Abstract
Hypertension caused by chronic infusion of angiotensin II (Ang II) in experimental animals is likely to be mediated, atleast in part, by an elevation of ongoing sympathetic nerve activity(SNA). However, the contribution of SNA relative to non-neural mechanisms in mediating Ang II-induced hypertension is an area of intense debate and remains unresolved. We hypothesize that sympathoexcitatory actions of Ang II are directly related to the level of dietary salt intake. To test this hypothesis, chronically instrumented rats were placed on a 0.1 (low), 0.4 (normal) or 2.0% NaCl diet (high) and, following a control period, administered Ang II (150 ng kg−1 min−1, s.c.) for 10–14 days. The hypertensive response to Ang II was greatest in rats on the high-salt diet (Ang II–salt hypertension), which was associated with increased ‘whole body’ sympathetic activity as measured by noradrenaline spillover and ganglionic blockade. Indirect and direct measures of organ-specific SNA revealed a distinct ‘sympathetic signature’ in Ang II–salt rats characterized by increased SNA to the splanchnic vascular bed, transiently reduced renal SNA and no change in SNA to the hindlimbs. Electrophysiological experiments indicate that increased sympathetic outflow in Ang II–salt rats is unlikely to involve activation of rostral ventrolateral medulla (RVLM) vasomotor neurons with barosensitive cardiac rhythmic discharge. Instead, another set of RVLM neurons that discharge in discrete bursts have exaggerated spontaneous activity in rats with Ang II–salt hypertension. Although their discharge is not cardiac rhythmic at resting levels of arterial pressure, it nevertheless appears to be barosensitive. Therefore, these burst-firing RVLM neurons presumably serve a vasomotor function, consistent with their having axonal projections to the spinal cord. Bursting discharge of these neurons is respiratory rhythmic and driven by the respiratory network. Given that splanchnic SNA is strongly coupled to respiration, we hypothesize that enhanced central respiratory–vasomotor neuron coupling in the RVLM could be an important mechanism that contributes to exaggerated splanchnic sympathetic outflow in Ang II–salt hypertension. This hypothesis remains to be tested directly in future investigations.
Overview
Hypertension caused by chronic infusion of angiotensin II (Ang II) in experimental animals is likely to be mediated, at least in part, by amplification of ongoing sympathetic nerve activity (SNA; Brody et al. 1980; Ferrario, 1983; Fink, 1997). However, the relative contribution of elevated SNA versus non-neural mechanisms in Ang II-induced hypertension is an intensely debated and unresolved issue.
Some of the disparate results between studies investigating the link between SNA and the hypertensive actions of Ang II may be due to differences in experimental design between laboratories. For example, we believe that the magnitude of the sympathoexcitatory actions of Ang II is directly related to the prevailing level of sodium chloride (salt) intake (Osborn & Fink, 2010), a factor that is often not discussed in the literature. Another issue is that the methods employed for measurement of SNA have not been consistent across studies, varying between indirect measures of ‘whole body’ sympathetic activity to direct and indirect measures of organ-specific SNA in both anaesthetized and conscious animals. This issue is further complicated by emerging evidence that the response of organ-specific SNA to Ang II appears to be differentially expressed; hence, recording the response of SNA to one organ does not always reliably predict the response of SNA to other organs. These, and other, differences in experimental approach (both known and unknown) make it difficult to reconcile disparate findings and arrive at a unified understanding of the neurogenic actions of circulating Ang II in hypertension.
In order to address this issue, we established the Neurogenic Cardiovascular Diseases Consortium (NCDC), linking five independent laboratories to systematically investigate, at all levels of biological control, the contribution of the sympathetic nervous system to the pathogenesis of Ang II-induced hypertension (Osborn et al. 2007a). The strategy of the NCDC is straightforward; to minimize sources of interlaboratory variability by implementing strict control of experimental protocols, thus allowing more effective integration of the results of investigations at the cellular, neural network and whole animal level. In this report we present and discuss our recent findings related to the response of peripheral sympathetic pathways to Ang II as well as sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM). Our results suggest that chronic Ang II administration increases SNA specifically to the splanchnic vascular bed only in rats consuming a high-salt diet, and this response is associated with increased activity of a specific phenotype of spinally projecting RVLM neurons.
Peripheral neural mechanisms of Ang II–salt hypertension: the ‘sympathetic signature’
The standardized protocol for producing the model has been described in detail (Osborn & Fink, 2010). Briefly, rats are placed on a low- (0.1%), normal-(0.4%) or high-NaCl diet (2.0%), instrumented with a radio-telemeter for continuous recording of arterial pressure and then, following a control period, Ang II is administered subcutaneously (150 ng kg−1 min−1) via an osmotic minipump (Alzet, Cupertino, CA, USA) for 10–14 days depending on the specific protocol.
