Abstract
Plasma FK506 was studied in 49 liver, 13 heart, 3 double-lung or heart-lung, and 21 kidney recipients. The levels were correlated with the drug doses used, kidney function, and liver function. In all varieties of recipients, there was an early rise in the FK506 plasma levels that occurred at the time of intravenous administration of the drug. At the same time or shortly after, there were increases in serum creatinine that were transitory except in liver recipients with continuing suboptimal graft function. The quality of hepatic function dominated all aspects of FK506 management in the liver recipients. Those who received well-functioning grafts could be given about the same drug doses as recipients of kidneys and the thoracic organs. Liver recipients with defective grafts had astronomical rises in plasma FK506, a high incidence of renal failure, and probably increased neurotoxicity. In kidney transplant recipients, the FK506 plasma levels and doses were essentially the same in patients with prompt versus delayed renal function. These studies have highlighted the necessity, first, of close pharmacologic monitoring of patients who are given FK506 in the presence of abnormal liver function, and second, of using smaller intravenous induction doses than in past practice.
FK506 has been in use clinically for almost 18 months (1–5). After liver transplantation, we have noticed that the quality of liver graft function dramatically influences the doses and trough levels of FK506, as well as the quality of renal function. We describe here the interrelation of these parameters after liver transplantation; transplantation of hearts, lungs, and heart-lungs; and after the transplantation of cadaver kidneys with either prompt or delayed function. From this study has come a better understanding of how management policies interrelate with the different kinds of transplantation.
MATERIALS AND METHODS
Case material
Eighty-seven adults who were 17–69 years old (45.4±13.6 SD) underwent transplantation between October 13, 1989 and May 17, 1990. Follow-ups were to August 1, 1990. The male/female ratio was 47/40. Patients were included only if there were frequent FK506 plasma trough determinations within the 60-day study period. Samples were drawn on Mondays and Thursdays in most patients, or at the time of outpatient visits after discharge. Liver recipients were not included for analysis if a postoperative diagnosis was made of hepatic artery thrombosis or stenosis, biliary leak, and biliary stricture.
Heart transplantation (n = 13)
Because one objective was to determine the effect of kidney and liver function on FK506 doses and plasma levels, the thoracic organ recipients provided the most complete information. None of them had known intrinsic renal or hepatic disease preoperatively. Four of the 13 adult recipients were from a series recently reported by Armitage et al. (5). Pretransplant mechanical circulatory support had been required in 6 (46%) of the 13 patients; 5 had the Novacor left ventricular assist device (LVAD)* and the other was maintained with an intraaortic balloon pump (IABP).
One other patient not included in the study group had been maintained for several weeks on a left ventricular assist device preoperatively. He had a cardiac arrest during preparation for transplantation and was administered cardiac massage until cardiopulmonary bypass could be instituted. His heart transplant functioned well, but the results from his FK506 studies as well as his postoperative clinical course were so different from the others that his case will be documented separately.
Double-lung (n = 2) and heart-lung (n = 1) transplantation
These patients were 26, 33, and 27 years old. Both double-lung recipients had cystic fibrosis, and the heart-lung recipient had Eisenmenger’s complex. These patients have been mentioned in other reports (3, 5).
Kidney transplantation (n = 21)
Fifteen patients underwent primary cadaveric transplantation and 6 were undergoing cadaveric retransplantation. None was known to have liver disease. They were stratified into those (n = 14) who had prompt graft function with sustained freedom from dialysis throughout the 2-month period of study, and those (n = 7) who had acute tubular necrosis requiring dialysis for 3– 20 days during graft recovery.
Liver transplantation (n = 49)
The 49 liver recipients were free of intrinsic chronic kidney disease, although 7 (14.3%) had hepatorenal syndrome not requiring dialysis and 4 more (8.2%) were on dialysis at the time of their liver replacement. Although 47 (95%) of the 49 patients survived, they could be stratified into 4 postoperative subgroups defined mainly by the quality of liver graft function and, to a lesser degree, by the rapidity of recovery from intensive care needs. Class I patients (n = 14) did not have evidence of major graft ischemia (SGPT <500 IU/ L in the first 5 days) and became jaundice-free with a serum bilirubin <2 mg% by 10 days. They were removed from ventilator assistance and extubated within 3 days. Class II patients (n = 17) had early rises in SGPT of >2000 IU/L with delayed resolution of jaundice until 10–20 days. Ventilator dependence was for 3 to 9 days. Class III (n = 10) patients had highly variable evidence of acute ischemic injury, and slow resolution of jaundice in that the serum bilirubin did not fall to <2 mg% until 20 to 45 days. Assisted ventilation was required for up to 30 days. The Class IV patients (n = 8) had abnormally high serum bilirubins throughout the 60-day period of study and remained ventilator-dependent throughout this time. All patients in Class III and IV required parenteral hyperalimentation. The 2 deaths were of group IV patients who died of multiple organ failure and sepsis after 40 and 47 days.
Immunosuppression
The beginning therapeutic regimen was relatively fixed for all of the organ recipients. Intravenous FK506 doses of 0.075 mg/kg infused over 4 hr were started in the operating or recovery rooms and repeated every 12 hr until oral intake was started. When the conversion was made from intravenous to oral dosing at 0.15 mg/ kg every 12 hr, administration by both routes frequently was overlapped for one or 2 doses. Doses were reduced if there were suspected adverse drug reactions, or which nephrotoxicity and neurotoxicity were the most common. The warning parameters were an increase in serum creatinine above 2 mg%, BUN above 100 mg% and/or changes in the mental status concomitant with significant tremors. These were considered particularly ominous in the patients with evident hepatic graft dysfunction or in those with clinical and/or bacteriological evidence of sepsis. Most upward dose adjustments were prompted by a clinical suspicion of rejection, the histopathologic diagnosis of rejection, or the demonstration of plasma FK506 levels <0.5 ng/ml. For most of the study period, the plasma levels were not available until 4 or 5 days after the samples were taken.
Prednisone usually was started at a daily dose of 20 mg, but in a number of the early patients a five-day steroid cycle was used, beginning at 200 mg perioperatively and finishing at 20 mg/day by the sixth postoperative day. By the end of two postoperative months, 13 (62%) and 27 (55%) of the kidney and liver recipients, respectively, were completely weaned from steroids as has been reported elsewhere in detail (6, 7). In contrast, only 2 (15%) of the cardiac recipients had steroids stopped in this period. The recipients of double lungs or a heart lung were not given steroids during the first 60 days. In these 3 patients, FK506 oral doses were increased until rises in creatinine were seen.
In addition to clinical criteria for guidance of therapy, protocol graft biopsies were obtained in the liver recipients after 10–14 days and again after 2 months. Biopsies in the heart recipients were obtained once a week throughout most of the first 2 months. Additional biopsies were obtained if there was evidence of rejection or graft dysfunction for unclear reasons. After kidney transplantation, the policy was to biopsy only for specific indications.
Persistent rejection was treated with an increase in FK506. When there was not a satisfactory response or when there was renal dysfunction (creatinine ≥2 mg%), which precluded augmented FK506 therapy, daily steroid doses were increased or steroid boluses were given as described elsewhere (2, 3, 5). In 12 (13.8%) of the 87 patients, a 3–5 day course of OKT3 was given (9 liver, 3 kidney, no hearts, lungs, or heart-lungs).
