Abstract
Fibronectin-binding proteins (FnBPs) of Staphylococcus aureus and Streptococcus pyogenes mediate invasion of human endothelial and epithelial cells in a process likely to aid the persistence and/or dissemination of infection. In addition to binding sites for the N-terminal domain (NTD) of fibronectin (Fn), a number of streptococcal FnBPs also contain an upstream region (UR) that is closely associated with an NTD-binding region; UR binds to the adjacent gelatin-binding domain (GBD) of Fn. Previously, UR was shown to be required for efficient streptococcal invasion of epithelial cells. Here we show, using a Streptococcus zooepidemicus FnBP, that the UR-binding site in GBD resides largely in the 8F19F1 module pair. We also show that UR inhibits binding of a peptide from the α1 chain of type I collagen to 8F19F1 and that UR binding to 8F1 is likely to occur through anti-parallel β-zipper formation. Thus, we propose that streptococcal proteins that contain adjacent NTD- and GBD-binding sites form a highly unusual extended tandem β-zipper that spans the two domains and mediates high affinity binding to Fn through a large intermolecular interface. The proximity of the UR- and NTD-binding sequences in streptococcal FnBPs is consistent with a non-linear arrangement of modules in the tertiary structure of the GBD of Fn.
Keywords: Bacteria, Collagen, Extracellular Matrix Proteins, Fibronectin, Protein-Protein Interactions
Introduction
Many cell surface-anchored bacterial proteins bind extracellular matrix proteins in the host. These proteins, also known as MSCRAMMs (microbial surface components recognizing adhesive matrix molecules) (1, 2), are likely to play roles in the establishment, dissemination, and/or persistence of infection by mediating interactions with host tissues. Fibronectin (Fn)7-binding proteins (FnBPs) from Staphylococcus aureus (FnBPA/FnBPB) (3) and Streptococcus pyogenes (SfbI/F1) (4) mediate adhesion to and invasion of host cells (5–9), including endothelial and epithelial cells. Fn is a large glycoprotein of ∼230 kDa (monomer molecular mass) which is present in human plasma in a soluble, dimeric form and in the extracellular matrix in an insoluble form. Fn has a modular structure, composed of type 1, type 2, and type 3 (F1, F2, and F3) modules that combine to form functional domains (10, 11). The N-terminal domain (NTD) is composed of five F1 modules (1–5F1), and the adjacent gelatin-binding domain (GBD) contains 6F11F22F27-8-9F1 (Fig. 1A). FnBPs from S. pyogenes, S. aureus, Streptococcus dysgalactiae, and Borrelia burgdorferi bind to the NTD through a tandem β-zipper (12–15). In other words, within these FnBPs, intrinsically disordered NTD-binding bacterial repeats (FnBRs) contain short motifs that form an anti-parallel β-strand along the triple-stranded β-sheet of consecutive F1 modules in the NTD. Structural evidence for this unusual mechanism of protein-protein recognition has been provided by both NMR spectroscopy and x-ray crystallography for all five F1 modules of the NTD and for several bacterial peptides (12, 14). Although FnBPs from staphylococci and streptococci contain multiple FnBRs (2), BBK32 from B. burgdorferi appears to contain only one (15).
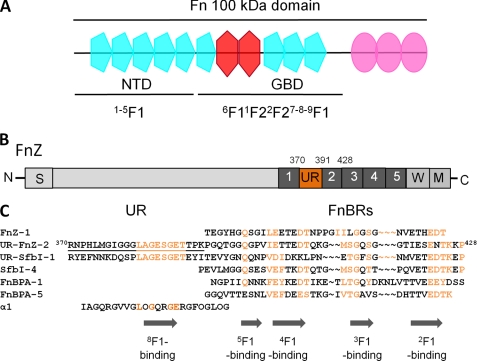
FIGURE 1.
FnZ and Fn. A, schematic of the 100-kDa N-terminal region of Fn showing F1 (blue), F2 (red), and F3 (pink) modules and showing the location of the NTD and GBD. B, schematic representation of FnZ showing putative functional domains. S, a signal sequence. Putative FnBRs (R1–R5) are colored dark gray, and the UR region is shown in orange. M and W, membrane- and wall-spanning regions, respectively. C, sequence alignment of putative 8F19F1/GBD binding (UR) and NTD-binding regions (FnBRs) from streptococcal and staphylococcal FnBPs and from the α1 chain of type I collagen. Residue numbers are given for FnZ, conserved residues in FnBRs and UR are highlighted in orange, and the location of F1-binding motifs based on published data is indicated. The FnZ peptide used in this study is underlined. Hydroxyproline residues in the collagen peptide are indicated with an O.
