin and adenomatous polyposis coli (APC) proteins, which facilitate β-catenin phosphorylation and its subsequent proteasomal degradation (18). The binding of Wnt proteins to their receptors Frizzled (FZD) and low density lipoprotein receptor-related protein-5 or -6 (LRP5/6) leads to β-catenin protein stabilization. Hypophosphorylated β-catenin protein translocates into the nucleus and activates its target genes (19). The activation of Wnt/β-catenin signaling leads to the repression of adipogenesis by blocking the induction of PPARγ and C/EBPα expression (20). On the other hand, PPARγ activation leads to proteasomal-dependent β-catenin degradation by stimulating the activity of GSK3β, a β-catenin kinase, and by interacting with phospho-β-catenin itself (20–22). A finely regulated balance between β-catenin activity and PPARγ expression is thus required for proper adipocyte differentiation (23).
We (7) and others (24) have shown that FXR is expressed in adipocyte, where it modulates adipocyte differentiation (7, 24). FXR expression was found to be decreased in adipose tissue of mouse models of dietary and genetic obesity (7). FXR expression is induced during adipocyte differentiation in 3T3-L1 cells and mouse embryonic fibroblasts (MEFs) (7, 24). MEFs isolated from FXR−/− mice (FXR−/− MEFs) display an impaired adipocyte differentiation with a delay in the expression of adipogenic genes and a decreased lipid droplet size (7). Additionally, FXR activation in 3T3-L1 cells during adipocyte differentiation by specific synthetic agonists increases mRNA expression of adipogenic genes, as well as insulin signaling and insulin-stimulated glucose uptake (7, 24).
In the present study, we provide in vivo evidence that FXR was necessary for a full response to PPARγ activation. We show that obese FXR−/−/ob/ob mice displayed an altered response to PPARγ activation by rosiglitazone. Retroviral re-expression of FXR in FXR−/− MEFs restored the adipogenic gene expression program in response to rosiglitazone. The delay in adipogenic differentiation in FXR−/− MEFs was associated with a sustained activation of Wnt/β-catenin signaling. All these results provide evidence for a crucial role of FXR in adipogenesis by promoting the PPARγ pathway and interfering with Wnt/β-catenin signaling.
EXPERIMENTAL PROCEDURES
Animals
Female and male ob/ob mice (B6.V-Lepob/J) from Charles River (Saint Aubin les Elseuf, France) were crossed with FXR+/+ and FXR−/− C57BL6/J mice to obtain FXR+/+/ob/ob and FXR−/−/ob/ob mice. 12-week-old FXR−/−/ob/ob female mice and their wild type littermates (n = 7/group) were housed on a 12-h light/12-h dark cycle with free access to water and were treated with the PPARγ agonist rosiglitazone (Avandia®, GlaxoSmithKline) (10 mg/kg of body weight) mixed with control diet (UAR A03, Villemoison/Orge, France) for 21 days.
Isolation and Culture of MEFs
MEFs were derived from 13.5-day-old FXR+/+ and FXR−/− embryos (C57BL6/N) (7). MEFs were plated in 6-well plates at 300,000 cells/well. Adipocyte differentiation was initiated 2 days after confluence with AmnioMAX-C100 medium (Invitrogen), 7.5% AmnioMAX-C100 supplement, 7.5% fetal bovine serum (FBS), 0.5 mm 3-isobutyl-1-methylxanthine, 1 μm dexamethasone, 5 μg/ml insulin. From days 3–8, cells were incubated with AmnioMAX-C100 medium with 5 μg/ml insulin and 1 μm rosiglitazone. At days 0, 4, and 8, cells were used for lipid metabolism studies (lipolysis, de novo lipogenesis, and triglyceride content), lysed and homogenized for RNA isolation, or fixed in 4% paraformaldehyde and stained with Oil Red O. All experiments were performed in triplicate.
Preadipocyte Isolation and Culture
Preadipocytes were isolated from inguinal fat pads of 20-week-old FXR−/−/ob/ob and their FXR+/+/ob/ob littermates. Adipose tissue was isolated, dissociated mechanically, and digested in Krebs buffer solution (118 mm NaCl, 5 mm KCl, 1.25 mm CaCl2, 1.2 mm KH2PO4, 1.2 mm MgSO4, 20 mm NaHCO3, 2 mm sodium pyruvate, 10 mm HEPES, 3% BSA, pH 7.4) containing 1.5 mg/ml collagenase A (Roche Diagnostics GmbH, Mannheim, Germany) for 1.5 h in a shaking water bath at 37 °C. After digestion, the mature adipocytes were separated from the stroma-vascular cells by centrifugation. The stroma-vascular pellet containing the preadipocytes was treated with erythrocyte lysis solution (154 mmol/liter NH4Cl, 10 mmol/liter KHCO3, 0.1 mmol/liter EDTA) for 5 min at room temperature and centrifuged. The preadipocyte-containing pellet was cultured in PromoCell® preadipocyte growth medium (PromoCell GmbH, Heidelberg, Germany) at 37 °C in a humidified 95% air and 5% CO2 incubator. Cultures were grown to confluence (days (−2)). Two days after confluence (day 0), the medium was changed to preadipocyte differentiation medium supplemented with 8 μg/ml d-biotin-4, 0.5 μg/ml bovine insulin, 400 ng/ml dexamethasone, 44 μg/ml 3-isobutyl-1-methylxanthine, 9 ng/ml l-thyroxine. From days 3–8, the medium was changed to adipocyte nutrition medium and was supplemented with rosiglitazone (1 μm). At days 0, 4, and 8, cells were lysed and homogenized for RNA isolation or fixed in 4% paraformaldehyde and stained with Oil Red O.
Measurement of Triglyceride Content
Cellular lipids were extracted with hexane/isopropyl alcohol (3:2 v/v). Lipids were then dried with nitrogen gas, redissolved in isopropyl alcohol, and quantified using the TG PAP 1000 kit (BioMérieux, Marcy l'Etoile, France).
Lipolysis Assay
Lipolysis experiments were performed at days 4 and 8 of MEF differentiation. Cells were washed with PBS and incubated with 300 μl of incubation solution (adipolysis assay kit, OB100, Millipore) with 2% BSA or containing 10 μm isoproterenol for 3 h. Glycerol was measured in the culture supernatant with free glycerol assay reagent (adipolysis assay kit, OB100, Millipore). Glycerol concentrations were normalized to total cellular protein content. All experiments were performed in triplicate.