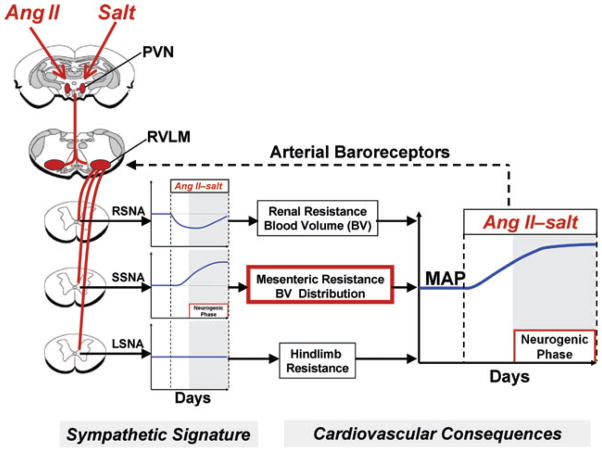
Our studies suggest that the salt sensitivity of this Ang II-induced model is, in large part, the result of a delayed activation of the sympathetic nervous system, but the increase in SNA is not uniformly distributed to all vascular beds (Osborn & Fink, 2010). Figure 1 summarizes our present viewpoint regarding the response to Ang II of region-specific SNA in rats consuming a high-salt diet, a pattern we have termed the ‘sympathetic signature of Ang II–salt hypertension’ (Osborn & Fink, 2010). Renal SNA is transiently decreased, as determined by direct long-term recordings (Yoshimoto et al. 2010), and renal denervation does not affect the steady-state phase of Ang II–salt hypertension (King et al. 2007). We hypothesize that the transient decrease in renal SNA during Ang II administration, possibly mediated by the baroreceptor reflex, buffers the initial hypertensive response to Ang II but that renal SNA plays no role in the steady-state phase of Ang II–salt hypertension. This hypothesis is consistent with a study in rabbits in which baroreceptor denervation abolished the decrease in renal SNA observed during chronic Ang II infusion but did not affect the steady-state response of arterial pressure (Barrett et al. 2005).
Figure 1. Sympathetic signature of Ang II–salt hypertension.
Proposed changes in renal (RSNA), splanchnic (SSNA) and muscle sympathetic nerve activity (LSNA) in Ang II–salt hypertension, based on studies from the authors’ laboratories and others, which result in long-term increases in mean arterial pressure (MAP). Shaded regions of graphs indicate the neurogenic phase. The dashed line from arterial baroreceptors represents a sympathoinhibitory input to sympathetic premotor neurons in the rostral ventrolateral medulla (RVLM). Excitatory drive to the RVLM by Ang II–salt is proposed to involve the paraventricular nucleus (PVN) of the hypothalamus.
In contrast to renal SNA, neither lumbar SNA (which largely targets the skeletal muscle vascular bed) nor hindlimb noradrenaline spillover is changed from control levels at any time during Ang II administration in rats consuming a high-salt diet (Yoshimoto et al. 2010). Others have reported that acute infusion of Ang II has no effect on SNA to skeletal muscle in rats (Xu & Sved, 2002) or humans (Matsukawa et al. 1991) in normal conditions but, when the buffering capacity of the baroreceptor reflex is prevented, a sympathoexcitatory response is observed. These observations suggest that the direct effect of circulating Ang II to increase skeletal muscle SNA is partly buffered by the arterial baroreceptor reflex.
A number of studies from our group suggest that this model of neurogenic hypertension is driven by increased splanchnic SNA (Osborn & Fink, 2010). Coeliac ganglionectomy (GGx), which interrupts sympathetic neurotransmission to the splanchnic vasculature, markedly attenuates Ang II-induced increases in arterial pressure (King et al. 2007), venomotor tone (King & Fink, 2006) and systemic arteriolar resistance (Osborn et al. 2007b) in rats on a high-salt diet. In addition, ganglionic blockade reverses the increase in venomotor tone in Ang II–salt rats (King et al. 2007). This hypothesis is also supported by a study in which splanchnic SNA was directly recorded and found to be increased in conscious rats following chronic Ang II administration (Luft, 1989).
As summarized in Fig. 1, we predict that the cardiovascular consequence of the Ang II–salt sympathetic signature is a redistribution of blood volume from the splanchnic vascular bed to the systemic arterial compartment and thus increased arterial pressure. This hypothesis remains to be tested by direct long-term recordings of splanchnic SNA in conjunction with measurements of blood volume distribution and splanchnic haemodynamics.
