Abstract
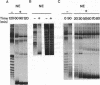
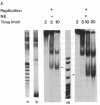
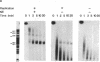
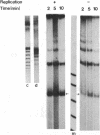
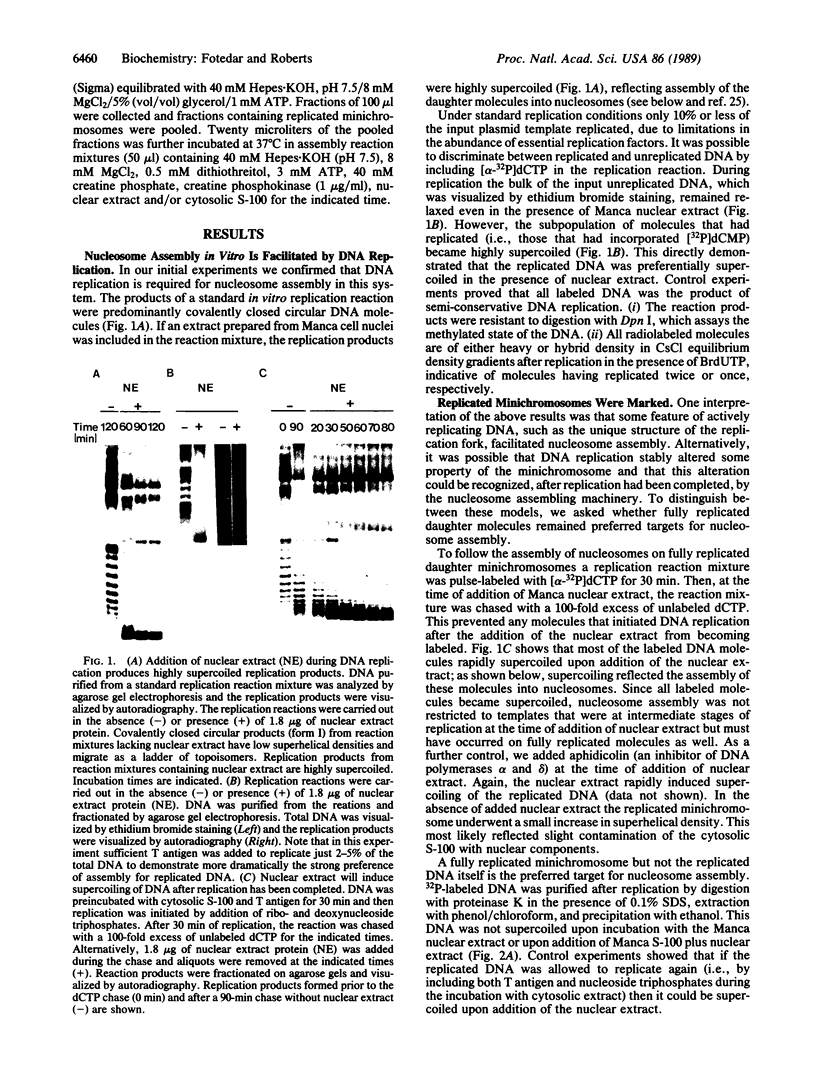
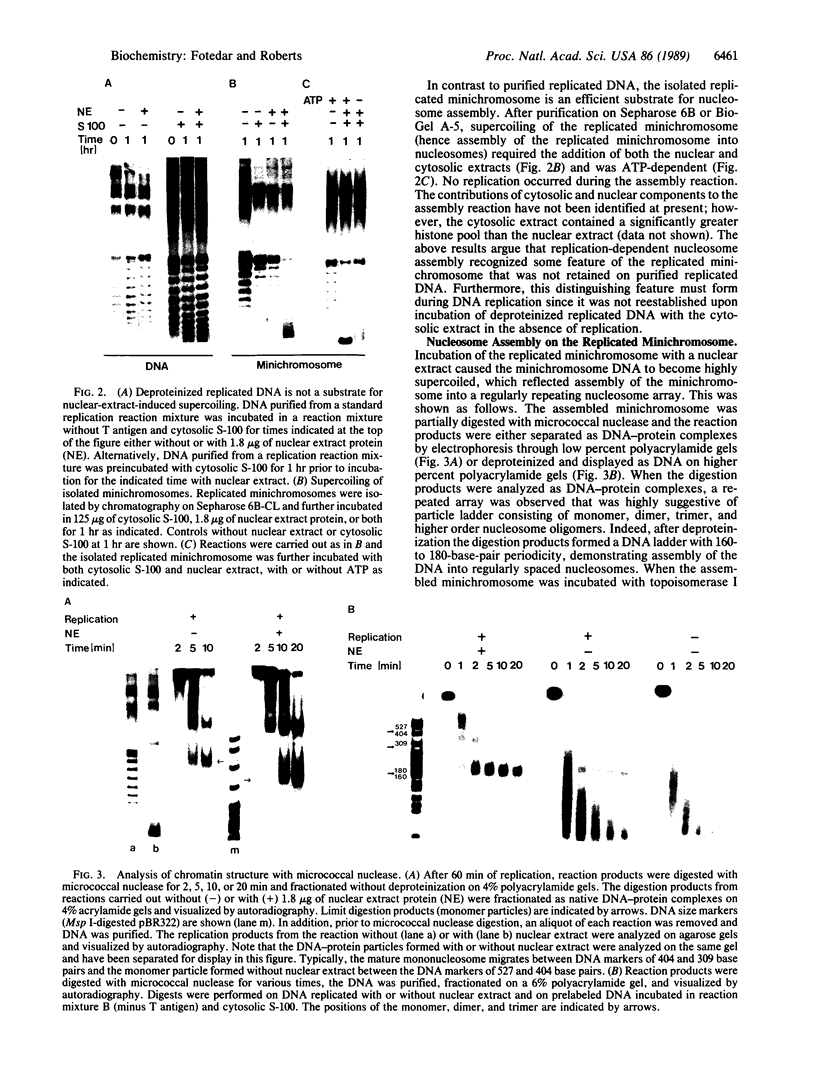
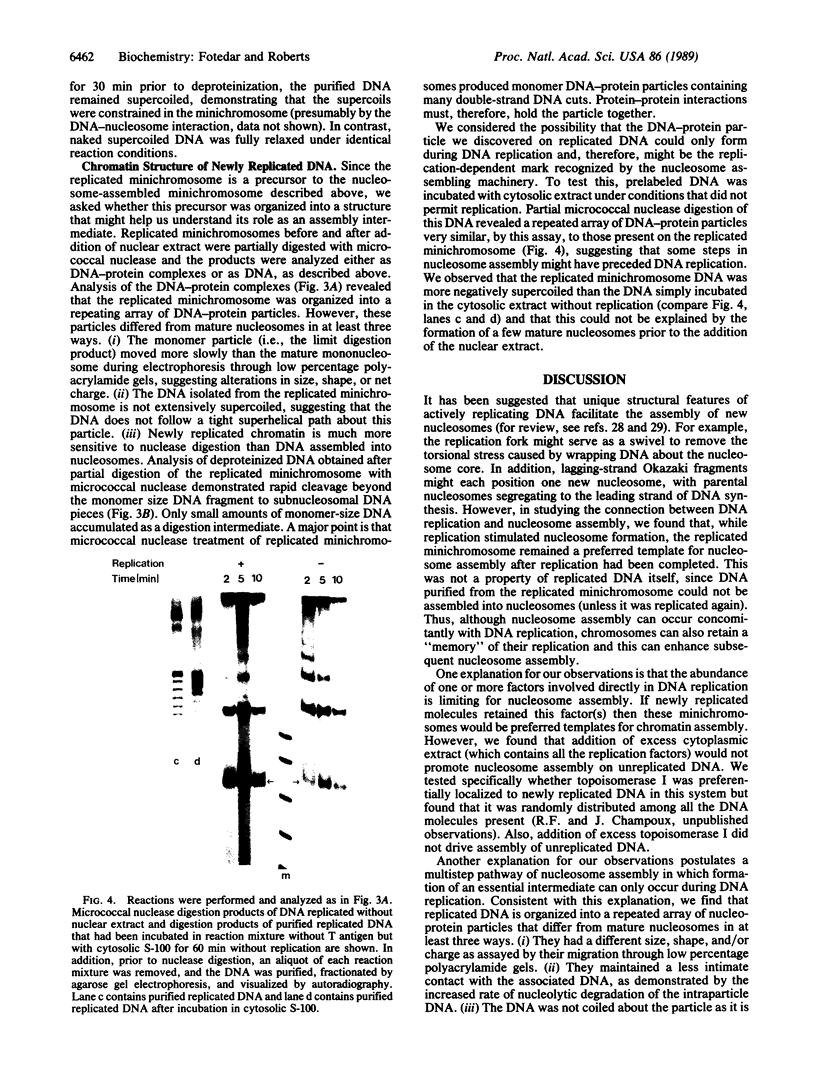
We have used cell-free DNA replication to study the relationship between DNA replication and chromatin assembly. As others have reported, we find that DNA replication facilitates nucleosome assembly. We show here that replication-dependent nucleosome assembly occurs in at least two steps. The first step requires replicating DNA; the second step occurs after replication has been completed and is promoted by a nuclear extract. Consistent with this multistep model, we observe that the replicated simian virus 40 minichromosome is organized into a repeating array of DNA-protein particles that are structurally distinct from mature nucleosomes. These particles may be precursors in a pathway of nucleosome assembly since in the second, replication-independent step the nuclear extract converts this nascent chromatin into nucleosomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgoyne L. A., Mobbs J. D., Marshall A. J. Chromatin structure: a property of the higher structures of chromatin and in the time course of its formation during chromatin replication. Nucleic Acids Res. 1976 Dec;3(12):3293–3304. doi: 10.1093/nar/3.12.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick M. E., Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: prenucleosomal deoxyribonucleic acid is rapidly excised from replicating simian virus 40 chromosomes by micrococcal nuclease. Biochemistry. 1981 Nov 10;20(23):6648–6658. doi: 10.1021/bi00526a020. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Dilworth S. M., Black S. J., Laskey R. A. Two complexes that contain histones are required for nucleosome assembly in vitro: role of nucleoplasmin and N1 in Xenopus egg extracts. Cell. 1987 Dec 24;51(6):1009–1018. doi: 10.1016/0092-8674(87)90587-3. