Abstract
To date there is no effective therapy for Alzheimer disease (AD). High levels of circulating high density lipoprotein (HDL) and its main protein, apolipoprotein A-I (apoA-I), reduce the risk of cardiovascular disease. Clinical studies show that plasma HDL cholesterol and apoA-I levels are low in patients with AD. To investigate if increasing plasma apoA-I/HDL levels ameliorates AD-like memory deficits and amyloid-β (Aβ) deposition, we generated a line of triple transgenic (Tg) mice overexpressing mutant forms of amyloid-β precursor protein (APP) and presenilin 1 (PS1) as well as human apoA-I (AI). Here we show that APP/PS1/AI triple Tg mice have a 2-fold increase of plasma HDL cholesterol levels. When tested in the Morris water maze for spatial orientation abilities, whereas APP/PS1 mice develop age-related learning and memory deficits, APP/PS1/AI mice continue to perform normally during aging. Interestingly, no significant differences were found in the total level and deposition of Aβ in the brains of APP/PS1 and APP/PS1/AI mice, but cerebral amyloid angiopathy was reduced in APP/PS1/AI mice. Also, consistent with the anti-inflammatory properties of apoA-I/HDL, glial activation was reduced in the brain of APP/PS1/AI mice. In addition, Aβ-induced production of proinflammatory chemokines/cytokines was decreased in mouse organotypic hippocampal slice cultures expressing human apoA-I. Therefore, we conclude that overexpression of human apoA-I in the circulation prevents learning and memory deficits in APP/PS1 mice, partly by attenuating neuroinflammation and cerebral amyloid angiopathy. These findings suggest that elevating plasma apoA-I/HDL levels may be an effective approach to preserve cognitive function in patients with AD.
Keywords: Alzheimer Disease, Amyloid, Apolipoproteins, High Density Lipoprotein (HDL), Mouse, Transgenic, Cerebral Amyloid Angiopathy, Cognitive Function, Learning and Memory, Neuroinflammation
Introduction
Alzheimer disease (AD)2 is currently estimated to affect over 5 million people in the United States alone. Each day, ∼1,200 new cases of AD develop, which translates to 450,000 new cases each year. Due to our aging population, it is expected that the prevalence of the disease will double in the next 40 years (1). There is no known cure for AD, and only a few treatments are available that slow the progression of symptoms. The current mantra in the field of AD research is that loss of neurons, increase in inflammation (activation of astrocytes and microglia), and concurrent decline in the cognitive abilities of AD patients are all caused by the amyloidogenic products of amyloid-β precursor protein (APP), amyloid-β peptides (Aβ). However, it is becoming increasingly evident that many factors contribute to the onset and continued aggressive progression of Aβ plaque formation and cognitive decline in AD patients. Included in this list are factors such as diet, obesity, diabetes, and cardiovascular disease (2–5). It is therefore imperative to identify other disease processes that affect the development of AD and any possible avenues that may be available to prevent AD and/or halt its progression.
With these considerations in mind, we focused on a protein that has been shown to play an important role in protecting against cardiovascular disease, apolipoprotein A-I (apoA-I) (6). ApoA-I is a major constituent of high density lipoprotein (HDL) in the plasma and plays a crucial role in reverse cholesterol transport, a process that removes excess cholesterol from peripheral tissues and carries it to the liver for excretion (7). It is well established that high levels of apoA-I/HDL and low levels of low density lipoprotein (LDL) in plasma decrease the risk of developing cardiovascular disease (8). In addition, apoA-I possesses anti-inflammatory and antioxidative properties and modulates immune function at the cellular level (9), contributing to its atheroprotective effects. Interestingly, low level expression of apoA-I from macrophages reduces atherosclerosis burden without increasing plasma HDL levels (10–13).
Although apoA-I is produced mainly in the liver and intestine, it is able to cross the blood-brain barrier and is found in the cerebrospinal fluid (CSF) and throughout the brain (14). It is associated with an HDL-like particle that also contains apoE in the CSF (15). Along with apoE, apoA-I is found to be associated with Aβ plaques in AD brains (16). ApoA-I has also been shown to bind Aβ in the CSF and plasma (16–18) and to bind full-length APP (19). In addition, apoA-I polymorphisms have been associated with AD and/or dementia (20, 21). Furthermore, epidemiological studies show a marked decrease of plasma apoA-I levels in human AD patients (22) and demonstrate that decreased HDL cholesterol and serum apoA-I concentrations are highly correlated with the severity of AD (23). Recently, results from the Honolulu-Asian Aging Study strongly suggest that apoA-I levels, more than HDL levels, inversely associate with the risk of AD (24). Therefore, we hypothesize that increasing plasma apoA-I levels provides protection against the development of AD.
To test this hypothesis, we generated a line of triple transgenic (Tg) mice overexpressing mutant forms of APP and PS1 (presenilin 1) as well as human apoA-I (AI) by cross-breeding two well described mouse models: APP/PS1 double Tg mice (25, 26) and human apoA-I Tg mice (27). Here we report that, compared with APP/PS1 double Tg mice, APP/PS1/AI triple Tg mice have a 2-fold increase in plasma HDL cholesterol levels. Although APP/PS1 mice develop age-related learning and memory deficits, APP/PS1/AI mice continue to perform normally in the Morris water maze test despite sustained global Aβ deposition in the brain during aging. The preservation of cognitive function in APP/PS1/AI mice appears to be related to the anti-inflammatory properties of human apoA-I/HDL and the reduction of cerebral amyloid angiopathy (CAA).
EXPERIMENTAL PROCEDURES
Animals
APP/PS1 double Tg mice (B6C3-Tg(APPswe, PSEN1dE9)85Dbo/J; stock number 004462) (25, 26) and human apoA-I Tg mice (C57BL/6-Tg(APOA1)1Rub/J; stock number 001927) (27) were purchased from the Jackson Laboratory (Bar Harbor, ME). APP/PS1 mice begin to develop Aβ plaques by 4–6 months of age and have robust plaques by 12 months old. The human AI Tg mice overexpress human apoA-I under the control of its natural promoter and have a 2-fold increase in plasma apoA-I/HDL and almost complete replacement of mouse apoA-I with human apoA-I (27). All transgenes are in the heterozygous state; therefore, breeding of APP/PS1 mice with AI Tg mice produced four genotypes of mice: APP/PS1/AI, APP/PS1, AI, and WT mice. Verification of genotypes was confirmed by PCR analysis of genomic DNA from tail biopsies. C57BL/6 mice (Jackson Laboratory) were used for organotypic hippocampal slice cultures. All animal procedures used for this study were prospectively reviewed and approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Behavioral Assessment
Spatial learning and memory abilities of mice were assessed in the Morris water maze as described previously (28–31). Briefly, the Morris water maze consists of a round basin filled with water. The room contained abundant extra-maze visual cues for orientation. The acquisition of the spatial task consists of placing the mice next to and facing the wall successively in north (N), east (E), south (S), and west (W) positions, with the escape platform hidden 1 cm beneath water level in the middle of the NE quadrant. In each trial, the mouse was allowed to swim until it finds the hidden platform or until 60 s had elapsed, at which point the mouse was guided to the platform, where it remained for 10 s before being returned to a cage containing paper towels to dry. The escape latency and swim path length were recorded by the SMART System (San Diego Instruments, San Diego, CA) for four trials daily for 5 days. The day after the acquisition phase, a probe trial was conducted by removing the platform and placing the mouse next to and facing the N side. The time spent in the previously correct (target) quadrant was measured in a single 60-s trial. Two hours later, the visible platform version of the test was performed with the escape platform lifted 1 cm above water level and shifted to the SE quadrant. A spire was added to the top of the escape platform as a viewing aid. This is used to evaluate the visual acuity of the mice. Mice exhibiting difficulty in finding the visible platform (escape latency greater than the mean plus two S.D. values within the group) were excluded from the final analysis.
CSF and Blood Collection and Brain Tissue Preparation
CSF was collected from the mice by a method described previously (32). Briefly, the mice were deeply anesthetized and securely placed into an immobilizing apparatus for stability, and an incision was made at the base of the skull. At this point, the musculature was gently removed from the skull and cervical vertebrae to reveal a small area of meninges overlying the cisterna magna. A small bore needle was used to pierce a hole in the meninges, and up to 20 μl of CSF was collected with a pipette. To ensure that there was no plasma contamination, the CSF samples were subjected to immunoblot analysis for apoB, which is abundant in the plasma but absent in the CSF. Following CSF collection, blood was collected by cardiac puncture with heparin as an anticoagulant. Following perfusion via the heart with ice-cold PBS, brains were cut sagittally into left and right hemispheres. The right hemisphere was fixed in buffered formalin for histological analysis. The left cerebrum was snap frozen in liquid nitrogen and stored at −80 °C for biochemical analysis.