As is clear from its name, the GBD has long been known to bind gelatin (denatured collagen) (16). Recently, evidence for a β-zipper interaction between an F1 module of the GBD and a synthetic peptide from the α1 chain of type I collagen was obtained using both NMR spectroscopy and x-ray crystallography. A crystal structure of a GBD module pair, 8F19F1, with peptide bound (17) revealed that the collagen peptide forms an anti-parallel β-strand along the C-terminal (E) strand of triple-stranded β-sheet of 8F1. The GBD of Fn has also been shown to be important for binding of pathogenic bacteria to Fn. Talay et al. (18) showed that SfbI from S. pyogenes binds to both NTD and GBD. Binding to GBD is mediated through a spacer domain (also known as an upstream region (UR)) on SfbI adjacent to and upstream of the FnBR region. Cell invasion assays demonstrated that the SfbI UR and FnBR regions are both required for efficient invasion of streptococci into the eukaryotic cell (18).
It is likely that bacterial peptides, in binding Fn, harness (and probably modify) its normal physiological activity. Thus, understanding the mechanism of interaction of FnBPs with Fn will lead to a better understanding not only of the role of FnBPs in infection but also of the activities of Fn itself and how they might be controlled. For example, an S. pyogenes UR-containing peptide has been shown to reduce Fn matrix polymerization (19) and to increase turnover of Fn (20) and collagen I (21), suggesting possible applications in reducing pathological matrix remodeling (22).
Streptococcus zooepidemicus (Streptococcus equi subspecies zooepidemicus) is a frequent cause of opportunistic pyogenic infections in horses and also, although rarely, can cause human infections (23). An FnBP from S. zooepidemicus, FnZ (Fig. 1B), has a sequence organization similar to SfbI from S. pyogenes (2, 24), but the UR-like sequence lies between the first and second of five putative FnBRs (2, 24) (Fig. 1B). The SfbI UR motif LAGESGET is conserved in the FnZ UR (Fig. 1C) (24). Because a similar motif is also present in the α1(I) collagen peptide (Fig. 1C), we hypothesized that bacterial peptides might bind to the GBD via the same mechanism as collagen and compete with collagen for Fn binding.
The aim of this work is to determine the mechanism of binding of streptococcal FnBPs to the GBD. First, we show that a S. zooepidemicus FnZ peptide binds both 8F19F1 and GBD. Second, data are presented that support an anti-parallel β-zipper mode of binding for FnZ to 8F19F1. Last, we show that the S. zooepidemicus FnZ peptide inhibits binding of the collagen peptide to 8F19F1, presumably due to overlap of the binding sites on 8F19F1. The role of conserved residues in 8F1-binding motifs and the consequences of the proximity of the NTD- and GBD-binding sites in the streptococcal proteins for the structure of Fn are also discussed.
EXPERIMENTAL PROCEDURES
Expression and Purification of Recombinant 8F19F1
Unlabeled and uniformly 15N-labeled 8F19F1 module pair (Fn precursor residues 516–608) was expressed in Pichia pastoris using a procedure similar to that described previously (25). Secreted protein was concentrated from fermentation media by cation exchange chromatography on a 5-ml SP-Sepharose Fast Flow column (GE Healthcare) at pH 3.0, eluting with a gradient from 0 to 1 m NaCl in 20 mm citric acid over 50 column volumes. Fractions containing protein were incubated with Endo Hf (New England Biolabs) at pH 5.5 to trim the sugars attached to glycosylation sites at Asn528 and Asn542 back to a single GlcNAc. 8F19F1 was further purified by gelatin affinity chromatography on an XK16 column (GE Healthcare) packed with 20 ml of gelatin-Sepharose 4B (GE Healthcare). Protein was loaded in phosphate-buffered saline (PBS) at pH 7.3 at 4 °C and eluted with a gradient from 0 to 4 m urea. The protein was finally purified by size exclusion chromatography on a HiLoad Superdex 75 gel filtration column (GE Healthcare) equilibrated in 40 mm NaH2PO4/K2HPO4, pH 7.3, 150 mm NaCl. The 8F19F1 concentration was determined from absorbance at 280 nm, and the molecular weight was confirmed by electrospray ionization mass spectrometry (ESI-MS). SDS-PAGE analysis of the purified protein showed a single band.
Expression and Purification of FnZ
DNA for residues 370–428 of FnZ from S. zooepidemicus (24) was produced synthetically (Entelechon) and cloned into a pGEX-6P-1 vector (GE Healthcare). The protein was expressed recombinantly in E. coli BL21 (DE3) as a GST-tagged fusion protein using autoinduction. The fusion was isolated from soluble bacterial cell lysates using a 5-ml GSTrap high performance column (GE Healthcare) equilibrated in PBS, pH 7.3, and eluted using 20 mm Tris-HCl, 50 mm NaCl, 10 mm reduced glutathione, pH 7.0. Appropriate fractions were pooled, and the glutathione was removed using dialysis. After cleavage of the affinity tag using 3C protease (Novagen) (leaving five vector residues, GPLGS, at the N terminus), the FnZ construct was further purified using reversed phase high performance liquid chromatography on a C4 Jupiter column (Phenomenex). Buffer A was 0.15% trifluoroacectic acid (TFA), and buffer B was 100% acetonitrile, 0.1% TFA. Protein was loaded onto the column equilibrated in 10% buffer B and eluted on a continuous linear gradient of 10–45% buffer B in 50 ml, and appropriate fractions were lyophilized. Concentration measurements were made based on mass, and the molecular weight was confirmed by ESI-MS.
