Abstract
While acute exposures to ozone (O3) can alter airway responsiveness, effects from long-term exposures at low concentrations are less clear. This study assessed whether such exposures could induce nonspecific hyperresponsiveness in nonatopic (nonsensitized) guinea pigs and/ or could exacerbate the pre-existing hyperresponsive state in atopic ( sensitized) animals, and whether gender was a factor modulating any effect of O3. Responsiveness was measured during and following exposures to 0.1 and 0.3 ppm O3 for 4 h/day, 4 days/ wk for 24 wk in male and female nonsensitized animals, those sensitized to allergen (ovalbumin) prior to initiation of O3 exposures, and those sensitized concurrently with exposures. Ozone did not produce hyperresponsiveness in nonsensitized animals, but did exacerbate hyperresponsiveness to both specific and nonspecific bronchoprovocation challenges in sensitized animals, an effect that persisted through at least 4 wk after exposures ended. Gender was not a factor modulating response to O3. Induced effects on responsiveness were not associated with numbers of eosinophils in the lungs nor with any chronic pulmonary inflammatory response, but were correlated with antigen-specific antibodies in blood. This study supports a role for chronic O3 exposure in the exacerbation of airways dysfunction in a certain segment of the general population, namely, those demonstrating atopy.
The tendency for airways to constrict in response to various stimuli is termed airway or bronchial responsiveness and is an essential component of respiratory-tract homeostasis. The relative sensitivity to such bronchoprovocation stimuli varies widely within the general population (Weiss et al., 1981), but when there is excessive reaction, a state of airway hyperresponsiveness (AHR) is considered to occur. This condition is often associated with atopy, a hypersensitivity to certain antigens mediated by specific antibodies. While both AHR and atopy have been linked to asthma (Peat et al., 1996; Peden, 2000; Wolfe et al., 2000), the pathogenetic relationship between AHR, atopy, and asthma remains unclear, since nonatopic individuals having no history of asthma or any other chronic lung disorder can show hyperresponsive airways (Josephs et al., 1990; Morgan & Reger, 1995; Paoletti et al., 1995; Smith & McFadden, 1995).
The etiology or expression of many respiratory-tract disorders involves environmental factors, one of which may be ambient air pollution. Evidence providing support for such a role of pollution is convincing, and specific links with ozone (O3), a ubiquitous pollutant, have been made in this regard. Population-based studies have noted an association between O3 and exacerbation of airway responsiveness and other asthma-related symptoms (Zwick et al., 1991; U.S. EPA, 1996; McDonnell et al., 1999; Thurston & Ito, 1999; Peden, 2000). A relationship between O3 exposure and airway responsiveness is also supported by controlled studies; concentrations ≥0.3 ppm have produced transient AHR in normal laboratory animals (Abraham et al., 1980; Holtzman et al., 1983; Gordon et al., 1984; Gross & Sargent, 1992), and clinical studies noted AHR following exposure of normal humans to as low as 0.08 ppm (Seltzer et al., 1986; Horstmann et al., 1990; Ying et al., 1990; Linn et al., 1994).
What remains unclear from both epidemiological and experimental exposure databases is whether O3 is involved in the induction of AHR, and whether atopic individuals are more susceptible than are normals to O3-induced alterations in airway function (Holtzman et al., 1979; Koenig et al., 1985; Kreit et al., 1989; McManus et al., 1990; Linn et al., 1992; Peden, 2000). Limited long-term exposure epidemiological studies seem to suggest that ambient O3 exposure may be involved in the development of asthma (Thurston & Ito, 1999), and there is some evidence that O3 can enhance the ability to become sensitized to inhaled antigens or at least can increase airway responsiveness to antigen exposure (Molfino et al., 1991; Jörres et al., 1996; Jenkins et al., 1999). There is also some indication that repeated O3 exposures may induce AHR; once-weekly 1 ppm O3 exposures produced persistent nonspecific AHR in nonatopic monkeys (Johnson et al., 1988).
Because the available database regarding the role of O3 in the induction/exacerbation of AHR involved largely acute exposures, the role of more realistic repeated exposures at relatively low concentrations remained uncertain. This study evaluated airway responsiveness during 24 wk of 4 h/day, 4 days/wk exposures to 0.1 and 0.3 ppm O3, as well as over an 8-wk postexposure period, using nonatopic and atopic guinea pigs of both genders. The aims were threefold: to determine if long-term repeated O3 exposure could induce a state of nonspecific AHR in normal nonatopic (nonsensitized) hosts; to determine if O3 exposure could exacerbate a pre-existing hyperresponsive state in atopic (sensitized) hosts; and to assess the modulating role of gender in any O3 exposure-related effects on airway responsiveness. In addition, the relationship between potential modulators of response to O3, such as specific cell types in bronchopulmonary lavage, systemic blood and lung tissue, and levels of antigen-specific antibodies in serum, was also evaluated.
METHODS
Animals
Viral antibody-free, Hartley guinea pigs (Charles River) were housed on corncob bedding in polycarbonate cages in a laminar-flow HEPA-filtered isolator unit in a temperature- and humidity-controlled room and were provided food and water ad libitum. Atopy was induced by inhalation exposure to 1% w/v ovalbumin (OA, in pyrogen-free normal saline; Sigma, St. Louis) for 0.5 h/day for 4 days (Herxheimer & West, 1955). To confirm sensitization, standard passive cutaneous anaphylaxis (PCA) was used to assess serum levels of immunoglobulin G (IgG, the major allergic antibody class in the guinea pig), and any IgE, initially in 1 male and 1 female subjected to the above regimen, as well as in 2 naive hosts; PCA was performed using intradermal injections of serial dilutions of test serum obtained 28 days after the first OA administration. Titers in the sensitized female and male were 200 and 100, respectively, indicating the presence of serum anti-OA IgG; control titers were <20. No blueing was observed after 10 days, indicating an absence of IgE. Additional 1-day titers were assessed with sera from other OA-challenged animals to confirm these initial findings. Thus, the OA challenge caused sensitization, and serum IgG was used as an index of this sensitization.
Ozone Exposure
All exposures were performed in 1.6-m3 stainless-steel chambers maintained at 25°C. Ozone was created by passing O2 (in argon) through an ultraviolet O3 generator (OREC model 03V1-0), and levels were measured with an ultraviolet photometer (Dasibi model 1003-PC). All dilution air in the exposure system was passed through an air-cleaning system to remove ambient pollutants, such as particles, sulfur dioxide, nitrogen oxides, and O3.
Experimental Protocols
This study involved three separate experimental protocols. To examine the effects of O3 exposure on airway responsiveness in normal animals, nonsensitized (nonatopic) guinea pigs were utilized (NS protocol). To examine the effects of O3 in atopic hosts, animals were either presensitized to OA, which involved the 4-day OA administration period followed by holding the animals for 28 days prior to initiating O3 exposures (PS protocol), or were administered the OA concurrently with the initiation of O3 exposures (CS protocol).
Within each protocol, 10 animals/gender were exposed 4 h/day, 4 days/wk for 24 wk to either clean air or 0.1 or 0.3 ppm O3. The actual mean exposure levels attained for all protocols ranged from 0.104 to 0.107 ppm and from 0.291 to 0.299 ppm (SE < .001). After the final exposure, 5 animals/gender/atmosphere were sacrificed; the remainder were maintained for an additional 8 wk (postexposure period) prior to sacrifice.
To assess airway responsiveness in relationship to O3 exposure, measurements of responsiveness were done at ~4-wk intervals during the course of the 24-wk exposure period and at 4- to 8-wk intervals during the postexposure period. One week after the end of the exposure or postexposure period (i.e., at 25 or 33 wk after initial exposure), a time lag needed in order to perform the final airway responsiveness tests, animals were euthanized by Nembutol overdose (150 mg/kg, ip), followed by cardiac puncture (to obtain blood for cell and immunoglobulin analyses) and exsanguination. The trachea and upper lung were then exposed, the right main bronchus clamped below the carina, and the left lung lavaged. Lungs were then removed; the left lung was fixed by airway perfusion of formalin solution and the right fixed with Carnoy’s solution to facilitate mast cell analysis.
Measurement of Airway Responsiveness
Airway responsiveness was assessed by bronchoprovocation challenge, wherein changes in specific airway conductance (sGaw) were measured following inhalation of increasing concentrations of bronchoconstrictor agents, that is, a nonspecific cholinergic agonist (acetylcholine, ACh) or a specific antigenic stimulus (OA). Both specific and nonspecific responsiveness were measured in sensitized hosts (PS and CS protocols), while only nonspecific responsiveness was measured in nonsensitized animals (NS protocol). While any changes in responsiveness during O3 exposure could reflect changes in the ability of the inhaled challenge agents to reach airway receptors due to effects on the epithelium (e.g., altered mucus secretion or epithelial permeability) rather than to an actual blunting or increased sensitivity of receptors, this would likely also occur in humans exposed in ambient air to O3 and then to any bronchoconstrictor challenges.
sGaw was assessed by a noninvasive method (Agrawal, 1981; Thompson et al., 1987) in unsedated, spontaneously breathing guinea pigs within a two-piece, whole-body constant-volume plethysmograph while breathing through a pneumotachograph. Prior to each provocation test, the stability of sGaw was assessed by measurements at 5-min intervals for 15 min. Baseline sGaw was then determined using a single 0.5-min inhalation of phosphate-buffered saline (PBS) generated by compressed medical-grade air nebulization. Animals were then administered the challenge agent. Responsiveness was quantitated in terms of PC50, the provocation concentration that resulted in a 50% decrease in sGaw from baseline; this value was obtained using log-linear interpolation of ACh or OA concentration-sGaw response relationships during each test.
To assess nonspecific responsiveness, animals were challenged with doubling doses of nebulized ACh aerosol, beginning at 0.05% and administered at 3-min intervals until sGaw was decreased 50% from baseline. Nonspecific responsiveness was always evaluated 24 h following an air or O3 exposure so as to minimize any potential acute effects from exposure on epithelial permeability to the challenge agents. To assess specific responsiveness, animals were challenged with OA. Because response to inhaled antigen in sensitized hosts is less rapid than is that to ACh, the interval between the end of each OA challenge and the start of the next was set at 10 min. OA challenge was performed 72 h after each ACh challenge test. This sequence (i.e., ACh prior to OA) was used to avoid the possibility of any residual effect from OA challenge influencing the response to ACh (Lewis & Broadley, 1995).
Prior to starting the O3 exposures, bronchoprovocation challenges were done in NS and PS animals to establish “wk 0” (preexposure) values. No such initial tests were done on CS hosts, as they were being exposed while undergoing sensitization (which precluded a preexposure OA test); an ACh test was not done due to the small size of the animals prior to start of exposures, which prevented an accurate measurement of responsiveness at that time.