De Novo Lipogenesis Assay
De novo lipogenesis was evaluated by measuring incorporation of radiolabeled acetate precursor into total cellular lipids. Cells were washed and incubated with compounds for 48 h in culture medium without FBS and with 3 mg/ml BSA. Cells were then washed and incubated in Krebs-Ringer buffer for 90 min in the presence of 1 μCi of [14C]acetate (Amersham Biosciences, Saclay, France). MEFs were washed, and total cellular lipids were extracted twice with hexane/isopropyl alcohol. The pooled organic fractions were transferred to scintillation vials, dried under nitrogen, and assessed for radioactivity by liquid scintillation counting. [14C]Acetate incorporation was normalized to total cellular protein content.
Real Time Quantitative Reverse Transcription-PCR
Total RNA was isolated from white adipose tissue using the acid guanidinium thiocyanate/phenol/chloroform method and from MEFs and differentiated preadipocytes using the TRIzol reagent (Invitrogen) and subsequently reverse-transcribed using Moloney murine leukemia virus (Applied Biosystems, Paris, France). cDNAs were quantified by quantitative PCR on a Mx4000 apparatus (Stratagene) using specific primers (supplemental Table 2). mRNA levels were subsequently normalized to those of cyclophilin. ΔCt was calculated as the difference between the Ct of the gene of interest and that of cyclophilin.
Plasmid and Retrovirus Infection
The retrovirus was constructed using the FXRα3 mouse cDNA. The sequences of the primers used were: 5′-atacgcggatccatggtgatgagtttcaggg-3′ and 5′-ctctagaccctacacgtcactcagctgcgcata-3′ (the initiation (atg) and stop (tag) codons are underlined). The FXR coding sequence was cloned into the mammalian expression vector pBabe-Puro (Invitrogen) in BamHI and SalI sites. Human embryonic kidney 293T modified packaging cells (Ecotropic Phoenix) were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% FBS and transiently transfected with a pBabe-puro-FXR chimera or a pBabe-puro vector alone as negative control. 48 h after transfection, the viral supernatant was harvested and used to infect the MEFs. Selection of MEFs that had incorporated the retrovirus was done by adding puromycin to the medium for 1 week. FXR expression was measured by quantitative PCR.
Treatment with sFRP1 Recombinant Protein
Recombinant human secreted Frizzled-related protein (sFRP) 1 was purchased from R&D Systems (Minneapolis, MN). FXR−/− MEFs were differentiated in the presence or absence of recombinant sFRP1 (75 nmol/liter) added at day 0.
Western Blot
Total cellular MEF protein was extracted using a radioimmune precipitation assay buffer (50 mm Tris-HCl, 150 mm NaCl, 1.0% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 1.0 mm EDTA, 0.1% (w/v) SDS, and 0.01% (w/v) sodium azide, pH:7.4). Protein concentration was determined by the Bradford method (Bio-Rad protein assay). Protein samples were denatured by heating to 90 °C in SDS-reducing buffer and resolved by electrophoresis on 10% SDS-polyacrylamide gels. After protein transfer, nitrocellulose membranes were incubated with a mouse monoclonal anti-β-catenin antibody (catalog number 610153, BD Transduction Laboratories) for 3 h and HRP-conjugated secondary antibody (Dako A/S, Glostrup, Denmark) for 1 h. Proteins were then visualized by chemiluminescence using an ECL detection kit (Amersham Biosciences, Orsay, France).
Adipocyte Size Determination
Inguinal adipose tissue was fixed in 4% neutral buffered paraformaldehyde, embedded in paraffin, cut into 7-μm sections, and stained with hematoxylin. Cell size was determined using the ImageJ software (National Institutes of Health, Bethesda, MD, rsb.info.nih.gov/ij).
Microarray Analysis
Total RNA was prepared from epididymal adipose tissue of FXR+/+ and FXR−/− mice, and the subsequent steps were performed as described elsewhere (25). Affymetrix raw data were normalized using the robust multiarray average (RMA) algorithm to obtain expression values in log2 (26). Log2-transformed expression values were fitted to a linear model according to Limma package methods (Biocoundctor). A model was established to identify probesets significantly differentially expressed between FXR+/+ and FXR−/− mice. False discovery rate correction was applied to take into account multiple testing hypotheses. Selection of relevant probe sets was based on a mean log2 expression value greater than 6.12 in a least one of the two compared conditions, a p value <10−5, and an absolute -fold change value of 1.5. Function of all clustering of regulated genes was performed by Ingenuity Pathway Analysis (Ingenuity).
Statistical Analysis
Statistical significance was analyzed using the unpaired Student's t test. All values are reported as means ± S.D. Values with p < 0.05 were considered significant.