Preparation of Fn Fragments and Synthetic Peptides
The GBD of Fn was obtained as a lyophilized proteolytic fragment (Sigma) and dialyzed into 10 mm NaH2PO4/K2HPO4 (pH 7.3) before use. A recombinant Fn 100-kDa N-terminal fragment (Fig. 1A) was expressed and purified as described elsewhere (26) and dialyzed against PBS (pH 7.3) prior to use. The S. zooepidemicus FnZ synthetic peptides (sequences RNPHLMGIGGGLAGESGETTPK and LAGESGET) were obtained from Alta Bioscience UK. The N and C termini of the shorter peptide were capped by acetylation and amidation, respectively; the termini of the longer peptide were uncapped. Peptide concentration measurements were made based on mass.
Isothermal Titration Calorimetry (ITC)
ITC experiments were carried out using a VP-ITC microcalorimeter (MicroCal Inc., Northampton, MA). In a typical experiment, the cell contained 1.4 ml of Fn fragment (8F19F1, GBD, or 100 kDa), and the syringe contained 277 μl of FnZ (synthetic peptide or recombinant protein) at a concentration 10–20 times higher than that of the protein in the cell. If possible, the cell concentration was chosen to correspond to a c value of 100–1000, where c = [protein]/predicted Kd (27). Titrations with 8F19F1 and GBD were carried out in 10 mm NaH2PO4/K2HPO4, pH 7.4, at 25 °C. Both the cell and syringe solutions were degassed at 20 °C for 15 min before use. Both sets of titrations were carried out as follows. One preinjection of 2 μl of syringe solution was followed by 39 injections of 7 μl at an injection speed of 0.5 μl/s. Stirring speed was 307 rpm, and there was a delay of 360 s between injections. For both titrations, a separate heat of dilution experiment was performed by injecting peptide into buffer. The averaged heats of dilution were subtracted from the main experiments. The titration with the 100-kDa Fn fragment was performed at 37 °C in PBS (pH 7.3) after degassing both cell and syringe solutions at 30 °C for 15 min. One preinjection of 2 μl of syringe solution was followed by 26 injections of 5 μl at an injection speed of 0.5 μl/s. Stirring speed was 302 rpm, and there was a delay of 300 s between injections. The heat of dilution was taken as the average heat of six points at the end of the titration. Data were analyzed using MicroCal Origin software (version 7), with data being fitted to a one-site binding model.
NMR Spectroscopy
All experiments were carried out at 25 °C on a Bruker Avance 700-MHz spectrometer. Samples were prepared by dissolving uniformly labeled 15N-8F19F1 to a concentration of 0.2 and 1.0 mm, for binding studies and confirmation of previous 8F19F1 assignments (25), respectively, in 90% H2O, 10% D2O containing 150 mm NaCl, 20 mm KH2PO4/Na2HPO4, 0.02% sodium azide with pH adjusted to 7.2. A series of heteronuclear single-quantum coherence (HSQC) experiments with excitation sculpting for solvent suppression and with sensitivity enhancement (28) were carried out with increasing concentrations of synthetic FnZ and LAGESGET peptides (0.05, 0.1, 0.15, 0.2, 1.0, 1.5, and 2.0 mol eq) for binding studies; three-dimensional 1H15N-HSQC-NOESY and 1H15N-HSQC-TOCSY experiments were employed for spectral assignment. HSQC peaks for most 8F19F1 residues were assigned de novo in both free and FnZ peptide-bound spectra, but for five peaks that were very weak in the spectra of the 8F19F1-peptide complex, assignments were based on assignments of the 8F19F1-collagen peptide complex (17) (BMRB entry 15986). Data processing and referencing were performed using NMRPipe (29), and spectral assignment was performed with CCPNMR Analysis version 1.0.15 (30).