FK506 monitoring
Trough plasma FK506 levels were determined with the enzyme immunoassay technique of Tamura et al. (8) twice per week or more often in complicated cases. This technique in our laboratory has a coefficient of variation of 22% at 0.5 ng/ml and 10% at 2.1 ng/ml. Most of the studies were performed during our learning curve with FK506, when optimal 12-hr trough levels were thought to be in the 0.5 to 1.5 ng/ml range. We now believe that the desirable range may be somewhat higher than this. However, results from the plasma determinations did not influence management because they were not available until days or even weeks later, especially in the first cases of the series. In most of the patients, the trough values were at 12 hr since dosing was twice a day. However, in a few class III or IV patients whose FK506 was given once per day or every other day, the troughs were at 24 or 48 hr.
Statistical analyses
The 60 days of the study were divided into 5-day intervals. For each case, the mean was calculated for results of the biochemical tests and other parameters during the 5-day period. These mean values were pooled for all cases in the various groups and the means (±SE) were calculated. Single variable comparison between two or more different groups (classes) at each point was done with the unpaired t test and Bonferroni (Dunn) test, respectively. Signed rank comparisons with Bonferroni adjustment were used for serial analysis of changes in each parameter at different times and Kendall Tau test for the correlation between two variables with and without a lag time. The correlation between the individual mean values of simultaneously obtained serum bilirubin, FK506 plasma level, and drug dose in liver recipients was calculated over all time points of the study period using Spearman rank correlations. These were computed using SAS/PC version 6.03 (SAS Inst. Inc., Gary, NC).
RESULTS
Heart transplantation
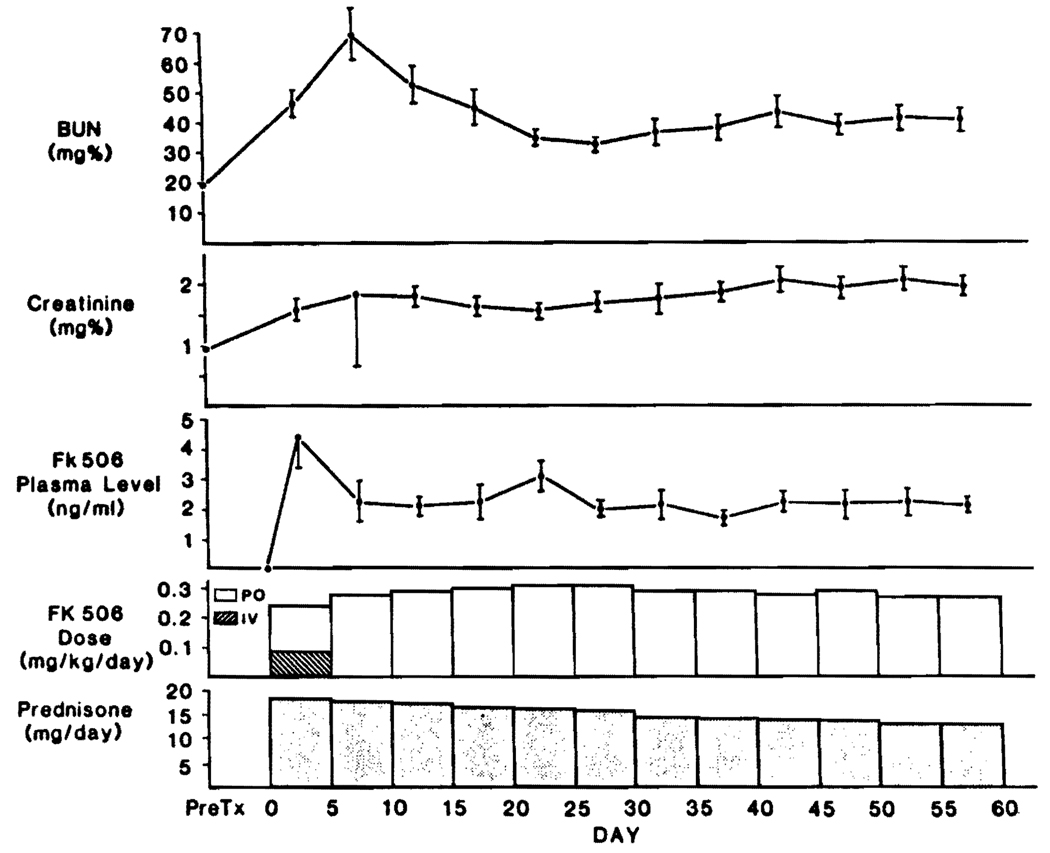
During the first 5 postoperative days, all 13 patients were converted from intravenous to oral FK506. During these 5 days, the average plasma trough levels of FK506 were higher (P>0.05) than at any subsequent time (Fig. 1). Oliguria or other evidence of renal dysfunction was common perioperatively, but maximal rises in the mean BUN or creatinine were delayed until the following 5-day period (Fig. 1). These improved subsequently, usually with minor adjustment of FK506 oral doses. None of the patients required dialysis. By the end of 60 days, the mean (±SD) of daily oral FK506 dose for all patients was 0.28±0.08 mg/kg, which was not significantly less than the planned dose of 0.3 mg/kg. However, in 4 of these patients who had elevated serum creatinine (≥2 mg%) the daily doses had been significantly (P<0.05) reduced, with a mean of 0.19 ± 0.06 mg/kg. In the meanwhile, the maintenance prednisone doses of 20 mg/day were slowly reduced for most patients throughout the entire 60-day period starting after the first 5 postoperative days (Fig. 1).
FIGURE 1.
Renal function (mean ± SE), FK506 doses, and plasma FK506 levels in 13 adult heart recipients.
