Abstract
BACKGROUND
Imatinib mesylate (Gleevec®) was evaluated as a treatment for Merkel cell carcinoma (MCC, neuroendocrine carcinoma of the skin) based on the identification of strong c-KIT staining of these neoplasms.
METHODS
Eligibility included patients with measurable metastatic or unresectable MCC, c-KIT (CD117) expression and a Zubrod performance status of 0–2. Imatinib 400 mg daily was administered orally in 28-day cycles to 23 patients.
RESULTS
Overall, imatinib was well tolerated with Grade 1 or 2 nausea, diarrhea, and hematologic toxicity as the most frequent side effects. A partial response was seen in 1 patient (4%; 95% CI: 0% – 22%). Median progression-free survival was 1 month (95% CI: 1–2 months). Median overall survival was 5 months (95% CI 2–8 months). One patient achieved a partial response and another had prolonged disease stabilization while receiving treatment.
CONCLUSIONS
The majority of patients progressed rapidly within 1–2 cycles of treatment. The observed progression-free survival and overall survival were not adequate to conclude that this agent was active in advanced MCC, and thus the planned second stage of patient accrual was not opened.
Introduction
Merkel cell carcinoma (MCC) was initially described as a rare neuroendocrine tumor of the skin by Toker in 1972.1 MCC generally presents initially as a small, firm, asymptomatic reddish or purplish skin nodule.1–4 Telangiectasia or ulceration may be seen. Initially the clinical behavior of these tumors was thought to be favorable despite their ominous small cell appearance on histologic sections.2 It has subsequently been established that this tumor is one of the most aggressive forms of skin cancer with a 5-year survival of less than 75%.5–7 MCC often recurs locally after surgery alone and has a high risk of both regional lymph node involvement and distant metastases.8
Although the term “cutaneous neuroendocrine carcinoma” (CNEC) is potentially a more precise description of these tumors,3, 4 these tumors are still frequently referred to as MCC and that nomenclature was retained in this study. Typically, the tumor presents in middle-aged to elderly patients, but younger patients in their teens and early adulthood have been described.1, 3, 4 The incidence in men and women appears to be similar. Risk factors for MCC appear to include both sun exposure and immunosuppression, including following organ transplantation and HIV infection.9 Despite elaborate pathological studies, it is still not certain that the Merkel cell is actually the cell of origin of this neoplasm.9 In fact, the early descriptions characterized this tumor as an undifferentiated carcinoma.1 Anatomically, Merkel cells have a different body distribution than the most common locations of primary tumors and they lack neurofilament proteins.9 The original Memorial Sloan-Kettering Cancer Center staging system described stages I–III.7 Stage I represented primary skin tumors (stage 1A ≤ 2 cm, stage 1B > 2 cm). Stage II represented regional lymph node involvement, and stage III was applied to systemic disease, including distant lymph node involvement. This staging system was subsequently modified to a TNM staging system for non-melanoma skin cancer, using the 4-tier format of the American Joint Committee on Cancer AJCC Sixth Edition 2002, by promoting stage IB to stage II.10
In addition, the trabecular appearance of MCC described by Toker, while often equated with MCC, actually classifies only a subset of the tumors. More recently, Gould described a classification of MCC based on predominant patterns of trabecular, intermediate or small cells.3 Most tumors are a mixture of these patterns although a large series from Memorial Sloan-Kettering identified the intermediate cell type as predominating in most of their patients.11 These subtypes are not yet known to have definite prognostic significance.
MCC frequently expresses c-KIT CD117.12–15 For example, Su et al. evaluated c-KIT expression in 22 biopsies of MCC, demonstrating expression in 95%.12 Imatinib mesylate (Gleevec®, formerly STI-571) is a small molecule that has been demonstrated to be a highly selective inhibitor of certain receptor tyrosine kinases (RTK), including c-KIT (CD117). We therefore designed a phase II trial of imatinib to test its clinical effectiveness in MCC. Since there are few effective treatments for patients with surgically incurable MCC, identification of an additional active drug would represent an important therapeutic advance. In addition, this study represented an attempt to establish whether multi-institutional trials in this rare disease are feasible in the United States.