Competition Experiment
Competition between the FnZ peptide and the collagen peptide for binding to 8F19F1 was demonstrated by fluorescence anisotropy measurements. Samples contained a 50 nm concentration (determined by absorbance at 280 nm) of an N-terminally 5-carboxyfluorescein-labeled α1(I) collagen peptide spanning residues 778–799, which has been shown to bind 8F19F1 with an affinity (Kd) of 4.5 μm (17); unlabeled 8F19F1 at concentrations of 10, 20, or 30 mm; and unlabeled FnZ peptide at increasing concentrations in a 20 mm Tris-HCl (pH 7.4), 150 mm NaCl buffer. Samples were excited at 485 nm with a 515 nm cut-off, and fluorescence was observed at 538 nm using an M5 fluorometer (Molecular Devices) at 25 °C. Differences in fluorescence anisotropy (FA) were simultaneously fit for all 8F19F1 concentrations using the formula,
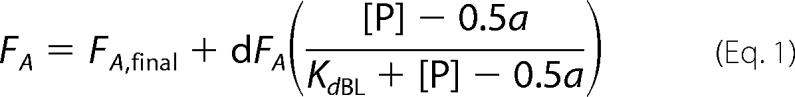 |
where
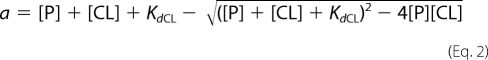 |
where FA is the fluorescence anisotropy measured at each point, FA,final is the fluorescence anisotropy at saturation of competing ligand (FnZ peptide), [P] is the protein (8F19F1) concentration, [CL] is the concentration of competing ligand, KdCL is the affinity of the competing ligand, and KdBL is the affinity of bound ligand (collagen peptide, 4.5 μm).
RESULTS
FnZ Binds the 100-kDa N-terminal Region of Fn with High Affinity
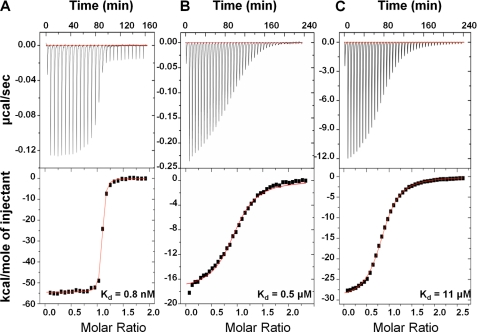
Residues 370–428 of FnZ contains a site with high sequence homology to NTD-binding FnBRs from S. pyogenes and S. aureus (2) and to the GBD-binding UR region of SfbI from S. pyogenes (18, 24) (Fig. 1C). ITC (Fig. 2A and Table 1) shows that FnZ(370–428) binds a recombinant 100-kDa N-terminal fragment of Fn containing both the NTD and GBD (in addition to three F3 modules; Fig. 1A) (26) with high affinity (Kd 0.8 ± 0.1 nm).
FIGURE 2.
ITC of FnZ binding to Fn domains. The binding isotherms for FnZ(370–428) with the 100-kDa fragment (A) and the interaction of FnZ peptide 370–391 with GBD (B) and 8F19F1 (C) are shown. The top panels show the heat differences upon injection of peptide, and the lower panels show the integrated heats of injection, with the best fit to a one-site binding model using MicroCal Origin. Thermodynamic parameters for these experiments are summarized in Table 1.
TABLE 1.
ITC analysis of interactions of FnZ with Fn domains
Thermodynamic parameters for the interaction of FnZ peptide with 8F19F1 and GBD and of FnZ(370–428) with the 100-kDa Fn fragment. Errors are from the curve fitting.
| Fn domain | [FnZ peptide] | [Fn domain] | ΔH | ΔS | Kd | n |
|---|---|---|---|---|---|---|
| mm | mm | kcal/mol | cal/mol K−1 | μm | ||
| 8F19F1 | 2.97 | 0.24 | −29.8 | −77.2 | 10.9 ± 0.3 | 0.86 |
| GBD | 0.11 | 0.01 | −17.7 | −30.8 | 0.54 ± 0.04 | 0.96 |
| [FnZ(370–428)] | [Fn domain] | ΔH | ΔS | Kd | n | |
|---|---|---|---|---|---|---|
| mm | mm | kcal/mol | cal/mol K−1 | μm | ||
| 100 kDa | 0.020 | 0.001 | −54.6 | −134.0 | 0.0008 ± 0.0001 | 1.06 |
The UR/spacer of FnZ binds 8F19F1
A sequence alignment of the UR sequences of FnZ and SfbI with the collagen I α1 peptide (residues 778–799; Fig. 1C) shows that the LAGESGET motif is conserved between FnZ and SfbI and has some homology with the collagen peptide. ITC experiments (Fig. 2 and Table 1) show that the peptide spanning residues 370–391 from FnZ (Fig. 1C) binds with micromolar affinity to both the GBD (Kd of 0.54 ± 0.04 μm; Fig. 2B) and 8F19F1 (Kd of 10.9 ± 0.3 μm; Fig. 2C). The relative affinities suggest that the majority of the FnZ peptide binding site is within 8F19F1, with some limited involvement (through direct binding or through stabilizing interdomain interfaces) of other residues in GBD.
