Abstract
Heme oxygenase-1 (HO-1) metabolizes heme to generate carbon monoxide (CO), biliverdin, and iron. Biliverdin is subsequently metabolized to bilirubin by biliverdin reductase. HO-1 has recently emerged as a promising therapeutic target in the treatment of vascular disease. Pharmacological induction or gene transfer of HO-1 ameliorates vascular dysfunction in animal models of atherosclerosis, post-angioplasty restenosis, vein graft stenosis, thrombosis, myocardial infarction, and hypertension, while inhibition of HO-1 activity or gene deletion exacerbates these disorders. The vasoprotection afforded by HO-1 is largely attributable to its end products: CO and the bile pigments, biliverdin and bilirubin. These end products exert potent anti-inflammatory, antioxidant, anti-apoptotic, and anti-thrombotic actions. In addition, CO and bile pigments act to preserve vascular homeostasis at sites of arterial injury by influencing the proliferation, migration, and adhesion of vascular smooth muscle cells, endothelial cells, endothelial progenitor cells, or leukocytes. Several strategies are currently being developed to target HO-1 in vascular disease. Pharmacological induction of HO-1 by heme derivatives, dietary antioxidants, or currently available drugs, is a promising near-term approach, while HO-1 gene delivery is a long-term therapeutic goal. Direct administration of CO via inhalation or through the use of CO-releasing molecules and/or CO-sensitizing agents provides an attractive alternative approach in targeting HO-1. Furthermore, delivery of bile pigments, either alone or in combination with CO, presents another avenue for protecting against vascular disease. Since HO-1 and its products are potentially toxic, a major challenge will be to devise clinically effective therapeutic modalities that target HO-1 without causing any adverse effects.
Keywords: heme oxygenase-1, carbon monoxide, biliverdin, bilirubin, atherosclerosis, thrombosis, myocardial infarction, hypertension
Introduction
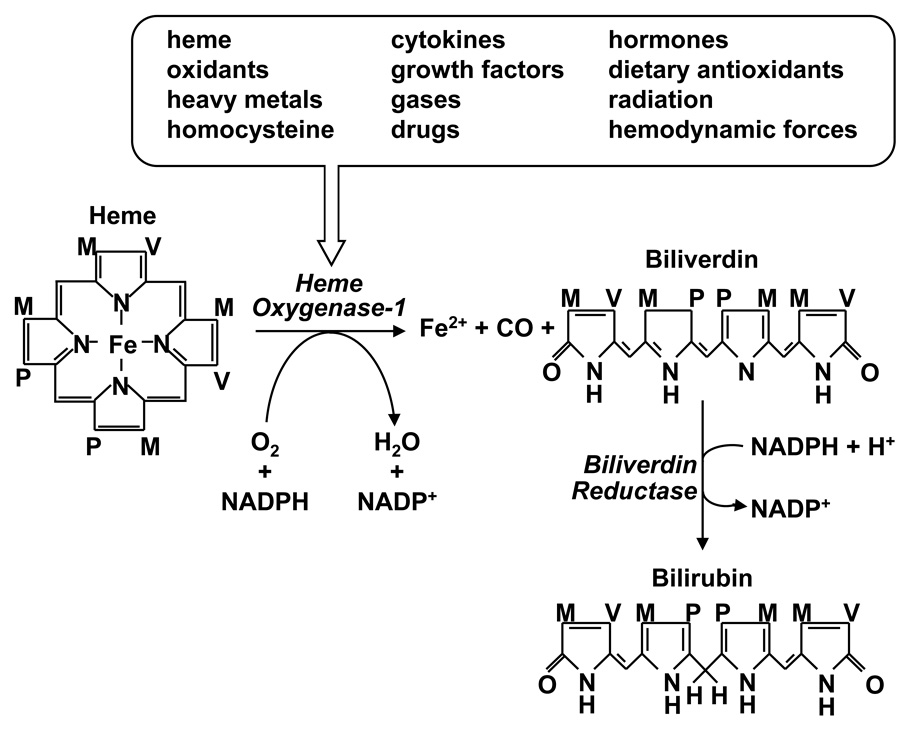
Heme oxygenase-1 (HO-1) is the inducible rate-limiting enzyme in the oxidative degradation of heme yielding equimolar amounts of carbon monoxide (CO), biliverdin, and ferrous iron (Figure 1). This reaction requires molecular oxygen, nicotinamide adenine dinucleotide phosphate, and the concerted action of cytochrome p450 reductase [1]. This catabolic pathway is inhibited by various metalloporphyrins, including zinc and tin protoporphyin-IX. Subsequently, biliverdin is metabolized to bilirubin by biliverdin reductase, and free iron is sequestered by ferritin and either excreted by cells or recycled for heme synthesis. HO-1 is a ubiquitously distributed, highly inducible enzyme. The expression of HO-1 is upregulated by numerous stimuli, including its substrate (heme), oxidants, heavy metals, cytokines, growth factors, gases, homocysteine, hormones, dietary antioxidants, radiation, hemodynamic forces, and by specific therapeutic agents (Figure 1). The control of HO-1 expression occurs primarily at the transcriptional level and is mediated by multiple signaling pathways and transcription factors. However, stress-activated transcription factors such as nuclear factor E2-related factor-2 (Nrf2), activator protein-1, and nuclear factor-κB play predominant roles and mediate the potent induction of HO-1 by agents that cause cellular stress [see 2,3]
Figure 1.