Materials and Methods
Patient eligibility
Patients enrolled in this trial were required to have biopsy proven MCC (Cutaneous Neuroendocrine Carcinoma) that was metastatic or unresectable. Tumor expression of c-KIT (by immunohistochemistry) and a history of a previous skin primary were required (to exclude metastatic small cell carcinoma of non-cutaneous origin). Patients with an unknown primary site were, therefore, not eligible. Patients were required to have at least one site of measurable disease. Patients with treated, stable, asymptomatic brain metastases were allowed on study. All radiotherapy, chemotherapy, biologic therapy or any other investigational drug treatment was required to be completed at least 28 days prior to registration. Patients were not allowed to have had major surgery within 14 days prior to registration. Additional eligibility requirements included: adequate hematologic, renal, and hepatic function and a Zubrod performance status ≤ 2. Patients with a second malignancy, as well as women who were pregnant or nursing were excluded from the study. Patients with Class 3/4 cardiac problems by the New York Heart Association Criteria were not eligible, nor were patients taking therapeutic doses of coumadin (warfarin) or those with severe and/or uncontrolled concurrent medical disease. All patients provided written acknowledgment of informed consent in accordance with institutional and federal guidelines.
Treatment schedule
Imatinib mesylate (Gleevec®) was administered at a dose of 400 mg p.o. daily. One cycle was defined as 28 days regardless of treatment delays. The intent was to administer imatinib continually without interruptions between cycles. Drug was supplied by the NCI through a CRADA collaborative agreement between the National Cancer Institute and Novartis Pharmaceuticals Corporation.
Toxicity assessment and dose modifications
Toxicity was defined by National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) Version 3.0. Dosage modifications were performed for hematologic and non-hematologic toxicities. Imatinib was held for a ANC <1,000, platelet count <50,000 or non-hematologic Grade 2 toxicity. Once toxicity resolved to Grade ≤ 1 treatment resumed at 400 mg per day. Dose reductions were implemented if neutropenia, thrombocytopenia or non-hematologic Grade 2 toxicity recurred with subsequent cycles. Following the second occurrence, imatinib was resumed at 300 mg per day after improvement to Grade ≤ 1. If neutropenia, thrombocytopenia or non-hematologic Grade 2 recurred, dosing was again held then resumed at 200 mg once resolved to Grade ≤ 1. For the first occurrence of non-hematologic Grade 3 or 4 toxicity, imatinib was held until resolved to Grade ≤ 1 and then restarted at 300 mg. If Grade 3 or 4 toxicity recurred at the reduced dose the imatinib was again held until toxicity resolved to Grade ≤ 1 then treatment resumed at 200 mg per day. The use of cytokines (G-CSF or GM-CSF) as well as epoietin alfa (EPO) was allowed at the discretion of the treating investigator. Initial response assessment was planned at 1 and 2 months following initiation of imatinib therapy.
Statistical methods
The primary goal of this study was to evaluate the true response probability (confirmed and unconfirmed, complete and partial responses as defined using RECIST criteria).
It was assumed that imatinib would not be of further interest if the true response probability was less than 5%, and would generate definite interest in further study if 20% or more. A two-stage enrollment was planned with 20 patients to be accrued initially. If more than one response was seen and toxicities appeared acceptable, an additional 20 patients would be accrued. Five or more responses out of 40 eligible patients would be sufficient evidence to warrant further study, providing other factors, such as toxicity, progression-free survival, and overall survival were also favorable. The design had a significance level of 5% and a power of 92%. Forty patients would be sufficient to estimate toxicity rates to within ± 15% (95% confidence interval). Any toxicity occurring with at least 5% probability was likely to be observed once (87% chance). Progression-free and overall survival probabilities were estimated using the method of Kaplan-Meier.16
Results
From December 2003 to October 2006, a total of 25 patients were accrued to this trial from 13 institutions with 6 accrued through Intergroup participation by the Eastern Cooperative Oncology Group. Two patients were found to be ineligible (one whose tumor was found not to express CD117 on central pathology review, and one without measurable disease). Therefore 23 patients were included in the analysis. The accrual rate compared favorably to SWOG’s previous trial in this population (S9716), which enrolled only 6 patients over 2 years, and suggests that future trials in this patient population are feasible. Patient demographics are included in Table 1. Median age was 77.1 (with a range of 56.9 to 91.9 years). Seventeen patients (74%) were men, 6 (26%) were women and all were Caucasian. It should be noted that this was a heavily pretreated patient population. Over 60% had progressed following prior chemotherapy. Only 4% of patients had received no prior chemo- or radiotherapy.