RESULTS
FXR Deficiency Results in a Partial Resistance to PPARγ Activation in Adipose Tissue in Vivo
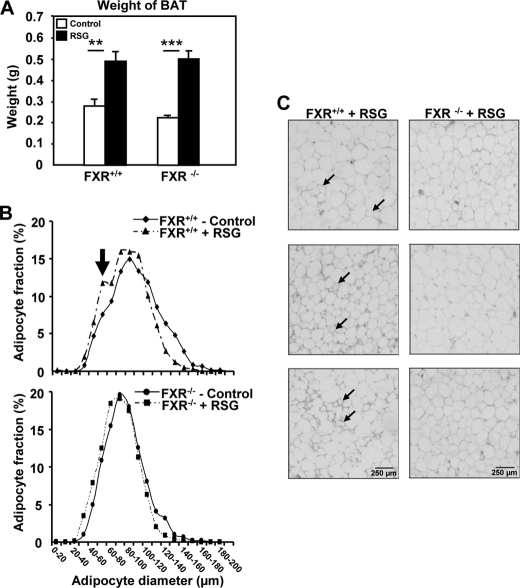
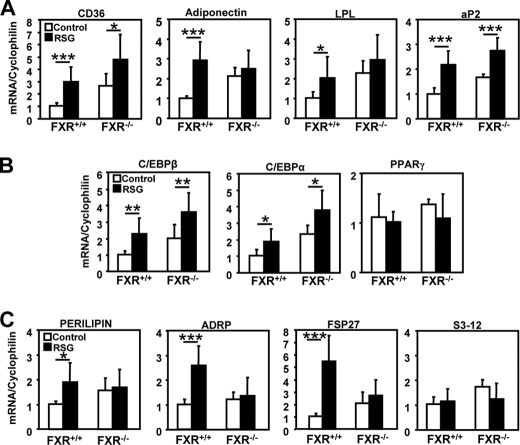
To explore whether the FXR and PPARγ pathways interact in vivo, genetically obese (ob/ob) FXR−/− and FXR+/+ mice were treated with rosiglitazone (10 mg/kg of body weight) for 21 days. As a positive control of in vivo PPARγ activation (27), mice from both genotypes displayed a comparable increase of brown adipose tissue mass (Fig. 1A), a tissue that does not express FXR (11, 28). As expected (7), adipose tissue of FXR−/− mice contained a larger proportion of small adipocytes (average diameter 78 ± 21 μm) than FXR+/+ adipose tissue (average diameter 90 ± 26 μm) (Fig. 1B and supplemental Table 1). Interestingly, although PPARγ activation by rosiglitazone led to the appearance of a subpopulation of smaller adipocytes in white adipose tissue of FXR+/+ mice, likely due to the induction of preadipocyte recruitment (29), this response was not observed in FXR−/− mice (Fig. 1, B and C, and supplemental Table 1). Moreover, the induction of some PPARγ target genes such as adiponectin and lipoprotein lipase (LPL) by rosiglitazone was abolished in adipose tissue from FXR−/− when compared with FXR+/+ mice (Fig. 2A). Although the induction of other PPARγ target genes (cd36 and aP2) was also reduced in FXR−/− mice, the expression of adipogenic genes, such as C/EBPα and C/EBPβ, was induced to the same level in both genotypes, and PPARγ expression itself was not modified (Fig. 2B). Most interestingly, the induction of genes involved in lipid droplet formation, such as perilipin, adipose differentiation-related protein (ADRP), and fsp27, by rosiglitazone was totally abolished in FXR−/− when compared with FXR+/+ mice, whereas s3-12 expression was not affected (Fig. 2C). These data demonstrate that FXR is necessary to ensure the full response to PPARγ activation in adipose tissue.
FIGURE 1.
Obese FXR−/−/ob/ob mice are resistant to the effects of PPARγ activation on adipocyte recruitment. A, increase of brown adipose tissue (BAT) mass in FXR−/− and FXR+/+/ob/ob mice after rosiglitazone (RSG) treatment. B, adipocyte size distribution in adipose tissue of FXR−/− and FXR+/+/ob/ob mice treated or not with RSG. 200 adipocytes were studied per section. Adipocyte size was measured using ImageJ. C, morphology of white adipocytes of FXR−/− and FXR+/+/ob/ob mice (n = 3/group) treated with RSG (10 mg/kg). Small adipocytes recruited after RSG treatment are indicated by the arrow. **, p < 0.01; ***, p < 0.001.
FIGURE 2.
FXR deficiency alters the expression profile of white adipose tissue genes following rosiglitazone treatment. A–C, mRNA expression of PPARγ target genes (A), adipogenic transcription factor genes (B), and lipid droplet genes (C) in white adipose tissue of FXR−/− and FXR+/+/ob/ob mice after rosiglitazone treatment. mRNA levels were measured by quantitative PCR. Values are normalized to the expression of cyclophilin and are expressed relative to control FXR+/+ mice. LPL, lipoprotein lipase. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FXR Deficiency Results in an Impaired Responsiveness to PPARγ Activation in MEFs in Vitro
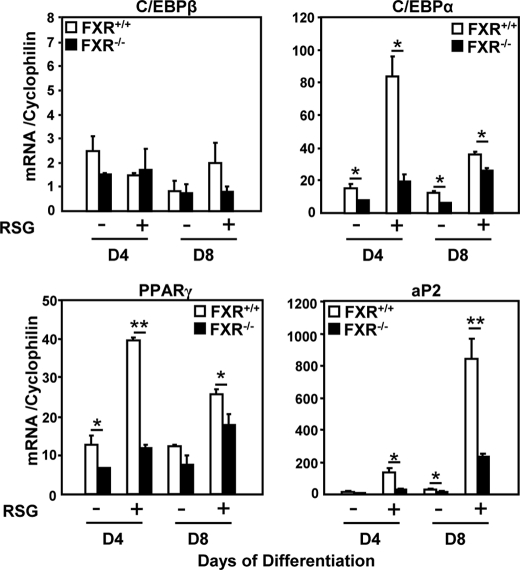
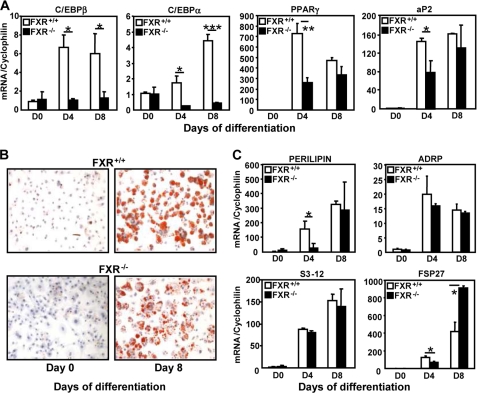
To study whether FXR modulates adipocyte PPARγ responsiveness in a cell-autonomous manner, MEFs were isolated from FXR+/+ and FXR−/− embryos and differentiated into adipocytes with or without rosiglitazone, and the expression of adipocyte differentiation markers was measured. As expected (7), FXR−/− MEFs were resistant to adipocyte differentiation with a lower expression of C/EBPα, PPARγ, and aP2 at day 4 of differentiation in the absence of rosiglitazone (Fig. 3). Upon PPARγ activation, the induction of C/EBPα, PPARγ, and aP2 following rosiglitazone treatment was drastically reduced at day 4, and to a lesser extent at day 8, in FXR−/− when compared with FXR+/+ MEFs (Fig. 3). These results show that FXR−/− MEFs are resistant to PPARγ activation, which is unable to induce the complete adipogenic differentiation program in these cells.
FIGURE 3.
FXR−/− MEFs are resistant to PPARγ activation. mRNA levels of adipogenic genes of 4- and 8-day-differentiated FXR−/− and FXR+/+ MEFs in the presence or absence of 1 μm RSG were measured by quantitative PCR. Values are normalized to the expression of cyclophilin and are expressed relative to those at day 0, which are arbitrarily set to 1. These results are representative of three experiments. *, p < 0.05; **, p < 0.01.