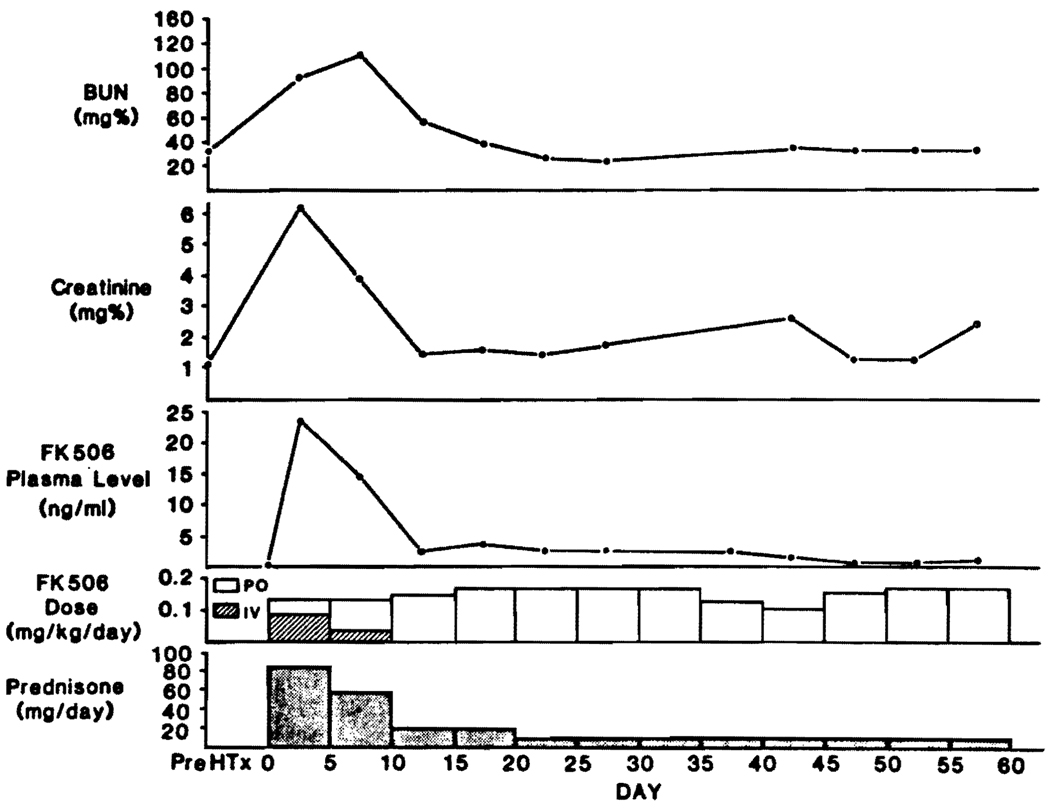
An extraordinary increase in perioperative plasma FK506 levels was seen in a 47-year-old patient (not included in the data of Fig. 1) who had been maintained on an LVAD for several weeks preoperatively and who had an irreversible cardiac arrest just before his heart transplantation. He was treated with cardiac massage and emergency placement of an IABP until the institution of cardiopulmonary bypass. Administration of the usual induction dose of i.v. FK506 (0.15 mg/kg/day) caused an astronomical increase in the trough levels to a peak of 33 ng/ml, with a mean in the first 5 days of 23.3 ng/ml (Fig. 2). Renal failure developed in the first postoperative day, necessitating 3 hemodialyses. He developed a paranoid psychosis at this time. With the reduced intravenous and oral FK506 doses (about half of those usually given) shown in Figure 2, renal function returned to normal and the psychosis resolved. Prednisone was tapered rapidly (Fig. 2). Liver function tests were normal in this and all other heart recipients.
FIGURE 2.
FK506 doses, high plasma FK506 levels, and renal dysfunction requiring 3 dialyses in a heart recipient who had a cardiac arrest and prolonged massage just before transplantation. The evolution was atypical for a heart recipient and probably was due to multiple-organ damage including the liver perioperatively. However, liver function tests were normal. Recovery was prompt and complete after a dose adjustment of FK506.
Lung and heart-lung recipients
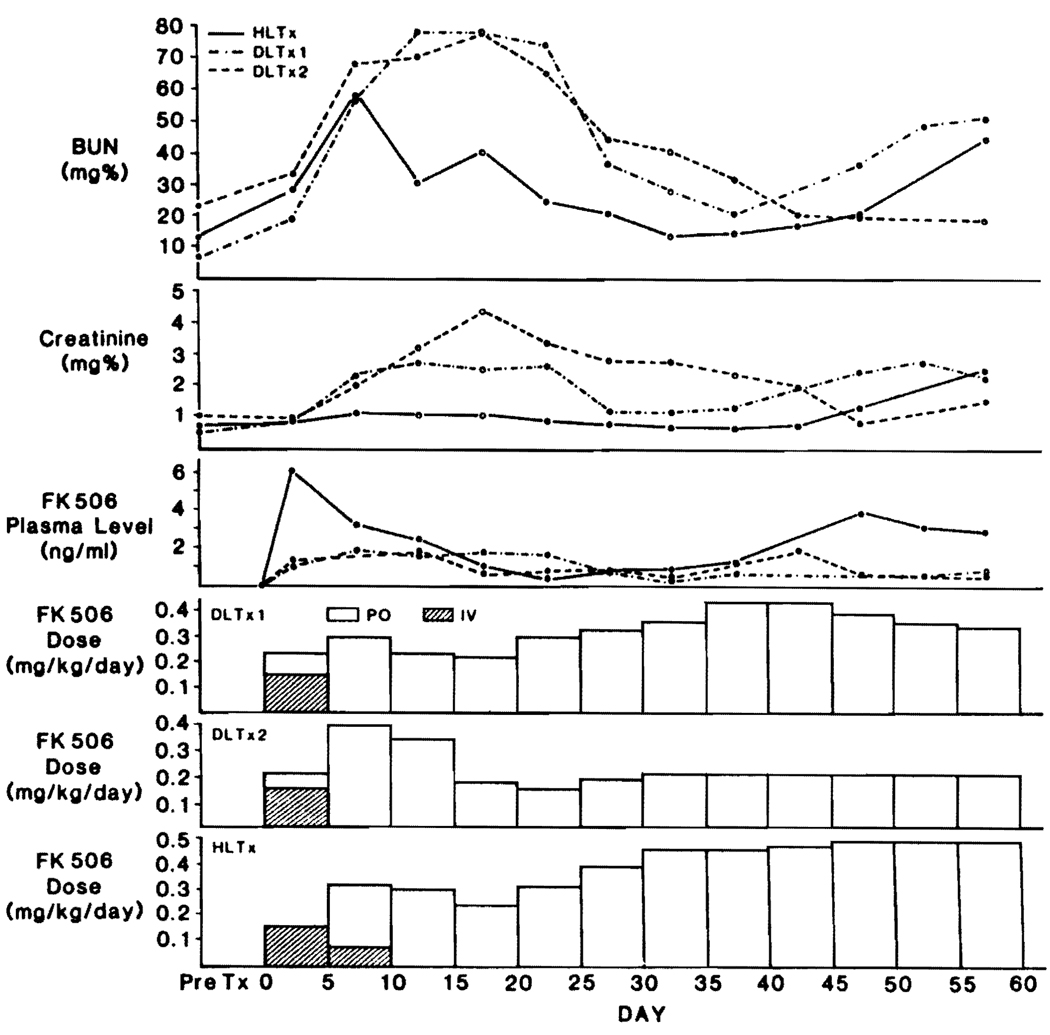
These 3 patients, who were on monotherapy with FK506, were able to take the drug orally within a few days (Fig. 3). Because steroids were avoided, the oral FK506 doses were increased to a limit imposed by renal dysfunction and presumed neurotoxicity. The creatinines and BUNs for the 3 patients are shown in Figure 3, as well as the FK506 doses. The principal acute rises in BUN and creatinine followed the intravenous treatment perioperatively (Fig. 3), but were later than in the heart recipients (Fig. 1). In 2 of the 3 patients, eventual maintenance doses (0.45–0.5 mg/kg) were considerably higher than those normally given and were well tolerated. Serious neurotoxicity and other adverse reactions were not seen.
FIGURE 3.
FK506 doses and plasma levels in 2 double-lung recipients and a heart-lung recipient who did not receive steroids. Rises in creatinine were used to determine the maximum FK506 doses.