Table 1.
Patient Characteristics
| Gender | Patients | Percent |
| Male | 17 | 74% |
| Female | 6 | 26% |
| Median age (yrs) (range) | 77 | (57 – 92) |
| Zubrod Performance Status | ||
| 0 | 11 | 48% |
| 1 | 9 | 39% |
| 2 | 3 | 13% |
| Prior Treatment | ||
| Surgery | 21 | 91% |
| Radiation Therapy | 16 | 70% |
| Chemotherapy | 14 | 61% |
| No prior treatment | 1 | 4% |
| Sites of disease at baseline | ||
| Primary Site | ||
| Limb/Extremity | 12 | 52% |
| Head and Neck | 9 | 39% |
| Trunk | 2 | 9% |
| Metastatic Involvement | ||
| Lymph Node/Skin/Soft Tissue | 17 | 74% |
| Lung | 5 | 22% |
| Liver | 5 | 22% |
| Bone | 1 | 4% |
| Other | 7 | 30% |
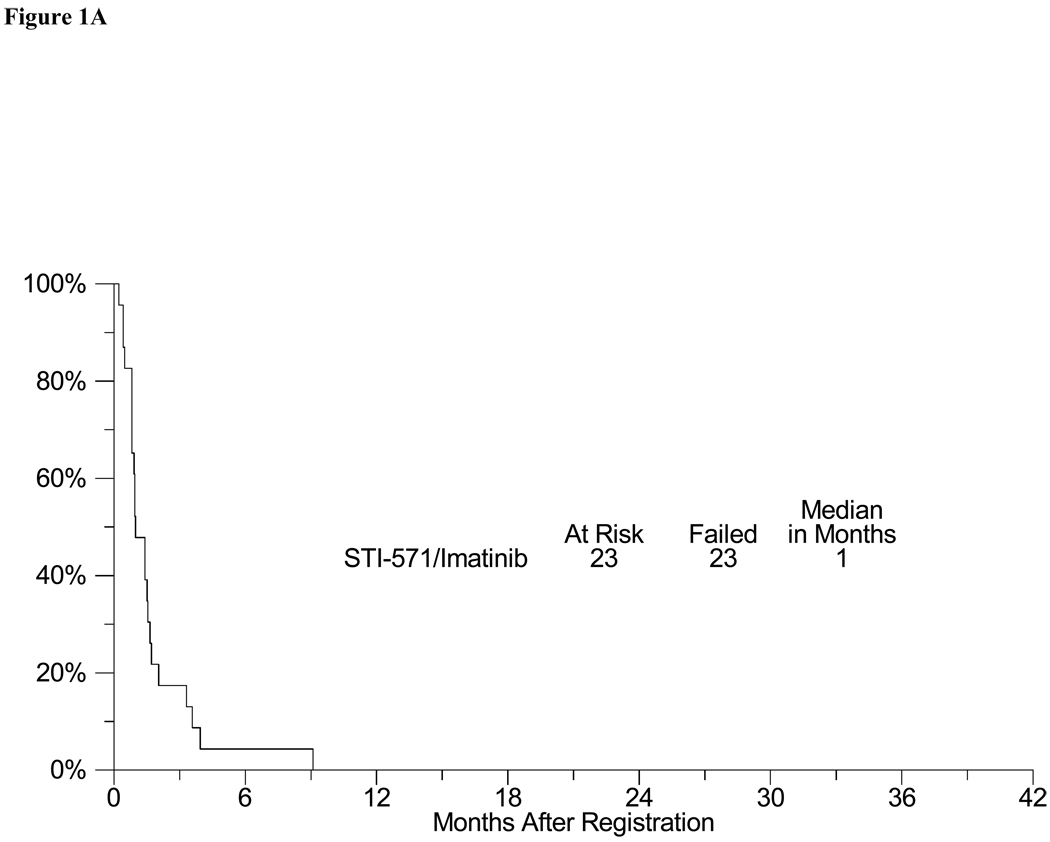
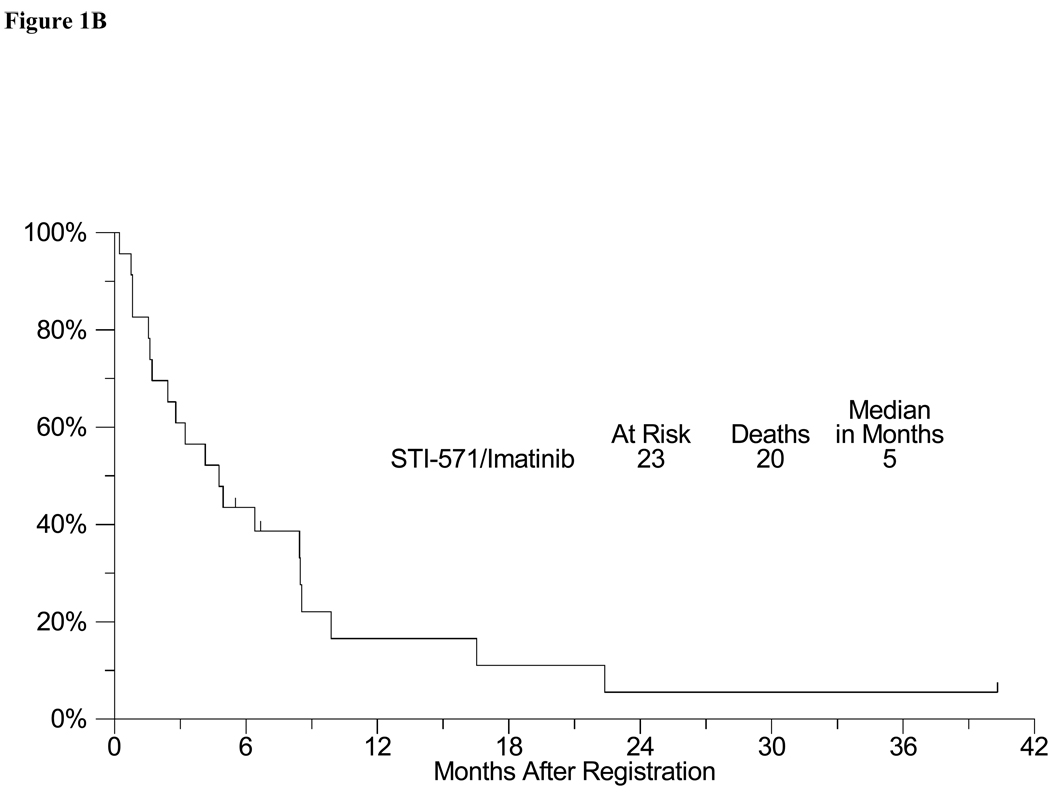
Toxicity in this trial was typical for clinical trials of imatinib (Table 2). There were 3 episodes of Grade 4 toxicities (one each of dyspnea, hyperglycemia and vomiting). Three patients experienced Grade 3 toxicities including one episode each of peripheral edema, fatigue, lymphopenia and rash. Grade 1–2 toxicities were mostly hematological and gastrointestinal, and included 4 patients with diarrhea, 6 patients with nausea, 12 with anemia (hemoglobin), 4 with leukopenia, and 4 patients with hypokalemia. There were no complete responses (0%) and 1 confirmed partial response (4%) in the 23 evaluable patients (4% objective response rate, CI 0 – 22%). In addition, stable disease was observed in 3 patients (9, 4 and 3 months). At the time of analysis, all evaluable patients had developed progressive disease. The estimated median progression-free survival was 1 month (95% CI: 1–2 months), with an estimated 6-month PFS of 4% (95% CI: 0% – 13%) (Figure 1A). The estimated median overall survival was 5 months (95% CI: 2–8 months)(Figure 1B). The estimated one-year overall survival was 17% (95% CI: 0% – 33%). There were three deaths on study, all were attributed to progressing tumor.