Plasma Lipoprotein Analysis
Plasma total and HDL cholesterol levels and profiles were determined using commercial reagents (Thermo Scientific, Victoria, Australia) as described previously (28–31). The profiles were further analyzed to obtain the area under the curve by the PeakFit software (SSI, San Jose, CA). For measuring plasma paraoxonase (PON) activity, protocols described previously (33, 34) were followed with minor modifications. Briefly, 5 μl of plasma HDL fraction was added to 200 μl of Tris/HCl buffer (100 mm, pH 8.0) containing 2 mm CaCl2 and 1 mm paraoxon (O,O-diethyl-O-p-nitrophenyl phosphate; Sigma) in duplicate in 96-well plates. The rate of generation of p-nitrophenol was determined every 2 min for 20 min at 405 nm, 25 °C, in a plate reader. Enzymatic activity was calculated from the molar extinction coefficient of 17,100 m−1 cm−1. One unit of PON activity is defined as 1 nmol of p-nitrophenol formed/min under the above assay conditions.
Organotypic Hippocampal Slice Culture
The hippocampal slice cultures were obtained from 7-day-old C57BL/6 pups and maintained in vitro as previously described (35). Briefly, the pups were beheaded, and brains were removed and placed into ice-cold dissecting medium containing Neurobasal-A medium (Invitrogen) plus d-glucose (36 mm) (Sigma) and antibiotics/antimycotics (1:100) (Invitrogen). Hippocampi were separated from the cortex and placed onto a 1.5% agar bed and sliced into 400-μm sections using a tissue slicer (Siskiyou Corp., Grants Pass, OR). The slices were then transferred to fresh ice-cold dissecting medium, separated, and incubated at 4 °C for 30 min. Following incubation, the slices were placed onto 0.4 μm pore size/12 mm diameter inserts (Millipore, Billerica, MA) in a 24-well plate (Falcon, Lincoln Park, NJ) containing 200 μl of culture medium consisting of Neurobasal-A, heat-inactivated horse serum (20%), B-27 supplement (1:50), antibiotics/antimycotics (1:100) (all from Invitrogen), and l-glutamine (1 mm) (Mediatech, Manassas, VA). The plates were then placed into an incubator and maintained at 37 °C, 5% CO2. After 4 days in culture, the slices were slowly weaned off of serum. The medium is removed and replaced with 10% serum-containing medium on day 4, 5% serum-containing medium on day 5, and serum-free medium on day 6.
On day 7, the slices were transduced with 20 μl of lentiviral vectors expressing either human apoA-I (lenti-AI) or green fluorescence protein (lenti-GFP) (2 × 106 IFU/μl) under the control of the CD 68 promoter (13). Seven days following transduction, the slices were treated with freshly prepared Aβ42 monomers as described previously (36). Briefly, monomeric Aβ was prepared by dissolving Aβ42 hexafluoroisopropyl alcohol peptide film in DMSO. The sample was vortexed, spun briefly in a microcentrifuge, and sonicated in a bath sonicator for 10 min. This solution was further diluted with ice-cold sterile double-distilled H2O to a concentration of 100 μm Aβ, and 10 μl was added to the slice culture for a final concentration of 5 μm. The slices/medium were harvested 48 h following Aβ treatment.
Microglial Cell Culture
BV2 cells (a murine microglial cell line generously provided by Dr. Hongwei Qin, University of Alabama at Birmingham) were propagated and maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, l-glutamine, and antibiotics/antimycotics. For experiments, 1.5 × 105 cells were plated in 24-well plates and grown to 80% confluence overnight. One hour before treatment, the medium was removed and replaced with serum-free DMEM supplemented with 0.2% fatty acid-free BSA, l-glutamine, and antibiotics/antimycotics. Purified human plasma apoA-I (37) was added to the cells, immediately followed by monomeric Aβ42. The cells were returned to the 37 °C incubator with 5% CO2 for 48 h. Following incubation, the medium was removed, and the cells were harvested for immunoblot analysis.
Immunoblot Analysis
Samples were prepared by procedures described previously (31). Briefly, frozen brain samples or microglial cells were homogenized and sonicated in SDS sample buffer (Invitrogen) containing protease inhibitor mixture (Roche Applied Science) and phosphatase inhibitor mixture (Sigma). Aliquots of samples were separated by SDS-PAGE and blotted to PVDF membranes. CSF samples were diluted with SDS sample buffer and, following separation by SDS-PAGE, transferred to nitrocellulose membranes for detection of specific proteins. The membranes were incubated with specific primary antibodies against human and mouse apoA-I (Brookwood Biomedical, Birmingham, AL), human Aβ (6E10, Signet, Dedham, MA), the carboxyl terminus of APP (CT695, Invitrogen), or IBA-1 (ionized calcium-binding adaptor molecule 1) (Wako, Richmond, VA), followed by biotinylated or HRP-conjugated secondary antibodies. Signal was detected by the Western LightningTM Chemiluminescence Reagent Plus (PerkinElmer Life Sciences) or AP Conjugate Substrate kit (Bio-Rad) and quantified by densitometric scanning using the LabWorks image acquisition and analysis software (UVP, Upland, CA). For a loading control when appropriate, the blots were stripped and reprobed with mouse anti-actin or anti-tubulin monoclonal antibody (Sigma).
Analysis of Brain Aβ and Cholesterol Levels and Chemokine/Cytokine Production in Organotypic Hippocampal Slice Culture Medium
Brain homogenates were prepared as described previously (31). Commercial ELISA kits (Invitrogen) were used to measure Aβ40 and Aβ42 levels (in PBS- and guanidine-soluble fractions) according to the manufacturer's protocol. Brain cholesterol content was measured as described previously (29). To determine the levels of chemokines/cytokines, MCP-1 (monocyte chemotactic protein-1), and IL-6 (interleukin-6), in the medium of organotypic hippocampal slice cultures, commercial ELISA kits (RayBiotech, Norcross, GA) were used according to the manufacturer's protocols.
Immunohistochemical Analysis and Quantification of Aβ Deposition, Activated Astrocytes, and Activated Microglia
Protocols for immunohistochemical analysis have been described previously (31). Briefly, formalin-fixed and paraffin-embedded tissue sections (5 μm) were subjected to immunostaining using an ABC kit (Vector, Burlingame, CA) to detect Aβ, activated microglia, and astrocytes. The primary antibody 6E10 (Signet) was used for assessing Aβ deposition, IBA-1 antibody (Wako) for assessing activated microglia, and glial fibrillary acidic protein (GFAP) antibody (Millipore) for assessing activated astrocytes. Following counterstaining with hematoxylin, immunoreactivity of Aβ, GFAP, and IBA-1 in the cortex and hippocampus of mouse brain was quantified using a histomorphometry system. Multiple images of 1 mm2 each were captured and analyzed from four coronal brain sections. A total area of 50 mm2 giving the highest total immunoreactivity was used to calculate the amyloid load and the degree of activation of microglia and astrocytes.
Congo Red Staining and Quantification of CAA
Protocols described in detail previously (38) were followed. The percentage areas occupied by total Congo Red (total amyloid load) and the Congo Red staining of blood vessels (CAA) were calculated.
Statistical Analysis
Data are expressed as means ± S.E. Comparison of different genotype groups was performed by Student's t test (for normally distributed data), the Mann-Whitney rank sum test (for non-normally distributed data), and repeated measures analysis of variance. The SigmaStat software (SPSS Science, Chicago, IL) was used for all statistical analyses. p < 0.05 was considered statistically significant.