Central nervous system (CNS) mechanisms of Ang II–salt hypertension
Although the mechanisms that ‘shape’ this sympathetic signature are presently unknown, we propose that a neural pathway originating in the forebrain lamina terminalis plays a pivotal role. Accordingly, neurons comprising sensory circumventricular organs (CVOs) that respond to circulating Ang II and elevated plasma sodium/hyperosmolality (Toney et al. 2003; Osborn et al. 2007a; Stocker et al. 2008) are postulated to excite downstream neuronal targets. These include the median preoptic nucleus whose efferent projections, together with direct projections from forebrain CVOs, activate the hypothalamic paraventricular nucleus (PVN; Stocker & Toney, 2005, 2007). Among various sympathetic regulatory connections of the PVN, those that directly innervate sympathetic premotor neurons in the RVLM (Osborn et al. 2007a) are considered to play a particularly important role in shaping the sympathetic signature.
Support for this concept derives from studies demonstrating, for example, that increased dietary salt ‘sensitizes’ sympathoexcitatory responses to Ang II (Adams et al. 2008) and excitatory amino acids (Ito et al. 1999) applied locally within the RVLM. Moreover, full manifestation of these responses has more recently been shown to depend on activity of neurons within the ventral forebrain lamina terminalis (Adams et al. 2009). The latter region represents the major sodium/osmolality sensor of the CNS, which transmits osmotic information to downstream sympathetic-regulatory regions of the hypothalamus and brainstem (Stocker et al. 2004; Shi et al. 2007; Adams et al. 2008).
In our investigation of CNS mechanisms underlying the sympathetic signature of Ang II–salt hypertension, we first focused on vasomotor neurons in the RVLM. After completing the standard NCDC protocol for Ang II–salt hypertension, rats were anaesthetized with a cocktail of urethane (800 mg kg−1, I.P.) and α-chloralose (80 mg kg−1, I.P.) and surgically instrumented to record arterial pressure, heart rate (HR; ECG lead I), renal or splanchnic SNA and expired PCO2. Using extracellular single unit recording techniques, standard criteria were applied to identify vasomotor neurons in the RVLM (Brown & Guyenet, 1985); they were spinally projecting based on antidromic activation from the T1–T3 spinal segment, had cardiac rhythmic spontaneous discharge (i.e. discharge entrained by arterial baroreceptor input), and could be inhibited by increasing arterial pressure (i.e. barosensitive). We found no differences in spontaneous discharge frequency or the firing probability across the cardiac cycle among these neurons from normotensive and Ang II–salt hypertensive rats. Though preliminary, these findings suggest that RVLM neurons with classical sympathoexcitatory vasomotor behaviour do not have exaggerated activity in rats with Ang II–salt hypertension. Accordingly, such neurons would be predicted not to play a significant role in supporting the specific pattern of SNA observed in our model of Ang II–salt hypertension, particularly the net increase of ongoing splanchnic SNA.
We next asked whether another population of neurons in the RVLM could potentially contribute to the patterning of SNA in our model. We identified a set of RVLM neurons whose axons project to the thoracic cord, but which did not exhibit ‘classical’ vasomotor discharge behaviour. Instead, these neurons had a bursting pattern of spontaneous discharge that was not cardiac rhythmic (Fig. 2A and B). We performed juxtacellular labelling of such neurons, and most were non-C1 cells located within the phenylethanolamine N-methyltransferase (PNMT)-positive C1 cluster, most often along its dorsolateral border (G.R.P. and G.M.T., unpublished observations).
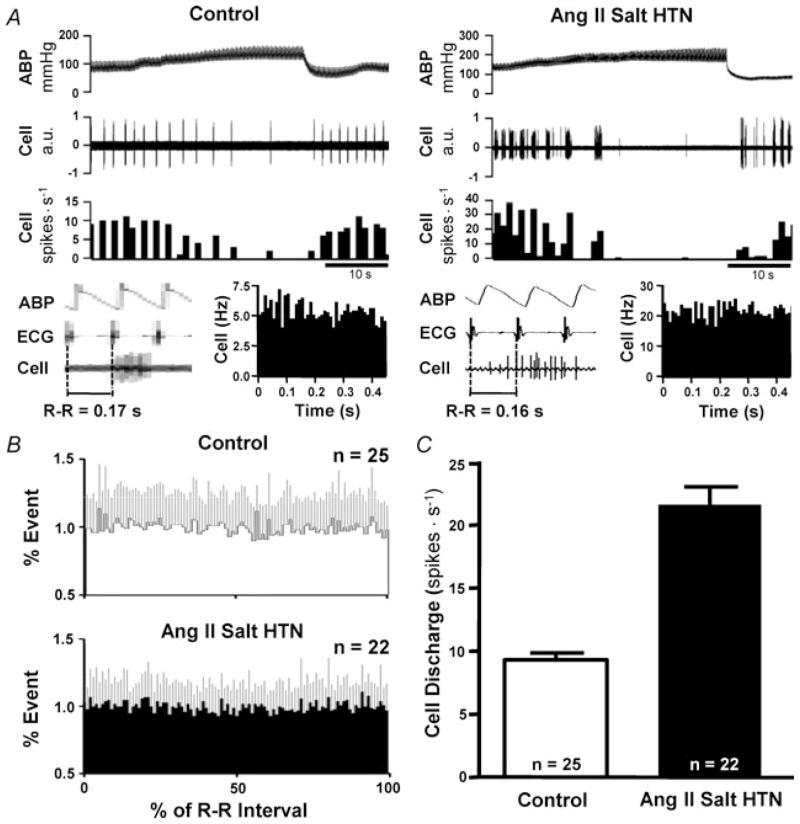
Figure 2. Neurons in RVLM with non-cardiac rhythmic barosensitive discharge exhibit exaggerated burst firing in rats with Ang II–salt hypertension.