Table 2.
Number of Patients with a Given Type and Grade of Adverse Event*
| Grade | ||||||
|---|---|---|---|---|---|---|
| ADVERSE EVENT | 0 | 1 | 2 | 3 | 4 | 5 |
| Anorexia | 20 | 1 | 2 | 0 | 0 | 0 |
| Constipation | 20 | 2 | 1 | 0 | 0 | 0 |
| Creatinine | 20 | 3 | 0 | 0 | 0 | 0 |
| Diarrhea | 19 | 4 | 0 | 0 | 0 | 0 |
| Dyspnea | 22 | 0 | 0 | 0 | 1 | 0 |
| Edema-limb | 20 | 1 | 1 | 1 | 0 | 0 |
| Fatigue | 20 | 2 | 0 | 1 | 0 | 0 |
| Hemoglobin | 11 | 7 | 5 | 0 | 0 | 0 |
| Hyperglycemia | 21 | 0 | 1 | 0 | 1 | 0 |
| Hypokalemia | 19 | 4 | 0 | 0 | 0 | 0 |
| Leukocytes | 19 | 3 | 1 | 0 | 0 | 0 |
| Lymphopenia | 20 | 1 | 1 | 1 | 0 | 0 |
| Nausea | 17 | 4 | 2 | 0 | 0 | 0 |
| Platelets | 20 | 3 | 0 | 0 | 0 | 0 |
| Rash | 22 | 0 | 0 | 1 | 0 | 0 |
| Vomiting | 20 | 2 | 0 | 0 | 1 | 0 |
| MAXIMUM GRADE ANY ADVERSE EVENT | ||||||
| Number | 3 | 7 | 7 | 3 | 3 | 0 |
Figure 1.
A: Progression free survival
B: Overall Survival
DNA sequencing of c-KIT was performed on tumor tissue from on a non-responding patient and the one patient with long-term stable disease (9 months). Neither demonstrated an activating mutation in c-KIT. Unfortunately, the patient with partial response withdrew consent for the study and DNA sequencing could not be performed.
Discussion
The majority of MCCs are located on the head and neck region.1, 2, 4, 11, 17 Other less frequent sites include the extremities (40%) and the trunk (10%). Anatomic distribution of MCC appears to correlate with chronic exposure to UV radiation. MCC has a high propensity to recur locally and to have both regional and distant metastases. Its biology is reminiscent of the behavior of other small cell neuroendocrine cancers.1, 3–5, 18 In one study, factors found to predict a lower survival rate included large tumor size, histologic small cell type, and high mitotic rate.19 Disseminated metastases occur in over 30% of the patients, and may involve liver, lung, bone, and brain.
Recently, the clonal integration of a new human polyoma virus, which was termed Merkel cell polyomavirus (MCPyV), has been reported in 8 of 10 MCC patients20. Kassem subsequently studied the formalin-fixed and paraffin- embedded tissue specimens of 39 MCC for the presence of MCPyV by PCR21. MCPyV was detected in 77% (n = 30) of MCC as confirmed by sequence analyses of the PCR products. The presence of MCPyV in the majority of MCC tissue specimens strongly indicates a possible role for MCPyV as an etiologic agent in the pathogenesis of MCC.
The significant frequency of distant metastases in MCC has elicited a series of chemotherapy reports based on small numbers of patients.17, 22–27 10–13 Selection of chemotherapy for MCC has usually been patterned after small cell lung cancer treatments since both the lung’s Kultchitzsky cells and the skin’s Merkel cells appear similar by light microscopy and are probably part of the amine precursor uptake and decarboxylation (APUD) system.2, 23 Although most studies include only a small number of patients, together they have documented frequent though brief responses to several drug combinations. Long term responses and complete remissions are rarely observed.