FnZ Residues 370–391 Bind to 8F1 via an Anti-parallel β-Zipper
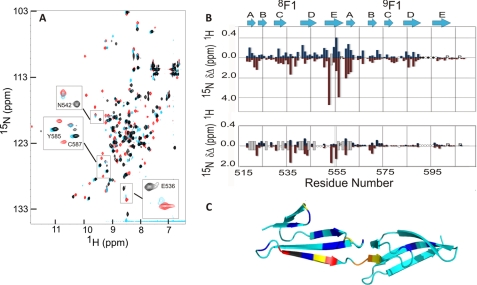
The interaction between 8F19F1 and the FnZ peptide was explored using NMR spectroscopy; a series of 1H15N-HSQC spectra were acquired for uniformly 15N-labeled 8F19F1 with increasing concentrations of peptide (Fig. 3A). The 1H15N-HSQC spectrum of 8F19F1 when bound to the FnZ peptide was assigned using three-dimensional 1H15N-HSQC-NOESY and 1H15N-HSQC-TOCSY experiments. A plot of chemical shift changes between the free and bound forms of 8F19F1 (Fig. 3B) shows that the most significant differences occur in the E-strand of 8F1 (Fig. 3C). Chemical shift changes were also observed in the A-strand of 9F1. In addition, four residues in the D-E loop of 9F1 could be assigned in the bound form, due to a sharpening of resonances compared with spectra of the unbound form of 8F19F1, where their assignment was not possible. This suggests that in the unbound form, this loop undergoes conformational exchange, resulting in line broadening that is reduced upon peptide binding. S. aureus and S. pyogenes FnBRs and the collagen α1(I) peptide bind along the E-strand of their respective F1 modules in an anti-parallel orientation. Fig. 3, A and B, shows that a shorter peptide (LAGESGET), from the C terminus of the FnZ 8F19F1-binding peptide, has no affect on 9F1 residues (e.g. Tyr585 and Cys587) that underwent chemical shift changes upon the addition of the longer peptide. Because 8F1 residues (e.g. Asn542 and Glu536) are affected by binding of both peptides, it is clear that FnZ also binds 8F19F1 in an anti-parallel orientation.
FIGURE 3.
Identification of the FnZ peptide binding site and binding orientation on 8F19F1 using NMR spectroscopy. A, overlay of the 1H15N HSQC spectra of free 8F19F1 (black) and of 8F19F1 bound to the FnZ (red) and LAGESGET (cyan) peptides. Highlighted residues are mentioned under “Results.” B, chemical shift perturbation map for 15N-labeled 8F19F1 upon binding to the FnZ (top) and LAGESGET (bottom) peptides. Blue and red bars indicate absolute chemical shift differences in backbone amide 1H and 15N nuclei, respectively, between free and peptide-bound 8F19F1. P, proline residues; ○, unassigned residues; ●, residues previously unassigned but now identified. The approximate location of previously defined secondary structure elements of the F1 modules are shown above. Peaks that disappear upon peptide binding from their chemical shift in free 8F19F1 but cannot be assigned in the peptide-bound spectrum are indicated by a gray bar of arbitrary size. C, location of combined chemical shift perturbations (square root of (ΔδHN2 + 0.2ΔδN2)) on FnZ peptide binding mapped onto the structure of 8F19F1 (17) (Protein Data Bank entry 3EJH). Small to large chemical shift changes are indicated by as follows: cyan < blue < yellow < orange < red and Trp553 (gray; prepared using PyMOL) (35).
The FnZ Peptide Binds Competitively to the Collagen-binding Site of 8F19F1
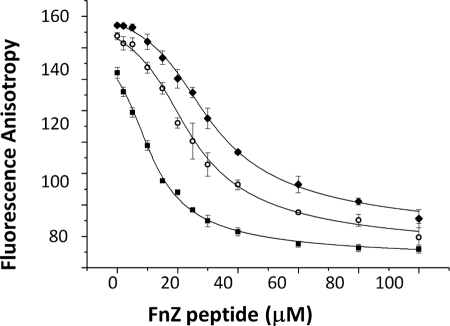
The ability of the FnZ peptide to compete with the collagen peptide for 8F19F1 binding was tested by fluorescence anisotropy. Fig. 4 shows that increasing concentrations of unlabeled FnZ peptide added to 8F19F1 plus fluorescently labeled collagen peptide led to a steady decrease in anisotropy, suggesting dissociation of the collagen peptide-8F19F1 complex. The Kd of FnZ peptide binding was determined from this experiment to be 1.9 ± 0.1 μm, indicating tighter binding to 8F19F1 than observed for the collagen peptide under similar conditions by fluorescence anisotropy (4.5 μm) (17). Together, these results suggest that residues 370–391 from FnZ bind to the same region of 8F19F1 as the collagen peptide and through a similar anti-parallel β-zipper mechanism.
FIGURE 4.
Competitive binding of FnZ peptide to 8F19F1 in presence of collagen peptide, demonstrated by fluorescence anisotropy measurements. Unlabeled FnZ peptide at increasing concentrations was incubated with unlabeled 8F19F1 at 10 (■), 20 (○), and 30 μm (♦) and the collagen peptide labeled with 5-carboxyfluorescein. Data were fit using Origin software. Error bars (S.D.) correspond to instrumental readout error and derive from triplicate measurements.