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Fowler E., Farb R., El-Saidy S. Distribution of the core histones H2A.H2B.H3 and H4 during cell replication. Nucleic Acids Res. 1982 Jan 22;10(2):735–748. doi: 10.1093/nar/10.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Chromatin structure: deduced from a minichromosome. Science. 1975 Mar 28;187(4182):1202–1203. doi: 10.1126/science.187.4182.1202. [DOI] [PubMed] [Google Scholar]
- Hewish D. Features of the structure of replicating and non-replicating chromatin in chicken erythroblasts. Nucleic Acids Res. 1977 Jun;4(6):1881–1890. doi: 10.1093/nar/4.6.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand C. E., Walters R. A. Rapid assembly of newly synthesized DNA into chromatin subunits prior to joining to small DNA replication intermediates. Biochem Biophys Res Commun. 1976 Nov 8;73(1):157–163. doi: 10.1016/0006-291x(76)90510-6. [DOI] [PubMed] [Google Scholar]
- Jackson V. Deposition of newly synthesized histones: new histones H2A and H2B do not deposit in the same nucleosome with new histones H3 and H4. Biochemistry. 1987 Apr 21;26(8):2315–2325. doi: 10.1021/bi00382a037. [DOI] [PubMed] [Google Scholar]
- Jorcano J. L., Ruiz-Carrillo A. H3.H4 tetramer directs DNA and core histone octamer assembly in the nucleosome core particle. Biochemistry. 1979 Mar 6;18(5):768–774. doi: 10.1021/bi00572a005. [DOI] [PubMed] [Google Scholar]
- Klempnauer K. H., Fanning E., Otto B., Knippers R. Maturation of newly replicated chromatin of simian virus 40 and its host cell. J Mol Biol. 1980 Feb 5;136(4):359–374. doi: 10.1016/0022-2836(80)90395-2. [DOI] [PubMed] [Google Scholar]
- Klevan L., Dattagupta N., Hogan M., Crothers D. M. Physical studies of nucleosome assemble. Biochemistry. 1978 Oct 17;17(21):4533–4540. doi: 10.1021/bi00614a027. [DOI] [PubMed] [Google Scholar]
- Leffak I. M., Grainger R., Weintraub H. Conservative assembly and segregation of nucleosomal histones. Cell. 1977 Nov;12(3):837–845. doi: 10.1016/0092-8674(77)90282-3. [DOI] [PubMed] [Google Scholar]