Kidney transplantation
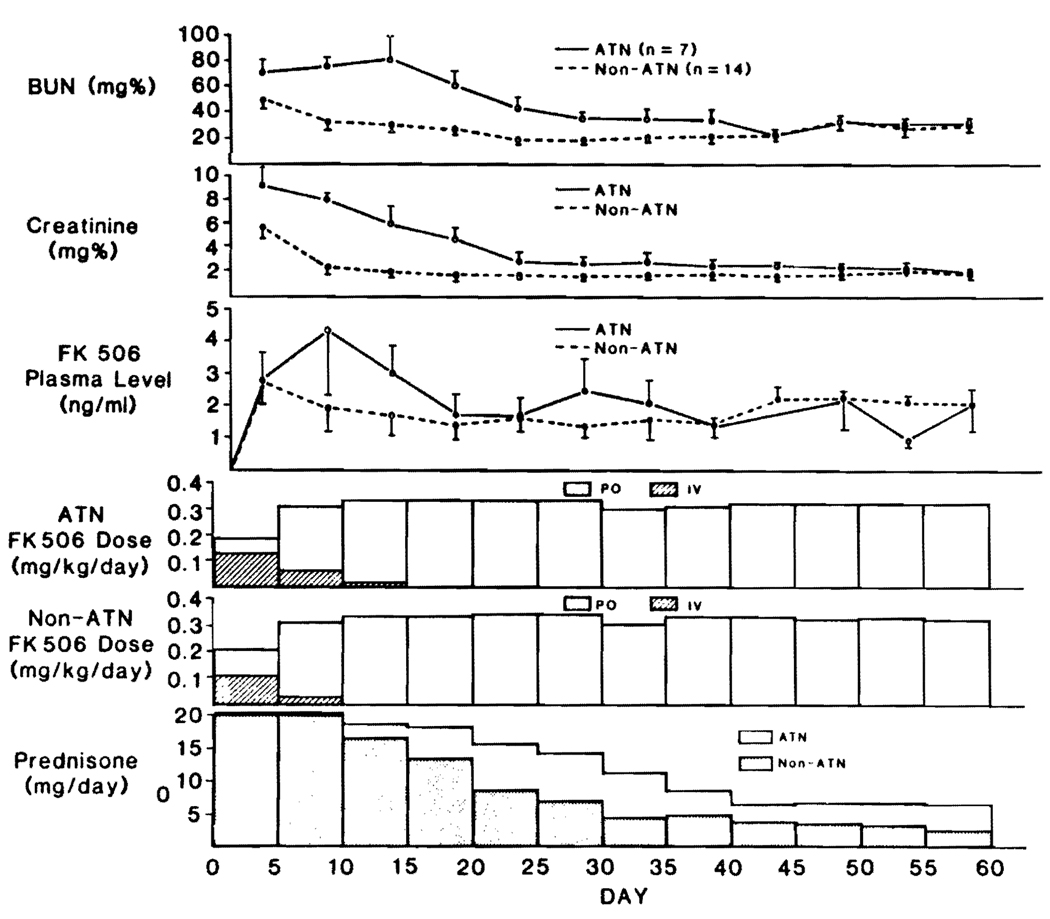
The postoperative evolution of plasma FK506 levels in kidney recipients was similar to that of the heart, lung, and heart-lung recipients. The plasma FK506 levels were highest during the perioperative intravenous induction and subsequently fell when the oral route was instituted (Fig. 4). These changes of plasma FK506 were not significantly different in 14 patients who had prompt renal graft function versus those (n = 7) who had ATN, with the exception of the 5–10 and 50–55 day values (Fig. 4). All of the patients eventually had satisfactory results, with almost identical final renal function tests. Prednisone was weaned at a more rapid rate in patients whose new kidneys functioned at once (Fig. 4), although the differences were not significant. By the end of 60 days, the mean (±SE) daily prednisone dose was 2.5±1.6 mg for non-ATN (n = 14) and 6.31±1.8 mg for ATN (n = 7) patients.
FIGURE 4.
Renal function (mean ± SE) plus dose and plasma levels in cadaver kidney recipients. Note that the presence or absence of ATN did not influence the FK506 kinetics insofar as this could be judged by trough levels.
Liver transplantation
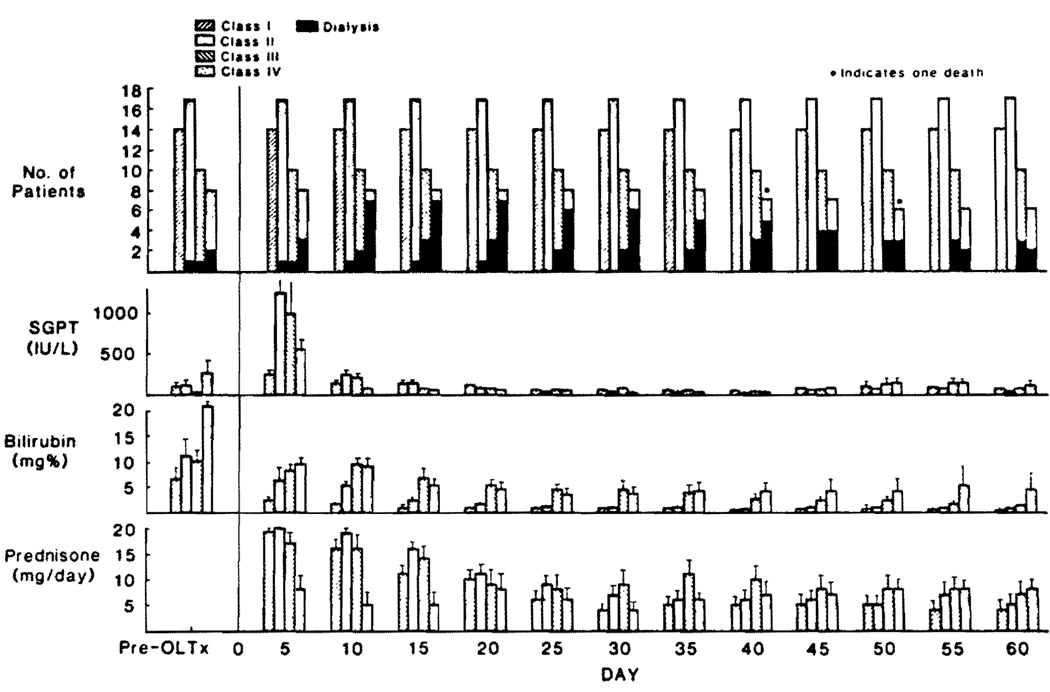
The number of patients, pre- and postoperative liver functions, and steroid use for each of classes I–IV are shown in Fig. 5.
FIGURE 5.
Stratification of liver recipients according to the graft function postoperatively. Categories were from the optimally functioning (class I) to the most seriously dysfunctional grafts (class IV). Note the shifting numbers (beginning with 4 before transplantation) of patients on dialysis.