A, example of RVLM neurons that fire in a bursting pattern in a normotensive rat (Control; left) and Ang II–salt hypertensive rat (Ang II Salt HTN; right). Note that discharge of both neurons lacks cardiac rhythm (bottom, left and right) and yet is inhibited by increasing ABP. B, summary phase histograms demonstrate the lack of cardiac rhythmic discharge among burst-firing RVLM neurons. Note that discharge probability does not vary across the cardiac cycle interval. C, summary data indicate that the average discharge of burst-firing neurons is considerably greater in rats with Ang II–salt hypertension compared with normotensive control animals.
In testing this group of neurons for responses to arterial baroreceptor input, we found that discharge was consistently reduced when arterial blood pressure (ABP) was acutely increased. In most cases, discharge was silenced (Fig. 2A, left and right). When considering their lack of cardiac rhythmicity together with having baro-inhibitable discharge, our first thought was that burst-firing neurons might receive baroreceptor inputs that are active only at levels of ABP above baseline. However, this does not appear to be the case because lowering ABP to unload arterial baroreceptors unexpectedly produced a very significant increase in discharge frequency (G.R.P. and G.M.T., unpublished observations). Therefore, the lack of cardiac rhythmic discharge among these neurons may be due to their receiving baroreceptor input that is not pulse rhythmic (i.e. like the discharge of most barosensitive neurons in the nucleus tractus solitarii; Mifflin, 2001) or they might be resistant to entrainment by pulse-rhythmic baroreceptor input. A potential caveat is that inhibition of discharge during acute increases of ABP could involve associated reduction of central respiratory drive to these cells (Brunner et al. 1982; Hopp & Seagard, 1998). At present, however, we do not consider this to be the dominant inhibitory mechanism because we have not observed cessation of phrenic bursting at levels of ABP that silence burst discharge of RVLM neurons (G.R.P. and G.M.T., unpublished observations). As noted above, lowing ABP produced a profound increase of discharge among burst-firing neurons. It is therefore possible that increased respiratory frequency associated with lowering arterial baroreceptor input could contribute to the increase of discharge (Hopp & Seagard, 1998). However, the increase in burst firing we have observed during baroreceptor unloading appears perhaps too large to be fully accounted for by the increase of respiratory activity reported to occur when carotid sinus pressure is lowered (Hopp & Seagard, 1998).
Whatever the explanation might be for pressure-sensitive discharge among non-cardiac rhythmic burst-firing neurons, an important observation was that the frequency of their spontaneous discharge was significantly greater in rats with Ang II–salt hypertension than in normotensive control animals (Fig. 2C). Exaggerated discharge was due to the neurons having greater peak discharge frequency within bursts and greater average burst duration. No difference in the interburst interval was detected between groups.
As shown in Fig. 3A, we noted that the average interburst interval of discharge was similar to the respiratory interval, as indicated by being phase locked with bursts of phrenic nerve activity (PNA). Indeed, time histograms of spontaneous discharge triggered by the onset of consecutive PNA bursts revealed tight respiratory coupling (Fig. 3A, right). Burst discharge typically began during the inspiratory period and continued through the early- to mid-expiratory phase of the respiratory cycle.