Biochemical and Cellular Assays of Lavage and Blood
The left lung was lavaged using a multiple-wash procedure (Schlesinger et al., 1992). Total recovered cell number and viability were determined by hemacytometer count and trypan blue exclusion, respectively. Relative percentages of cell types were assessed by differential staining with Diff-Quik. Lavage fluid lactate dehydrogenase (LDH; an index of general cytotoxicity or cell membrane damage) and total soluble protein (a measure of serum protein transudation reflecting damage to the barrier between airways/alveoli and circulation) levels were analyzed in the first wash supernate using standard kits (Sigma). Differential counts were determined from Wright–Giemsa-stained blood smears. OA-specific IgG1 and IgG2 levels in atopic host blood were assessed by enzyme-linked immunosorbent assay (ELISA) (Fraser et al., 1998) and expressed as the ratio of anti-OA IgG1 or IgG2 to that in an IgG standard.
Histopathology
Fixed lungs were embedded in paraffin and sectioned along the plane of the main airway axis. Sections from the formalin-fixed left lung were stained with Giemsa for eosinophil identification; those from the Carnoy’s solution-fixed right lung were stained with 0.1% alcian blue for mast-cell identification. Quantitative analysis of cellular infiltration was performed on the main intrapulmonary bronchus and small noncartilagenous bronchioles chosen at random in each section. Mast-cell and eosinophil numbers in epithelial and subepithelial layers of each airway were quantitated per unit cross-sectional area with light microscopy. Ten 20× fields were counted for each airway examined for each animal. Sampling sites were chosen only where the airway examined had been sectioned longitudinally. Sections were also scanned for evidence of inflammation.
Statistical Analysis
Airway responsiveness (as PC50) and sGaw were analyzed using multivariate profile analyses. These were performed independently for each protocol and, within each protocol, independently for ACh and OA challenge results. Postexposure-period data were analyzed separately from exposure data within each protocol. Prior to analyses, data were checked for homogeneity of variance using Bartlett’s test. Based on this test, PC50 values were normalized using log10 transformation, while sGaw values were subjected to a square-root transformation.
The vector of dependent variables for profile analyses consisted of either measurements made during the 24-wk exposure period (including preexposure values for NS and PS animals) or those obtained at wk 24, 28 and 32, with the latter two obtained during the postexposure period, and the 24-wk value representing the last measurement during exposure. Independent variables were O3 concentration, gender, and the interaction between O3 concentration and gender. Statistical significance (at p < .05) for the profile analysis was determined by the Hotelling–Lawley trace.
If any statistically significant interaction between the time vector of dependent variables and the independent variables was detected, univariate analyses of variance (ANOVAs) for each time point were performed. Factors in ANOVAs were also O3 concentration, gender, and the interaction between O3 concentration and gender. For any ANOVA, statistical significance was evaluated by the F-test (p < .05). A significant factor F-test led to subtesting for exposure or for gender-by-exposure interactions. No subtests were required for a significant gender effect. Subtesting was performed by uncorrected t-tests of the least squares means.
Results of lavage, blood cell, immunoglobulin, and airway cell assays were analyzed by three-way ANOVA; factors were gender, O3 concentration, and sacrifice time, that is, after the exposure or postexposure periods. All possible interactions were also tested. Statistically significant factor or interaction effects (p < .05) were subtested using uncorrected t-tests. Prior to analysis, all data were tested using Bartlett’s test. Based on this test, lavage and systemic blood cell counts were normalized using an arcsine transformation while IgG data were normalized by a log10 transformation. While systemic blood cell counts were obtained for relative eosinophil, neutrophil (PMN), lymphocyte, basophil, and monocyte numbers, as the latter two types were noted only in low numbers if at all, they were excluded from analysis.
Additional statistical analyses were performed to evaluate the strength of linear relationships between particular parameters. To address if variability in airway responsiveness could be explained by OA-specific antibody levels, Pearson correlation coefficients were calculated for the relationship between IgG1 and IgG2 at sacrifice and final PC50 measures with OA or ACh challenge prior to sacrifice. Since PC50 was measured at repeated time points, but IgG only at sacrifice, and as PS and CS protocols had different temporal relationships between sensitization and exposures, these analyses were done using only air controls (pooled). The ratio of PC50 obtained in the final provocation challenge normalized (i.e., divided) by that at wk 0 was also examined in this manner.
To evaluate any relationship between eosinophils in lavage and the degree of AHR, a Pearson correlation coefficient was calculated for the eosinophil fraction with PC50 obtained at 24 wk. Another analysis was done to evaluate if variability in the fraction of blood eosinophils may be associated with the degree of sensitization, as measured by IgG levels. Pearson coefficients were calculated for eosinophil fraction with IgG1 and IgG2 using pooled data from PS and CS protocol air controls. A Pearson coefficient was also obtained for the relationship between total eosinophil numbers in airway sections and the percentage in lavage fluid for each PS and CS animal to determine if the numbers of the cells recovered in lavage were representative of those in the lungs. Finally, correlation analysis was done to determine the strength of the relationship between lung tissue mast-cell numbers and airway responsiveness.
RESULTS
The various data sets obtained in this study were statistically analyzed as already described. The approach employed for describing the data was to use these analyses as the basis to evaluate results for each endpoint within each protocol in terms of an overall consistency of pattern or trend that could be related to O3 exposure, rather than focusing on individual statistical differences at or between specific time points.
Specific Airway Conductance
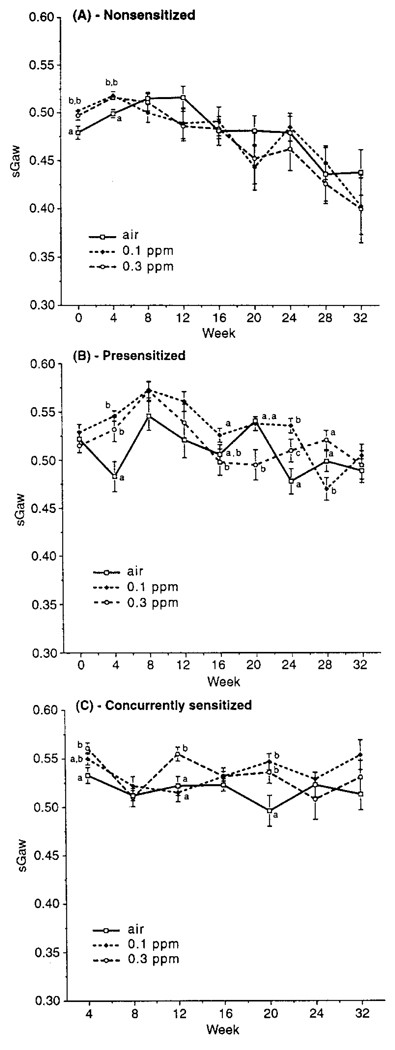
Baseline sGaw values were obtained prior to challenge with ACh for animals in each protocol (Figure 1). sGaw was also measured in PS and CS animals prior to each OA challenge as well; these values were essentially identical to those in Figure 1. In each protocol, within-gender values remained relatively consistent throughout the exposure and postexposure periods. There was no overall pattern or trend of O3 effect on sGaw, although there were some significant differences between air- and O3-exposed hosts at specific time points. This suggests that observed effects reflected random variability rather than any biologically significant effect on sGaw relatable to exposure. Overall, PS and CS animal sGaw values were similar to each other and generally greater than those of NS hosts. sGaw values in males were generally lower than in females at most time points for all protocols, although the gender differences did not always reach statistical significance; in any case, O3 exposure had no effect on this difference (data not shown).
FIGURE 1.
Baseline specific airway conductance (sGaw) as a function of time from start of exposures to O3 or air for (A) NS, (B) PS, and (C) CS animals. Each point is the mean (±SE) for all animals at each time point. Significant differences between atmospheres at each time point are indicated by differing letter designations. Group sizes are 20/time point/atmosphere through wk 24, and 10/time point/atmosphere for wk 28–32.
Airway Responsiveness
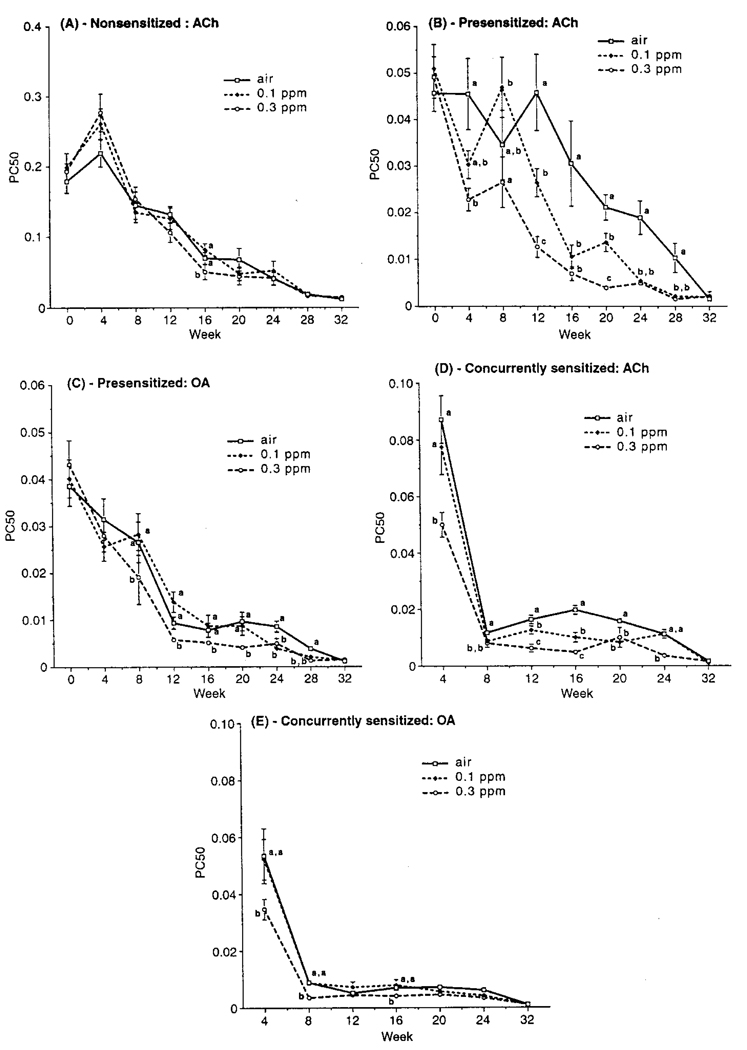
A comparison of PC50 values for NS air controls with PS and CS air control values shows that the latter groups were indeed sensitized (Figure 2). PC50 values at wk 0 in PS and at wk 4 in CS hosts were much lower than that for their time-matched NS counterparts, indicating that the airways in the atopic animals were hyperresponsive.
FIGURE 2.