DISCUSSION
UR sequences are found in FnBPs from pathogenic streptococci (2, 18) and in BBK32, an FnBP from B. burgdorferi (2, 31). The UR binding site in SfbI from S. pyogenes had been located to the GBD (18). Here we localized the FnZ UR binding site within GBD by showing that it binds primarily to the 8F19F1 module pair.
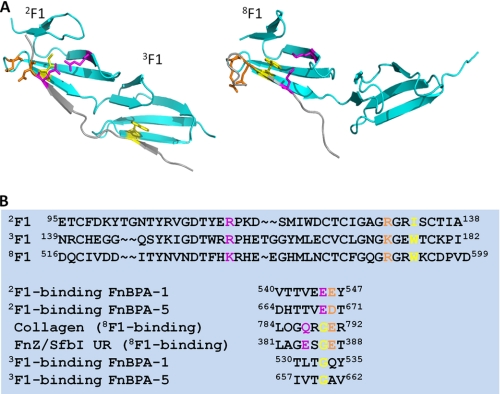
We were unable to determine the structure of the 8F19F1-FnZ peptide complex using either NMR spectroscopy or x-ray crystallography. However, by close comparison of the UR and collagen peptide sequences with previously identified F1-binding motifs from S. aureus and S. pyogenes FnBRs and the available F1 module-peptide structures (Fig. 5), it is possible to understand the role of conserved residues in the 8F1-binding motif of UR. For example, the glycine residue in the GET (UR) or GER (collagen peptide) appears to be conserved to avoid a steric clash with Trp553 in the E-strand of 8F1 (Fig. 5A). This is similar to the role of a conserved glycine residue in S. aureus FnBR 3F1-binding motifs (Fig. 5A) (14) because the E-strand of 3F1 contains a tryptophan side chain in a similar orientation to the 8F1 E-strand tryptophan (Fig. 5). This E-strand tryptophan residue is not conserved in 2F1, so here the equivalent peptide residue in the FnBR is the glutamate of the EDT motif (Fig. 5B), which forms a salt bridge with an arginine residue in the C-strand of the F1 module (Fig. 5A). In the collagen peptide-8F19F1 structure, due to the requirement for the glycine, this interaction is shifted two residues along the peptide and is formed by the glutamine in GQR (Fig. 5A) and thus in UR by the glutamate in GES. In 3F1-binding motifs, there is no equivalent negatively charged or polar residue to interact with the C-strand arginine, and in isolation, this motif binds 3F1 only very weakly (13). In 2F1 binding, the aspartate of the EDT makes polar contacts with an arginine in the loop between the D and E strands. The sequence of this loop is very similar in 8F1, and the glutamate in the collagen peptide GER makes a similar interaction (Fig. 5A) as, we suggest, does the glutamate in the GET in UR. The 3F1-binding motif lacks the negatively charged residue that could interact with the lysine residue in the D-E loop of 3F1. We predict that the threonine in the GET UR motif interacts with backbone atoms in the B-strand and D-E loop of the F1 module (as observed for homologous hydroxyl-containing residues in 2F1- and 4F1-binding motifs from S. aureus FnBPA (14)). The leucine in the LAG (UR) sequence is likely to be involved in hydrophobic contacts similar to those previously observed for the leucine in the LPG sequence in the collagen peptide (17). The role of the glycine in these motifs is less clear and will require further investigation.
FIGURE 5.
Comparison of F1-binding motifs and the role of conserved residues. A, structure of 2F13F1 in complex with the 2F13F1-binding motif from FnBPA-5 of S. aureus FnBPA (14) (Protein Data Bank entry 3CAL) and of 8F19F1 in complex with the peptide from the α1 chain of type I collagen (17) (Protein Data Bank entry 3EJH). Conserved side chains mentioned under “Results” are shown. B, sequence alignments of 2F1, 3F1, and 8F1 and of F1-binding motifs. Coloring of side chains is as in A. Sequence numbering is according to UniProtKB accession codes P02751 (fibronectin), P14738 (FnBPA), P72416 (FnZ), and P02452 (collagen α1(I) chain after subtraction of signal and propeptide sequences).
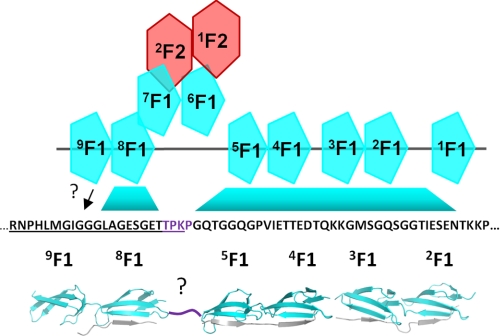
These findings have important implications for the structure of the GBD. Fig. 6 shows a model of the 70-kDa region of Fn that contains both the NTD and GBD and the sequence of UR FnZ-2 (Fig. 1C). The binding sites for F1 modules in the FnZ sequence are indicated, based on our previous work studying homologous proteins from S. pyogenes (12) (13) and S. aureus (14) and on the work presented here. What is immediately striking is that there are only four residues between the most N-terminal 5F1-binding residue and the most C-terminal 8F1-binding residue based on crystal structures of S. aureus FnBPA-1 and FnBPA-5 peptides in complex with 4F15F1 (14) and on the 8F19F1-collagen peptide complex (17). Thus, as suggested previously by the hairpin arrangement of modules in the solution structure of 6F11F22F2 (32), the GBD modules appear to have a non-linear arrangement in the complex. In fact, the FnZ sequence suggests that the 70-kDa (NTD + GBD) fragment of Fn presents a surface that is largely composed of F1 modules from both the NTD and GBD and that streptococcal FnBPs bind across this surface through a highly extended tandem β-zipper, forming a large protein-protein interface.