- Levy A., Jakob K. M. Nascent DNA in nucleosome like structures from chromatin. Cell. 1978 Jun;14(2):259–267. doi: 10.1016/0092-8674(78)90112-5. [DOI] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6973–6977. doi: 10.1073/pnas.81.22.6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Miller O. L., Jr Electron microscopic analysis of chromatin replication in the cellular blastoderm Drosophila melanogaster embryo. Cell. 1977 Nov;12(3):795–804. doi: 10.1016/0092-8674(77)90278-1. [DOI] [PubMed] [Google Scholar]
- Murphy R. F., Wallace R. B., Bonner J. Altered nucleosome spacing in newly replicated chromatin from Friend leukemia cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5903–5907. doi: 10.1073/pnas.75.12.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pospelov V., Russev G., Vassilev L., Tsanev R. Nucleosome segregation in chromatin replicated in the presence of cycloheximide. J Mol Biol. 1982 Mar 25;156(1):79–91. doi: 10.1016/0022-2836(82)90460-0. [DOI] [PubMed] [Google Scholar]
- Riley D., Weintraub H. Conservative segregation of parental histones during replication in the presence of cycloheximide. Proc Natl Acad Sci U S A. 1979 Jan;76(1):328–332. doi: 10.1073/pnas.76.1.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. M., D'Urso G. An origin unwinding activity regulates initiation of DNA replication during mammalian cell cycle. Science. 1988 Sep 16;241(4872):1486–1489. doi: 10.1126/science.2843984. [DOI] [PubMed] [Google Scholar]
- Ruberti I., Worcel A. Mechanism of chromatin assembly in Xenopus oocytes. J Mol Biol. 1986 Jun 5;189(3):457–476. doi: 10.1016/0022-2836(86)90317-7. [DOI] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Jorcano J. L., Eder G., Lurz R. In vitro core particle and nucleosome assembly at physiological ionic strength. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3284–3288. doi: 10.1073/pnas.76.7.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaeger E. J., Klempnauer K. H. The structure of chromatin replicated in vitro. Eur J Biochem. 1978 Sep 1;89(2):567–574. doi: 10.1111/j.1432-1033.1978.tb12561.x. [DOI] [PubMed] [Google Scholar]