A variety of chemotherapy regimens have been employed in patients with metastatic MCC, with a significant frequency of clinical responses. These regimens have included cyclophosphamide, methotrexate and 5-fluorouracil (CMF), etoposide and cisplatin, cisplatin plus doxorubicin, and cyclophosphamide, doxorubicin and vinblastine.17, 25, 27, 28 Accurate estimates of median progression free and overall survival for chemotherapy treatment of metastatic MCC are difficult to ascertain. This is because most reports lump a variety disease stages and treatment regimens into the same report. Unfortunately, most chemotherapy responses have proven short-lived, although rare long-term remissions have been reported.10 Since many of the individuals that develop MCC are upwards of 70 years age (mean age of onset is 69), multi-agent chemotherapy with anthracyclines and platinum compounds is often poorly tolerated, and must be closely monitored. Thus, identification of novel agents to treat MCC is desirable.
MCC frequently expresses c-KIT CD117, in contrast to a lower incidence of expression in SCLC.12–15 Su et al. evaluated c-KIT expression in 22 biopsies of MCC.12 This study found that 21 of 22 MCC biopsies (95%) expressed CD117. Intensity of CD117 expression did not appear to correlate with aggressive behavior. While the incidence of activating mutations in c-KIT in MCC was not evaluated in this report, its pathogenic role in other malignant neoplasms suggests the possibility of a similar role in MCC.15
Imatinib mesylate (Gleevec®, formerly STI-571) is a small molecule that has been demonstrated to be a highly selective inhibitor of certain receptor tyrosine kinases (RTK), including 1) Abl and the chimeric BCR-Abl fusion protein found in certain leukemias such as chronic myeloid leukemia (CML); 2) the platelet-derived growth factor (PDGF) receptor; and 3) KIT, the product of the c-KIT proto-oncogene.29, 30 Imatinib inhibits the KIT RTK at an IC50 of approximately 100 nM, which is similar to that required for inhibiting the tyrosine kinase activity associated with BCR-Abl and the PDGF receptor.31 The selectivity of imatinib is important as it does not affect other members of the Type III receptor tyrosine kinase family, such as Flt-3 and the receptor for M-CSF (the product of the c-fms proto-oncogene).17 Imatinib has been extensively tested in Philadelphia chromosome-positive leukemia patients where the main target is inhibition of the dysregulated kinase activity associated with the chimeric BCR-Abl fusion protein.32 Additionally, single center and multicenter Phase trials have now documented significant activity of imatinib in gastrointestinal stromal tumors (GIST), a solid tumor type that usually expresses gain-of-function somatic mutation of the KIT RTK.33 Our data has demonstrated that imatinib has minimal activity against MCC, despite expression of CD117 (c-KIT) protein. This clinical result has subsequently been explained by molecular studies. MCCs generally fail to express activating mutations in c-KIT.34, 35 This was retrospectively confirmed in the tumor of two patients with c-KIT expression in the current study. Neither of these patients (including one with protracted disease stabilization) had activating mutations in c-KIT. Further efforts are therefore needed to identify agents that are active and tolerable for treatment of patients with this rare, but aggressive skin cancer. It is hoped that the identification of genomic integration of the MCC-associated polyoma virus will provide important clues as to the pathophysiology, as well as aid in the identification of new treatment approaches.20
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA35090, CA073590, CA20319), CA45377, CA35178, CA35176, CA46113, CA46441, CA42777, CA86780, CA58861, CA46282, CA35119, CA15488, CA21115
References
- 1.Toker C. Trabecular carcinoma of the skin. Arch Dermatol. 1972;105(1):107–110. [PubMed] [Google Scholar]