RESULTS
Generation of APP/PS1/AI Triple Transgenic Mice and Characteristics of Their Plasma Lipoproteins
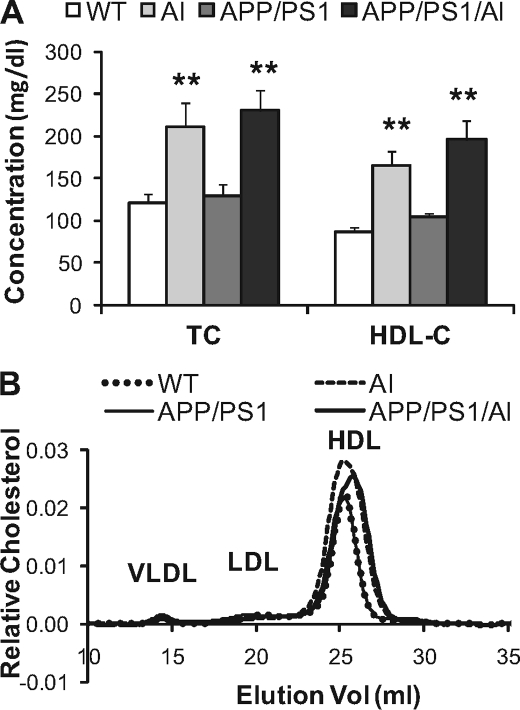
By breeding APP/PS1 double Tg mice (25, 26) with human apoA-I Tg mice (27), we produced APP/PS1/AI triple Tg mice, along with APP/PS1, AI, and WT mice. No significant differences in birth rates, appearance, general health status, and grooming behavior were observed between mice of different genotypes. To confirm that APP/PS1/AI mice indeed have elevated levels of HDL, blood samples were collected, and plasma total and HDL cholesterol levels were determined. As the results show (Fig. 1A), similar to AI mice, APP/PS1/AI mice had about a 2-fold increase in HDL cholesterol, which accounts for most of the cholesterol in the plasma of these mice. Analysis of plasma lipoprotein cholesterol profiles further confirms the elevation of HDL in AI and APP/PS1/AI mice (Fig. 1B). In the profile, although the difference in the height of HDL peaks looks relatively small, the area under the curve for HDL fractions from AI and APP/PS1/AI mice (0.071 arbitrary units) is about twice as large as the one from WT and APP/PS1 mice (0.036 arbitrary units). It is known that expression of human apoA-I in mice converts the mouse monodisperse HDL to human-like HDL profiles with two major HDL fractions, HDL2 and HDL3 (27). However, our chromatography system apparently was not sensitive enough to separate the two HDL fractions. Instead, they show up as a single wider peak.
FIGURE 1.
Plasma lipoprotein cholesterol levels and profiles. A, plasma total cholesterol (TC) and HDL cholesterol (HDL-C) levels were increased about 2-fold in mice overexpressing human AI alone (age 9.8 ± 0.1 months, n = 13 (8 male, 5 female)) and in triple transgenic APP/PS1/AI mice (age 10.0 ± 0.1 months, n = 16 (9 male, 7 female)) compared with WT (age 10.1 ± 0.1 months, n = 15 (8 male, 7 female)) and double transgenic APP/PS1 mice (age 10.0 ± 0.2 months, n = 10 (8 male, 2 female)). B, representative lipoprotein cholesterol profiles. Plasma samples from four mice (2 male, 2 female) of each genotype were pooled for the analysis. Cholesterol was increased mainly in the HDL fraction. Analysis of the area under the curve indicates that the area for HDL fractions from AI and APP/PS1/AI mice (0.071 arbitrary units) is about twice as large as the one from WT and APP/PS1 mice (0.036 arbitrary units). **, p < 0.01. Error bars, S.E.
Because the sex of the mice affects plasma lipid levels (39), we analyzed plasma cholesterol levels separately in males and females. As shown in supplemental Fig. S1, A and B, plasma total/HDL cholesterol levels are higher in males than in females. In particular, in AI and APP/PS1/AI mice, the increase of total/HDL cholesterol is much more prominent in males than in females. However, this difference did not appear to affect HDL function because the HDL-associated paraoxonase (PON) activity was not different between males and females in the same genotype of mice (supplemental Fig. S1C). Also, there was no difference in performance between male and female APP/PS1/AI mice in the Morris water maze test (supplemental Fig. S2). Therefore, both male and female were included in subsequent experiments.
APP/PS1/AI Mice Are Resistant to the Development of Learning and Memory Deficits
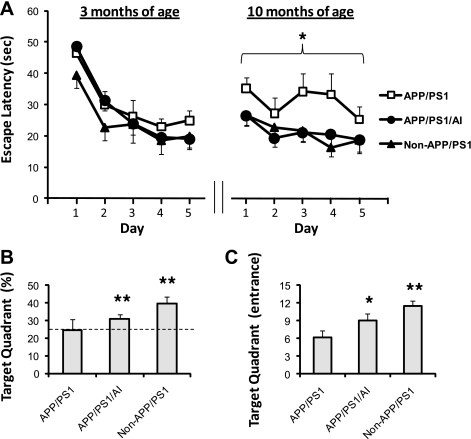
It has been shown that APP/PS1 mice develop age-dependent learning and memory deficits (31, 40). To determine whether overexpression of human apoA-I affects learning and memory function, APP/PS1/AI mice, together with APP/PS1 and non-APP/PS1 (AI and WT) mice, were subjected to the Morris water maze test at 3 months of age (before Aβ deposition) and again at 10 months of age (following Aβ plaque formation). AI and WT mice were combined because there was no difference in their performance in the Morris water maze test (supplemental Fig. S3). As shown in Fig. 2A, all groups of mice performed normally at 3 months of age during the acquisition phase of the test. However, by 10 months of age, APP/PS1 mice showed learning deficits, whereas APP/PS1/AI mice continued to perform as well as non-APP/PS1 controls.
FIGURE 2.
Preservation of spatial learning and memory function of APP/PS1/AI mice in the Morris water maze test. A, escape latencies during the acquisition phase of the test. At 3 months of age (before Aβ deposition), both APP/PS1 and APP/PS1/AI mice performed normally as non-APP/PS1 controls (WT and AI mice combined). When tested again at 10 months of age (following Aβ plaque formation), whereas APP/PS1 mice exhibited learning deficits, APP/PS1/AI mice continued to perform as well as non-APP/PS1 controls. B, in the probe trial following acquisition at 10 months of age, APP/PS1 mice only spent the chance level (25%, indicated by a dashed line) of time in the target quadrant, whereas APP/PS1/AI and non-APP/PS1 controls spent significantly more than the 25% chance level. C, during the probe trial, APP/PS1/AI mice, as did non-APP/PS1 mice, made significantly more entries to the target quadrant than APP/PS1 mice. For clarity, error bars are shown in only one direction. n = 11 (6 male, 4 female) for APP/PS1 mice; n = 11 (5 male, 6 female) for APP/PS1/AI mice; n = 10 (6 male, 4 female) for non-APP/PS1 mice. *, p < 0.05; **, p < 0.01. Error bars, S.E.
To assess memory retention following acquisition, the mice were subjected to a single probe trial 24 h later. Our findings in the acquisition test are bolstered by the finding that whereas APP/PS1 mice only spent 24.7 ± 5.8% of the time (not different from the 25% chance level) in the target quadrant (Fig. 2B), APP/PS1/AI and non-APP/PS1 controls spent significantly more time in this location (30.8 ± 2.4% and 39.7 ± 3.7%, respectively). In addition, APP/PS1/AI mice, as controls, made significantly more entries to the target quadrant than APP/PS1 mice during the probe trial (Fig. 2C). These findings indicate that APP/PS1/AI mice are protected from the development of learning and memory deficits that occur in APP/PS1 mice.
In the visible platform test, the escape latencies for APP/PS1/AI and APP/PS1 mice did not differ (10.8 ± 2.7 versus 11.1 ± 2.6 s). To exclude the possibility that different groups of mice may have different swimming abilities, the path length was recorded during the Morris water maze test for the calculation of swimming speed. The results show that there was no difference between APP/PS1/AI and APP/PS1 mice in swimming speed (18.0 ± 0.8 versus 17.3 ± 1.0 cm/s). These data further indicate that improvement of learning and memory in APP/PS1/AI mice was not caused by changes in non-cognitive parameters.