PC50 as a function of time from the start of exposures to O3 or air for (A) NS ACh, (B) PS ACh, (C) PS OA, (D) CS ACh, and (E) CS OA challenge hosts. Each point is the mean (±SE) for all animals at each time point. Significant differences between atmospheres at each time point are indicated by differing letter designations. Group size/time point/atmosphere for (A), (C), and (D), 20 through wk 24 and 10 for wk 28–32; for (B) and (E), 20 for wk 0 and 8–24, and 20 for wk 4, 28, and 32.
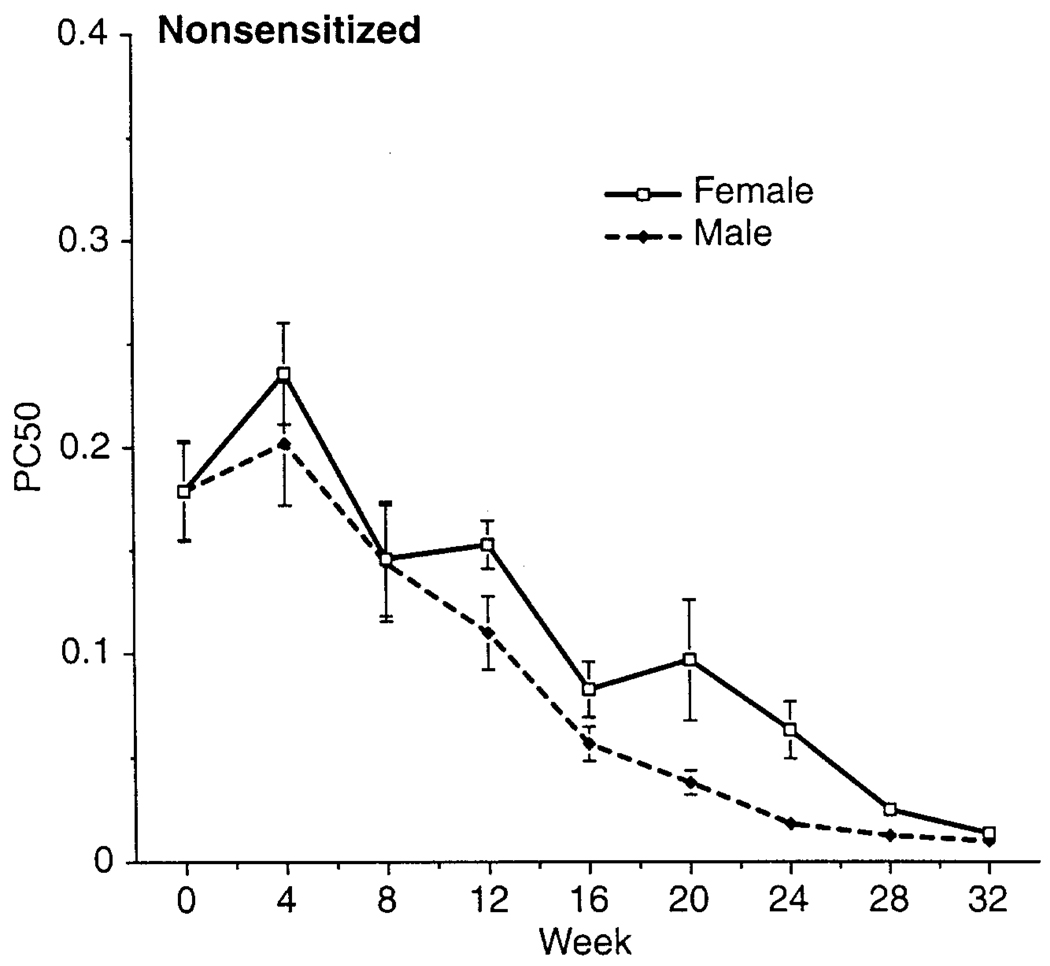
In general, NS animals showed no consistent pattern or trend of O3-induced effect on ACh PC50 during either exposure or postexposure periods (Figure 2A). However, there was a clear gender difference in responsiveness in these animals (Figure 3); except for wk 0, PC50 values for males were generally significantly lower than those for females, indicating that males had normally more responsive airways.
FIGURE 3.
Gender comparison of ACh PC50 for air-exposed NS hosts. Each point is the mean (±SE) for all animals at each time point.
Ozone-exposed PS animals had lower ACh PC50 values than air controls; these differences were significant at most time points during the 24-wk exposure period (Figure 2B). While the effect of exposure on nonspecific responsiveness was often statistically comparable for both O3 levels, there was evidence of an O3 concentration-related trend for responsiveness, that is, an overall pattern of decreasing PC50 with increasing O3 level. The O3-related increased airway responsiveness versus that with air was maintained 4 wk into the postexposure period, that is, at 28 wk, but was no longer evident by 32 wk. PS animals challenged with OA (Figure 2C) showed somewhat less of a pattern of O3 concentration-related effect on PC50; values for air- and 0.1 ppm O3-exposed hosts were not significantly different until wk 24 of exposure. However, PC50 values for 0.3 ppm O3 animals were generally lower than for air- or 0.1 ppm-exposed hosts, and significantly so, during most of the exposure period. Significant differences compared to air controls for both 0.1 and 0.3 O3-exposed animals extended 4 wk into the postexposure period. PS animals did not show any consistent pattern of gender difference in the relationship between PC50 (with either ACh or OA) and O3 exposure, although, as in the NS animals, males had significantly more responsive airways than females at all times except wk 0.
As in PS hosts, CS animals generally displayed significant effects of O3 on ACh or OA PC50 values (Figure 2, D and E). A pattern of increasing airway responsiveness with increasing O3 concentration was noted; ACh PC50 values for 0.3 ppm-exposed hosts were consistently lower than in air and 0.1 ppm counterparts, and those for 0.1 ppm-exposed hosts were generally significantly lower than in air controls at most time points. This was, however, not the case with OA. Unlike with ACh, where 0.3 ppm-exposed host PC50 values were significantly different from air controls at most time points during exposures, OA PC50 values of these animals only differed from controls at early time points. As with PS hosts, an O3 concentration-response pattern was no longer evident by wk 32 and PC50 values for males were generally lower than for females. In any case, no gender-related differences in response to O3 were noted.
Thus, overall, NS animals exhibited no biologically significant effect of O3 on airway responsiveness. Exposure of PS or CS hosts, with already hyperresponsive airways, resulted in a further increase in responsiveness to ACh and OA, in an O3 concentration-related manner. Furthermore, there was no differential effect of O3 on responsiveness for either gender. On the other hand, there did appear to be an age dependence of responsiveness in the NS and PS animals.
Lavage Parameters
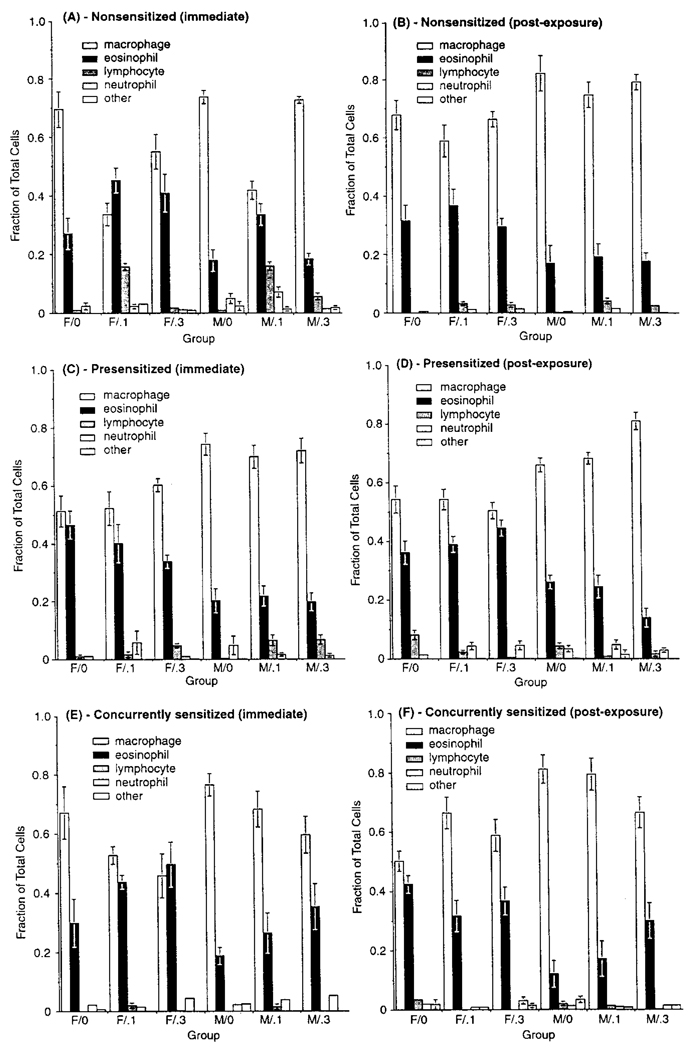
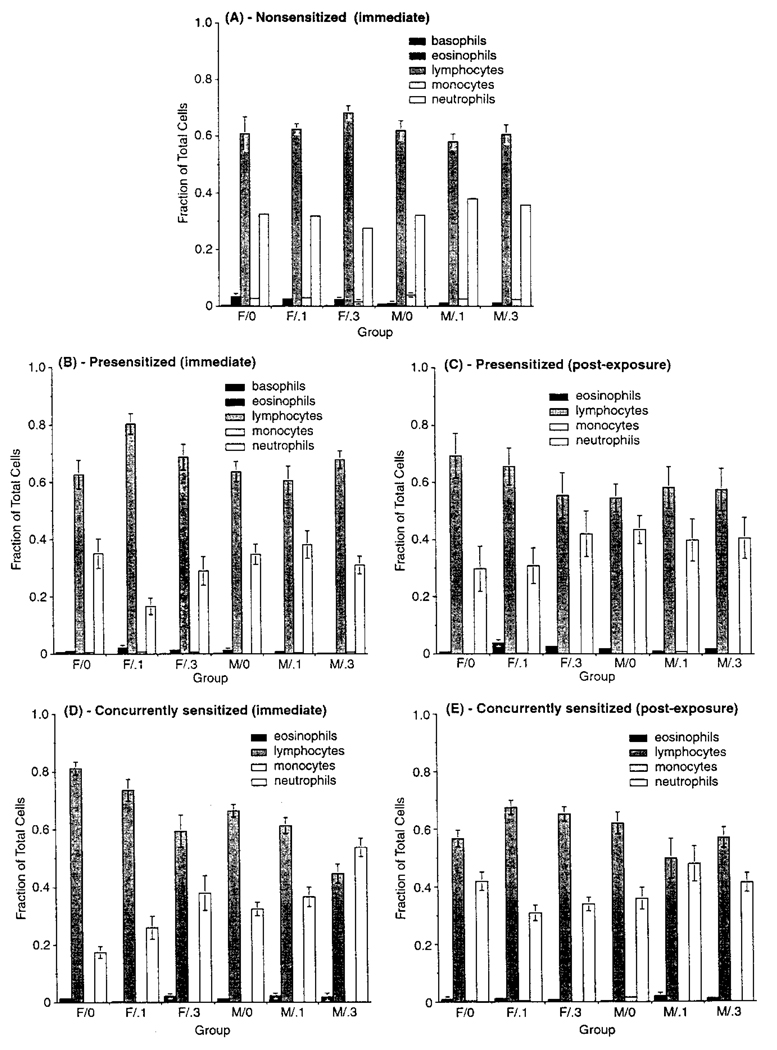
Analysis of various lavage assays indicated no consistent pattern for total or relative cell counts or viability relatable to O3 exposure in any protocol. A gender-related difference was noted for eosinophils; relative percentages in females in each protocol were generally significantly higher (and macrophage levels lower) than in males at each sacrifice point (Figure 4). There was no consistent interaction between O3 level and gender, suggesting that any gender differences were unrelated to exposure. In addition, the relative within-gender eosinophil percentage was similar for nonsensitized and sensitized hosts. The correlation coefficient for the relationship between eosinophil fraction and PC50 (r = .061, p = .82) indicated no significant association. Differences in LDH and total protein content were noted in O3-exposed hosts compared to controls, but were inconsistently related to exposure and considered biologically insignificant (data not shown).