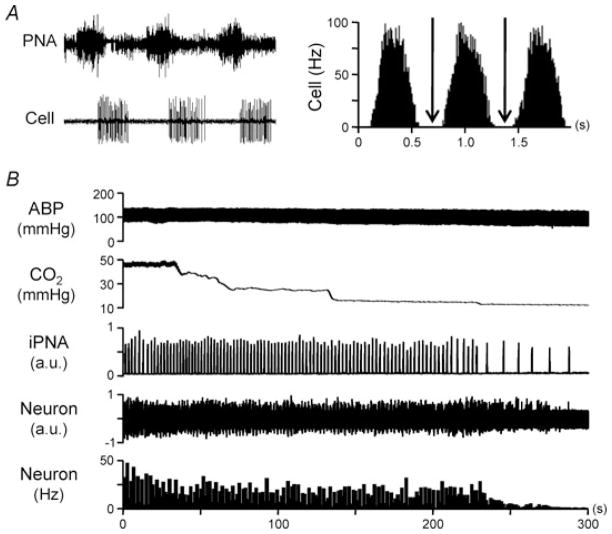
Figure 3. Burst discharge of non-cardiac rhythmic barosensitive RVLM neurons is respiratory rhythmic.
A, simultaneous recording of phrenic nerve activity (PNA) and an individual burst-firing RVLM neuron reveals that spontaneous discharge consistently occurs during late inspiration through early expiration (left). Phrenic nerve activity-triggered time histogram analysis further demonstrates the respiratory rhythmic discharge of this neuron (right). Note that discharge falls to zero (arrows) between respiratory rhythmic bursts (right). B, reducing expired CO2 and, therefore, central respiratory drive by hyperventilation causes a gradual and parallel cessation of both PNA and neuronal discharge. Thus, burst firing appears to be dependent on input from the central respiratory network. iPNA, integrated phrenic nerve activity.
Interestingly, discharge of these neurons in rats with and without Ang II–salt hypertension was consistently silenced by hyperventilation (Fig. 3B). This indicates that at the prevailing level of ABP suprathreshold depolarizations and spiking arise exclusively from respiratory network input. This conclusion is supported by time histograms of discharge triggered by PNA, which revealed that discharge most often fell to zero between bursts (Fig. 3A, right, arrows). Thus, exaggerated respiratory rhythmic RVLM burst discharge in rats with Ang II–salt hypertension would seem to result from one or a combination of the following: (1) greater excitatory synaptic input from the central respiratory network; (2) intrinsic adaptations that increase neuronal excitability such that even a normal level of respiratory input causes an exaggerated postsynaptic response; or (3) subthreshold synaptic inputs or local neuromodulators that facilitate responses to respiratory input.
Our experiments have yet to address the question of whether Ang II–salt hypertension is accompanied by an increase in the strength of central respiratory drive at rest, though preliminary telemetry data indicate no effect on the frequency of spontaneous respiration. Studies have also not investigated possible mechanisms leading to enhanced intrinsic excitability of respiratory rhythmic neurons. Instead, we have sought to determine possible chemical mediators of enhanced respiratory–sympathetic coupling. We have focused on actions of Ang II in the RVLM in light of recent evidence that local Ang II plays an enhanced sympathoexcitatory role in rats consuming high salt (Adams et al. 2008), an effect that could involve greater Ang II-induced formation of superoxide anions (Braga, 2010). In preliminary experiments, a noticeable reduction of inspiratory splanchnic SNA burst amplitude was observed following bilateral RVLM microinjection of the Ang II type 1 (AT1) receptor antagonist losartan in rats with Ang II–salt hypertension, with no effect observed in normotensive control animals. The effect of losartan in the RVLM did not appear to be secondary to a reduction in the strength of respiratory drive, since the amplitude of PNA-triggered averages of integrated PNA was unaffected in both groups (Fig. 4).
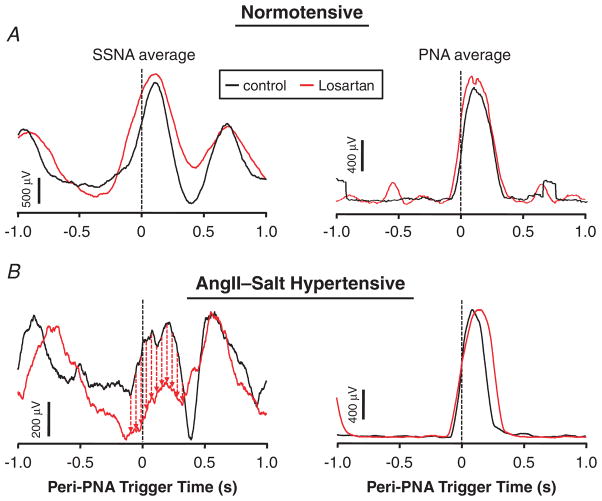
Figure 4. Blockade of Ang II AT1 receptors in the RVLM bilaterally reduces inspiratory splanchnic SNA burst amplitude in Ang II–salt hypertensive rats without affecting the strength of central respiratory drive.