Regulation of heme metabolism by heme oxygenase-1 (HO-1). HO-1 is induced by numerous biochemical and biophysical stimuli and catalyzes the oxidative degradation of heme into equimolar amounts ferrous iron (Fe2+), carbon monoxide (CO), and biliverdin. Biliverdin is subsequently metabolized to bilirubin by biliverdin reductase. M, P, and V represent methyl, propionyl, and vinyl groups, respectively; NADPH, nicotinamide adenine dinucleotide phosphate.
Although interest in HO-1 initially focused on the heme degrading properties of the enzyme, research in the past two decades has shifted and currently center on its protective functions. It is now well established that the induction of HO-1 provides a fundamental cellular defense mechanism against tissue injury. The cytoprotection afforded by HO-1 is mediated by several different mechanisms, including the catabolism of pro-oxidant heme to the antioxidant bile pigments biliverdin and bilirubin; the co-ordinate induction of ferritin, which chelates free iron; and the liberation of CO, which exerts significant anti-inflammatory and anti-apoptotic effects [see 4–6]. Compelling evidence indicates that HO-1 also protects against the development of cardiovascular disease. Genetic deficiency of HO-1 is associated with oxidative tissue damage, anemia, chronic inflammation, thrombosis, and increased susceptibility to atherosclerosis in both mice and humans, while overexpression of HO-1 improves vascular dysfunction in numerous animal models [7–12]. In addition, functional polymorphisms in the promoter region of the HO-1 gene that are linked to impaired inducibility are associated with several cardiovascular pathologies [see 13]. Further evidence for a beneficial role for HO-1 is provided by clinical studies demonstrating that low serum concentrations of the heme metabolite, bilirubin, are correlated with an increased risk of coronary and peripheral artery disease [14–16].
This article will review the effects of HO-1 in the circulation and discuss cellular and molecular mechanisms that contribute to the vasoprotective properties of this enzyme. In addition, it will highlight potential therapeutic strategies targeting HO-1 or its end products in the treatment or prevention of vascular disease.
HO-1 and Atherosclerosis
Considerable evidence suggests that HO-1 plays a beneficial role in atherosclerosis. HO-1 is highly expressed in the endothelium, macrophage, and foam cells of atherosclerotic plaques in both humans and animals [17]. HO-1 expression is observed throughout the development of lesions from early fatty streaks to advanced complex atherosclerotic lesions. Atherectomy biopsy samples of patients with coronary artery disease reveals that HO-1 expression closely correlates with features of the vulnerable plaque: HO-1 is specifically upregulated in human vulnerable atherosclerotic lesions with lipid and macrophage accumulation and low collagen and vascular smooth muscle cell content [18]. Topographical distribution of HO-1-positive cells shows a significantly higher density of cells in the shoulder region and fibrous cap of complex lesions [19]. Interestingly, HO-1 expression is more prevalent in carotid atherosclerotic plaques obtained from asymptomatic compared with symptomatic patients [20], suggesting a possible role for HO-1 in blocking plaque rupture. Significantly, the only identified human case of HO-1 deficiency displayed hyperlipidemia and early development of fatty streaks and fibrous plaque in the aorta [9,10]. Moreover, studies assessing polymorphisms in the 5’-flanking region of the human HO-1 gene suggests a beneficial role for HO-1 in atherosclerosis. In particular, a long (GT)n microsatellite polymorphism in the human HO-1 promoter that is linked to reduced expression is associated with susceptibility to coronary artery disease in some patient populations [21–23]. Furthermore, a single nucleotide polymorphism in the HO-1 promoter, T(−413)A, that elevates basal promoter activity correlates with a reduced frequency of ischemic heart disease in a cohort of Japanese subjects [24].