FIGURE 4.
Cell differentials in lavage from NS, PS, and CS hosts sacrificed at wk 24 (A, C, and E, respectively) or after the postexposure period (B, D, and F, respectively). Each bar is the mean (±SE) for five male or five female guinea pigs exposed to the indicated atmosphere. Due to the various statistical comparisons within and between exposure cohorts, for simplicity, symbol designations for significance are not included in the figure. Details of statistical results are described in the text.
Systemic Blood Cell Differentials
Analyses of individual cell types, expressed as fractions of total cells counted, indicated no significant effects of O3 in NS animals (Figure 5). A gender difference was seen for eosinophils, with a higher fraction in females than in males; this was similar to the pattern in lavage, although the relative fraction of these cells was lower. Postexposure PS animals showed significant differences in fractions compared to those sacrificed at wk 24, with eosinophils and PMNs being increased while lymphocytes were generally decreased. Gender differences were noted in both cohorts, with females having higher eosinophil and lymphocyte, but lower PMN, levels. A gender-by-O3 interaction was noted for eosinophils, with levels being higher in females. As this was not observed for other cell types, and increases in one type should be offset by decreases in others (as fractions must sum to one), the biologic significance is unclear. CS animal eosinophil numbers were unaffected by experimental factors. There was a difference in lymphocyte and PMN fractions related to exposure that was related to sacrifice time. In hosts analyzed at wk 24, lymphocytes decreased, and PMNs increased, as a function of O3 concentration. In postexposure animals, no changes in either type were relatable to O3 level. As with PS animals, CS females had higher lymphocyte, but lower PMN, levels regardless of O3 level or sacrifice time. Gender differences differed for the various O3 level–sacrifice time combinations for PMN, but not for lymphocytes. In those assessed at wk 24, an O3 level-response gradient was noted for both genders. No gradient was evident in postexposure hosts; males and females had different response patterns as a function of O3 level, suggesting no biologically meaningful trend.
FIGURE 5.
Cell differentials in blood from NS, PS, and CS hosts sacrificed at wk 24 (A, B, and D, respectively) or after the postexposure period (C and E, respectively). There was no postexposure blood differentials performed with the NS animals. Each bar is the mean (±SE) for five male or five female guinea pigs exposed to the indicated atmosphere. Due to the various statistical comparisons within and between exposure cohorts, for simplicity, symbol designations for significance are not included in the figure. Details of statistical results are described in the text.
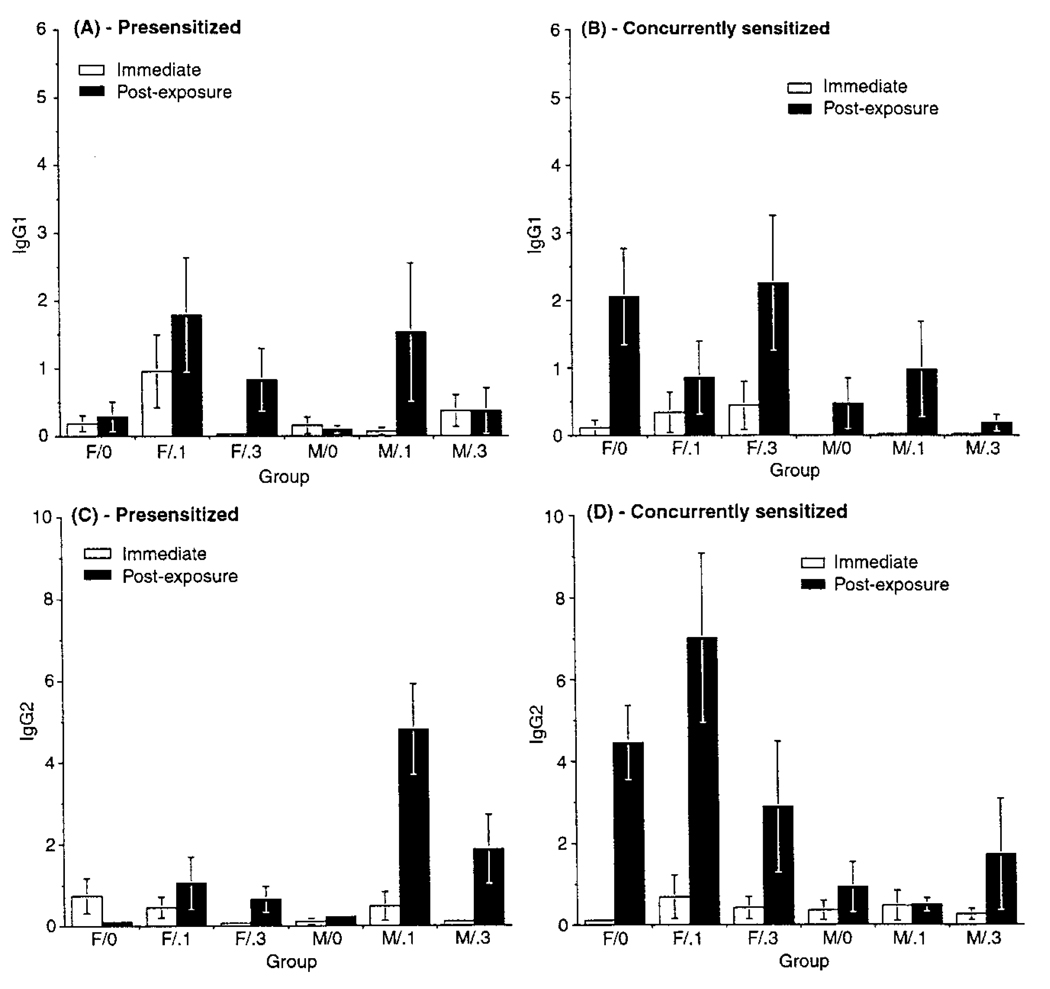
Antigen-Specific Antibodies
OA-specific IgG1 levels in PS and CS hosts were unaffected by O3 (Figure 6). Two general observations were that among CS (but not PS) animals, IgG1 levels were significantly higher in postexposure hosts than in those sacrificed at wk 24, and levels in females were higher than in males. A gender–sacrifice time interaction was also noted in CS hosts; males sacrificed at wk 24 had IgG1 levels (regardless of O3 level) significantly lower than in any postexposure group. In contrast, postexposure females had the highest IgG1 levels. For IgG2, a statistically significant O3-exposure-related effect was noted in PS (but not CS) animals. The highest IgG2 levels were seen in hosts exposed to 0.1 ppm O3, while those for animals exposed to 0.3 ppm were between air and 0.1 ppm O3 levels, and not significantly different from either group.
FIGURE 6.
OA-specific immunoglobulin levels in blood from animals sacrificed at wk 24 or after the postexposure period: (A) IgG1 in PS, (B) IgG1 in CS, (C) IgG2 in PS, and (D) IgG2 in CS animals. Values represent IgG concentration in blood relative to that of an IgG standard. Each bar is the mean (±SE) for five male or five female guinea pigs exposed to the indicated atmosphere. Due to the various statistical comparisons within and between exposure cohorts, for simplicity, symbol designations for significance are not included in the figure. Details of statistical results are described in the text.
Analyses were done to determine if blood IgG levels could be reflective of the degree of AHR (Table 1). Correlations between PC50 with either ACh or OA and IgG1 or IgG2 were all negative, indicating that increased IgG levels are associated with decreased PC50, that is, increased airway responsiveness. All correlations were statistically significant and accounted for ~34–50% of the linear association between IgG and PC50. There were no substantial differences in interpretation between IgG1 and IgG2 with ACh challenge, or IgG2 with OA challenge. These three correlation sets are similar to one another and explain about 10% less variability than that by correlations between IgG1 and OA challenge. In analyses to evaluate if variability in the fraction of blood eosinophils may be associated with the degree of sensitization, correlation coefficients were 0.051 (p = .76) and −0.027 (p = .87) for IgG1 and IgG2, respectively, indicating no statistical relationship between these parameters.
TABLE 1.
Correlation of airway responsiveness and serum antigen-specific antibodies
| IgG1 |
IgG2 |
|||
|---|---|---|---|---|
| PC50 | Correlation coefficient |
p Value | Correlation coefficient |
p Value |
| OA challenge | ||||
| At 24 wka | −0.417 | .008 | −0.340 | .032 |
| Ratiob | −0.496 | .005 | −0.381 | .034 |
| ACh challenge | ||||
| At 24 wka | −0.352 | 026 | −0.365 | .021 |
| Ratiob | −0.429 | .006 | −0.416 | .008 |
Analysis using PC50 (measured after 24 wk of exposure) with noted challenge agent. This analysis involved data from both the PS and CS protocols.
Analysis using ratio of PC50 at 24 wk to that at 0 wk.
Histopathology
Lung sections revealed no evidence of inflammatory response in any animal. Statistical comparison of eosinophils in airway subepithelium to that in lavage resulted in a significant correlation coefficient (r = .7, p < .05), indicating that the number of such cells in lavage likely reflected the levels in lung. Consistent with lavage results, O3 exposure did not influence airway eosinophil levels. Analyses of airway mast cells indicated no significant difference in cell number that could be related to O3 exposure in any protocol (data not shown), nor was there any significant correlation between mast cell number and airway responsiveness (r = .12, p = .73).