Classes I and II
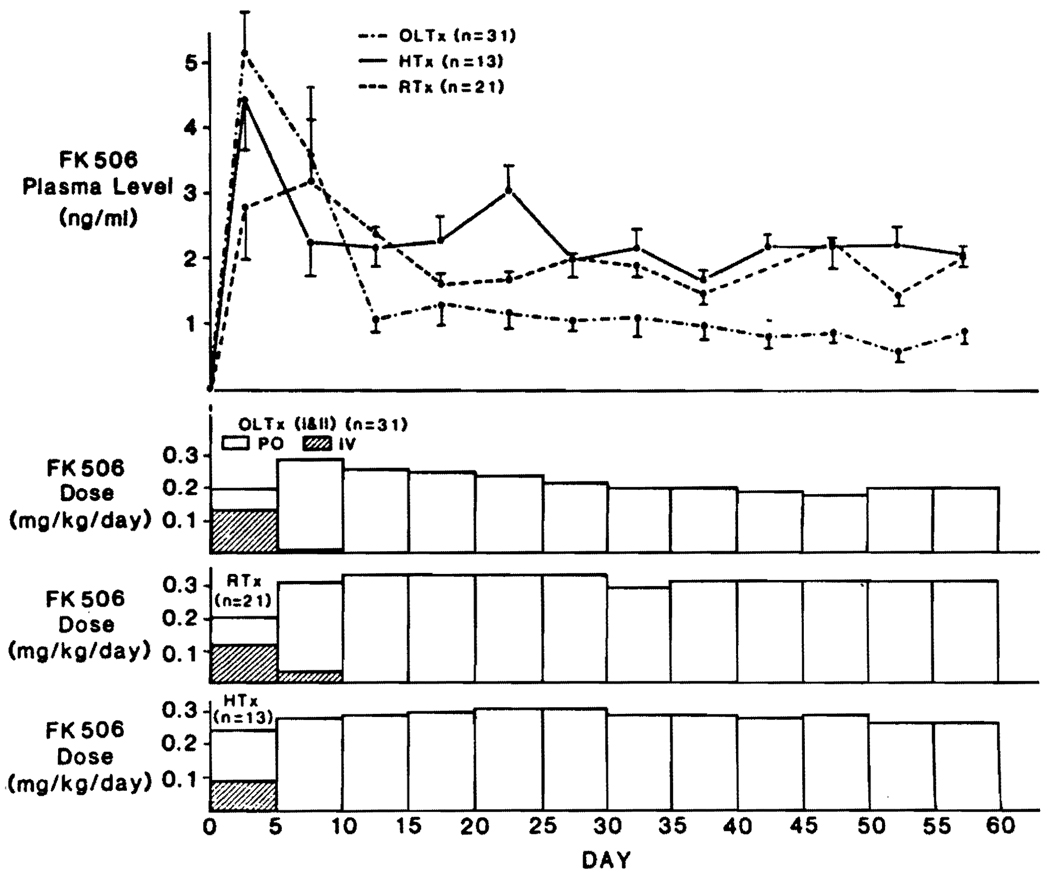
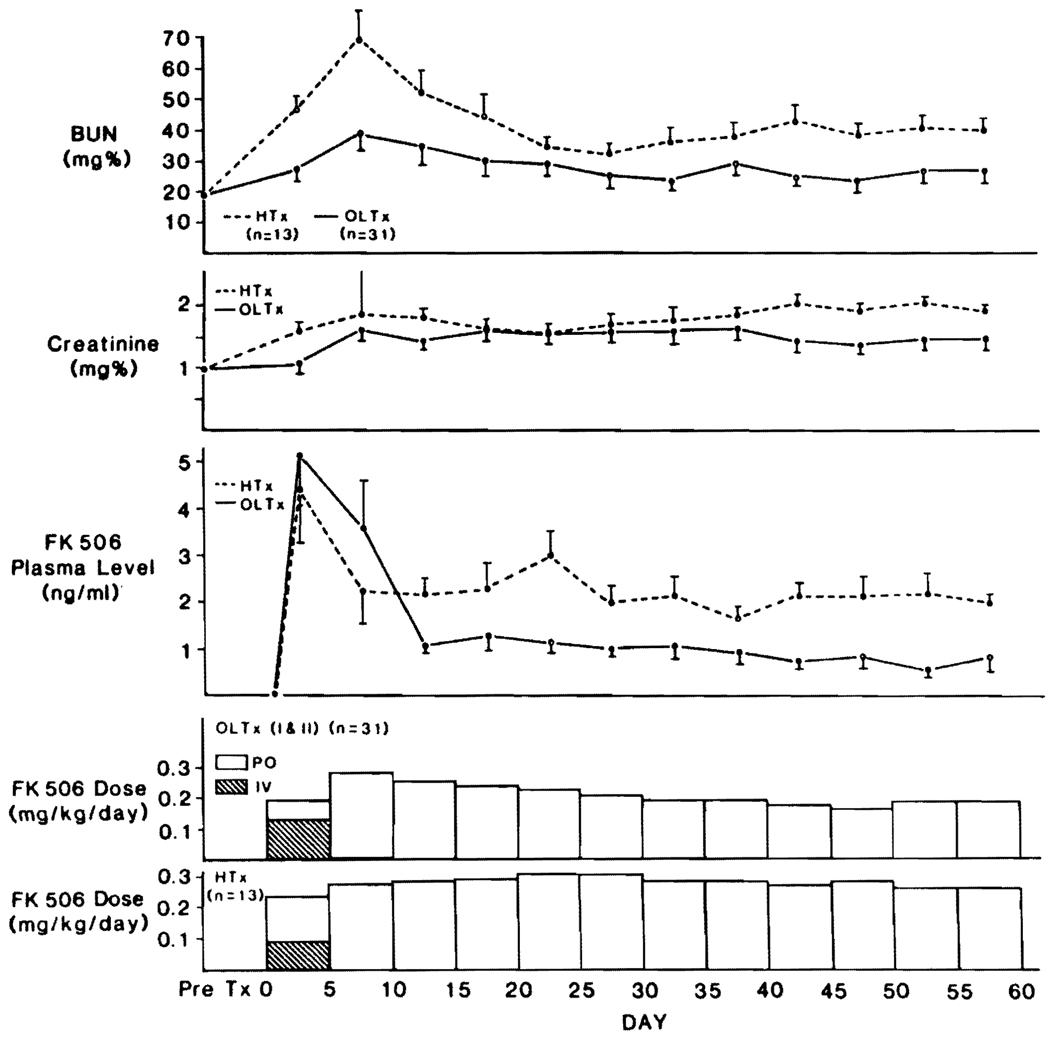
Patients who had excellent or good liver graft function, short-term need for i.v. medication, and short stays in the intensive care units (class I and II) had an evolution of FK506 doses and trough levels similar to heart, lung, and renal recipients (Fig. 6). As with the other kinds of organ transplantation, the plasma levels were highest during i.v. administration. However, the eventual oral doses for these class I and II liver recipients were significantly less (P<0.05) than after renal and cardiac transplantation, which was reflected in lower plasma FK506 levels after day 10 (Fig. 6). The mean BUN and creatinine levels in the class I and II liver patients also were significantly lower (P<0.05) than in the cardiac recipients, especially in the early and late postoperative study period (Fig. 7). There was no new perioperative dialysis requirement in the class I and II liver recipients and in fact, a previously dialysis-bound patient in class II had restoration of renal function 21 days after transplant (Fig. 5).