FXR−/− MEFs Exhibit a Reduced Lipid Storage Capacity upon PPARγ Activation
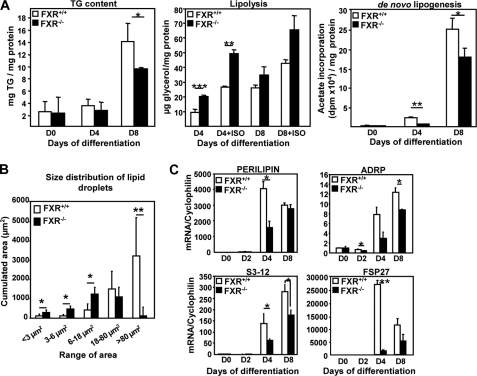
The functional consequences of the resistance to PPARγ activation was studied by comparing the phenotype of FXR−/− and FXR+/+ MEFs differentiated in the presence of rosiglitazone. The increase in TG content at day 8 of differentiation was significantly lower in FXR−/− MEFs when compared with FXR+/+ MEFs (Fig. 4A). This reduction in TG content can be the consequence of either an increase in TG degradation (lipolysis) and/or a decrease in TG synthesis (lipogenesis). Therefore, basal and β-adrenergically (isoproterenol)-induced lipolysis was assessed by measuring glycerol release into the cell culture medium. Rosiglitazone-treated FXR−/− MEFs displayed an enhanced lipolysis when compared with FXR+/+ MEFs under both basal conditions and after β-adrenergic stimulation (Fig. 4A), which was statistically significant at day 4 of differentiation. Analysis of [14C]acetate incorporation demonstrated a significant decrease of fatty acid synthesis (de novo lipogenesis) in rosiglitazone-treated FXR−/− when compared with FXR+/+ MEFs at days 4 and 8 of differentiation (Fig. 4A). Moreover, PPARγ-activated FXR−/− MEFs also exhibited an abnormal morphology with lipid droplets of smaller size (Fig. 4B). Accordingly, rosiglitazone-treated FXR−/− MEFs showed decreased expression of several lipid droplet genes at day 4, such as perilipin, ADRP, s3-12, three members of the Perilipin, ADRP, and TIP47 (PAT) protein family (30), and fsp27 (31), a member of the recently identified cell death-inducing DFF45-like effector (CIDE) protein family (Fig. 4C). Although perilipin gene expression did not differ between the two genotypes at day 8, ADRP and s3-12 gene expression was still significantly reduced (Fig. 4C). These results indicate that FXR plays a critical role in developing a full lipid storage capacity during adipocyte differentiation by controlling the PPARγ-mediated formation of lipid droplets.
FIGURE 4.
FXR deficiency alters triglyceride storage, lipolysis, de novo lipogenesis, and the expression of lipid droplet genes in MEFs during differentiation to adipocytes. A, TG content in FXR−/− and FXR+/+ MEFs at days 0, 4, and 8 of differentiation treated with 1 μm rosiglitazone; lipolysis was measured in FXR−/− and FXR+/+ MEFs at days 0, 4, and 8 as glycerol release under basal and stimulated (isoproterenol: ISO) conditions. De novo lipogenesis in FXR−/− and FXR+/+ MEFs was measured at days 0, 4, and 8 of differentiation. The results are representative of three experiments and are presented as means ± S.D. B, quantification of the lipid droplet size of 8-day-differentiated FXR−/− and FXR +/+ MEFs. C, mRNA expression of genes coding for lipid droplet proteins in FXR−/− and FXR+/+ MEFs during differentiation measured by quantitative PCR. Values are normalized to the expression of cyclophilin and are expressed relative to those at day 0, which are arbitrarily set to 1. The results are representative of three experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
FXR−/− Preadipocytes Display Impaired Adipocyte Differentiation upon PPARγ Activation
To determine whether FXR deficiency also impairs primary adipocyte differentiation upon PPARγ activation, preadipocytes were isolated from the stromal fraction of inguinal adipose tissue of FXR−/−/ob/ob and FXR+/+/ob/ob littermates and differentiated into adipocytes in the presence of rosiglitazone. C/EBPβ and C/EBPα mRNA levels were clearly lower at days 4 and 8 of differentiation in FXR−/− when compared with FXR+/+ cells, whereas the reduction of PPARγ and aP2 expression reached significance only at day 4 (Fig. 5A). As in differentiated MEFs, 8-day-differentiated rosiglitazone-treated FXR−/− adipocytes exhibited an abnormal morphology with smaller lipid droplets when compared with FXR+/+ cells (Fig. 5B). This phenotype was associated with a transiently reduced expression of the perilipin and fsp27 genes at day 4, whereas ADRP and S3-12 gene expression was not affected (Fig. 5C). Surprisingly, FSP27 mRNA levels were found to be increased at day 8 in FXR−/− adipocytes. Altogether, these results show that FXR−/− preadipocytes display altered adipocyte differentiation even when PPARγ is activated.
FIGURE 5.
FXR deficiency impairs PPARγ-induced differentiation and lipid droplet formation of primary preadipocytes. A and C, expression of adipogenic marker (A) and lipid droplet protein (C) genes in FXR−/− when compared with FXR+/+ preadipocytes treated with 1 μm rosiglitazone. Preadipocytes were isolated from white adipose tissue of obese FXR−/−/ob/ob and FXR+/+/ob/ob mice. mRNA levels were measured by quantitative PCR. Values (± S.D.) are normalized to the expression of cyclophilin and are expressed relative to those at day 0, which are arbitrarily set to 1. B, smaller size of lipid droplets in FXR−/− when compared with FXR+/+ preadipocytes. Representative Oil Red O staining of FXR−/− and FXR+/+ preadipocytes at days 0 and 8 of differentiation is shown (×20 magnification). *, p < 0.05; **, p < 0.01; ***, p < 0.001.