The onset of 200 consecutive PNA bursts was used to construct PNA-triggered averages of splanchnic SNA (left) and integrated PNA (right). A, in a normotensive control rat there was no obvious change in either averaged waveform 15 min after microinjection of the Ang II AT1 receptor antagonist losartan bilaterally (1.0 nmol in 60 nl per side) into the RVLM. B, in a rat with Ang II–salt hypertension, losartan in the RVLM reduced inspiratory splanchnic SNA burst amplitude (left) without affecting the amplitude of the PNA burst (right). Thus, blockade of AT1 receptors in the RVLM appears to reduce the strength of coupling between respiration and splanchnic SNA without affecting the strength of central respiratory drive.
Does enhanced respiratory–sympathetic coupling contribute to the sympathetic signature of Ang II–salt hypertension?
Functional coupling between the central respiratory network and cardiovascular end organs has been recognized for more than a century (Traube, 1865; Hering, 1869). In 1932, Adrian et al. determined that functional respiratory modulation probaby involved respiratory network modulation of SNA. Indeed, they showed the presence of respiratory rhythmic burst discharge in direct electrical recordings of SNA (Adrian et al. 1932). Since then, studies have revealed numerous detailed interactions between neurons comprising the pontomedullary central respiratory network and sympathetic regulatory neurons in the brainstem (McAllen, 1987; Haselton & Guyenet, 1989; Mandel & Schreihofer, 2006) and spinal cord (Czyzyk-Krzeska & Trzebski, 1990b; Habler et al. 1994a,b; Johnson & Gilbey, 1996; Gilbey, 2001).
Studies have also investigated the possibility that chronic elevations of SNA in cardiovascular diseases might involve exaggerated respiratory–sympathetic coupling (for review see Zoccal et al. 2009). In spontaneously hypertensive rats (SHR), a model of neurogenic arterial hypertension, studies have revealed an enhanced respiratory rhythmic patterning of SNA compared with normotensive Wistar–Kyoto (WKY) rats (Czyzyk-Krzeska & Trzebski, 1990a). Moreover, a recent study by Simms et al. (2009) convincingly demonstrated that the strength of respiratory–sympathetic coupling is increased in young, prehypertensive SHR compared with age-matched, normotensive WKY rats. Importantly, they showed that this enhanced coupling is very likely to have a functional impact, because the amplitude of PNA synchronous Traube–Hering ABP waves was larger in SHR than in WKY rats and recovery of resting arterial pressure was more pronounced after hypocapnia-induced apnoea (Simms et al. 2009). These findings appear to extend beyond juvenile SHR, since adult Sprague–Dawley rats made hypertensive by exposure to chronic intermittent hypoxia have been reported to have increased respiratory rhythmic thoracic SNA bursting along with a late-expiratory burst of nerve activity (Zoccal et al. 2008).
In summary, our studies indicate that hypertension in rats treated with Ang II and a high-salt diet is associated with a specific pattern of peripheral SNA, i.e. a sympathetic signature. Data suggest that the splanchnic vascular bed may be a primary neural target supporting increased vascular tone and ABP. Our electrophysiological data further suggest that exaggerated SNA in Ang II–salt hypertension is unlikely to involve activation of cardiac rhythmic RVLM vasomotor neurons. Instead, data raise the possibility that the hypertension could involve exaggerated activity of RVLM neurons that discharge in discrete respiratory rhythmic bursts. Given evidence presented here that the strength of respiratory–splanchnic SNA coupling may be differentially regulated by Ang II in the RVLM of hypertensive and normotensive rats, together with evidence from the literature that splanchnic SNA is strongly coupled to respiration (Miyawaki et al. 2002), we propose that enhanced central respiratory–vasomotor neuron coupling in the RVLM could be an important mechanism contributing to exaggerated splanchnic sympathetic outflow and increased vascular resistance in Ang II–salt hypertension.
Acknowledgments
Studies by the authors’ laboratories were supported by NIH grant RO1 HL076312 to the Neurogenic Cardiovascular Diseases Consortium.