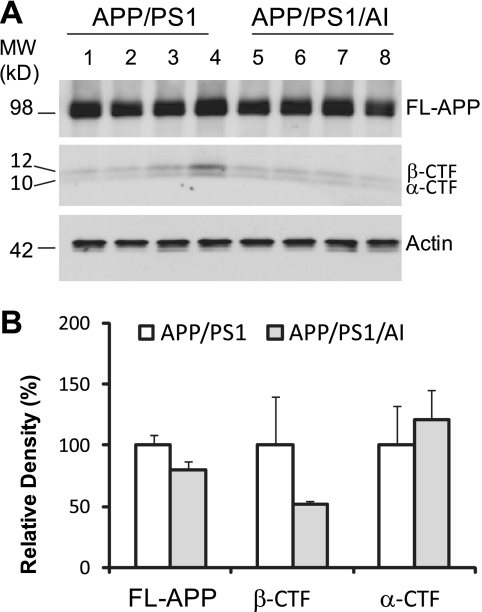
Overexpression of Human ApoA-I Does Not Alter APP Processing or the Steady State Level of or Deposition of Aβ in the Brain
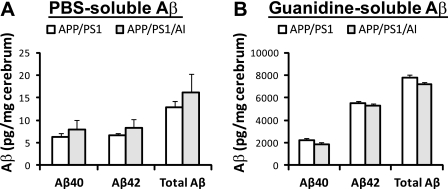
To determine if overexpression of human apoA-I affects the extent of cerebral amyloidosis in the brain of APP/PS1 mice, three independent assays were conducted. First, we used immunoblot analysis to quantify full-length APP and its carboxyl-terminal fragments (CTF) in the cerebral homogenate. The results show that there were no significant differences in the amount of full-length APP, α-CTF, and β-CTF between APP/PS1 and APP/PS1/AI mice (Fig. 3), indicating that overexpression of apoA-I does not affect the expression of APP and the processing of APP by α- and β-secretases significantly. Next, we used Aβ40- and Aβ42-specific ELISA to quantify the levels of PBS- and guanidine-soluble Aβ in the cerebral homogenate. The results show that the steady state levels of either PBS- or guanidine-soluble Aβ40 and Aβ42 were not different between APP/PS1 and APP/PS1/AI mice (Fig. 4). Next, we used immunohistochemical analysis to quantify Aβ deposition in brain sections. Consistent with the data on APP processing and Aβ levels, the results show that cerebral Aβ deposition did not differ significantly between APP/PS1 and APP/PS1/AI mice (Fig. 5). In addition, total cholesterol content in brain was not different between APP/PS1 and APP/PS1/AI mice (14.9 ± 0.4 versus 15.1 ± 0.6 μg/mg brain wet weight). These data indicate that overexpression of human apoA-I and/or elevation of plasma HDL do not affect the total Aβ load and that preservation of learning and memory function in APP/PS1/AI mice is independent of general β-amyloidosis status in the brains of these mice.
FIGURE 3.
Overexpression of human apoA-I does not affect steady state levels of APP expression and the production of α- and β-CTF. A, immunoblot analysis of full-length APP (FL-APP) and α- and β-CTF of APP. B, densitometric analysis of immunoblots (normalized by the amount of actin) with the levels in the control group set as 100%. There were no significant differences between APP/PS1 and APP/PS1/AI mice at 10 months of age. Error bars, S.E.
FIGURE 4.
Overexpression of human apoA-I does not affect steady state levels of cerebral Aβ. Aβ40 and Aβ42 levels in the cerebral homogenate of APP/PS1 and APP/PS1/AI mice at 10 months of age were measured by ELISA. A, PBS-soluble Aβ40 and Aβ42 levels were not different between APP/PS1 and APP/PS1/AI mice. B, there was a trend for a reduced level of guanidine-soluble Aβ in APP/PS1/AI mice, but the difference did not reach statistical significance. n = 9 (6 male, 3 female) for APP/PS1/AI mice; n = 10 (7 male, 3 female) for APP/PS1/AI mice. Error bars, S.E.
FIGURE 5.
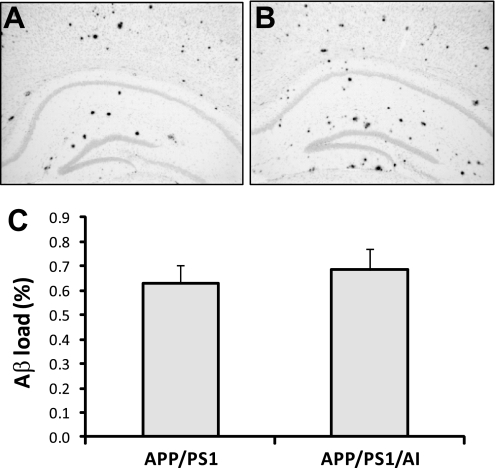
Overexpression of human apoA-I does not alter total Aβ load in the brain. A and B, representative images of Aβ immunostained brain sections from an APP/PS1 and APP/PS1/AI mouse, respectively. C, cerebral Aβ loads determined by immunohistochemical and morphometric analyses. There was no significant difference in cerebral Aβ load between APP/PS1 (n = 9 (6 male, 3 female)) and APP/PS1/AI (n = 10 (7 male, 3 female)) mice at 10 months of age. Error bars, S.E.
CAA Is Reduced in APP/PS1/AI Mice
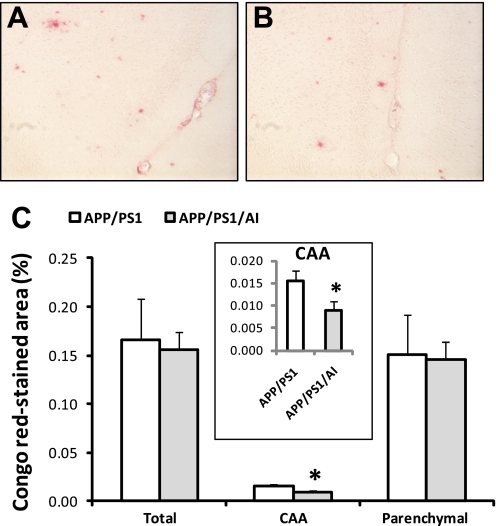
Although there was no change in total Aβ load in the brains of APP/PS1/AI mice, it is possible that overexpression of human apoA-I may affect local Aβ deposition in cerebral vessels (CAA) because apoA-I is closely involved in modulating vascular functions. To quantify CAA, Congo Red staining (38) was performed on brain sections from APP/PS1/AI and APP/PS1 mice to detect vascular and parenchymal Aβ deposits (Fig. 6). The results show that the level of CAA was significantly decreased (by 44%) in APP/PS1/AI mice compared with that in APP/PS1 mice (p < 0.05). Consistent with data from Aβ immunohistochemical analysis (Fig. 5), there were no significant changes in total or parenchymal Aβ deposition between different genotypes of mice.
FIGURE 6.
Overexpression of human apoA-I reduces CAA. A and B, representative images of Congo Red-stained brain sections from an APP/PS1 and APP/PS1/AI mouse, respectively. C, percentage of Congo Red-stained areas quantified by morphometric analysis. The level of CAA was significantly reduced in APP/PS1/AI mice (age 10.7 ± 0.4 months, n = 7 (4 male, 3 female)) compared with that in APP/PS1 mice (age 10.6 ± 0.4 months, n = 6 (4 male, 2 female)), although there were no differences in total or parenchymal staining between APP/PS1 and APP/PS1/AI mice. *, p < 0.05. Error bars, S.E.
HDL-associated Paraoxonase Activity Is Increased in APP/PS1/AI Mice
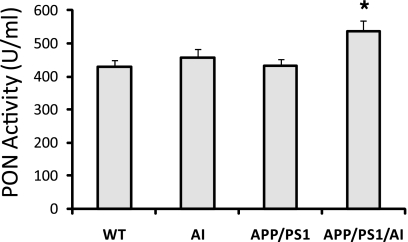
To determine if apoA-I overexpression alters HDL function in APP/PS1/AI mice, we measured PON activity. PON is an HDL-associated protein that exhibits a wide range of important hydrolytic activities, including drug metabolism and detoxification of nerve agents (41). Recent studies have also shown that PON, by inhibiting the oxidation of LDL, contributes to the antioxidative and anti-inflammatory properties of HDL and plays a crucial role in protection against atherosclerosis (41, 42). We conducted an in vitro assay for PON activity in the HDL fraction of the plasma from mice with different genotypes. The results show that in APP/PS1/AI mice, the PON activity was increased significantly compared with that in APP/PS1 mice (Fig. 7). Interestingly, PON activity was not increased in AI mice, in which the level of HDL was similarly elevated as in APP/PS1/AI mice. It has been shown that PON activity is not always correlated with apoA-I/HDL levels (43). Also, an increase of PON is observed in response to an increase of oxidative stress in the atherosclerotic lesion (44). Our data suggest that the increase of PON activity may be a compensatory response to the oxidative stress and inflammation present in APP/PS1 mice but not in AI mice.