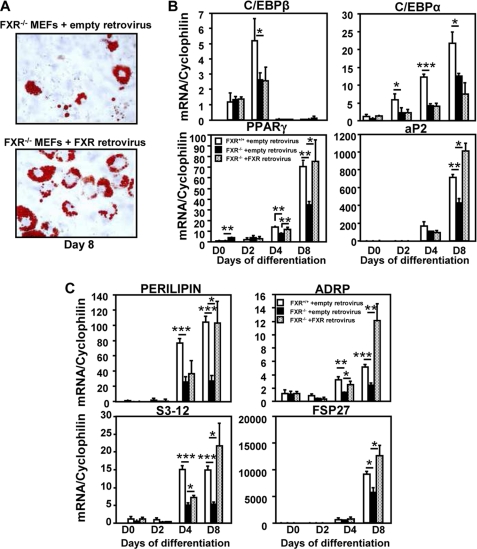
Reintroduction of FXR in FXR−/− MEFs Restores the Expression of Adipogenic and Lipid Droplet Genes and Increases the Number of Differentiated Adipocyte Clusters
To determine whether the altered adipocyte differentiation observed in rosiglitazone-treated FXR−/− MEFs was strictly FXR-dependent, FXR was exogenously (re-)expressed in FXR−/− and FXR+/+ MEFs using a retrovirus encoding FXRα3, which, together with FXRα4, is expressed in MEF cells (data not shown). Infected cells were subsequently subjected to adipogenic differentiation in the presence of rosiglitazone. Retroviral infection led to a pronounced mRNA expression of FXRα3 in FXR−/− MEFs during adipocyte differentiation, with a 3-fold higher expression at day 8 when compared with FXR+/+ MEFs infected with empty retrovirus (data not shown). Importantly, reintroduction of FXR resulted in an increased number of differentiated adipocyte clusters (Fig. 6A) and a restoration of PPARγ and aP2 expression at day 8, whereas neither the expression of C/EBPβ nor the expression of C/EBPα was affected in FXR−/− MEFs (Fig. 6B). Moreover, the expression of all lipid droplet genes was significantly increased at day 8 (Fig. 6C). Thus, re-expression of FXR in FXR−/− MEFs restores the capacity of MEFs to differentiate into adipocytes upon rosiglitazone treatment.
FIGURE 6.
Re-expression of FXR reverses the impaired adipocyte differentiation of rosiglitazone-treated FXR−/− MEFs. FXR−/− MEFs were infected with a retrovirus coding for FXRα3 or the empty vector, subjected to adipogenic differentiation, and treated with 1 μm rosiglitazone. A, increased number of differentiated cell clusters after FXR retroviral infection of rosiglitazone-treated FXR−/− MEFs. Representative Oil Red O staining of FXR−/− and FXR+/+ MEFs at day 8 of differentiation is shown (×20 magnification). B and C, mRNA levels of adipogenic markers (B) and lipid droplet protein (C) genes in empty retrovirus- and FXR retrovirus-infected rosiglitazone-treated FXR−/− MEFs measured by quantitative PCR. Empty retrovirus-transfected FXR+/+ MEFs were used as reference. Values are normalized to cyclophilin mRNA and are expressed relative to those at day 0, which are arbitrarily set to 1. The results are representative of three experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
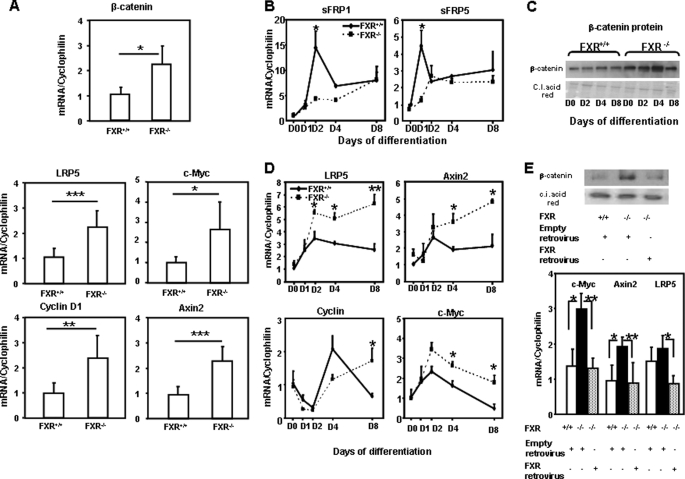
FXR Deficiency Results in a Dysregulation of the Wnt/β-Catenin Pathway in Adipose Tissue and Adipocytes
To unravel the mechanisms underlying the impaired adipocyte differentiation linked to FXR deficiency, a microarray analysis was performed in white adipose tissue from FXR−/− versus FXR+/+ mice to identify pathways with altered expression (data not shown). One of those identified is the Wnt/β-catenin signaling pathway known to inhibit adipocyte differentiation at least in part by inhibiting PPARγ activity (19). Indeed, mRNA levels of β-catenin were increased in adipose tissue of FXR−/− versus FXR+/+ obese mice (Fig. 7A). In accordance with an increased Wnt/β-catenin signaling, mRNA levels of lrp5 and Axin2 (18), both target genes and components of the Wnt/β-catenin pathway, and the target genes cyclin D1 and c-myc (32, 33), were higher in adipose tissue of FXR−/− when compared with FXR+/+ mice (Fig. 7A).
FIGURE 7.
Up-regulation of the Wnt/β-catenin signaling pathway in adipose tissue of FXR−/−/ob/ob mice and FXR−/− MEFs. A, mRNA expression of β-catenin, LRP5, c-Myc, Axin2, and cyclin D1 in inguinal adipose tissue of FXR−/− and FXR+/+/ob/ob mice. mRNA levels were measured by quantitative PCR. Values are normalized to the expression of cyclophilin and are expressed relative to FXR+/+ mice, which are arbitrarily set to 1. The results are presented as means ± S.D. B, mRNA expression of sFRP1 and sFRP5 during differentiation of FXR−/− and FXR+/+ MEFs treated with 1 μm rosiglitazone. C, β-catenin protein levels during FXR−/− and FXR+/+ MEF differentiation. D, mRNA levels of Wnt/β-catenin target genes during differentiation of FXR−/− and FXR+/+ MEFs. E, top, β-catenin protein levels. Bottom, mRNA levels of c-Myc, Axin2, and LRP5 in FXR+/+ and FXR−/− MEFs transfected for 2 days with empty of FXR retrovirus as indicated. mRNA levels were measured by quantitative PCR. Values are normalized to the expression of cyclophilin and are expressed relative to those at day 0, which are arbitrarily set to 1. The results are representative of two experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001. C.I. acid red, Ponceau S.