- 2.De Wolff-Peeters C, Marien K, Mebis J, Desmet V. A cutaneous APUDoma or Merkel cell tumor? A morphologically recognizable tumor with a biological and histological malignant aspect in contrast with its clinical behavior. Cancer. 1980 Oct 15;46(8):1810–1816. doi: 10.1002/1097-0142(19801015)46:8<1810::aid-cncr2820460819>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Gould VE, Moll R, Moll I, Lee I, Franke WW. Neuroendocrine (Merkel) cells of the skin: hyperplasias, dysplasias, and neoplasms. Lab Invest. 1985 Apr;52(4):334–353. [PubMed] [Google Scholar]
- 4.Wick MR, Goellner JR, Scheithauer BW, Thomas JRr, Sanchez NP, Schroeter AL. Primary neuroendocrine carcinomas of the skin (Merkel cell tumors). A clinical, histologic, and ultrastructural study of thirteen cases. Am J Clin Pathol. 1983 Jan;79(1):6–13. doi: 10.1093/ajcp/79.1.6. [DOI] [PubMed] [Google Scholar]
- 5.Shaw JH, Rumball E. Merkel cell tumour: clinical behaviour and treatment. Br J Surg. 1991 Feb;78(2):138–142. doi: 10.1002/bjs.1800780205. [DOI] [PubMed] [Google Scholar]
- 6.Raaf JH, Urmacher C, Knapper WK, Shiu MH, Cheng EW. Trabecular (Merkel cell) carcinoma of the skin. Treatment of primary, recurrent, and metastatic disease. Cancer. 1986 Jan 1;57(1):178–182. doi: 10.1002/1097-0142(19860101)57:1<178::aid-cncr2820570134>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Yiengpruksawan A, Coit DG, Thaler HT, Urmacher C, Knapper WK. Merkel cell carcinoma. Prognosis and management. Arch Surg. 1991 Dec;126(12):1514–1519. doi: 10.1001/archsurg.1991.01410360088014. [DOI] [PubMed] [Google Scholar]
- 8.Hitchcock CL, Bland KI, Laney RG, Franzini D, Harris B, Copeland EM. Neuroendocrine (Merkel cell) carcinoma of the skin. Its natural history, diagnosis, and treatment. Ann Surg. 1988 Feb;207(2):201–207. doi: 10.1097/00000658-198802000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goessling W, McKee PH, Mayer RJ. Merkel cell carcinoma. J Clin Oncol. 2002 Jan 15;20(2):588–598. doi: 10.1200/JCO.2002.20.2.588. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg SR, Neifeld JP, Frable WJ. Prognostic value of tumor thickness in patients with Merkel cell carcinoma. J Surg Oncol. 2007;95(8):618–622. doi: 10.1002/jso.20737. [DOI] [PubMed] [Google Scholar]
- 11.Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23(10):2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 12.Su LD, Fullen DR, Lowe L, Uherova P, Schnitzer B, Valdez R. CD117 (KIT receptor) expression in Merkel cell carcinoma. Am J Dermatopathol. 2002;24(4):289–293. doi: 10.1097/00000372-200208000-00001. [DOI] [PubMed] [Google Scholar]
- 13.Bobos M, Hytiroglou P, Kostopoulos I, Karkavelas G, Papadimitriou CS. Immunohistochemical distinction between merkel cell carcinoma and small cell carcinoma of the lung. Am J Dermatopathol. 2006;28(2):99–104. doi: 10.1097/01.dad.0000183701.67366.c7. [DOI] [PubMed] [Google Scholar]
- 14.Strong S, Shalders K, Carr R, Snead DR. KIT receptor (CD117) expression in Merkel cell carcinoma. Br J Dermatol. 2004;150(2):384–385. doi: 10.1111/j.1365-2133.2003.05779.x. [DOI] [PubMed] [Google Scholar]
- 15.Feinmesser M, Halpern M, Kaganovsky E, Brenner B, Fenig E, Hodak E, et al. c-kit expression in primary and metastatic merkel cell carcinoma. Am J Dermatopathol. 2004;26(6):458–462. doi: 10.1097/00000372-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 17.Tai PT, Yu E, Winquist E, Hammond A, Stitt L, Tonita J, et al. Chemotherapy in neuroendocrine/Merkel cell carcinoma of the skin: case series and review of 204 cases. J Clin Oncol. 2000;18(12):2493–2499. doi: 10.1200/JCO.2000.18.12.2493. [DOI] [PubMed] [Google Scholar]
- 18.Tai PT, Yu E, Tonita J, Gilchrist J. Merkel cell carcinoma of the skin. J Cutan Med Surg. 2000 Oct;4(4):186–195. doi: 10.1177/120347540000400403. [DOI] [PubMed] [Google Scholar]