DISCUSSION
A main goal of this study was to evaluate effects of long-term, repeated exposures to ozone (O3) on airway responsiveness in both nonatopic and atopic hosts. This study was the first to examine specific and nonspecific responsiveness, and the potential modulating effect of gender on these parameters, with such an exposure regime and using relatively low O3 levels. While O3 did not induce AHR in nonatopic animals, it exacerbated AHR, in an O3-level-dependent but gender-independent manner, in atopic hosts, and this effect persisted for at least 4 wk after exposures ended. Finally, O3 did not alter baseline specific airway conductance, implying that normal airway caliber was not affected.
Previous repeated O3 exposure studies that examined AHR in nonsensitized hosts have been equivocal. Reduced responsiveness was noted in guinea pigs exposed to 1 ppm for 3 h/day for 4 days (Sun & Chung, 1997), while no change was seen in those exposed to 0.15 ppm for 4 h/day, 5 days/wk for 4 mo (Kagawa et al., 1989) or in cynomologus monkeys to 1 ppm for 6 h/day, 5 days/wk for 12 wk (Biagini et al., 1986). Conversely, increased nonspecific airway responsiveness occurred in rhesus monkeys following 19 wk of single, 2-h weekly exposures to 1 ppm O3 and in guinea pigs through 24 days of exposure to 0.3 ppm for 4 h/day, 6 days/wk (Johnson et al., 1988; Vargas et al., 1998). Coupled with results of numerous studies of acute O3 exposure (e.g., reviewed in Ryan, 2000), the database indicates that while O3 can produce increased airway responsiveness in nonsensitized individuals of various species, including humans, levels that consistently resulted in this response were higher than those used herein; exposures to ≤0.3 ppm O3 had been associated with inconsistent effects.
The few studies of sensitized hosts exposed to O3, most of which used acute exposures at levels ranging upward from 0.6 ppm, generally also showed increased airway responsiveness to nonspecific provocation challenge (Holtzman et al., 1979; Thorne & Broadley, 1994; Sun et al., 1997). Results herein with specific challenge are also consistent with reported studies, although all involved short-term O3 exposure. In one, both sensitized and nonsensitized dogs were exposed for 2 h to 3 ppm O3, after which antigen was administered; O3 did not affect responsiveness to antigen in nonsensitized dogs, but did increase it in sensitized ones (Yanai et al., 1990). In a study of atopic adult human asthmatics, it was found that 1 h of exposure to 0.12 ppm O3 potentiated the response to subsequently inhaled allergen (Molfino et al., 1991). Similar effects were noted with acute exposures at higher levels (i.e., 0.16–0.25 ppm O3) as well (Jörres et al., 1996; Jenkins et al., 1999; Peden, 1999). Thus, the current study provides evidence for exacerbation of AHR with repeated O3 exposures at a level below that previously shown to produce such a response.
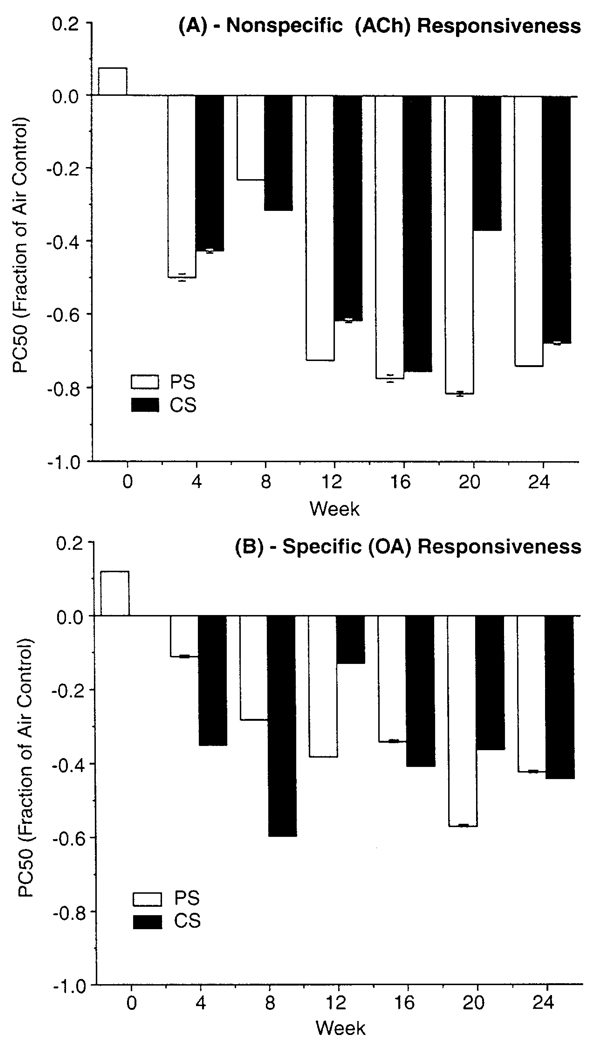
The design of this study does not allow for statistical comparison of responsiveness between the protocols, as each was designed to address a specific question and differed in some experimental detail that precluded such direct evaluation. Still, results can be compared in a qualitative manner. One comparison of interest is in response to provocation challenge in O3-exposed hosts, to see whether it differed between the two groups of sensitized animals, that is, those presensitized versus those sensitized during start of exposures. Figure 7 shows PC50 values obtained with ACh challenge in sensitized animals exposed to 0.3 ppm O3 (level that had greatest effect on responsiveness) expressed as fractional change from that in air controls. O3-induced exacerbation of AHR is quite comparable in both protocols and thus appears relatively independent of whether such exposure occurred in already-sensitized animals, or of whether initial exposure to allergen and O3 occurred within the same time frame.
FIGURE 7.
Comparison of (A) nonspecific and (B) specific airway responsiveness in PS and CS hosts exposed to 0.3 ppm O3. PC50 is expressed as the fraction of respective air controls. Each value is the mean (±SE) for 10 guinea pigs/protocol.
Many biological responses evaluated with repeated O3 exposures are characterized by adaptation, an attenuation of response with continued exposure. While such attenuation may occur with some endpoints and not others, it is not clear whether it is a factor in pollutant-induced changes in airway responsiveness. For example, no adaptation in responsiveness was noted in humans with 6.6-h/day; 5-day exposures to 0.12 ppm O3 (Folinsbee et al., 1994), although 2-h/day, 3-day exposures to 0.4 ppm resulted in an initially increased responsiveness that returned to pre-exposure levels by day 3 (Dimeo et al., 1981). These cited studies involved relatively short exposure durations; it is likely that the specific length of total exposure could affect development of adaptation. For example, in a study by Vargas et al. (1998) where nonatopic guinea pigs were exposed to 0.3 ppm O3 for 4 h/day, increased airway responsiveness evident through 24 days of exposure was no longer noted by 48 days, suggesting an adaptive phenomenon after a certain length of repeated exposures. The current study, which lasted 24 wk, did not show any evidence for adaptation in O3-induced exacerbation of AHR.
In this study, animals were evaluated over a timeframe during which they matured from prepubertal (about 4 wk of animal age) through adolescence into adulthood, beginning at about 16 wk of animal age (Gaultier et al., 1984). It is evident that within given protocols, normal airway responsiveness varied with the age of the animal. This effect is clearly demonstrated by the PC50 patterns of the NS and PS air controls; as these guinea pigs matured, their airway responsiveness increased dramatically. However, this age-responsiveness relationship does not seem to be a universal attribute. Unlike the air controls in the PS and NS studies, with CS animals, responsiveness remained fairly constant from wk 8 of exposure onward. As both the CS and PS hosts had their first contact with OA at the same age (i.e., at 4 wk old), it might be speculated that the presence of the 28-day lag period prior to initiating exposures of the PS hosts may have introduced the variable of a primary versus secondary immune response-related effect into the measurements. However, while this could explain, in part, the differences between PS and CS animals’ PC50 patterns of response to OA, it would not provide a clear basis as to why the differential response patterns arose when the animals received the nonspecific ACh challenge. As such, for now, the underlying reason for this difference in age-responsiveness pattern between the sensitized animal groups remains unclear.
Results of this investigation did show there to be often large intragroup variability, even within a gender, in measured airway responsiveness. Many species, including guinea pigs, have previously been shown to display a large range of sensitivity to bronchoprovocation agents, and to the extent of resultant measured airway responsiveness (Turner & Martin, 1997). This variability may reflect interindividual differences in airway receptor number or sensitivity (Abraham et al., 1980; Ahmed et al., 1980). Susceptibility to O3-induced alterations in airway responsiveness has also been shown to be variable within any particular exposure group (Douglas et al., 1977; Snapper et al., 1978; Habib et al., 1979; Abraham et al., 1980).
A potential factor underlying variability in airway responsiveness among individuals could be differences in the extent of sensitization, as measured by blood antigen-specific antibody levels. It was found that IgG levels correlated with PC50, but that the relationship accounted for <50% of the linear association between the parameters. Allergic IgE levels in humans have been shown to have a low correlation with increased nonspecific AHR (Palmer et al., 2000); in asthmatics and nonasthmatics, for example, ~30% of individual variance in airway responsiveness could be attributed to total serum IgE levels (Burrows et al., 1989; Sunyer et al., 1995). Thus, to a limited extent, the degree of sensitization may be reflected in extent of AHR.
This study also evaluated gender differences in airway responsiveness in relationship to O3 exposure. The effect of O3 was similar for both males and females; there was no evidence for any gender-based difference in functional response to O3. Previously reported studies, all involving humans, have provided conflicting results in terms of gender differences in lung functional responses to O3. Some have found no differences, while others suggested that females may show greater responses (Adams et al., 1981; Lauritzen & Adams, 1985; Dreschler-Parks et al., 1987). Such variations might have been due to differential doses and pollutant-to-lung volume ratios resulting from differences in lung size/ventilatory parameters. However, no gender difference in lung functional indices was found in humans exposed acutely to 0.35 ppm O3 when adjustments were made to assure similar ventilation (Weinmann et al., 1995). In the present study, similar effects occurred in both genders in spite of differences in body and lung size between male and female guinea pigs.
While our study showed no gender dependence of O3 effect on airway responsiveness, what is evident is that the general degree of responsiveness did differ between males and females. Within each protocol, both genders had similar responsiveness at the start of exposure, that is, at wk 0; after this, female guinea pigs showed less responsive airways. Similarly, Wanner et al. (1990) noted that male and female guinea pigs were equally reactive in early weeks of life, but gender differences began to appear at a later age. A temporal divergence of airway responsiveness between genders is also consistent with a study in humans, in which no gender difference was noted below 12 yr of age but began to emerge beyond this (Forastiere et al., 1996).