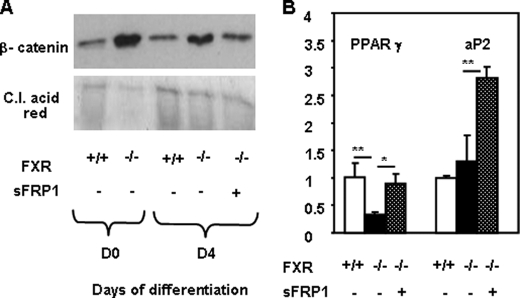
The expression of regulators and components of the Wnt/β-catenin pathway was also analyzed during the differentiation of FXR+/+ and FXR−/− MEFs into adipocytes. Interestingly, the expression of sFRP1 and sFRP5, negative regulators of the Wnt pathway, was lower in FXR−/− MEFs during early adipocyte differentiation, at days 2 and 1, respectively (Fig. 7B). In parallel, Western blot analysis showed that β-catenin protein expression was higher in FXR−/− MEFs with a peak at day 4 when compared with FXR+/+ MEFs (Fig. 7C). In accordance with an increased Wnt/β-catenin signaling, the expression of lrp5, Axin2, cyclin D1, and c-myc, four target genes of this pathway, was increased in FXR−/− when compared with FXR+/+ MEFs (Fig. 7D). Moreover, restoration of FXR expression in FXR−/− MEFs using the FXRα3-encoding retrovirus resulted in a decrease of β-catenin protein expression (Fig. 7E) along with the improvement of adipocyte differentiation (Fig. 6B). Moreover, mRNA expression of the Wnt/β-catenin pathway genes c-myc, Axin2, and lrp5 decreased upon FXR reintroduction in FXR−/− MEFs (Fig. 7E). To further assess whether the anti-adipogenic effect of FXR deficiency is mediated by the activation of the Wnt/β-catenin signaling pathway, we investigated whether the secreted Wnt antagonist sFRP1 could reverse this effect. The elevated β-catenin protein expression in FXR−/− when compared with FXR+/+ MEFs was reduced upon incubation with recombinant sFRP1 protein (Fig. 8A). Concomitantly, sFRP1 treatment of FXR−/− MEFs increased the expression of adipogenic markers, such as PPARγ and aP2 (Fig. 8B). These results demonstrate that the Wnt/β-catenin pathway, which inhibits adipocyte differentiation and PPARγ function, is activated in FXR-deficient adipocytes in vivo and in vitro and that restoration of FXR expression inhibits the Wnt signaling pathway.
FIGURE 8.
sFRP1 reduces β-catenin protein (A) and increases PPARγ and aP2 gene expression in FXR−/− MEFs (B). FXR−/− MEFs were differentiated in the presence or absence of recombinant sFRP1 (75 nmol/liter). A, β-catenin protein levels. B, mRNA levels of adipogenic genes measured by quantitative PCR. Values are normalized to the expression of cyclophilin and are expressed relative to those in FXR+/+ MEFs, which are arbitrarily set to 1. *, p < 0.05; **, p < 0.01. C.I. acid red, Ponceau S.
DISCUSSION
The results of this study identify a role for the nuclear receptor FXR as a modulator of the PPARγ and Wnt signaling pathways in adipocyte differentiation. FXR−/− mice displayed an impaired response to PPARγ activation, and FXR−/− MEFs were resistant to the induction of adipogenic differentiation by PPARγ activation. Our results show that lipid storage is impaired in FXR−/− MEFs even after PPARγ activation due to decreased de novo lipogenesis and increased lipolysis. Microarray analysis of adipose tissue from FXR+/+ and FXR−/− mice allowed us to identify that FXR deficiency alters mRNA expression of genes implicated in the Wnt/β-catenin signaling pathway. FXR−/− adipose tissue and FXR−/− MEFs exhibited an overactivation of the Wnt/β-catenin signaling pathway, an inhibitor of adipocyte differentiation and PPARγ function. These results demonstrate that FXR is critical for full adipocyte differentiation by promoting PPARγ activation and interfering with the Wnt/β-catenin signaling pathways.
Our study shows that FXR deficiency led to impaired adipocyte differentiation of MEFs, as well as preadipocytes isolated from the stromal fraction of FXR−/− adipose tissue, with a clear alteration of lipid droplet gene expression after differentiation in the presence of rosiglitazone. This alteration of mRNA expression in FXR−/− cells was already detectable at days 2 and 4, whereas the functional consequences reflected in lipid droplet morphology were observed later at day 8 of differentiation. This alteration of adipocyte differentiation induced by rosiglitazone was strictly FXR-dependent because FXR reintroduction improved differentiation of FXR−/− MEFs with an increased expression of adipogenic markers, such as PPARγ and aP2, and an increased number of differentiated cell clusters. Intriguingly, C/EBPα and C/EBPβ mRNA expression was not restored. We have currently no explanation for the lack of response of these two genes.
Rosiglitazone-treated FXR−/− MEFs displayed an alteration of triglyceride storage that was correlated with a combination of a decrease of de novo lipogenesis and an increase of lipolysis. In parallel, the expression of lipogenic genes was decreased in FXR−/− MEFs (data not shown). This result is in agreement with those obtained in the 3T3-L1 cell line showing an increase of lipogenic gene expression after treatment with FXR agonists (24).
FXR−/− MEFs exhibited an increase of both basal and β-adrenergically induced lipolysis. However, the extent of β-adrenergically mediated induction was similar between FXR−/− and FXR+/+ MEFs, suggesting that downstream pathways are not influenced by FXR. However, the expression of lipid droplet genes such as perilipin, ADRP, s3-12, and fsp27, which play a role in lipid droplet formation and lipolysis, is altered even after PPARγ activation. Because FXR−/− MEFs and preadipocytes present a decreased lipid droplet size, it would be interesting to determine the localization of the adipose triacylglycerol lipase (ATGL) and hormone-sensitive lipase (HSL) proteins before and after β-adrenergic stimulation. Indeed, a recent study proposes that these two enzymes are preferentially associated with small lipid droplets (34) and thus could contribute to the increase of lipolysis in FXR−/− adipocytes.