The induction of HO-1 also co-localizes with oxidized phospholipids in atherosclerotic lesions [25,26]. Given that oxidized lipids are potent inducers of HO-1, they may contribute to the induction of HO-1 in vascular lesions [17,27]. However, numerous other atherogenic molecules, including peroxynitrite, homocysteine, inflammatory cytokines, growth factors, and hypochlorous acid, have been implicated in stimulating HO-1 expression in atherosclerotic lesions [28–33]. Interestingly, the induction of HO-1 by many of these atherogenic agents is mediated through Nrf2 but the upstream signaling pathways that trigger the activation of Nrf2 differ between stimuli. Protein kinase C, mitogen-activated protein kinases, and phosphatidylinositol-3-kinase have all been implicated in the direct activation of Nrf2 while oxidants and peroxynitrite may indirectly mobilize Nrf2 by interfering with its Keap1-mediated sequestration and/or degradation. Thus, atherogenic factors are capable of activating multiple signaling pathways that stimulate Nrf2-mediated HO-1 gene transcription.
Animal studies provide further proof for the protective role of HO-1 in atherosclerosis. Long-term inhibition of HO activity by metalloporphyrins promotes lesion formation in atherogenic mice and rabbits [26,34]. Similarly, deletion of HO-1 in apolipoprotein E (apoE)-deficient mice fed a western diet results in larger and more advanced lesions despite comparable increases in circulating cholesterol [8]. Alternatively, pharmacological induction of HO-1 decreases lesion size in low-density lipoprotein (LDL)-receptor-knockout mice fed a high fat diet [26]. In addition, intraventricular delivery of an HO-1 adenovirus significantly retards lesion formation in apoE-deficient mice [12]. Similarly, adenoviral-mediated gene transfer of HO-1 inhibits graft arteriosclerosis in both rat aortic and cardiac transplants [35–37]. More recent work indicates that HO-1 may also regulate plaque phenotype. Using a vulnerable plaque model, Cheng et al [18] demonstrated that pharmacological induction or gene delivery of HO-1 prevents vulnerable plaque formation in apoE-deficient mice resulting in lesions with a diminished necrotic core, increased fibrous cap thickness, reduced lipid levels, and elevated intimal smooth muscle cell content. In contrast, inhibition of HO-1 by zinc protoporphyrin-IX elicits an opposite effect and triggers plaque destabilization. The specific expression of HO-1 in macrophages also plays a beneficial role in atherosclerosis by decreasing the inflammatory component of vascular lesions [38]. Bone marrow transplantation experiments performed in lethally irradiated LDL-receptor-null mice indicate that animals reconstituted with bone marrow from HO-1-deficient mice exhibit atherosclerotic lesions with greater macrophage content compared to animals reconstituted with bone marrow from wild-type mice. Collectively, clinical and experimental studies strongly support the hypothesis that HO-1 protects against the development of coronary artery disease by reducing plaque size and stabilizing plaque phenotype.
There are several potential mechanisms by which HO-1 prevents the development of atherosclerosis. Since increases in iron deposition are closely associated with the progression of atherosclerosis [39], the ability of HO-1 to reduce iron overload in aortic lesions of apoE-knockout mice is highly significant and may contribute to the anti-atherogenic properties of this protein [12]. Although the precise mechanism whereby HO-1 decreases iron deposition in atherosclerosis is not known, HO-1-mediated increases in iron efflux from cells may be involved [40]. In addition, the increase in ferritin that accompanies HO-1 induction may chelate intracellular free iron and maintain it in a less reactive form. Other beneficial effects of HO-1 may derive from the antioxidant property of this enzyme. Consistent with this proposal, induction of HO-1 decreases plasma hydroperoxide levels in LDL-receptor knockout mice and hyperlipidemic rabbits while HO inhibition increases circulating and tissue hydroperoxide concentrations [26,34]. Bile pigments are likely involved in the antioxidant actions of HO-1 since bilirubin oxidative metabolites are detected in atherosclerotic lesions [41]. However, CO may also contribute to the antioxidant actions of HO-1 by stimulating the expression of antioxidant genes and inhibiting the activity of pro-oxidant enzymes [42,43].