Although the effect of O3 on airway responsiveness does not appear to differ between the two groups of sensitized animals, the question remains of whether O3 affected sensitization—that is, did O3 coexposure during sensitization result in a different degree or extent of sensitization than in animals exposed to O3 after sensitization? The extent of sensitization was evaluated by comparing blood levels of antigen-specific IgG in animals in the PS and CS protocols. There was a statistically significant O3-exposure-related effect on IgG2 in animals sensitized prior to exposure, a pattern that was generally maintained in CS animals, although not at a significant level. It appears that increased levels of OA-specific antibody were produced in both groups of sensitized animals exposed to O3 compared to animals exposed to air, with PS animals showing a somewhat stronger effect. The ability of O3 to affect allergic sensitization had been addressed in a study by Osebold et al. (1980); in mice repeatedly exposed to OA and then to 0.5 or 0.8 ppm O3 for 24 h/day for 3–4 days, the extent of sensitization was enhanced compared to controls, a result consistent with that reported here.
Potential modulators of airway responsiveness in relation to O3 exposure are lung inflammatory cells. Studies of atopic animals and asthmatic humans suggest that AHR may be associated with increased numbers of eosinophils, monocytes, macrophages, PMNs, mast cells, and lymphocytes within airway subepithelium (Larsen, 1991). Bronchial lavage studies have shown an influx of eosinophils and PMNs into airways of asthmatic patients after allergen inhalation challenge that was associated with development of AHR (DeMonchy et al., 1985; Beasley et al., 1989); nonatopic guinea pigs exposed repeatedly to 1 ppm O3 had increased numbers of macrophages, eosinophils, and PMNs obtained by lavage at time points even when O3-induced AHR was returning to control levels (Sun & Chung, 1997).
A cell type particularly associated with airway dysfunction is the eosinophil. In our study, however, there was no significant relationship found between levels of these cells in lavage and the degree of AHR measured following the last exposure at 24 wk. In general, a causal association between eosinophilia and AHR appears equivocal. While some studies have found a relationship between numbers of eosinophils in lavage and AHR after antigen challenge in sensitized guinea pigs (Dunn et al., 1988; Hutson et al., 1988; Sanjar et al., 1990b; Santing et al., 1994), others noted that eosinophilia is not accompanied by AHR, suggesting there may be no causal relationship between these phenomena (Sun et al., 1997; Sanjar et al., 1990a, 1990b; Chapman et al., 1991; Kips et al., 1992; Pretolani et al., 1994).
To explain these disparate results, it has been suggested that animals sensitized with low doses of allergen exhibit airway eosinophilia in the absence of AHR, while those sensitized with higher doses exhibit both eosinophilia and AHR. However, if data from individual animals were examined in this regard, no correlation between the two parameters could be detected, consistent with results in our study. It has been suggested that eosinophil activation, rather than accumulation per se, is the constitutive factor in development of AHR in sensitized guinea pigs (Pretolani et al., 1994); however, cell activation was not measured in our study. The uncertain relationship between eosinophils and AHR in guinea pigs is similar to that in humans. Thus, while increased numbers of eosinophils in peripheral blood have been associated with human AHR (Rijcken et al., 1993), asthmatics can have eosinophilia without AHR (Chapman et al., 1991), and AHR has been shown to occur independently of eosinophil levels or total amount of IgE (Desjardins et al., 1988).
Another cell type associated with airways dysfunction is the PMN. While a consistent temporal association between onset of O3-induced AHR and PMN influx into airways has been found in studies with other species (Hotzman et al., 1983; Seltzer et al., 1986), no such temporal effect was seen here. While this may reflect interspecies differences in the relationship between these responses and O3 exposure, current indications are that, like eosinophilia, AHR and neutrophilia are not necessarily correlated.
The mast cell is also suggested to play a role in AHR and in atopic asthma (Wardlaw et al., 1988; van den Toorn et al., 2000). Increased numbers of such cells could result in enhanced responsiveness due to increased release of bronchoconstricting histamine. No increase in mast cells with exposure was noted in our study, consistent with other reports showing no relationship between mast cell numbers and AHR (Smith & McFadden, 1995).
As noted earlier, animals in this study were sacrificed ~7 days following their final test of airway responsiveness after the 24-wk exposure or 8-wk postexposure periods. This time frame may have impacted on some cellular results described here. For example, guinea pigs sensitized to OA and subsequently challenged by OA inhalation showed a peak response of eosinophilia in lavage at 24 h postchallenge (Underwood et al., 1992). Thus, it is possible that any early post-OA exposure eosinophilia, neutrophilia, or other measure of inflammation could have resolved by the time guinea pigs were lavaged. Conversely, a lack of a consistent correlation between intensity of inflammation, number of inflammatory cells/mediators, and degree of airway responsiveness in this and other studies undermines the assumption that AHR is driven by inflammation. It has alternatively been suggested that O3-induced AHR may be due instead to a loss of normal epithelial function (Matsubara et al., 1995). In a study of OA-sensitized guinea pigs, increased response to ACh challenge was found without any light-microscopic evidence of epithelial damage (Masaki et al., 1994); such increased nonspecific AHR was suggested to be due to altered epithelial function, which included changes in neural reflexes and in the regulation of mediators of airway caliber. The results of our study, including a lack of evidence for pulmonary inflammation in lung sections, provide some support for this potential mechanism.
The prevalence and severity of airway diseases such as asthma are increasing worldwide, and while the factors involved are likely to be complex, various environmental influences, including air pollution, are recognized as potentially playing a role in their genesis/exacerbation. Exacerbation of AHR following exposure to an inhaled pollutant has a practical health impact, which can range from minor airway constriction to severe asthmatic attack. Since asthmatics generally have lower levels of lung function and increased airway responsiveness compared to most normals, the clinical consequences of any O3 exposure-induced effects on airway function may be quite serious.
This study has shown that long-term repeated exposures to O3 did not result in the actual induction of AHR in nonsensitized animals. Thus, while a limited number of long-term exposure epidemiological studies suggest that O3 is involved in induction of asthma, our study does not support this, at least in terms of the development of AHR in nonatopic hosts. On the other hand, our study demonstrated persistent exacerbation of AHR, to both nonspecific and specific stimuli, in sensitized animals. This suggests that the subpopulation which incorporates individuals with hyperresponsive airways may be at increased risk from exposure to air pollutants, such as O3, supporting epidemiological evidence that ambient O3 is associated with the aggravation of preexisting pulmonary diseases such as asthma, and that O3 adversely affects atopic humans.
Acknowledgments
This study was supported by a research contract (94-12) from the Health Effects Institute and is part of a Center Program (ES00260) supported by NIEHS.
Footnotes
Copyright of Inhalation Toxicology is the property of Taylor & Francis Ltd and its content may not be copied or emailed of multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
Contributor Information
Richard B. Schlesinger, Department of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, USA
Mitchell D. Cohen, Department of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, USA
Terry Gordon, Department of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, USA.
Christine Nadziejko, Department of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, USA.
Judith T. Zelikoff, Department of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, USA
Maureen Sisco, Department of Environmental Medicine, New York University School of Medicine, Tuxedo, New York, USA.
Jean F. Regal, Department of Pharmacology, University of Minnesota, Duluth, Minnesota, USA
Margaret G. Ménache, Department of Pediatrics, University of New Mexico Health Sciences Center, Albuquerque, New Mexico, USA
REFERENCES
- Abraham W, Januszkiewicz AJ, Mingle M, Welker M, Wanner A, Sackner A. Sensitivity of bronchoprovocation and tracheal mucous velocity in detecting airway response to O3. J. Appl. Physiol. 1980;48:789–793. doi: 10.1152/jappl.1980.48.5.789. [DOI] [PubMed] [Google Scholar]
- Adams WC, Savin W, Christo AE. Detection of ozone toxicity during continuous exercise via the effective dose concept. J. Appl. Physiol. 1981;51:415–422. doi: 10.1152/jappl.1981.51.2.415. [DOI] [PubMed] [Google Scholar]
- Agrawal KP. Specific airway conductance in guinea pigs: Normal values and histamine induced fall. Respir. Physiol. 1981;43:23–30. doi: 10.1016/0034-5687(81)90085-2. [DOI] [PubMed] [Google Scholar]