- Seale R. L. Nucleosomes associated with newly replicated DNA have an altered conformation. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2717–2721. doi: 10.1073/pnas.75.6.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale R. L. Studies on the mode of segregation of histone nu bodies during replication in HeLa cells. Cell. 1976 Nov;9(3):423–429. doi: 10.1016/0092-8674(76)90087-8. [DOI] [PubMed] [Google Scholar]
- Seidman M. M., Levine A. J., Weintraub H. The asymmetric segregation of parental nucleosomes during chrosome replication. Cell. 1979 Oct;18(2):439–449. doi: 10.1016/0092-8674(79)90063-1. [DOI] [PubMed] [Google Scholar]
- Senshu T., Fukuda M., Ohashi M. Preferential association of newly synthesized H3 and H4 histones with newly replicated DNA. J Biochem. 1978 Oct;84(4):985–988. doi: 10.1093/oxfordjournals.jbchem.a132213. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Camerini-Otero R. D., Felsenfeld G. An octamer of histones H3 and H4 forms a compact complex with DNA of nucleosome size. Nucleic Acids Res. 1978 Dec;5(12):4805–4818. doi: 10.1093/nar/5.12.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogo J. M., Stahl H., Koller T., Knippers R. Structure of replicating simian virus 40 minichromosomes. The replication fork, core histone segregation and terminal structures. J Mol Biol. 1986 May 5;189(1):189–204. doi: 10.1016/0022-2836(86)90390-6. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Gluzman Y. Replication and supercoiling of simian virus 40 DNA in cell extracts from human cells. Mol Cell Biol. 1985 Aug;5(8):2051–2060. doi: 10.1128/mcb.5.8.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. Chromatin assembly during SV40 DNA replication in vitro. Cell. 1986 May 23;45(4):555–565. doi: 10.1016/0092-8674(86)90287-4. [DOI] [PubMed] [Google Scholar]
- Stockley P. G., Thomas J. O. A nucleosome-like particle containing an octamer of the arginine-rich histones H3 and H4. FEBS Lett. 1979 Mar 1;99(1):129–135. doi: 10.1016/0014-5793(79)80264-1. [DOI] [PubMed] [Google Scholar]
- Worcel A., Han S., Wong M. L. Assembly of newly replicated chromatin. Cell. 1978 Nov;15(3):969–977. doi: 10.1016/0092-8674(78)90280-5. [DOI] [PubMed] [Google Scholar]