FIGURE 6.
FK506 doses and FK506 plasma levels in heart, kidney, and class I and II liver recipients. (OLTX) liver; (RTX) kidney; (HTX) heart.
FIGURE 7.
FK506 doses, plasma level, and kidney function in liver (class I and II) and heart transplant patients.
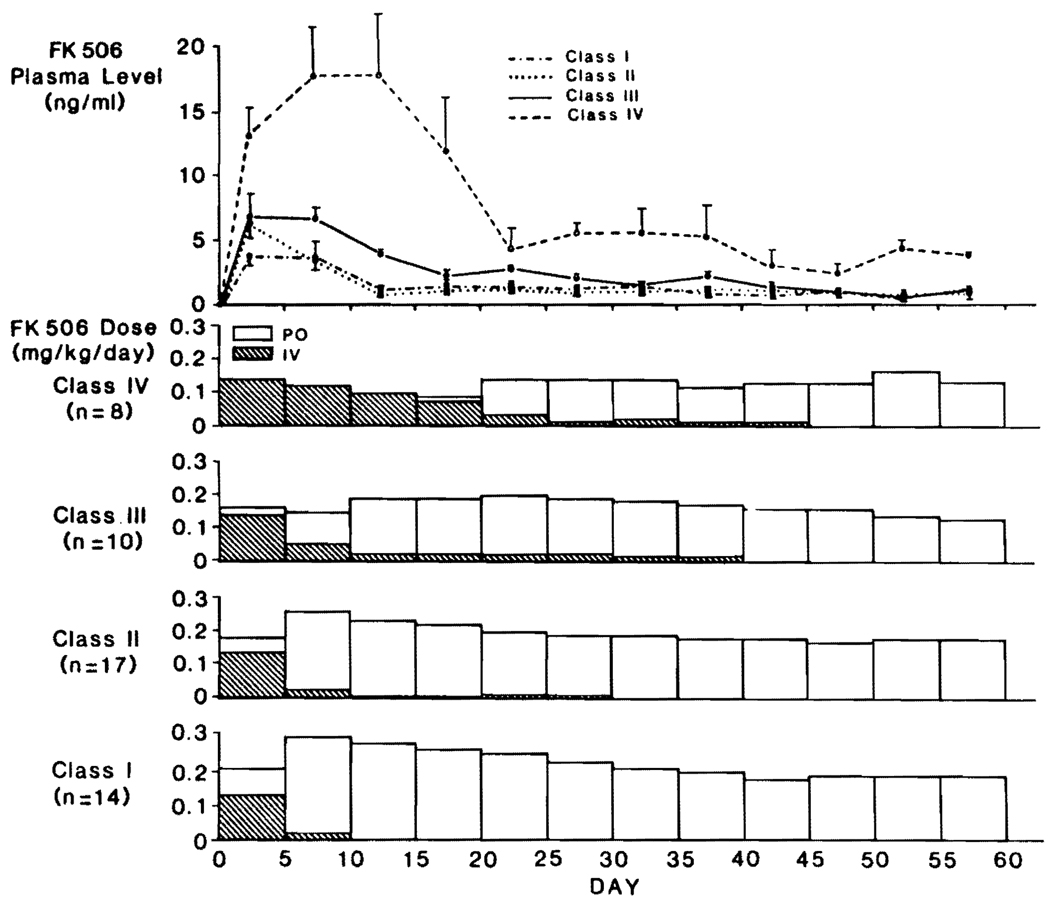
Classes III and IV
The therapeutic and plasma patterns in class I and II liver patients are shown separately in Figure 8 and compared with the less-favored class III liver recipients and the severely ill class IV members who had protracted recovery of the graft and concurrent multiple organ dysfunction. FK506 was given intravenously for much longer times in class III and IV patients. Their plasma trough levels remained high even when downward dose adjustments were made. In class IV patients who all remained jaundiced for protracted periods, plasma FK506 levels were astonishingly high for most of the first month, and up to 60 days they remained significantly elevated (P<0.05) compared with class I, II, and III patients (Fig. 8). Renal failure severe enough to require dialysis for the first time developed in 4 of the class III and 5 of the class IV patients, although this usually was reversible (Fig. 5). At the end of 60 days, 5 of the 13 patients who required early dialysis (including 4 on dialysis preoperatively) still needed this treatment (Fig. 5). Four of the 5 have subsequently recovered from renal failure, leaving only one who currently is being weaned from dialysis.
FIGURE 8.
The effect of postoperative liver function on FK506 doses and plasma levels. Note that the lowest doses and highest plasma levels were in the patients with unsatisfactory early hepatic function.
How ill class III and IV patients were in other ways was shown by the need for tracheostomy and ventilator dependence for many weeks or months in 12 of the 18 patients. However, 16 of them survived, the only deaths being from multiple organ failure at 40 and 47 days in 2 class IV cases. Both were in a coma throughout most of their course. There were no deaths in the class I, II, or III patients.
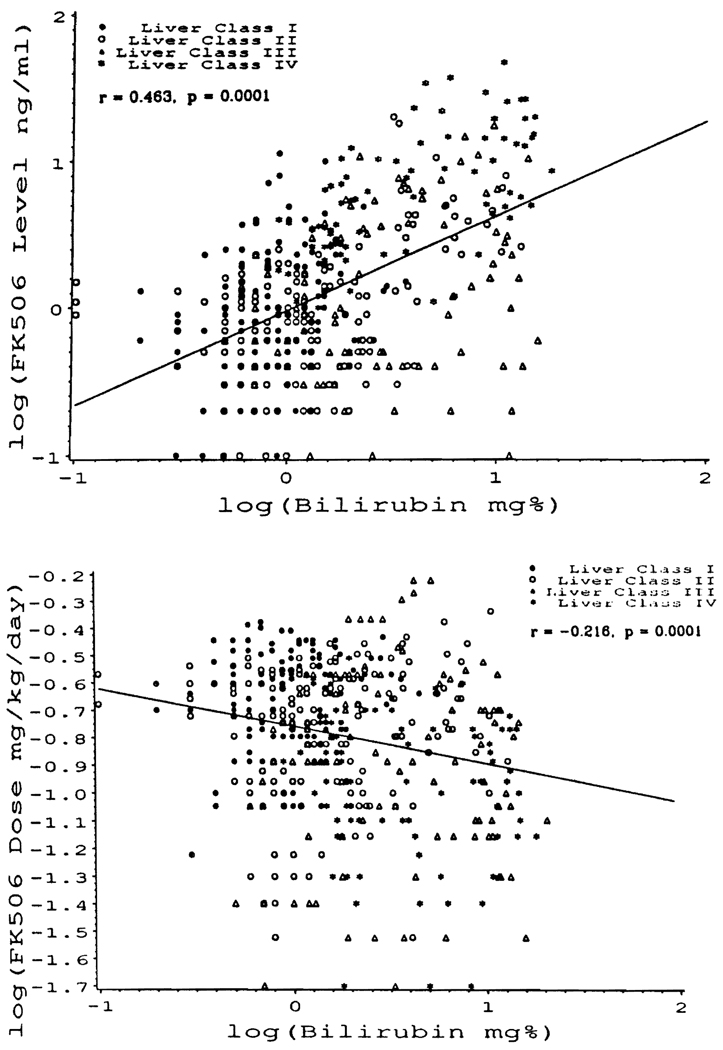
Correlations of bilirubin, FK506 plasma levels, and drug dose: The correlation of bilirubin with plasma FK506 levels drawn on the same day is shown in Fig. 9A for patients of the combined classes I–IV. Although there was considerable scatter, the correlation (r = 0.463) of these 2 parameters was significant (P = 0.0001).