Microarray analysis of white adipose tissue of FXR+/+ and FXR−/− mice showed that FXR deficiency is associated with impaired expression of regulators and components of the Wnt/β-catenin signaling pathway (35). Interestingly, the expression of sFRP5, an inhibitor of the Wnt/β-catenin signaling pathway, was down-regulated in vivo (data not shown) and in vitro in FXR−/− MEFs. Conversely, β-catenin, a gene implicated in inhibition of adipocyte differentiation and adipose tissue formation (35–37), was up-regulated in adipose tissue of FXR−/− mice. This observation is consistent with studies showing that there is an increase of β-catenin in the intestine of FXR−/− mice (38).
sFRP5 mRNA levels correlate with adiposity (39). Thus, the observed decrease of sFRP5 mRNA expression is in agreement with the description of lower adipose tissue mass in FXR−/− mice (7). Moreover, the expression of sFRP1, which, when overexpressed, induces spontaneous adipocyte differentiation (40), was decreased in FXR−/− MEFs. mRNA levels of sFRP1 and sFRP5 were decreased respectively at days 2 and 1 of MEFs differentiation, which correlates with the increased β-catenin protein levels especially at day 4 of FXR−/− MEF differentiation. Moreover, incubation of FXR−/− MEFs with sFRP1 decreased β-catenin protein and increased expression of adipogenic genes. These results show that FXR acts at an early stage of the adipogenic program, at least in part by controlling the expression of negative regulators of Wnt/β-catenin signaling pathway.
Several lines of evidence indicate that the Wnt/β-catenin signaling pathway is increased in FXR−/− adipose tissue and MEF cells. The expression of the receptor, LRP5, the regulator of β-catenin stability and phosphorylation, Axin2, and the target genes of the Wnt/β-catenin signaling pathway, cyclin D1 and c-myc, were up-regulated in the absence of FXR. Cyclin D1 and c-Myc both inhibit adipocyte differentiation by decreasing PPARγ activity through histone deacetylase recruitment on the promoter of its target genes (32) and by suppressing C/EBPα and p21 gene expression (41). These results suggest that FXR interferes with the activation of the Wnt/β-catenin signaling pathway to promote adipocyte differentiation. This interference could be mediated by a decrease of β-catenin protein stability via a feedback regulation affecting the expression and protein level of Axin2 or LRP5 or via the induction of GSK3β, the kinase that phosphorylates the β-catenin protein.
Another argument for the modulation of the Wnt/β-catenin signaling pathway is the observation that FXR deficiency results in an impaired responsiveness to PPARγ activation in vitro and in vivo. First, FXR deficiency altered PPARγ expression in MEFs, and FXR reintroduction in FXR−/− MEFs increased mRNA expression of PPARγ. Moreover, FXR−/− MEFs displayed a resistance to the induction of adipocyte differentiation by rosiglitazone treatment. In the same line, FXR−/−/ob/ob mice exhibited an impaired responsiveness to PPARγ activation reflected by altered expression of several PPARγ target genes, including lipid droplet genes. These results further corroborate the existence of a cross-talk between FXR and PPARγ activities, as has been proposed in hepatocytes where FXR increases PPARγ expression, thereby regulating the antifibrotic activity of FXR in rodent liver (42).
In summary, FXR is necessary for a proper response to PPARγ activation, suggesting a cross-talk between FXR and PPARγ. Moreover, FXR contributes to the induction of adipocyte differentiation by interfering with the Wnt/β-catenin signaling pathway. These results identify a critical role for FXR in adipose tissue to ensure the accurate and complete course of adipocyte differentiation.
Supplementary Material
This work was supported by European Union Grant HEPADIP 018734, COST Action Grant BM0602, and Agence Nationale de la Recherche Grant A05056GS. This work was also supported by a grant from the Fondation pour la Recherche Médicale (to M. A.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables 1 and 2.
- FXR
- farnesoid X receptor
- PPARγ
- peroxisome proliferator-activated receptor-γ
- MEF
- mouse embryonic fibroblast
- TG
- triglyceride(s)
- C/EBP
- CAAT/enhancer-binding protein
- sFRP
- secreted Frizzled-related protein
- RSG
- rosiglitazone
- ADRP
- adipose differentiation-related protein.
REFERENCES
- 1.Lefebvre P., Cariou B., Lien F., Kuipers F., Staels B. (2009) Physiol. Rev. 89, 147–191 [DOI] [PubMed] [Google Scholar]
- 2.Makishima M., Okamoto A. Y., Repa J. J., Tu H., Learned R. M., Luk A., Hull M. V., Lustig K. D., Mangelsdorf D. J., Shan B. (1999) Science 284, 1362–1365 [DOI] [PubMed] [Google Scholar]
- 3.Wang H., Chen J., Hollister K., Sowers L. C., Forman B. M. (1999) Mol. Cell 3, 543–553 [DOI] [PubMed] [Google Scholar]
- 4.Parks D. J., Blanchard S. G., Bledsoe R. K., Chandra G., Consler T. G., Kliewer S. A., Stimmel J. B., Willson T. M., Zavacki A. M., Moore D. D., Lehmann J. M. (1999) Science 284, 1365–1368 [DOI] [PubMed] [Google Scholar]
- 5.Sinal C. J., Tohkin M., Miyata M., Ward J. M., Lambert G., Gonzalez F. J. (2000) Cell 102, 731–744 [DOI] [PubMed] [Google Scholar]
- 6.Ma K., Saha P. K., Chan L., Moore D. D. (2006) J. Clin. Invest. 116, 1102–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cariou B., van Harmelen K., Duran-Sandoval D., van Dijk T. H., Grefhorst A., Abdelkarim M., Caron S., Torpier G., Fruchart J. C., Gonzalez F. J., Kuipers F., Staels B. (2006) J. Biol. Chem. 281, 11039–11049 [DOI] [PubMed] [Google Scholar]