FIGURE 7.
HDL-associated PON activity is increased in APP/PS1/AI mice. Although AI mice had a similar level of HDL as APP/PS1/AI mice, HDL-associated PON activity was not increased in the plasma of AI mice. Samples from the same mice as in Fig. 1 were used. *, p < 0.05. Error bars, S.E.
Human ApoA-I Replaces Endogenous Mouse ApoA-I in the CSF of APP/PS1/AI Mice
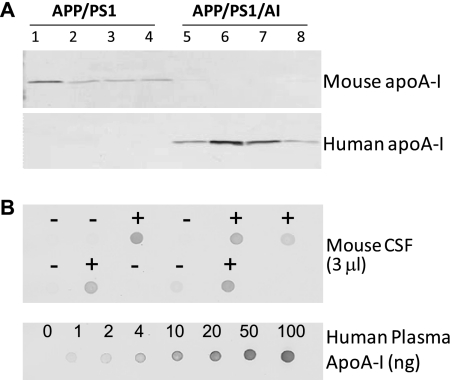
The human apoA-I Tg mice used in this study overexpress human apoA-I under the control of its natural promoter and have a 2-fold increase in plasma apoA-I/HDL levels (27) (Fig. 1). Although apoA-I is produced predominantly in the liver and the intestine, plasma apoA-I can cross the blood-brain barrier and enter the CSF (14). To ensure the availability of human apoA-I to the brain in APP/PS1/AI mice, CSF was collected from APP/PS1/AI and APP/PS1 mice and subjected to immunoblot analysis with specific antibodies against human and mouse apoA-I, respectively. The results show that endogenous mouse apoA-I decreased to undetectable levels and was replaced by human apoA-I in the CSF of APP/PS1/AI mice (Fig. 8A). To determine the level of human apoA-I in the CSF of APP/PS1/AI and AI mice, CSF samples were subjected to a semiquantitative immuno-dot-blot analysis using purified human plasma apoA-I as standards (Fig. 8B). The results show that CSF samples from APP/PS1/AI and AI Tg mice have human apoA-I at the level of 0.3 ± 0.1 mg/dl, which is comparable with the level of apoA-I in human CSF (0.2–0.4 mg/dl) (15, 45).
FIGURE 8.
Human apoA-I in the CSF. A, immunoblot analyses of CSF samples from APP/PS1 and APP/PS1/AI mice at 10 months of age with specific antibodies against mouse and human apoA-I. The endogenous mouse apoA-I was undetectable and was replaced by human apoA-I in the CSF of APP/PS1/AI mice. B, representative immuno-dot-blot analysis for human apoA-I in the CSF of different genotypes of mice (+, APP/PS1/AI or AI mice; −, APP/PS1 or WT mice). Purified human plasma apoA-I was used to construct a standard curve. The results show that CSF samples from APP/PS1/AI and AI Tg mice (n = 9 (3 male, 6 female)) have human apoA-I at a level of 0.3 ± 0.1 mg/dl, which is comparable with the level of apoA-I in human CSF.
Overexpression of Human ApoA-I Attenuates Neuroinflammation
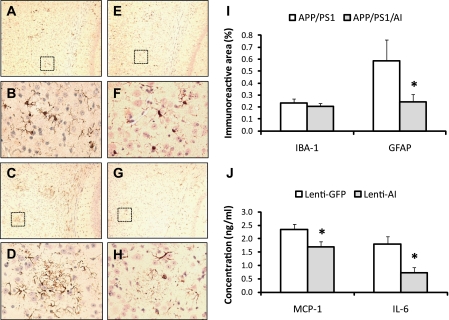
Human apoA-I/HDL possesses anti-inflammatory properties (9). Increase of PON activity in the HDL of APP/PS1/AI mice indicates a systemic enhancement of antioxidative and anti-inflammatory potential in these mice. To determine if overexpression of apoA-I affects neuroinflammation, we measured the level of microglial and astrocyte activation in the brains of APP/PS1 and APP/PS1/AI mice by immunohistochemical analyses. The results show a trend for a reduced level of microglial activation (IBA-1) in the brain of APP/PS1/AI mice, which did not reach statistical significance (Fig. 9). The extent of astrocyte activation (GFAP), however, was significantly decreased in the cortex and hippocampus of APP/PS1/AI mice compared with APP/PS1 mice (Fig. 9).
FIGURE 9.
Microglial and astrocyte activation in the mouse brain and Aβ-induced proinflammatory chemokine/cytokine production in the mouse organotypic hippocampal slice culture. A and E, representative images of IBA-1-immunostained brain sections from an APP/PS1 and APP/PS1/AI mouse, respectively, under low magnification. B and F, high magnification of the area indicated by the box in A and E, respectively. C and G, representative images of GFAP-immunostained brain sections from an APP/PS1 and APP/PS1/AI mouse, respectively, under low magnification. D and H, high magnification of the area indicated by the box in C and G, respectively. I, quantification of IBA-1 and GFAP immunoreactivity. There was a trend for a reduced level of IBA-1 immunoreactivity in APP/PS1/AI mice, but it did not reach statistical significance. The level of GFAP immunoreactivity was significantly decreased in the brains of APP/PS1/AI mice compared with that in APP/PS1 mice. J, organotypic hippocampal slice cultures were prepared from C57BL/6 mice and transduced with lentiviral vectors to express human apoA-I or GFP as control, followed by the treatment with 5 μm monomeric Aβ. Then the levels of proinflammatory chemokine/cytokine, MCP-1 and IL-6, in the culture medium were measured by ELISA. The Aβ-induced production of MCP-1 and IL-6 was significantly reduced in the culture expressing human apoA-I compared with the control culture expressing GFP (n = 12 slices/4 mice for each treatment). *, p < 0.05. Error bars, S.E.
To further study the role of human apoA-I in modulating neuroinflammation, we utilized an in vitro system, mouse organotypic hippocampal slice cultures. Lentiviral vectors (13) were used to express human apoA-I in the slice cultures, with cultures expressing GFP as controls. Following expression of human apoA-I and GFP, the slice cultures were treated with monomeric Aβ. Then culture media were collected and subjected to ELISA for the measurement of proinflammatory chemokine/cytokine, MCP-1, and IL-6. The results show that in the cultures expressing human apoA-I, the levels of both MCP-1 and IL-6 were significantly reduced compared with those in GFP control cultures (Fig. 9J).
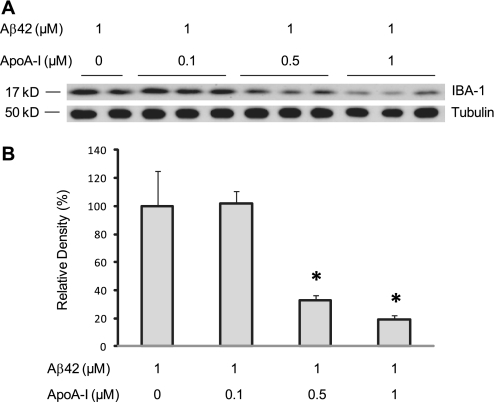
Because MCP-1 and IL-6 are produced by both astrocytes and microglia (46, 47), these results suggest that human apoA-I could inhibit the activation of both cell types. Because we observed a significant reduction of astrocyte activation but only a trend of reduction in microglial activation in APP/PS1/AI mice, we hypothesize that the amount of human apoA-I in the brains of APP/PS1/AI mice might not be sufficient to attenuate microglial activation significantly. To test this hypothesis, we treated BV2 cells, a murine microglial cell line, with Aβ42 and determined the level of microglial activation (IBA-1) in response to different concentrations of human apoA-I in the medium. As shown in Fig. 10, in the presence of 0.1 μm (equivalent to 0.3 mg/dl in the CSF of APP/PS1/AI mice) human apoA-I, the level of IBA-1 was not different from that of Aβ42 controls in the absence of human apoA-I. However, in the presence of higher levels of human apoA-I, the level of IBA-1 was decreased significantly in an apoA-I dose-dependent manner.
FIGURE 10.
Dose-dependent response of Aβ-induced microglial activation to human apoA-I. BV2 cells were co-treated with 1 μm Aβ42 and different concentrations of human apoA-I for 48 h followed by immunoblot analyses. A, immunoblot analysis of IBA-1 and tubulin. B, densitometric analysis of IBA-1 immunoblots (normalized by the amount of tubulin) with the level in control treatment (Aβ42 alone) set as 100%. The results show that human apoA-I reduces the level of IBA-1 in a dose-dependent manner. *, p < 0.05. Error bars, S.E.