FIGURE 9.
Scatter plots for simultaneously determined serum bilirubin, FK506 plasma level, and drug dose on log10 scale in all liver recipients. Serum bilirubin has a significant positive correlation with FK506 plasma level (A), and negative correlation with FK506 dose (B).
The foregoing association between high serum bilirubin and high plasma FK506 levels could not be explained by increased FK506 doses, as might have been the case if the elevated serum bilirubins were ascribed to rejection. On the contrary, there was a significant (P = 0.0001) inverse correlation (r = −0.216) between the serum bilirubins and the FK506 doses (Fig. 9B), as well as a weaker correlation (r = 0.154) between the FK506 plasma levels and FK506 doses, with P = 0.001 (data not shown graphically).
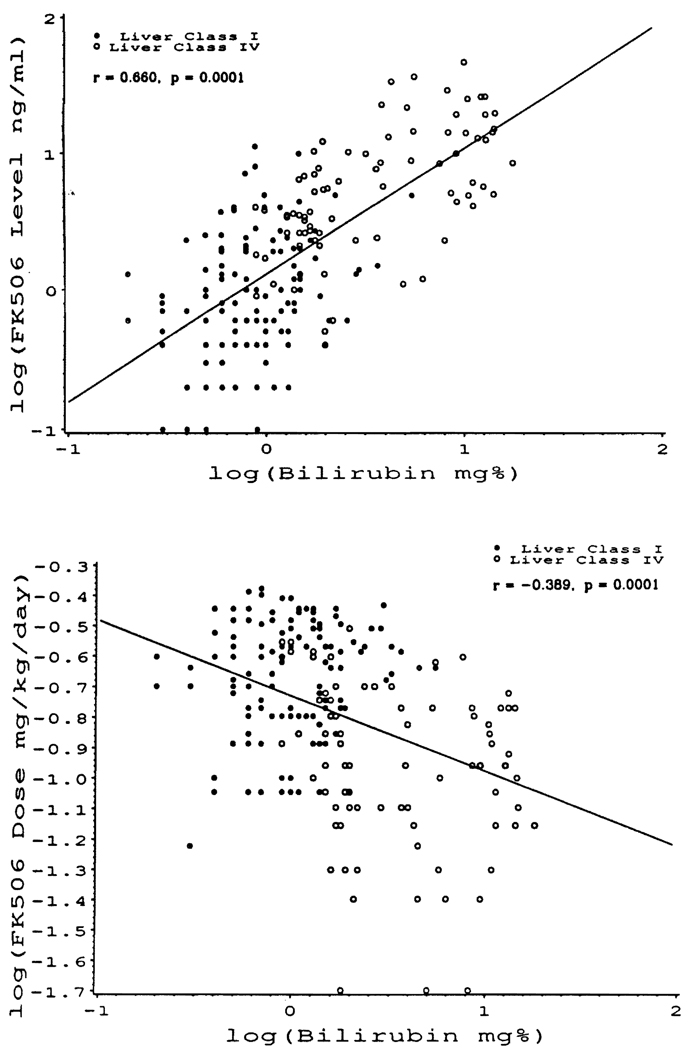
The foregoing correlations in the pooled class I–IV patients were even more significant when the patients of class I and IV were considered alone (Fig. 10, A and B).
FIGURE 10.
The same correlations as in Figure 9, but only in the class I and class IV patients. The correlation coefficients were even more significant than in Figure 9 when comparisons were between optimally functioning (class I) and seriously dysfunctional grafts (class IV).
DISCUSSION
The pharmacokinetics of FK506 already are reasonably well understood in large animals and humans (9, 10). Following an intravenous dose in normal subjects, the terminal half-life after a redistribution phase is about 9 hours (9). FK506 is metabolized mainly by the liver. However, its clearance is high and actually exceeds total hepatic blood flow, implying significant extrahepatic clearance. In contrast, cyclosporine is a low-clearance drug (11). With oral dosing, the nature of FK506 absorption and timing of peak blood (or plasma) levels also appear to differ from cyclosporine. In contrast to cyclosporine, bile is not needed for FK506 absorption (10).6 Because FK506 may be absorbed over many hours with peak plasma levels that are blunted in comparison with cyclosporine (11), its oral formulation may have “slow release” qualities with fewer fluctuations in plasma concentrations. As far as is known, FK506 has no hepatotoxicity (2, 12) or cardiac toxicity (13).
Most of the foregoing information was used in planning the schedules for FK506 dosing and monitoring, which have been highly successful in our clinical trials (1–5). However, the extent to which graft dysfunction should be taken into consideration for management decisions has not been examined previously. This factor was predicted to be particularly important if the organ grafted was the liver.
The present study has confirmed this expectation. The functional state of the liver graft dominated all postoperative pharmacokinetic variables. If the hepatic graft functioned relatively normally from the outset (class I) or even if it was damaged significantly but recovered quickly (class II), the same starting intravenous and oral doses as those used for kidney, heart, and lung transplantation were well tolerated. Plasma FK506 trough levels rose during the period of intravenous drug administration, but the levels promptly receded in the average patient as the liver recovered and if oral dosing could be started. Because FK506 augments liver regeneration (14) and has other hepatotrophic qualities (12), its presence may have facilitated hepatic recovery from perioperative liver damage.
In contrast, when perioperative hepatic graft dysfunction did not resolve promptly as in class III and IV liver recipients, and particularly if FK506 was continued intravenously, the plasma levels rose astronomically and often stayed high for days or weeks in spite of FK506 dose reductions or discontinuance. These patients had a high rate of renal failure, often leading to dialysis, which seemed associated with the high plasma levels of FK506. Incongruously, this association was not statistically significant at any time point, a failure that may reflect the heterogeneity of the cases and sample sizes. Most of these patients had variable degrees of multiple organ failure in a complex setting in which the hepatorenal syndrome, hypertension, multiple blood transfusions, sepsis, endotoxemia, and the need for nephrotoxic antibiotics could have been independent or collaborating factors with elevated FK506 blood levels, in the genesis of renal failure (15, 16). Recovery from this syndrome has not been possible very often in the past without resorting to hepatic retransplantation (17).
FK506 nephrotoxicity could be studied with discrimination only in the thoracic organ recipients who did not have preexisting kidney or liver disease. The transient rises in plasma FK506 levels during intravenous treatment and associated rises in BUN or creatinine were reversed partly when the FK506 doses were reduced or changed to the oral route. In the lung or heart-lung recipients, in whom the sole immunosuppression was with FK506, an FK506 dose ceiling dictated by nephrotoxicity was sought. It was not hard to give enough oral drug to reach this level. The consequent slow rises in creatinine and BUN were quickly responsive to dose reduction.