FIGURE 6.
Model of the 70-kDa (NTD + GBD) domain of Fn in complex with FnZ(370–428) highlighting the unusual extended tandem β-zipper proposed to form across NTD and GBD. The locations of F1-binding motifs in FnZ based on previous data from SfbI-Fn and FnBPA-Fn interactions and from data in the current work are indicated. The question marks indicate that the location of the binding site on 9F1 is uncertain, as is the conformation of the four residues (purple) between the 5F1 and 8F1-binding motifs. The model was assembled from Protein Data Bank entries 3CAL, 2RLO (14), and 3EJH (17).
The UR region in SfbI from S. pyogenes is essential for efficient invasion of bacteria into epithelial cells, resulting in a 10-fold increase in invasion compared with bacteria expressing constructs that lacked this region and contained only the NTD-binding FnBRs (18). The precise molecular mechanism of this effect is unknown. We and others (15, 19) have previously suggested that the role of bacterial FnBRs in adhesion and invasion might be to disrupt intramolecular Fn-Fn interactions that maintain the compact conformation that is observed in solution, thus exposing the integrin binding site in 10F3. The NTD has been implicated in such intramolecular interactions and was shown recently to interact with F3 modules (26, 33). It is less clear whether the GBD modules are involved in intramolecular interactions outside the GBD, but the high affinity and large intermolecular interface formed by FnBPs that bind both the NTD and GBD might be particularly efficient in disrupting intramolecular Fn-Fn interactions. In addition, the suggestion that IGD sequences within the GBD of Fn might bind to the αvβ3 integrin (34) means that UR binding to the GBD could have a more direct effect on the cell binding activity of Fn.
We showed (Fig. 4) that the FnZ peptide inhibited binding of a peptide from the α1 chain of type I collagen to 8F19F1. Further work would be required to determine if FnBPs can inhibit intact collagen-Fn interactions. Recently, it was shown that collagen I matrix turnover is regulated by Fn (21), suggesting that the interaction between polymerized Fn and collagen stabilizes collagen within the extracellular matrix and reduces endocytosis and degradation of collagen. pUR4, a GBD/NTD-binding region from SfbI with homology to FnZ (Fig. 1C), inhibits Fn polymerization and enhances collagen endocytosis and degradation (21). The identification of the UR binding site within GBD and of the role of conserved residues will aid further studies of the function of the GBD in both physiological and pathological processes involving Fn and how bacterial FnBPs might modify or exploit these functions during infection.
This research was supported by the BBSRC.
- Fn
- fibronectin
- FnBP
- fibronectin-binding protein
- NTD
- N-terminal domain
- GBD
- gelatin-binding domain
- FnBR
- fibronectin binding repeat
- UR
- upstream region
- ESI
- electrospray ionization
- ITC
- isothermal calorimetry
- HSQC
- heteronuclear single-quantum coherence
- NOESY
- nuclear Overhauser effect spectroscopy
- TOCSY
- total correlation spectroscopy.