- Ahmed T, Eyre P, Januszkiewicz J, Wanner A. Role of H1- and H2-receptors in airway reactions to histamine in conscious sheep. J. Appl. Physiol. 1980;49:826–833. doi: 10.1152/jappl.1980.49.5.826. [DOI] [PubMed] [Google Scholar]
- Beasley R, Roche WM, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am. Rev. Respir. Dis. 1989;139:806–817. doi: 10.1164/ajrccm/139.3.806. [DOI] [PubMed] [Google Scholar]
- Biagini RE, Moorman WJ, Lewis TR, Bernstein IL. Ozone enhancement of platinum asthma in a primate model. Am. Rev. Respir. Dis. 1986;134:719–725. doi: 10.1164/arrd.1986.134.4.719. [DOI] [PubMed] [Google Scholar]
- Burrows B, Martinez F, Halonen M, Barbee R, Cline M. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N. Engl. J. Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- Chapman ID, Boubekeur K, Morley J. New drugs for asthma therapy. Basel: Birkhauser Verlag; 1991. Airway eosinophilia and airway hyperreactivity are parallel rather than sequential events in the guinea pig; pp. 445–456. [PubMed] [Google Scholar]
- DeMonchy JG, Kauffman HF, Venge P, Koëter GH, Jansen HM, Sluiter HJ, DeVries K. Bronchoalveolar eosinophilia during allergen-induced late asthmatic reactions. Am. Rev. Respir. Dis. 1985;131:373–376. doi: 10.1164/arrd.1985.131.3.373. [DOI] [PubMed] [Google Scholar]
- Desjardins A, de Luca S, Cartier P. Nonspecific bronchial hyperresponsiveness to inhaled histamine and hyperventilation of cold dry air in subjects with respiratory symptoms of uncertain etiology. Am. Rev. Respir. Dis. 1988;137:1020–1025. doi: 10.1164/ajrccm/137.5.1020. [DOI] [PubMed] [Google Scholar]
- Dimeo MJ, Glenn M, Holtzman M, Sheller J, Nadel J, Boushey HA. Threshold concentration of ozone causing an increase in bronchial reactivity in humans and adaptation with repeated exposures. Am. Rev. Respir. Dis. 1981;124:245–248. doi: 10.1164/arrd.1981.124.3.245. [DOI] [PubMed] [Google Scholar]
- Douglas JS, Ridgway P, Brink C. Airway response of the guinea pig in vivo and in vitro. J. Pharmacol. Exp. Ther. 1977;202:116–124. [PubMed] [Google Scholar]
- Drechsler-Parks DM, Bedi JF, Horvath SM. Pulmonary function responses of older men and women to ozone exposure. Exp. Gerontol. 1987;22:91–101. doi: 10.1016/0531-5565(87)90044-1. [DOI] [PubMed] [Google Scholar]
- Dunn CJ, Elliott GA, Oostveen JA, Richards IM. Development of a prolonged eosinophil-rich inflammatory leukocyte infiltration in the guinea-pig asthmatic response to oval-bumin inhalation. Am. Rev. Respir. Dis. 1988;137:541–547. doi: 10.1164/ajrccm/137.3.541. [DOI] [PubMed] [Google Scholar]
- Folinsbee LJ, Horstman DH, Kehrl HR, Harder S, Abdul-Salaan S, Ives PJ. Respiratory responses to repeated prolonged exposure to 0.12 ppm ozone. Am. J. Respir. Crit. Care Med. 1994;149:98–105. doi: 10.1164/ajrccm.149.1.8111607. [DOI] [PubMed] [Google Scholar]
- Forastiere F, Corbo GM, Dell’orco V, Pistelli R, Agabiti N, Kriebel D. A longitudinal evaluation of bronchial responsiveness to methacholine in children: Role of baseline lung function, gender, and change in atopic status. Am. J. Respir. Crit. Care Med. 1996;153:1098–1104. doi: 10.1164/ajrccm.153.3.8630551. [DOI] [PubMed] [Google Scholar]
- Fraser DG, Graziano FM, Larsen CP, Regal JF. Role of IgG1 and IgG2 in trimel-litic anhydride-induced allergic response in guinea pig lung. Toxicol. Appl. Pharmacol. 1998;150:218–227. doi: 10.1006/taap.1998.8419. [DOI] [PubMed] [Google Scholar]
- Gaultier CL, Harf A, Lorino AM, Atlan G. Lung mechanics in growing guinea pigs. Respir. Physiol. 1984;56:217–228. doi: 10.1016/0034-5687(84)90105-1. [DOI] [PubMed] [Google Scholar]
- Gordon T, Drazen JM, Amdur MO, Venugopalan CS. Ozone-induced airway hyperreactivity in guinea pigs. J. Appl. Physiol. 1984;57:1034–1038. doi: 10.1152/jappl.1984.57.4.1034. [DOI] [PubMed] [Google Scholar]
- Gross KB, Sargent NE. Increases in bronchial responsiveness induced by ozone inhalation and the effects of selected non-steroidal antiinflammatory agents. Am. Rev. Respir. Dis. 1992;145:A428. [Google Scholar]
- Habib MP, Pare PD, Engel LA. Variability of airway responses to inhaled histamine in normal subjects. J. Appl. Physiol. 1979;47:51–58. doi: 10.1152/jappl.1979.47.1.51. [DOI] [PubMed] [Google Scholar]
- Herxheimer H, West T. Sensitization of guinea pigs by inhalation. J. Physiol. 1955;127:564–571. doi: 10.1113/jphysiol.1955.sp005277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman MJ, Cunningham JH, Sheller JR, Irsigler GB, Nadel JA, Boushey HA. Effect of ozone on bronchial reactivity in atopic and non-atopic subjects. Am. Rev. Respir. Dis. 1979;120:1059–1067. doi: 10.1164/arrd.1979.120.5.1059. [DOI] [PubMed] [Google Scholar]
- Holtzman MJ, Fabbri LM, O’Byrne PM, Gold BD, Aizawa H, Walters EH, Alpert SE, Nadel JA. Importance of airway inflammation for hyperresponsiveness induced by ozone. Am. Rev. Respir. Dis. 1983;127:686–690. doi: 10.1164/arrd.1983.127.6.686. [DOI] [PubMed] [Google Scholar]
- Horstmann DH, Folinsbee LJ, Ives PJ, Abdul-Salaam S, McDonnell WF. Ozone concentration and pulmonary response relationships for 6.6-hour exposures with five hours of moderate exercise to 0.08, 0.10, and 0.12 ppm. Am. Rev. Respir. Dis. 1990;142:1158–1163. doi: 10.1164/ajrccm/142.5.1158. [DOI] [PubMed] [Google Scholar]
- Hutson PA, Church MK, Clay TP, Miller P, Holgate SP. Early and late-phase bronchoconstriction after allergen challenge of nonanesthetized guinea pigs. I. The association of disordered airway physiology to leukocyte infiltration. Am. Rev. Respir. Dis. 1988;137:548–557. doi: 10.1164/ajrccm/137.3.548. [DOI] [PubMed] [Google Scholar]
- Jenkins HS, Devalia JL, Mister RL, Bevan AM, Rusznak C, Davies RJ. The effect of exposure to ozone and nitrogen dioxide on the airway response of atopic asthmatics to inhaled allergen. Dose- and time-dependent effects. Am. J. Respir. Crit. Care Med. 1999;160:33–39. doi: 10.1164/ajrccm.160.1.9808119. [DOI] [PubMed] [Google Scholar]
- Johnson HG, Stout BK, Ruppel PL. Inhibition of the 5-lipoxygenase pathway with piripost (U-60,257) protects normal primates from ozone-induced methacholine hyperresponsive small airways. Prostaglandins. 1988;35:459–466. doi: 10.1016/0090-6980(88)90136-0. [DOI] [PubMed] [Google Scholar]
- Jörres R, Nowak D, Magnussen H. The effect of ozone exposure on allergen responsiveness in subjects with asthma or rhinitis. Am. J. Respir. Crit. Care Med. 1996;153:56–64. doi: 10.1164/ajrccm.153.1.8542163. [DOI] [PubMed] [Google Scholar]
- Josephs LK, Gregg I, Holgate ST. Eur. Respir. J. Vol. 3. 1990. Does non-specific bronchial responsiveness indicate the severity of asthma? pp. 220–227. [PubMed] [Google Scholar]
- Kagawa J, Haga M, Miyazaki M. Effects of repeated exposure to 0.15 ppm O3 for four months on bronchial reactivity in guinea pigs (4 hrs/d, 5 d/wk) In: Schneider T, Lee SD, Wolters GJ, Grant LD, editors. Atmospheric ozone research and its policy implications: Proceedings of the 3rd US–Dutch international symposium. Amsterdam: Elsevier; 1989. pp. 545–552. [Google Scholar]
- Kips JC, Cuvelier CA, Pauwels RA. Effect of acute and chronic antigen inhalation on airway morphology and responsiveness in actively sensitized rats. Am. Rev. Respir. Dis. 1992;145:1306–1310. doi: 10.1164/ajrccm/145.6.1306. [DOI] [PubMed] [Google Scholar]
- Koenig JQ, Covert DS, Morgan MS. Acute effects of 0.12 ppm ozone or 0.12 ppm nitrogen dioxide on pulmonary function in healthy and asthmatic adolescents. Am. Rev. Respir. Dis. 1985;132:648–651. doi: 10.1164/arrd.1985.132.3.648. [DOI] [PubMed] [Google Scholar]
- Kreit JW, Gross KB, Moore TB, Lorenzen TJ, D’Arcy J, Eschembacher WL. Ozone-induced changes in pulmonary function and bronchial responsiveness in asthmatics. J. Appl. Physiol. 1989;66:217–222. doi: 10.1152/jappl.1989.66.1.217. [DOI] [PubMed] [Google Scholar]
- Larsen GL. Experimental models of reversible airway obstruction. In: Crystal RG, West JB, editors. The lung: Scientific foundations. New York: Raven Press; 1991. pp. 913–966. [Google Scholar]
- Lauritzen SK, Adams WC. Ozone inhalation effects consequent to continuous exercise in females: Comparison to males. J. Appl. Physiol. 1985;59:1601–1606. doi: 10.1152/jappl.1985.59.5.1601. [DOI] [PubMed] [Google Scholar]
- Lewis CA, Broadley KJ. Airway hyper- or hyporeactivity to inhaled spasmogens 24 h after ovalbumin challenge of sensitized guinea-pigs. Br. J. Pharmacol. 1995;116:2351–2358. doi: 10.1111/j.1476-5381.1995.tb15079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linn WS, Shamoo DA, Anderson KR, Peng RC, Avol EL, Hackney JD. Responses of asthmatics in prolonged, repeated exposures to ozone and sulfuric acid. Am. Rev. Respir. Dis. 1992;145:A428. [Google Scholar]
- Linn WS, Shamoo DA, Anderson KR, Peng RC, Avol EL, Hackney JD. Effects of prolonged, repeated exposure to ozone, sulfuric acid and the combination in healthy and asthmatic volunteers. Am. J. Respir. Crit. Care Med. 1994;150:431–440. doi: 10.1164/ajrccm.150.2.8049826. [DOI] [PubMed] [Google Scholar]
- Masaki Y, Munakata M, Amishima M, Homma Y, Kawakami Y. In vivo, in vitro correlation of acetylcholine airway responsiveness in sensitized guinea pig. Role of modified epithelial function. Am. J. Respir. Crit. Care Med. 1994;149:1494–1498. doi: 10.1164/ajrccm.149.6.8004304. [DOI] [PubMed] [Google Scholar]
- Matsubara S, Fushimi K, Kaminuma O, Kikkawa H, Shimazu N, Iwasaki H, Ikezawa K. Importance of impairment of the airway epithelium for ozone-induced airway hyperresponsiveness in guinea pigs. Jpn. J. Pharmacol. 1995;67:375–382. doi: 10.1254/jjp.67.375. [DOI] [PubMed] [Google Scholar]
- McDonnell WF, Abbey DE, Nishino N, Lebowitz MD. Long-term ambient ozone concentration and the incidence of asthma in nonsmoking adults: The Ahsmog study. Environ. Res. 1999;80:110–121. doi: 10.1006/enrs.1998.3894. Sect. A. [DOI] [PubMed] [Google Scholar]