Finally, it was important to note that renal failure itself did not alter either the daily FK506 doses or the plasma levels. The purest information came from the kidney recipients with and without postoperative ATN. Not only was the basic FK506 management the same, but at the end of 60 days, the kidney function was identical in patients with immediate versus delayed graft function. The observations were consonant with information from other sources that essentially no FK506 is eliminated by the kidney (9).
Taken together, the observations from the different kinds of organ transplants permit reasonably clear conclusions and recommendations about management. First, the greatest risk of either acute or prolonged over-dosage, as judged by renal dysfunction and by excessive plasma FK506 levels, has been in patients with defective drug metabolism caused by hepatic dysfunction. The need is clear to consider early dose reductions under such circumstances along with frequent monitoring of plasma FK506 levels. Second, over-dosing, as defined by high FK506 plasma levels, is possible even with normal liver function, and was associated strongly with perioperative intravenous administration. This became a serious problem only when conversion to the oral route could not be done promptly. With this conversion, the daily doses of 0.15 mg/kg intravenously were increased to 0.3 mg/kg orally. In terms of FK506 availability, this change was considered to be an absolute dose reduction since only about 20–30% of FK506 is absorbed from the intestine (9). In future cases, smaller doses of FK506 for the intravenous induction are advisable—at least in selected cases but probably in all. In addition, we are evaluating continuous intravenous infusion instead of a 4-hr infusion twice a day, as was our practice during this study. Such an approach has been previously recommended for cyclosporine (18).
The demonstration that FK506 kinetics is drastically altered by hepatic dysfunction is a clear warning against over-dosage in patients with failing liver grafts under conventional immunosuppression who are switched to FK506 under so-called “rescue” circumstances (19). Failure to accurately monitor plasma FK506 levels in these patients and to make appropriate dose adjustments accordingly could lead systematically to toxicity. We have considered nephrotoxicity in this study, but serious neurotoxicity was also observed in one of our cardiac patients and has been reported in liver recipients (20). The extent of drug-related neuropsychiatric problems may be impossible to identify in patients dying of liver failure since they are prone to such complications in any event (20, 21).
Footnotes
This work was supported by Research Grants from the Veterans Administration and by Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD.
Abbreviations: IABP, intraaortic balloon pump; LV AD, left ventricular assist device.
Imventarza O, Furukawa H, Venkataramanan R, et al. The effect of bile duct ligation and bile diversion on FK506 pharmacokinetics in dogs. (Submitted for publication.)
REFERENCES
- 1.Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramanan R, Jain A. FK 506 for human liver, kidney, and pancreas transplantation. Lancet. 1989;2:1000. doi: 10.1016/s0140-6736(89)91014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Starzl TE, Fung J, Jordan M, et al. Kidney transplantation under FK 506. JAMA. 1990;264:63. [PMC free article] [PubMed] [Google Scholar]
- 3.Todo S, Fung JJ, Starzl TE, et al. Liver, kidney, and thoracic organ transplantation under FK 506. Ann Surg. 1990;212:295. doi: 10.1097/00000658-199009000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fung JJ, Todo S, Jain A, et al. Conversion of liver allograft recipients with cyclosporine related complications from cyclosporine to FK 506. Transplant Proc. 1990;22:6. [PMC free article] [PubMed] [Google Scholar]
- 5.Armitage JM, Kormos RL, Fung J, et al. Preliminary experience with FK506 in thoracic transplantation. Transplantation. doi: 10.1097/00007890-199107000-00038. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain AB, Fung JJ, Todo S, et al. Incidence of treatment of rejection episodes in primary orthotopic liver transplantation under FK 506. Transplant Proc. 1991;23:928. [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro R, Jordan M, Fung J, et al. Kidney transplantation under FK 506 immunosuppression. Transplant Proc. 1991;23:920. [PMC free article] [PubMed] [Google Scholar]
- 8.Tamura K, Kobayashi M, Hashimoto K, et al. A highly sensitive method to assay FK-506 levels in plasma. Transplant Proc. 1987;19 suppl 6:823. [PubMed] [Google Scholar]
- 9.Venkataramanan R, Jain A, Cadoff E, et al. Pharmacokinetics of FK 506: preclinical and clinical studies. Transplant Proc. 1990;22:52. [PMC free article] [PubMed] [Google Scholar]
- 10.Jain AB, Venkataramanan R, Cadoff E, et al. Effect of hepatic dysfunction and T tube clamping on FK 506 pharmacokinetics and trough concentrations. Transplant Proc. 1990;22:57. [PMC free article] [PubMed] [Google Scholar]
- 11.Ptachcinski RJ, Venkataramanan R, Burckard GJ. Clinical pharmacokinetics of cyclosporine. Clin Pharmacokinet. 1986;11:107. doi: 10.2165/00003088-198611020-00002. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE, Porter KA, Mazzaferro V, Todo S, Fung J, Francavilla A. Hepatotrophic effects of FK506 in dogs. Transplantation. 1991;51:67. doi: 10.1097/00007890-199101000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang Y, Mazer MA, DeWolf AM, et al. Acute hemodynamic effects of FK 506 during and after orthotopic liver transplantation. Transplant Proc. 1990;22:21. [PMC free article] [PubMed] [Google Scholar]
- 14.Francavilla A, Barone M, Todo S, Zeng Q, Porter KA, Starzl TE. Augmentation of rat liver regeneration by FK 506 compared with cyclosporine. Lancet. 1989;1:1248. doi: 10.1016/s0140-6736(89)91853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCauley J, Van Thiel D, Starzl TE, Puschett JB. Acute and chronic renal failure after liver transplantation. Nephron. 1990;55:121. doi: 10.1159/000185938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCauley J, Fung JJ, Jain A, Todo S, Starzl TE. The effects of FK 506 upon renal function after liver transplantation. Transplant Proc. 1990;22:17. [PMC free article] [PubMed] [Google Scholar]
- 17.Starzl TE, Demetris AJ. Liver transplantation. Chicago, IL: Year Book; 1990. pp. 43–56. 57–70, 85. [Google Scholar]
- 18.Kahan BD, Givelel J. Optimization of cyclosporine therapy in renal transplantation by a pharmacokinetic strategy. Transplantation. 1988;46:631. doi: 10.1097/00007890-198811000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Fung JJ, Todo S, Tzakis A, et al. Conversion of liver allograft recipients from cyclosporine to FK 506 based immunosuppression: benefits and pitfalls. Transplant Proc. 1991;23:14. [PMC free article] [PubMed] [Google Scholar]
- 20.Reyes J, Gayowski T, Fung J, Todo S, Alessiani M, Starzl TE. Expressive dysplasia possibly related to FK506 in 2 liver transplantation recipients. Transplantation. 1990;50:1043. doi: 10.1097/00007890-199012000-00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Starzl TE, Schneck SA, Mazzoni G, et al. Acute neurological complications after liver transplantation with particular reference to intraoperative cerebral air embolus. Ann Surg. 1978;187:236. doi: 10.1097/00000658-197803000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]