REFERENCES
- 1.Joh H. J., House-Pompeo K., Patti J. M., Gurusiddappa S., Höök M. (1994) Biochemistry 33, 6086–6092 [DOI] [PubMed] [Google Scholar]
- 2.Schwarz-Linek U., Höök M., Potts J. R. (2006) Microbes Infect. 8, 2291–2298 [DOI] [PubMed] [Google Scholar]
- 3.Signäs C., Raucci G., Jönsson K., Lindgren P. E., Anantharamaiah G. M., Höök M., Lindberg M. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 699–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talay S. R., Valentin-Weigand P., Jerlström P. G., Timmis K. N., Chhatwal G. S. (1992) Infect. Immun. 60, 3837–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock S. J., Foster T. J., Cameron B. J., Berendt A. R. (1999) Microbiology 145, 3477–3486 [DOI] [PubMed] [Google Scholar]
- 6.Massey R. C., Kantzanou M. N., Fowler T., Day N. P., Schofield K., Wann E. R., Berendt A. R., Höök M., Peacock S. J. (2001) Cell Microbiol. 3, 839–851 [DOI] [PubMed] [Google Scholar]
- 7.Kintarak S., Whawell S. A., Speight P. M., Packer S., Nair S. P. (2004) Infect. Immun. 72, 5668–5675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jett B. D., Gilmore M. S. (2002) Infect. Immun. 70, 4697–4700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed S., Meghji S., Williams R. J., Henderson B., Brock J. H., Nair S. P. (2001) Infect. Immun. 69, 2872–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hynes R. (1990) Fibronectins, Springer-Verlag, Berlin, Germany [Google Scholar]
- 11.Potts J. R., Campbell I. D. (1994) Curr. Opin. Cell Biol. 6, 648–655 [DOI] [PubMed] [Google Scholar]
- 12.Schwarz-Linek U., Werner J. M., Pickford A. R., Gurusiddappa S., Kim J. H., Pilka E. S., Briggs J. A., Gough T. S., Höök M., Campbell I. D., Potts J. R. (2003) Nature 423, 177–181 [DOI] [PubMed] [Google Scholar]
- 13.Schwarz-Linek U., Pilka E. S., Pickford A. R., Kim J. H., Höök M., Campbell I. D., Potts J. R. (2004) J. Biol. Chem. 279, 39017–39025 [DOI] [PubMed] [Google Scholar]
- 14.Bingham R. J., Rudiño-Piñera E., Meenan N. A., Schwarz-Linek U., Turkenburg J. P., Höök M., Garman E. F., Potts J. R. (2008) Proc. Natl Acad. Sci. U.S.A. 105, 12254–12258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raibaud S., Schwarz-Linek U., Kim J. H., Jenkins H. T., Baines E. R., Gurusiddappa S., Höök M., Potts J. R. (2005) J. Biol. Chem. 280, 18803–18809 [DOI] [PubMed] [Google Scholar]
- 16.Forastieri H., Ingham K. C. (1985) J. Biol. Chem. 260, 10546–10550 [PubMed] [Google Scholar]
- 17.Erat M. C., Slatter D. A., Lowe E. D., Millard C. J., Farndale R. W., Campbell I. D., Vakonakis I. (2009) Proc. Natl Acad. Sci. U.S.A. 106, 4195–4200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Talay S. R., Zock A., Rohde M., Molinari G., Oggioni M., Pozzi G., Guzman C. A., Chhatwal G. S. (2000) Cell Microbiol. 2, 521–535 [DOI] [PubMed] [Google Scholar]
- 19.Tomasini-Johansson B. R., Kaufman N. R., Ensenberger M. G., Ozeri V., Hanski E., Mosher D. F. (2001) J. Biol. Chem. 276, 23430–23439 [DOI] [PubMed] [Google Scholar]
- 20.Sottile J., Chandler J. (2005) Mol. Biol. Cell 16, 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi F., Harman J., Fujiwara K., Sottile J. (2010) Am. J. Physiol. Cell Physiol. 298, C1265–C1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiang H. Y., Korshunov V. A., Serour A., Shi F., Sottile J. (2009) Arterioscler. Thromb. Vasc. Biol. 29, 1074–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timoney J. F. (2004) Vet. Res. 35, 397–409 [DOI] [PubMed] [Google Scholar]
- 24.Lindmark H., Jacobsson K., Frykberg L., Guss B. (1996) Infect. Immun. 64, 3993–3999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millard C. J., Campbell I. D., Pickford A. R. (2005) FEBS Lett. 579, 4529–4534 [DOI] [PubMed] [Google Scholar]
- 26.Vakonakis I., Staunton D., Ellis I. R., Sarkies P., Flanagan A., Schor A. M., Schor S. L., Campbell I. D. (2009) J. Biol. Chem. 284, 15668–15675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiseman T., Williston S., Brandts J. F., Lin L. N. (1989) Anal. Biochem. 179, 131–137 [DOI] [PubMed] [Google Scholar]
- 28.Palmer A. G., Cavanagh J., Wright P. E., Rance M. (1991) J. Magn. Reson. 93, 151–170 [Google Scholar]
- 29.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 30.Vranken W. F., Boucher W., Stevens T. J., Fogh R. H., Pajon A., Llinas M., Ulrich E. L., Markley J. L., Ionides J., Laue E. D. (2005) Proteins Struct. Funct. Bioinf. 59, 687–696 [DOI] [PubMed] [Google Scholar]
- 31.Probert W. S., Kim J. H., Höök M., Johnson B. J. (2001) Infect. Immun. 69, 4129–4133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pickford A. R., Smith S. P., Staunton D., Boyd J., Campbell I. D. (2001) EMBO J. 20, 1519–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vakonakis I., Staunton D., Rooney L. M., Campbell I. D. (2007) EMBO J. 26, 2575–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schor S. L., Ellis I., Banyard J., Schor A. M. (1999) J. Cell Sci. 112, 3879–3888 [DOI] [PubMed] [Google Scholar]
- 35.Delano W. L. (2002) The PyMOL Molecular Graphics System, DeLano Scientific, Palo Alto, CA [Google Scholar]