- 8.Duran-Sandoval D., Cariou B., Percevault F., Hennuyer N., Grefhorst A., van Dijk T. H., Gonzalez F. J., Fruchart J. C., Kuipers F., Staels B. (2005) J. Biol. Chem. 280, 29971–29979 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Lee F. Y., Barrera G., Lee H., Vales C., Gonzalez F. J., Willson T. M., Edwards P. A. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 1006–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duran-Sandoval D., Mautino G., Martin G., Percevault F., Barbier O., Fruchart J. C., Kuipers F., Staels B. (2004) Diabetes 53, 890–898 [DOI] [PubMed] [Google Scholar]
- 11.Cariou B., Bouchaert E., Abdelkarim M., Dumont J., Caron S., Fruchart J. C., Burcelin R., Kuipers F., Staels B. (2007) FEBS Lett. 581, 5191–5198 [DOI] [PubMed] [Google Scholar]
- 12.Halberg N., Wernstedt-Asterholm I., Scherer P. E. (2008) Endocrinol. Metab. Clin. North Am. 37, 753–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tontonoz P., Hu E., Spiegelman B. M. (1994) Cell 79, 1147–1156 [DOI] [PubMed] [Google Scholar]
- 14.Lin F. T., Lane M. D. (1994) Proc. Natl. Acad. Sci. U.S.A. 91, 8757–8761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Z., Bucher N. L., Farmer S. R. (1996) Mol. Cell. Biol. 16, 4128–4136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefterova M. I., Lazar M. A. (2009) Trends Endocrinol. Metab. 20, 107–114 [DOI] [PubMed] [Google Scholar]
- 17.Christodoulides C., Lagathu C., Sethi J. K., Vidal-Puig A. (2009) Trends Endocrinol. Metab. 20, 16–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Logan C. Y., Nusse R. (2004) Annu. Rev. Cell Dev. Biol. 20, 781–810 [DOI] [PubMed] [Google Scholar]
- 19.Angers S., Moon R. T. (2009) Nat. Rev. Mol. Cell Biol. 10, 468–477 [DOI] [PubMed] [Google Scholar]
- 20.Liu J., Farmer S. R. (2004) J. Biol. Chem. 279, 45020–45027 [DOI] [PubMed] [Google Scholar]
- 21.Sharma C., Pradeep A., Wong L., Rana A., Rana B. (2004) J. Biol. Chem. 279, 35583–35594 [DOI] [PubMed] [Google Scholar]
- 22.Farmer S. R. (2005) Int. J. Obes. (Lond.) 29, Suppl. 1, S13–S16 [DOI] [PubMed] [Google Scholar]
- 23.Moldes M., Zuo Y., Morrison R. F., Silva D., Park B. H., Liu J., Farmer S. R. (2003) Biochem. J. 376, 607–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rizzo G., Disante M., Mencarelli A., Renga B., Gioiello A., Pellicciari R., Fiorucci S. (2006) Mol. Pharmacol. 70, 1164–1173 [DOI] [PubMed] [Google Scholar]
- 25.Slonim D. K., Koide K., Johnson K. L., Tantravahi U., Cowan J. M., Jarrah Z., Bianchi D. W. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 9425–9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Irizarry R. A., Bolstad B. M., Collin F., Cope L. M., Hobbs B., Speed T. P. (2003) Nucleic Acids Res. 31, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarenko O. P., Rzonca S. O., Hogue W. R., Swain F. L., Suva L. J., Lecka-Czernik B. (2007) Endocrinology 148, 2669–2680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe M., Houten S. M., Mataki C., Christoffolete M. A., Kim B. W., Sato H., Messaddeq N., Harney J. W., Ezaki O., Kodama T., Schoonjans K., Bianco A. C., Auwerx J. (2006) Nature 439, 484–489 [DOI] [PubMed] [Google Scholar]
- 29.Yamauchi T., Kamon J., Waki H., Terauchi Y., Kubota N., Hara K., Mori Y., Ide T., Murakami K., Tsuboyama-Kasaoka N., Ezaki O., Akanuma Y., Gavrilova O., Vinson C., Reitman M. L., Kagechika H., Shudo K., Yoda M., Nakano Y., Tobe K., Nagai R., Kimura S., Tomita M., Froguel P., Kadowaki T. (2001) Nat. Med. 7, 941–946 [DOI] [PubMed] [Google Scholar]
- 30.Londos C., Brasaemle D. L., Schultz C. J., Segrest J. P., Kimmel A. R. (1999) Semin. Cell Dev. Biol. 10, 51–58 [DOI] [PubMed] [Google Scholar]
- 31.Puri V., Konda S., Ranjit S., Aouadi M., Chawla A., Chouinard M., Chakladar A., Czech M. P. (2007) J. Biol. Chem. 282, 34213–34218 [DOI] [PubMed] [Google Scholar]
- 32.Fu M., Rao M., Bouras T., Wang C., Wu K., Zhang X., Li Z., Yao T. P., Pestell R. G. (2005) J. Biol. Chem. 280, 16934–16941 [DOI] [PubMed] [Google Scholar]
- 33.He T. C., Sparks A. B., Rago C., Hermeking H., Zawel L., da Costa L. T., Morin P. J., Vogelstein B., Kinzler K. W. (1998) Science 281, 1509–1512 [DOI] [PubMed] [Google Scholar]
- 34.Bezaire V., Mairal A., Ribet C., Lefort C., Girousse A., Jocken J., Laurencikiene J., Anesia R., Rodriguez A. M., Ryden M., Stenson B. M., Dani C., Ailhaud G., Arner P., Langin D. (2009) J. Biol. Chem. 284, 18282–18291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennell J. A., MacDougald O. A. (2005) J. Biol. Chem. 280, 24004–24010 [DOI] [PubMed] [Google Scholar]
- 36.Bennett C. N., Ross S. E., Longo K. A., Bajnok L., Hemati N., Johnson K. W., Harrison S. D., MacDougald O. A. (2002) J. Biol. Chem. 277, 30998–31004 [DOI] [PubMed] [Google Scholar]
- 37.Ross S. E., Hemati N., Longo K. A., Bennett C. N., Lucas P. C., Erickson R. L., MacDougald O. A. (2000) Science 289, 950–953 [DOI] [PubMed] [Google Scholar]
- 38.Modica S., Murzilli S., Salvatore L., Schmidt D. R., Moschetta A. (2008) Cancer Res. 68, 9589–9594 [DOI] [PubMed] [Google Scholar]
- 39.Lagathu C., Christodoulides C., Virtue S., Cawthorn W. P., Franzin C., Kimber W. A., Nora E. D., Campbell M., Medina-Gomez G., Cheyette B. N., Vidal-Puig A. J., Sethi J. K. (2009) Diabetes 58, 609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhat R. A., Stauffer B., Komm B. S., Bodine P. V. (2004) Protein Expr. Purif. 37, 327–335 [DOI] [PubMed] [Google Scholar]
- 41.Freytag S. O., Geddes T. J. (1992) Science 256, 379–382 [DOI] [PubMed] [Google Scholar]
- 42.Fiorucci S., Rizzo G., Antonelli E., Renga B., Mencarelli A., Riccardi L., Morelli A., Pruzanski M., Pellicciari R. (2005) J. Pharmacol. Exp. Ther. 315, 58–68 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.