References
- Adams JM, Bardgett ME, Stocker SD. Ventral lamina terminalis mediates enhanced cardiovascular responses of rostral ventrolateral medulla neurons during increased dietary salt. Hypertension. 2009;54:308–314. doi: 10.1161/HYPERTENSIONAHA.108.127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams JM, McCarthy JJ, Stocker SD. Excess dietary salt alters angiotensinergic regulation of neurons in the rostral ventrolateral medulla. Hypertension. 2008;52:932–937. doi: 10.1161/HYPERTENSIONAHA.108.118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED, Bronk DW, Phillips G. Discharges in mammalian sympathetic nerves. J Physiol. 1932;74:115–133. doi: 10.1113/jphysiol.1932.sp002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett CJ, Guild SJ, Ramchandra R, Malpas SC. Baroreceptor denervation prevents sympathoinhibition during angiotensin II-induced hypertension. Hypertension. 2005;46:168–172. doi: 10.1161/01.HYP.0000168047.09637.d4. [DOI] [PubMed] [Google Scholar]
- Braga VA. Dietary salt enhances angiotensin-II-induced superoxide formation in the rostral ventrolateral medulla. Auton Neurosci. 2010 doi: 10.1016/j.autneu.2009.12.007. in press. [DOI] [PubMed] [Google Scholar]
- Brody MJ, Haywood JR, Touw KB. Neural mechanisms in hypertension. Ann Rev Physiol. 1980;42:441–453. doi: 10.1146/annurev.ph.42.030180.002301. [DOI] [PubMed] [Google Scholar]
- Brown DL, Guyenet PG. Electrophysiological study of cardiovascular neurons in the rostral ventrolateral medulla in rats. Circ Res. 1985;56:359–369. doi: 10.1161/01.res.56.3.359. [DOI] [PubMed] [Google Scholar]
- Brunner MJ, Sussman MS, Greene AS, Kallman CH, Shoukas AA. Carotid sinus baroreceptor reflex control of respiration. Circ Res. 1982;51:624–636. doi: 10.1161/01.res.51.5.624. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Trzebski A. Respiratory-related discharge pattern of sympathetic nerve activity in the spontaneously hypertensive rat. J Physiol. 1990a;426:355–368. doi: 10.1113/jphysiol.1990.sp018142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Trzebski A. Respiratory modulation of the cutaneous somatosympathetic reflex in normotensive (WKY) and in spontaneously hypertensive rats (SHR): species and strain-dependent patterns. Acta Neurobiol Exp (Wars) 1990b;50:47–60. [PubMed] [Google Scholar]
- Ferrario CM. Neurogenic actions of angiotensin II. Hypertension. 1983;5:V73–V79. doi: 10.1161/01.hyp.5.6_pt_3.v73. [DOI] [PubMed] [Google Scholar]
- Fink GD. Long-term sympathoexcitatory effect of angiotensin II: a mechanism of spontaneous and renovascular hypertension. Clin Exp Pharmacol Physiol. 1997;24:91–95. doi: 10.1111/j.1440-1681.1997.tb01789.x. [DOI] [PubMed] [Google Scholar]
- Gilbey MP. Multiple oscillators, dynamic synchronization and sympathetic control. Clin Exp Pharmacol Physiol. 2001;28:130–137. doi: 10.1046/j.1440-1681.2001.03414.x. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Krummel M, Peters OA. Reflex patterns in postganglionic neurons supplying skin and skeletal muscle of the rat hindlimb. J Neurophysiol. 1994a;72:2222–2236. doi: 10.1152/jn.1994.72.5.2222. [DOI] [PubMed] [Google Scholar]
- Habler HJ, Janig W, Michaelis M. Respiratory modulation in the activity of sympathetic neurones. Prog Neurobiol. 1994b;43:567–606. doi: 10.1016/0301-0082(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Guyenet PG. Central respiratory modulation of medullary sympathoexcitatory neurons in rat. Am J Physiol Regul Integr Comp Physiol. 1989;256:R739–R750. doi: 10.1152/ajpregu.1989.256.3.R739. [DOI] [PubMed] [Google Scholar]
- Hering E. Über den Einfluß der Atmung auf den Kreislauf. Erste Mitteilung. Über Atembewegungen der Gefaßsysteme. Sitzungsber Akad Wiss Wien, Math-Naturw (Abt 2) 1869;60:829–856. [Google Scholar]
- Hopp FA, Seagard JL. Respiratory responses to selective blockade of carotid sinus baroreceptors in the dog. Am J Physiol Regul Integr Comp Physiol. 1998;275:R10–R18. doi: 10.1152/ajpregu.1998.275.1.R10. [DOI] [PubMed] [Google Scholar]
- Ito S, Gordon FJ, Sved AF. Dietary salt intake alters cardiovascular responses evoked from the rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1600–R1607. doi: 10.1152/ajpregu.1999.276.6.R1600. [DOI] [PubMed] [Google Scholar]
- Johnson CD, Gilbey MP. On the dominant rhythm in the discharges of single postganglionic sympathetic neurones innervating the rat tail artery. J Physiol. 1996;497:241–259. doi: 10.1113/jphysiol.1996.sp021764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ, Fink GD. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension. 2006;48:927–933. doi: 10.1161/01.HYP.0000243799.84573.f8. [DOI] [PubMed] [Google Scholar]
- King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50:547–556. doi: 10.1161/HYPERTENSIONAHA.107.090696. [DOI] [PubMed] [Google Scholar]