DISCUSSION
In the present study, we describe a novel mouse model, APP/PS1/AI triple transgenic mice, to investigate the role of human apoA-I in AD. We observed a 2-fold increase of HDL in the plasma and a complete replacement of endogenous mouse apoA-I by human apoA-I in the CSF of APP/PS1/AI mice. Behavioral tests demonstrated that overexpression of human apoA-I prevented the development of age-related learning and memory deficits despite continued Aβ deposition in APP/PS1/AI mice. Enzymatic analysis showed that HDL-associated PON activities were increased in the HDL fraction of APP/PS1/AI mice, indicating augmentation of antioxidative and anti-inflammatory functions of HDL in these mice. Consistent with this finding, we observed reduced activation of astrocytes in vivo and decreased chemokine/cytokine levels in response to Aβ treatment in vitro in the presence of human apoA-I. Taken together, these findings suggest that one of the mechanisms by which human apoA-I preserves cognitive function is by attenuating neuroinflammation. Also, although total Aβ load was not changed, the level of CAA was decreased significantly in APP/PS1/AI mice, presenting another mechanism for apoA-I to improve brain function.
Although the role of apoA-I in the periphery (e.g. in the development of cardiovascular disease) has been studied extensively, little is known about its function in the central nervous system. There have only been a limited number of studies on the potential role of apoA-I/HDL in AD, and the results from those studies are inconsistent. Some epidemiological studies show a marked decrease of plasma apoA-I levels in human AD patients (22) and a strong correlation between decreased HDL/apoA-I levels and the severity of AD (23). Recently, results from the Honolulu-Asian Aging Study strongly suggest that apoA-I levels, more than HDL cholesterol levels, negatively associate with the risk of AD (24). The study shows that low levels of apoA-I are a risk factor for dementia in both apoE4 and non-apoE4 carriers. Other epidemiological studies, however, show no connections between apoA-I/HDL levels and AD status (48, 49). In contrast, two other studies report that increased levels of apoA-I/HDL may increase the risk of AD; one shows that increased HDL cholesterol levels are associated with an increasing number of amyloid plaques in human brain (50), whereas the other shows that a polymorphism in the promoter of the apoA-I gene that increases plasma apoA-I level is associated with an earlier onset of non-familial AD (20). However, a more recent study reports no association between this polymorphism and AD (21). Although in vitro studies demonstrate that human apoA-I directly interacts with APP and inhibits Aβ aggregation and toxicity (19, 51, 52), a lack of endogenous mouse apoA-I had no effect on AD-like amyloidosis in a mouse model (53). The reason for the discrepancies among these studies is not known. We speculate that differential structural and functional properties of apoA-I/HDL (e.g. human versus mouse apoA-I or anti- versus proinflammatory HDL) may account for some of the discrepancies (54).
Consistent with findings in apoA-I-deficient mice (53), the level of plasma apoA-I/HDL cholesterol did not affect the total level and deposition of Aβ in the brain of APP/PS1/AI mice in the present study, although the level of CAA was decreased. This lack of effect on global brain β-amyloidosis may be related to the lack of an increase in apoA-I concentration in the brain. Although plasma apoA-I can cross the blood-brain barrier, access is relatively limited. In humans, the concentration of apoA-I in the CSF is only 0.26% of plasma levels (∼141 mg/dl) (15). In the CSF of APP/PS1/AI mice, human apoA-I replaced mouse endogenous apoA-I and measured 0.3 mg/dl on average. Although this level is similar to the apoA-I concentration in human CSF (15), it is in stark contrast to the level (245 mg/dl) in the plasma of human apoA-I transgenic mice (27). Therefore, this level of apoA-I in the brain may not be sufficient to affect the process of β-amyloidosis. Determination of whether elevating apoA-I expression locally in the brain could modulate Aβ pathology awaits further investigation.
Although we did not observe significant changes in the total level and deposition of Aβ in the brains of APP/PS1/AI mice, overexpression of human apoA-I in the periphery prevented the development of learning and memory deficits in this model, indicating a protective role of human apoA-I in cognitive function independent of the status of general β-amyloidosis. It was not known if a lack of mouse endogenous apoA-I had any detrimental effects on learning and memory performance because behavioral functions were not assessed in the apoA-I-deficient mouse model of AD (53). Recently, Lefterov et al. (68) assessed behavioral functions in apoA-I-deficient APP/PS1 (APP/PS1/AIKO) mice. Consistent with our data, APP/PS1/AIKO mice exhibit learning and memory deficits without affecting total Aβ load in the brain. In addition, clinical studies have shown that plasma HDL levels are highly and positively correlated with cognitive function in centenarians (55, 56). On the other hand, low HDL levels have been found to be associated with poor memory and decline in memory in middle-aged adults (57).
How apoA-I/HDL modulates memory function is not fully understood. Several potential mechanisms have been proposed (58, 59). One of the major protective functions of apoA-I/HDL relates to its antioxidative and anti-inflammatory properties. In AD, neuroinflammation is chronic (60). As Aβ is produced and deposited, a cascade of signaling events occurs (61), which activates microglia and increases the production of proinflammatory cytokines and chemokines that lead to the activation of astrocytes. In APP/PS1/AI mice, plasma HDL-associated PON activity was increased, indicating an augmentation of antioxidative and anti-inflammatory functions of HDL. Consistently, the level of activation of astrocytes was attenuated in the brains of APP/PS1/AI mice. There was also a trend for a reduced level of activation of microglia, but it did not reach statistical significance. Results from experiments in BV2 cells indicate that the amount of human apoA-I in the brains of APP/PS1/AI mice was not sufficient to attenuate microglial activation in vivo. Higher concentrations of human apoA-I were able to inhibit Aβ-induced microglial activation significantly in BV2 cells. Consistently, in organotypic hippocampal slice cultures treated with Aβ, the production of proinflammatory chemokine/cytokine, MCP-1 and IL-6, was significantly reduced in the presence of human apoA-I. These results are significantly relevant because previous studies have shown that production of both MCP-1 and IL-6 are increased in response to Aβ treatment in vitro and in human AD brain samples (46, 47). In addition, Lefterov et al. (68) show that human apoA-I forms SDS-stable complexes with Aβ, preventing the formation of toxic high molecular weight oligomers and fibrils. Furthermore, our findings are in agreement with recent reports that a human apoA-I mimetic peptide inhibits inflammation in the brain and improves cognitive performance in Western diet-fed LDL receptor-deficient mice (62) and in APP/PS1 mice with co-treatment of a statin drug (pravastatin) (63). Furthermore, in APP/PS1/AI mice, although total Aβ deposition is not changed, the level of CAA is significantly decreased. This finding is consistent with data from a recent study by Lefterov et al. (68), in which lack of apoA-I increases CAA in APP/PS1 mice. Therefore, human apoA-I may improve cerebral vascular functions by reducing CAA in APP/PS1/AI mice, contributing to the prevention of cognitive impairment.
How apoA-I/HDL regulates inflammation has been under intensive investigation. Recently, Yvan-Charvet et al. (64) reviewed evidence that apoA-I/HDL exerts anti-inflammatory functions by modulating sterol/lipid levels and organization in the cell membrane and via Toll-like receptor signaling. Interestingly, Smoak et al. (65) also reported that apoA-I signals in the macrophage through Toll-like receptors. However, in contrast to previous findings, apoA-I was shown to be proinflammatory (activates nuclear factor-κB and induces cytokines) instead of anti-inflammatory. The authors suggest that the form of apoA-I (lipid-free versus lipid-associated), the presence or absence of infections, the period of exposure (acute versus chronic), and the model system (in vitro versus in vivo) may all affect the outcome of apoA-I/HDL-related studies. Clearly, how apoA-I/HDL regulates inflammatory response is more complicated than previously thought and warrants further investigations.