- 19.Skelton HG, Smith KJ, Hitchcock CL, McCarthy WF, Lupton GP, Graham JH. Merkel cell carcinoma: analysis of clinical, histologic, and immunohistologic features of 132 cases with relation to survival. J Am Acad Dermatol. 1997 Nov;37(5 Pt 1):734–739. doi: 10.1016/s0190-9622(97)70110-5. [DOI] [PubMed] [Google Scholar]
- 20.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319(5866):1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kassem A, Schopflin A, Diaz C, Weyers W, Stickeler E, Werner M, et al. Frequent detection of Merkel cell polyomavirus in human Merkel cell carcinomas and identification of a unique deletion in the VP1 gene. Cancer Res. 2008;68(13):5009–5013. doi: 10.1158/0008-5472.CAN-08-0949. [DOI] [PubMed] [Google Scholar]
- 22.Crown J, Lipzstein R, Cohen S, Goldsmith M, Wisch N, Paciucci PA, et al. Chemotherapy of metastatic Merkel cell cancer. Cancer Invest. 1991;9(2):129–132. doi: 10.3109/07357909109044222. [DOI] [PubMed] [Google Scholar]
- 23.Feun LG, Savaraj N, Legha SS, Silva EG, Benjamin RS, Burgess MA. Chemotherapy for metastatic Merkel cell carcinoma. Review of the M.D. Anderson Hospital's experience. Cancer. 1988 Aug 15;62(4):683–685. doi: 10.1002/1097-0142(19880815)62:4<683::aid-cncr2820620406>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 24.Fenig E, Brenner B, Njuguna E, Katz A, Schachter J, Sulkes A. Oral etoposide for Merkel cell carcinoma in patients previously treated with intravenous etoposide. Am J Clin Oncol. 2000 Feb;23(1):65–67. doi: 10.1097/00000421-200002000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Voog E, Biron P, Martin JP, Blay JY. Chemotherapy for patients with locally advanced or metastatic Merkel cell carcinoma. Cancer. 1999 Jun 15;85(12):2589–2595. doi: 10.1002/(sici)1097-0142(19990615)85:12<2589::aid-cncr15>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 26.Bajetta E, Rimassa L, Carnaghi C, Seregni E, Ferrari L, Di Bartolomeo M, et al. 5-Fluorouracil, dacarbazine, and epirubicin in the treatment of patients with neuroendocrine tumors. Cancer. 1998 Jul 15;83(2):372–378. doi: 10.1002/(sici)1097-0142(19980715)83:2<372::aid-cncr23>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Fenig E, Lurie H, Sulkes A. The use of cyclophosphamide, methotrexate, and 5-fluorouracil in the treatment of Merkel cell carcinoma. Am J Clin Oncol. 1993 Feb;16(1):54–57. doi: 10.1097/00000421-199302000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Poulsen M, Rischin D, Walpole E, Harvey J, Mackintosh J, Ainslie J, et al. High-risk Merkel cell carcinoma of the skin treated with synchronous carboplatin/etoposide and radiation: a Trans-Tasman Radiation Oncology Group Study--TROG 96:07. J Clin Oncol. 2003;21(23):4371–4376. doi: 10.1200/JCO.2003.03.154. [DOI] [PubMed] [Google Scholar]
- 29.Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001 Apr 5;344(14):1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 30.Savage DG, Antman KH. Imatinib mesylate--a new oral targeted therapy. N Engl J Med. 2002 Feb 28;346(9):683–693. doi: 10.1056/NEJMra013339. [DOI] [PubMed] [Google Scholar]
- 31.Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996 May;2(5):561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 32.O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003 Mar 13;348(11):994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 33.Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002 Aug 15;347(7):472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 34.Swick BL, Ravdel L, Fitzpatrick JE, Robinson WA. Merkel cell carcinoma: evaluation of KIT (CD117) expression and failure to demonstrate activating mutations in the C-KIT proto-oncogene - implications for treatment with imatinib mesylate. J Cutan Pathol. 2007;34(4):324–329. doi: 10.1111/j.1600-0560.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 35.Kartha RV, Sundram UN. Silent mutations in KIT and PDGFRA and coexpression of receptors with SCF and PDGFA in Merkel cell carcinoma: implications for tyrosine kinase-based tumorigenesis. Mod Pathol. 2008;21(2):96–104. doi: 10.1038/modpathol.3800980. [DOI] [PubMed] [Google Scholar]