- Luft FC. Salt and hypertension: recent advances and perspectives. J Lab Clin Med. 1989;114:215–221. [PubMed] [Google Scholar]
- McAllen RM. Central respiratory modulation of subretrofacial bulbospinal neurones in the cat. J Physiol. 1987;388:533–545. doi: 10.1113/jphysiol.1987.sp016630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel DA, Schreihofer AM. Central respiratory modulation of barosensitive neurones in rat caudal ventrolateral medulla. J Physiol. 2006;572:881–896. doi: 10.1113/jphysiol.2005.103622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S-I, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol Regul Integr Comp Physiol. 1991;261:R690–R696. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- Mifflin SW. What does the brain know about blood pressure? News Physiol Sci. 2001;16:266–271. doi: 10.1152/physiologyonline.2001.16.6.266. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Goodchild AK, Pilowsky P. Evidence for a tonic GABA-ergic inhibition of excitatory respiratory-related afferents to presympathetic neurons in the rostral ventrolateral medulla. Brain Res. 2002;924:56–62. doi: 10.1016/s0006-8993(01)03025-6. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Fink GD. Region-specific changes in sympathetic nerve activity in angiotensin II–salt hypertension in the rat. Exp Physiol. 2010;95:61–68. doi: 10.1113/expphysiol.2008.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007a;9:228–235. doi: 10.1007/s11906-007-0041-3. [DOI] [PubMed] [Google Scholar]
- Osborn JW, Guzman PP, King A, Fink G. Celiac ganglionectomy abolishes the chronic vasoconstrictor responses to angiotensin II (AngII) in conscious rats consuming a high salt diet. FASEB J. 2007b;21:899–893. [Google Scholar]
- Shi P, Stocker SD, Toney GM. Organum vasculosum laminae terminalis contributes to increased sympathetic nerve activity induced by central hyperosmolality. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2279–R2289. doi: 10.1152/ajpregu.00160.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms AE, Paton JF, Pickering AE, Allen AM. Amplified respiratory–sympathetic coupling in the spontaneously hypertensive rat: does it contribute to hypertension? J Physiol. 2009;587:597–610. doi: 10.1113/jphysiol.2008.165902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Cunningham JT, Toney GM. Water deprivation increases Fos immunoreactivity in PVN autonomic neurons with projections to the spinal cord and rostral ventrolateral medulla. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1172–R1183. doi: 10.1152/ajpregu.00394.2004. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating Ang II and baroreceptor input in the rat. J Physiol. 2005;568:599–615. doi: 10.1113/jphysiol.2005.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Toney GM. Vagal afferent input alters the discharge of osmotic and ANG II-responsive median preoptic neurons projecting to the hypothalamic paraventricular nucleus. Brain Res. 2007;1131:118–128. doi: 10.1016/j.brainres.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Osborn JL, Carmichael SP. Fore-brain osmotic regulation of the sympathetic nervous system. Clin Exp Pharmacol Physiol. 2008;35:695–700. doi: 10.1111/j.1440-1681.2007.04835.x. [DOI] [PubMed] [Google Scholar]
- Toney GM, Chen QH, Cato MJ, Stocker SD. Central osmotic regulation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:43–55. doi: 10.1046/j.1365-201X.2003.01046.x. [DOI] [PubMed] [Google Scholar]
- Traube L. Uber periodische Thatigkeis-Aeusserungen des vasomotorischen und Hemmungs-Nervencentrums. Centralbl medic Wissensch. 1865;56:881–885. [Google Scholar]
- Xu L, Sved AF. Acute sympathoexcitatory action of angiotensin II in conscious baroreceptor-denervated rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R451–R459. doi: 10.1152/ajpregu.00648.2001. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M, Miki K, Fink GD, King A, Osborn JW. Chronic angiotensin II infusion causes differential responses in regional sympathetic nerve activity in rats. Hypertension. 2010;55:644–651. doi: 10.1161/HYPERTENSIONAHA.109.145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccal DB, Paton JF, Machado BH. Do changes in the coupling between respiratory and sympathetic activities contribute to neurogenic hypertension? Clin Exp Pharmacol Physiol. 2009;36:1188–1196. doi: 10.1111/j.1440-1681.2009.05202.x. [DOI] [PubMed] [Google Scholar]
- Zoccal DB, Simms AE, Bonagamba LG, Braga VA, Pickering AE, Paton JF, Machado BH. Increased sympathetic outflow in juvenile rats submitted to chronic intermittent hypoxia correlates with enhanced expiratory activity. J Physiol. 2008;586:3253–3265. doi: 10.1113/jphysiol.2008.154187. [DOI] [PMC free article] [PubMed] [Google Scholar]