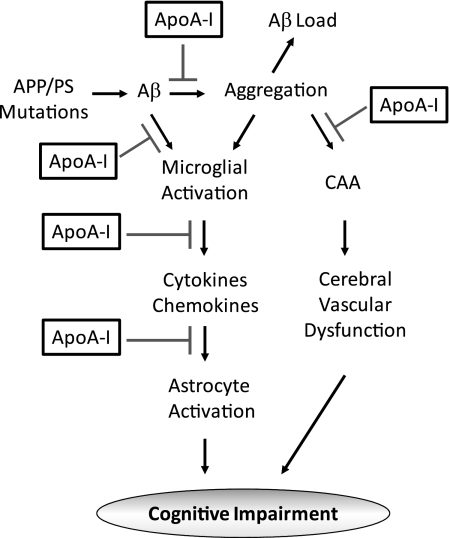
In summary, although peripheral overexpression of human apoA-I is not sufficient to significantly affect the total level and deposition of Aβ in the brains of APP/PS1/AI mice, it attenuates Aβ-associated neuroinflammation and CAA, leading to the preservation of cognitive function in these mice (Fig. 11).
FIGURE 11.
Schematic presentation of potential mechanisms by which apoA-I prevents the development of cognitive impairment. Human apoA-I reduces the production of proinflammatory cytokines/chemokines, such as IL-6 and MCP-1, and attenuates the activation of microglia and astrocytes. In addition, apoA-I inhibits Aβ aggregation and reduces its toxicity. Furthermore, although apoA-I does not change total Aβ load, it ameliorates CAA, potentially improving cerebral vascular functions.
Increasing evidence indicates that the disease process begins prior to Aβ and Tau pathologies in AD. Memory impairment is one of the earliest symptoms of the disease. Aβ plaques and Tau tangles are most likely the consequence rather than the cause of the disease process. This concept is supported by a recent report that Aβ plaque removal does not lead to improved cognitive function (66). Thus, it seems necessary to intercede early to halt and/or reverse the disease process. Elevating the level of apoA-I/HDL has emerged as a promising strategy for early intervention in cardiovascular diseases (67). Our findings in APP/PS1/AI mice suggest that increasing apoA-I/HDL levels could also be an effective approach to prevent the development of cognitive impairment in AD.
Supplementary Material
Acknowledgments
We thank Drs. David Holtzman and John Cirrito for training in the collection of CSF from mice. We thank Drs. Lucas Pozzo-Miller and Gaston Calfa for assistance in mouse organotypic hippocampal slice culture preparation and Dr. Hongwei Qin for providing BV2 cells.
This work was supported, in whole or in part, by National Institutes of Health Grants AG031846, HL65709, HL086988, and AG03012801. This work was also supported by American Health Assistance Foundation Grant A2010328, Alzheimer's Association Grants IIRG-09-131791 and ZEN-08-99900, American Heart Association Grant 0455304B, University of Illinois Chicago Center for Clinical and Translational Science Pilot Grant UL1RR029879, and anonymous philanthropic foundations.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- AD
- Alzheimer disease
- Aβ
- amyloid-β protein
- AI
- human apoA-I
- APP
- amyloid-β precursor protein
- CAA
- cerebral amyloid angiopathy
- CSF
- cerebrospinal fluid
- GFAP
- glial fibrillary acidic protein
- PON
- paraoxonase
- CTF
- carboxyl-terminal fragment(s)
- Tg
- transgenic.
REFERENCES
- 1.Alzheimer's Association (2010) Alzheimers Dement. 6, 158–194 [DOI] [PubMed] [Google Scholar]
- 2.Scarmeas N., Luchsinger J. A., Schupf N., Brickman A. M., Cosentino S., Tang M. X., Stern Y. (2009) JAMA 302, 627–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naderali E. K., Ratcliffe S. H., Dale M. C. (2009) Am. J. Alzheimers Dis. Other Demen. 24, 445–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Craft S. (2009) Arch. Neurol. 66, 300–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fillit H., Nash D. T., Rundek T., Zuckerman A. (2008) Am. J. Geriatr. Pharmacother. 6, 100–118 [DOI] [PubMed] [Google Scholar]
- 6.Linsel-Nitschke P., Tall A. R. (2005) Nat. Rev. Drug Discov. 4, 193–205 [DOI] [PubMed] [Google Scholar]
- 7.Wang X., Rader D. J. (2007) Curr. Opin. Cardiol. 22, 368–372 [DOI] [PubMed] [Google Scholar]
- 8.Segrest J. P., Anantharamaiah G. M. (1994) Curr. Opin. Cardiol. 9, 404–410 [PubMed] [Google Scholar]
- 9.Barter P. J., Nicholls S., Rye K. A., Anantharamaiah G. M., Navab M., Fogelman A. M. (2004) Circ. Res. 95, 764–772 [DOI] [PubMed] [Google Scholar]
- 10.Ishiguro H., Yoshida H., Major A. S., Zhu T., Babaev V. R., Linton M. F., Fazio S. (2001) J. Biol. Chem. 276, 36742–36748 [DOI] [PubMed] [Google Scholar]
- 11.Major A. S., Dove D. E., Ishiguro H., Su Y. R., Brown A. M., Liu L., Carter K. J., Linton M. F., Fazio S. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 1790–1795 [DOI] [PubMed] [Google Scholar]
- 12.Su Y. R., Ishiguro H., Major A. S., Dove D. E., Zhang W., Hasty A. H., Babaev V. R., Linton M. F., Fazio S. (2003) Mol. Ther. 8, 576–583 [DOI] [PubMed] [Google Scholar]
- 13.Su Y. R., Blakemore J. L., Zhang Y., Linton M. F., Fazio S. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1439–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dietschy J. M., Turley S. D. (2001) Curr. Opin. Lipidol. 12, 105–112 [DOI] [PubMed] [Google Scholar]
- 15.Koch S., Donarski N., Goetze K., Kreckel M., Stuerenburg H. J., Buhmann C., Beisiegel U. (2001) J. Lipid Res. 42, 1143–1151 [PubMed] [Google Scholar]
- 16.Harr S. D., Uint L., Hollister R., Hyman B. T., Mendez A. J. (1996) J. Neurochem. 66, 2429–2435 [DOI] [PubMed] [Google Scholar]
- 17.Golabek A., Marques M. A., Lalowski M., Wisniewski T. (1995) Neurosci. Lett. 191, 79–82 [DOI] [PubMed] [Google Scholar]
- 18.Koudinov A. R., Berezov T. T., Kumar A., Koudinova N. V. (1998) Clin. Chim. Acta. 270, 75–84 [DOI] [PubMed] [Google Scholar]
- 19.Koldamova R. P., Lefterov I. M., Lefterova M. I., Lazo J. S. (2001) Biochemistry 40, 3553–3560 [DOI] [PubMed] [Google Scholar]
- 20.Vollbach H., Heun R., Morris C. M., Edwardson J. A., McKeith I. G., Jessen F., Schulz A., Maier W., Kölsch H. (2005) Ann. Neurol. 58, 436–441 [DOI] [PubMed] [Google Scholar]
- 21.Helbecque N., Codron V., Cottel D., Amouyel P. (2008) Dement. Geriatr. Cogn. Disord. 25, 97–102 [DOI] [PubMed] [Google Scholar]
- 22.Kawano M., Kawakami M., Otsuka M., Yashima H., Yaginuma T., Ueki A. (1995) Clin. Chim. Acta 239, 209–211 [DOI] [PubMed] [Google Scholar]
- 23.Merched A., Xia Y., Visvikis S., Serot J. M., Siest G. (2000) Neurobiol. Aging. 21, 27–30 [DOI] [PubMed] [Google Scholar]
- 24.Saczynski J. S., White L., Peila R. L., Rodriguez B. L., Launer L. J. (2007) Am. J. Epidemiol. 165, 985–992 [DOI] [PubMed] [Google Scholar]
- 25.Jankowsky J. L., Slunt H. H., Ratovitski T., Jenkins N. A., Copeland N. G., Borchelt D. R. (2001) Biomol. Eng. 17, 157–165 [DOI] [PubMed] [Google Scholar]
- 26.Jankowsky J. L., Fadale D. J., Anderson J., Xu G. M., Gonzales V., Jenkins N. A., Copeland N. G., Lee M. K., Younkin L. H., Wagner S. L., Younkin S. G., Borchelt D. R. (2004) Hum. Mol. Genet. 13, 159–170 [DOI] [PubMed] [Google Scholar]