- McManus MS, Gong H, Clark JJ. Tolerance to repeated daily ozone exposures in asthmatic subjects. Am. Rev. Respir. Dis. 1990;141:A70. [Google Scholar]
- Molfino NA, Wright SC, Katz I, Tarlo S, Silverman F, McClean PA, Szalai JP, Raizenne M, Slutsky AS, Zamel N. Effect of low concentrations of ozone on inhaled allergen responses in asthmatic subjects. Lancet. 1991;338:199–203. doi: 10.1016/0140-6736(91)90346-q. [DOI] [PubMed] [Google Scholar]
- Morgan WK, Reger RB. Chronic airflow limitation and occupation. In: Cherniack NS, editor. Chronic obstructive pulmonary disease. Philadelphia: W. B. Saunder; 1991. pp. 270–285. [Google Scholar]
- Osebold JW, Gershwin LJ, Zee YC. Studies on the enhancement of allergic lung sensitization by inhalation of ozone and sulfuric acid aerosol. J. Environ. Pathol. Toxicol. Oncol. 1980;3:221–234. [PubMed] [Google Scholar]
- Palmer LJ, Burton PR, Faux JA, James AL, Musk AW, Coodson WO. Independent inheritance of serum immunoglobulin E concentrations and airway responsiveness. Am. J. Crit. Care Med. 2000;161:1836–1843. doi: 10.1164/ajrccm.161.6.9805104. [DOI] [PubMed] [Google Scholar]
- Paoletti P, Carrozi L, Viegi G, Modena P, Ballerin L, Pede F, Grado L, Baldacci S, Pedreschi M, Vellutini M. Distribution of bronchial responsiveness in a general population: Effect of sex, age, smoking and level of pulmonary function. Am. J. Respir. Crit. Care Med. 1995;151:1770–1777. doi: 10.1164/ajrccm.151.6.7767519. [DOI] [PubMed] [Google Scholar]
- Peat J, Salome C, Xuan W. On adjusting measurements of airway responsiveness for lung size and airway caliber. Am. J. Respir. Crit. Care Med. 1996;154:870–875. doi: 10.1164/ajrccm.154.4.8887577. [DOI] [PubMed] [Google Scholar]
- Peden D. Controlled exposures of asthmatics to air pollutants. In: Holgate ST, Samet JM, Koren HS, Maynard RL, editors. Air pollution and health. San Diego: Academic Press; 1999. pp. 865–880. [Google Scholar]
- Peden D. Development of atopy and asthma: Candidate environmental influences and important periods of exposure. Environ. Health Perspect. 2000;108 suppl. 3:475–482. doi: 10.1289/ehp.00108s3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretolani M, Ruffié C, Joseph D, Campos MG, Church MK, Lefort J, Vargaftig BB. Role of eosinophil activation in the bronchial reactivity of allergic guinea pigs. Am. J. Respir. Crit. Care Med. 1994;149:L1167–L1174. doi: 10.1164/ajrccm.149.5.8173756. [DOI] [PubMed] [Google Scholar]
- Rijcken B, Schouten JP, Mensinga TT, Weiss ST, De Vries K, van der Lende R. Factors associated with bronchial responsiveness to histamine in a population sample of adults. Am. Rev. Respir. Dis. 1993;147:1447–1453. doi: 10.1164/ajrccm/147.6_Pt_1.1447. [DOI] [PubMed] [Google Scholar]
- Ryan L. Ozone. In: Cohen MD, Zelikoff JT, Schlesinger RB, editors. Pulmonary immunotoxicology. Boston: Kluwer; 2000. pp. 301–336. [Google Scholar]
- Sanjar S, Aoki S, Boubekeur K, Chapman ID, Smith D, Kings MA, Morley J. Eosinophil accumulation in pulmonary airways of guinea-pigs induced by exposure to an aerosol of platelet activating factor: Effect of anti-asthma drugs. Br. J. Pharmacol. 1990a;99:267–272. doi: 10.1111/j.1476-5381.1990.tb14692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjar S, Aoki S, Kristersson A, Smith D, Morley J. Antigen challenge induces pulmonary airway eosinophil accumulation in sensitized guinea pigs: The effects of anti-asthma drugs. Br. J. Pharmacol. 1990b;99:679–686. doi: 10.1111/j.1476-5381.1990.tb12989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santing RE, Hoekstra Y, Pasman Y, Zaagsma J, Meurs H. The importance of eosinophil activation for the development of allergen-induced bronchial hyperreactivity in conscious, unrestrained guinea-pigs. Clin. Exp. Allergy. 1994;24:1157–1163. doi: 10.1111/j.1365-2222.1994.tb03322.x. [DOI] [PubMed] [Google Scholar]
- Schlesinger RB, Gorczynski JE, Dennison J, Richards L, Kinney PL, Bosland MC. Long-term intermittent exposures to sulfuric acid aerosol, ozone, and their combination: Alterations in tracheobronchial mucociliary clearance and epithelial secretory cells. Exp. Lung Res. 1992;18:505–534. doi: 10.3109/01902149209064343. [DOI] [PubMed] [Google Scholar]
- Seltzer J, Bigby BG, Stulbarg M, Holtzman MJ, Nadel JA, Ueki IF, Leikauf G, Goetzl EJ, Boushey HA. O3-Induced change in bronchial reactivity to methacholine and airway inflammation in humans. J. Appl. Physiol. 1986;60:1321–1326. doi: 10.1152/jappl.1986.60.4.1321. [DOI] [PubMed] [Google Scholar]
- Smith L, McFadden ER., Jr Bronchial hyperreactivity revisited. Allergy Asthma Immunol. 1995;74:454–469. [PubMed] [Google Scholar]
- Snapper JR, Drazen JM, Loring SH, Schneider W, Ingram RH., Jr Distribution of pulmonary responsiveness to aerosol histamine in dogs. J. Appl. Physiol. 1978;44:738–742. doi: 10.1152/jappl.1978.44.5.738. [DOI] [PubMed] [Google Scholar]
- Sun J, Chung F. Airway inflammation despite loss of bronchial hyperresponsiveness after multiple ozone exposures. Respir. Med. 1997;91:47–55. doi: 10.1016/s0954-6111(97)90136-0. [DOI] [PubMed] [Google Scholar]
- Sun J, Koto H, Chung KF. Interaction of ozone and allergen challenges, bronchial responsiveness and inflammation in sensitized guinea pigs. Int. Arch. Allergy Immunol. 1997;112:191–195. doi: 10.1159/000237453. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Anto JM, Sabria J, Roca J, Morell F, Rodriguez-Roisin R, Rodrigo MJ. Relationship between serum IgE and airway responsiveness in adults with asthma. J. Allergy Clin. Immunol. 1995;95:699–706. doi: 10.1016/s0091-6749(95)70175-3. [DOI] [PubMed] [Google Scholar]
- Thompson JE, Gordon T, Scypinski LA, Sheppard D. Tachykinins mediate toluene diisocyanate-induced airway hyperresponsiveness. Am. Rev. Respir. Dis. 1987;136:1118–1121. doi: 10.1164/ajrccm/136.1.43. [DOI] [PubMed] [Google Scholar]
- Thorne JR, Broadley KJ. Adenosine-induced bronchoconstriction in conscious hyperresponsive and sensitized guinea pigs. Am. J. Respir. Crit. Care Med. 1994;149:392–399. doi: 10.1164/ajrccm.149.2.8306036. [DOI] [PubMed] [Google Scholar]
- Thurston GD, Ito K. Epidemiological studies of ozone exposure effects. In: Holgate ST, Samet JM, Koren HS, Maynard RL, editors. Air pollution and health. San Diego: Academic Press; 1999. pp. 485–510. [Google Scholar]
- Turner DJ, Martin JG. Animal models. In: Barnes PJ, Grunstein MM, Leff AR, Woolcock AJ, editors. Asthma. Philadelphia: Lippincott-Raven; 1997. pp. 261–274. [Google Scholar]
- Underwood DC, Osborn RR, Hand JM. Lack of late-phase airway responses in conscious guinea pigs after a variety of antigen challenges. Agents Actions. 1992;37:191–194. doi: 10.1007/BF02028106. [DOI] [PubMed] [Google Scholar]
- U.S. Environmental Protection Agency. Air quality criteria for ozone and related photochemical oxidants. Washington, DC: U.S. Environmental Protection Agency, Office of Research and Development; 1996. EPA/600/P-93/0041F. [Google Scholar]
- van den Toorn LM, Prins JB, Overbeek SE, Hoogsteden HC, de Jongste JC. Adolescents in clinical remission of atopic asthma have elevated exhaled nitric oxide levels and bronchial hyperresponsiveness. Am. J. Respir. Crit. Care Med. 2000;162:953–957. doi: 10.1164/ajrccm.162.3.9909033. [DOI] [PubMed] [Google Scholar]
- Vargas MH, Romero L, Sommer B, Zamudio P, Gustin P, Montano LM. Chronic exposure to ozone causes tolerance to airway hyperresponsiveness in guinea pigs: Lack of SOD role. J. Appl. Physiol. 1998;84:1749–1755. doi: 10.1152/jappl.1998.84.5.1749. [DOI] [PubMed] [Google Scholar]
- Wanner A, Abraham WM, Douglas JS, Drazen JM, Richerson HB, Ram JS. Models of airway hyperresponsiveness. Am. Rev. Respir. Dis. 1990;142:253–257. doi: 10.1164/ajrccm/141.1.253. [DOI] [PubMed] [Google Scholar]
- Wardlaw AJ, Dunnette S, Gleich GH, Collins JV, Kay AB. Eosinophils and mast cells in bronchoalveolar lavage in subjects with mild asthma. Am. Rev. Respir. Dis. 1988;137:62–69. doi: 10.1164/ajrccm/137.1.62. [DOI] [PubMed] [Google Scholar]
- Weinmann GG, Weidenbach-Gerbase M, Foster WM, Zacur H, Frank R. Evidence for ozone-induced small-airway dysfunction: Lack of menstrual-cycle and gender effects. Am. J. Respir. Crit. Care Med. 1995;152:988–996. doi: 10.1164/ajrccm.152.3.7663815. [DOI] [PubMed] [Google Scholar]
- Weiss ST, Tager IB, Weiss JW, Speizer FE, Ingram RH., Jr The distribution of airways responsiveness in a population sample of adults and children. Am. Rev. Respir. Dis. 1981;123 suppl.:130. doi: 10.1164/arrd.1984.129.6.898. [DOI] [PubMed] [Google Scholar]
- Wolfe R, Carlin JB, Oswald H, Olinsky A, Phelan PD, Robertson CF. Association between allergy and asthma from childhood to middle adulthood in an Australian cohort study. Am. J. Respir. Crit. Care Med. 2000;162:2177–2181. doi: 10.1164/ajrccm.162.6.9812019. [DOI] [PubMed] [Google Scholar]
- Yanai M, Ohrui T, Aikawa T, Okayama H, Sekizawa K, Maeyama K, Sasaki H, Takishima T. Ozone increases susceptibility to antigen inhalation in allergic dogs. J. Appl. Physiol. 1990;68:2267–2273. doi: 10.1152/jappl.1990.68.6.2267. [DOI] [PubMed] [Google Scholar]
- Ying RL, Gross KB, Terzo TS, Eschenbacher WL. Indomethacin does not inhibit the ozone-induced increase in bronchial responsiveness in human subjects. Am. Rev. Respir. Dis. 1990;142:817–821. doi: 10.1164/ajrccm/142.4.817. [DOI] [PubMed] [Google Scholar]
- Zwick H, Popp W, Wagner C, Reiser K, Schmoger J, Bock A, Herkner K, Hadunsky K. Effects of ozone on the respiratory health, allergic sensitization, and cellular immune system in children. Am. Rev. Respir. Dis. 1991;144:1075–1079. doi: 10.1164/ajrccm/144.5.1075. [DOI] [PubMed] [Google Scholar]