- 27.Rubin E. M., Ishida B. Y., Clift S. M., Krauss R. M. (1991) Proc. Natl. Acad. Sci. U.S.A. 88, 434–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L., Cao D., Garber D. W., Kim H., Fukuchi K. (2003) Am. J. Pathol. 163, 2155–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Cao D., Kim H., Lester R., Fukuchi K. (2006) Ann. Neurol. 60, 729–739 [DOI] [PubMed] [Google Scholar]
- 30.Cao D., Fukuchi K., Wan H., Kim H., Li L. (2006) Neurobiol. Aging 27, 1632–1643 [DOI] [PubMed] [Google Scholar]
- 31.Cao D., Lu H., Lewis T. L., Li L. (2007) J. Biol. Chem. 282, 36275–36282 [DOI] [PubMed] [Google Scholar]
- 32.DeMattos R. B., Bales K. R., Parsadanian M., O'Dell M. A., Foss E. M., Paul S. M., Holtzman D. M. (2002) J. Neurochem. 81, 229–236 [DOI] [PubMed] [Google Scholar]
- 33.Abbott C. A., Mackness M. I., Kumar S., Boulton A. J., Durrington P. N. (1995) Arterioscler. Thromb. Vasc. Biol. 15, 1812–1818 [DOI] [PubMed] [Google Scholar]
- 34.Paragh G., Balla P., Katona E., Seres I., Egerházi A., Degrell I. (2002) Eur. Arch. Psychiatry Clin. Neurosci. 252, 63–67 [DOI] [PubMed] [Google Scholar]
- 35.Chapleau C. A., Carlo M. E., Larimore J. L., Pozzo-Miller L. (2008) J. Neurosci. Methods 169, 182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stine W. B., Jr., Dahlgren K. N., Krafft G. A., LaDu M. J. (2003) J. Biol. Chem. 278, 11612–11622 [DOI] [PubMed] [Google Scholar]
- 37.Li L., Chen J., Mishra V. K., Kurtz J. A., Cao D., Klon A. E., Harvey S. C., Anantharamaiah G. M., Segrest J. P. (2004) J. Mol. Biol. 343, 1293–1311 [DOI] [PubMed] [Google Scholar]
- 38.Wilcock D. M., Gordon M. N., Morgan D. (2006) Nat. Protoc. 1, 1591–1595 [DOI] [PubMed] [Google Scholar]
- 39.Vergnes L., Baroukh N., Ostos M. A., Castro G., Duverger N., Nanjee M. N., Najib J., Fruchart J. C., Miller N. E., Zakin M. M., Ochoa A. (2000) Arterioscler. Thromb. Vasc. Biol. 20, 2267–2274 [DOI] [PubMed] [Google Scholar]
- 40.Reiserer R. S., Harrison F. E., Syverud D. C., McDonald M. P. (2007) Genes Brain Behav. 6, 54–65 [DOI] [PubMed] [Google Scholar]
- 41.Navab M., Hama S. Y., Hough G. P., Hedrick C. C., Sorenson R., La Du B. N., Kobashigawa J. A., Fonarow G. C., Berliner J. A., Laks H., Fogelman A. M. (1998) Curr. Opin. Lipidol. 9, 449–456 [DOI] [PubMed] [Google Scholar]
- 42.Shih D. M., Lusis A. J. (2009) Curr. Opin. Lipidol. 20, 288–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durrington P. N., Mackness B., Mackness M. I. (2001) Arterioscler. Thromb. Vasc. Biol. 21, 473–480 [DOI] [PubMed] [Google Scholar]
- 44.Mackness B., Hunt R., Durrington P. N., Mackness M. I. (1997) Arterioscler. Thromb. Vasc. Biol. 17, 1233–1238 [DOI] [PubMed] [Google Scholar]
- 45.Fagan A. M., Younkin L. H., Morris J. C., Fryer J. D., Cole T. G., Younkin S. G., Holtzman D. M. (2000) Ann. Neurol. 48, 201–210 [PubMed] [Google Scholar]
- 46.Szczepanik A. M., Rampe D., Ringheim G. E. (2001) J. Neurochem. 77, 304–317 [DOI] [PubMed] [Google Scholar]
- 47.Prat E., Baron P., Meda L., Scarpini E., Galimberti D., Ardolino G., Catania A., Scarlato G. (2000) Neurosci. Lett. 283, 177–180 [DOI] [PubMed] [Google Scholar]
- 48.Song H., Saito K., Seishima M., Noma A., Urakami K., Nakashima K. (1997) Neurosci Lett. 231, 175–178 [DOI] [PubMed] [Google Scholar]
- 49.Reitz C., Tang M. X., Luchsinger J., Mayeux R. (2004) Arch. Neurol. 61, 705–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Launer L. J., White L. R., Petrovitch H., Ross G. W., Curb J. D. (2001) Neurology 57, 1447–1452 [DOI] [PubMed] [Google Scholar]
- 51.Maezawa I., Jin L. W., Woltjer R. L., Maeda N., Martin G. M., Montine T. J., Montine K. S. (2004) J. Neurochem. 91, 1312–1321 [DOI] [PubMed] [Google Scholar]
- 52.Paula-Lima A. C., Tricerri M. A., Brito-Moreira J., Bomfim T. R., Oliveira F. F., Magdesian M. H., Grinberg L. T., Panizzutti R., Ferreira S. T. (2009) Int. J. Biochem. Cell. Biol. 41, 1361–1370 [DOI] [PubMed] [Google Scholar]
- 53.Fagan A. M., Christopher E., Taylor J. W., Parsadanian M., Spinner M., Watson M., Fryer J. D., Wahrle S., Bales K. R., Paul S. M., Holtzman D. M. (2004) Am. J. Pathol. 165, 1413–1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Navab M., Ananthramaiah G. M., Reddy S. T., Van Lenten B. J., Ansell B. J., Hama S., Hough G., Bachini E., Grijalva V. R., Wagner A. C., Shaposhnik Z., Fogelman A. M. (2005) Ann. Med. 37, 173–178 [DOI] [PubMed] [Google Scholar]
- 55.Atzmon G., Gabriely I., Greiner W., Davidson D., Schechter C., Barzilai N. (2002) J. Gerontol. A Biol. Sci. Med. Sci. 57, M712–M715 [DOI] [PubMed] [Google Scholar]
- 56.Barzilai N., Atzmon G., Derby C. A., Bauman J. M., Lipton R. B. (2006) Neurology 67, 2170–2175 [DOI] [PubMed] [Google Scholar]
- 57.Singh-Manoux A., Gimeno D., Kivimaki M., Brunner E., Marmot M. G. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1556–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kontush A., Chapman M. J. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 1418–1420 [DOI] [PubMed] [Google Scholar]
- 59.Scarmeas N. (2007) Am. J. Epidemiol. 165, 993–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eikelenboom P., van Exel E., Hoozemans J. J., Veerhuis R., Rozemuller A. J., van Gool W. A. (2010) Neurodegener Dis. 7, 38–41 [DOI] [PubMed] [Google Scholar]
- 61.Glass C. K., Saijo K., Winner B., Marchetto M. C., Gage F. H. (2010) Cell 140, 918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Buga G. M., Frank J. S., Mottino G. A., Hendizadeh M., Hakhamian A., Tillisch J. H., Reddy S. T., Navab M., Anantharamaiah G. M., Ignarro L. J., Fogelman A. M. (2006) J. Lipid Res. 47, 2148–2160 [DOI] [PubMed] [Google Scholar]
- 63.Handattu S. P., Garber D. W., Monroe C. E., van Groen T., Kadish I., Nayyar G., Cao D., Palgunachari M. N., Li L., Anantharamaiah G. M. (2009) Neurobiol Dis. 34, 525–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yvan-Charvet L., Wang N., Tall A. R. (2010) Arterioscler. Thromb. Vasc. Biol. 30, 139–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smoak K. A., Aloor J. J., Madenspacher J., Merrick B. A., Collins J. B., Zhu X., Cavigiolio G., Oda M. N., Parks J. S., Fessler M. B. (2010) Cell. Metab. 11, 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holmes C., Boche D., Wilkinson D., Yadegarfar G., Hopkins V., Bayer A., Jones R. W., Bullock R., Love S., Neal J. W., Zotova E., Nicoll J. A. (2008) Lancet 372, 216–223 [DOI] [PubMed] [Google Scholar]
- 67.Duffy D., Rader D. J. (2006) Circulation 113, 1140–1150 [DOI] [PubMed] [Google Scholar]
- 68.Lefterov I., Fitz N. F., Cronican A. A., Fogg A., Lefterov P., Kodali R., Wetzel R., Koldamova R. (2010) J. Biol. Chem. 285, 36945–36957 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.