HO-1 may also protect against atherosclerosis by preserving vascular cell function and survival. Endothelial cell dysfunction manifested by impaired endothelium-dependent vasodilation and endothelial nitric oxide (NO) synthesis is one of the earliest changes associated with the development of atherosclerosis. Interestingly, overexpression of HO-1 improves endothelium-dependent vascular relaxation and restores endothelial NO synthase expression in various animal models [44,45]. Both bilirubin and CO have been implicated in this protective response [45,46]. Apoptosis of endothelial cells also plays a pivotal role in the progression of atherosclerosis. Many of the classical pro-atherogenic stimuli are potent inducers of endothelial cell apoptosis, and apoptotic endothelial cells have been directly detected in human atherosclerotic plaques [47]. Significantly, HO-1 gene delivery protects cultured endothelial cells from apoptosis in response to both extrinsic and intrinsic pathways of apoptosis and affords endothelial protection in preclinical models of transplant arteriosclerosis [48,49]. The anti-apoptotic action of HO-1 in endothelial cells appears to be predominantly mediated by CO which modulates multiple steps of the apoptotic cascade [48,50]. However, we recently demonstrated that biliverdin and bilirubin can also inhibit endothelial cell apoptosis by preserving mitochondrial membrane potential [29]. Interestingly, the induction of HO-1 also prevents apoptosis of vascular smooth muscle cells [51,52]. In this case, the anti-apoptotic action of HO-1 appears to be mediated exclusively by CO [51]. Since vascular smooth muscle cell apoptosis has recently been identified as a critical process in mediating plaque rupture [53], the ability of HO-1 to inhibit smooth muscle cell apoptosis in the vulnerable shoulder region of plaques may provide an important mechanism by which HO-1 promotes plaque stability.
Another important mechanism by which HO-1 exerts an anti-atherogenic effect is by arresting inflammation. HO-1 inhibits monocyte chemotaxis and leukocyte adhesion to activated vascular endothelium [26,54]. HO-1 overexpression also attenuates the production of inflammatory cytokines from activated endothelial cells and macrophages [55]. These anti-inflammatory actions of HO-1 are largely mediated through the formation of CO and bile pigments. In this respect, CO elicits divergent regulatory effects on the production of cytokines: CO inhibits the generation of the pro-inflammatory cytokines tumor necrosis factor-α, interleukin-1, and macrophage inflammatory protein-1β while increasing the synthesis of the anti-inflammatory cytokine interleukin-10 [55]. Furthermore, CO downregulates the inflammatory response by blocking inducible NO synthase activity and the expression of granulocyte-macrophage colony stimulating factor, which is known to promote the production of inflammatory mediators and the differentiation of hematopoietic progenitor cells into macrophages and neutrophils [56,57]. Similarly, the HO-1 product biliverdin stimulates the production of interleukin-10 while suppressing the synthesis of inflammatory cytokines [58]. In addition, bilirubin diminishes monocyte chemotaxis and the expression of adhesion receptors on endothelial cells, leading to inhibition of leukocyte rolling, adhesion, and infiltration into the vessel wall [26,54,59]. Thus, HO-1 is able to alleviate vascular inflammation in a multifold manner through the production of CO and biliverdin/bilirubin.
HO-1 and Vascular Occlusion
HO-1 may also exert a salutary effect on the pathologic remodeling response that frequently occurs following percutaneous transluminal angioplasty or coronary artery bypass surgery. These surgical procedures are commonly used to treat atherosclerotic disease; however, they are prone to early occlusion due to thrombosis and late-onset occlusion as a result of vascular smooth muscle cell proliferation and intimal thickening. Interestingly, HO-1 protects against both of these negative clinical outcomes. In an experimental vein graft model where a segment of jugular vein is patched into a defect created in the autologous carotid artery of the mouse, HO-1-knockout mice develop large smooth muscle cell-rich intimal lesions ten days after surgery compared to wild-type animals, suggesting an essential protective role for HO-1 in graft stenosis [8]. Prior induction of HO-1 by hemin also retards neointima formation following balloon injury of rat carotid arteries, whereas inhibition of HO activity by metalloporphyrins augments lesion formation [60–62]. In addition, localized adenovirus-mediated HO-1 gene delivery immediately after arterial injury attenuates neointimal hyperplasia in rat carotid and pig femoral arteries [63,64]. Furthermore, deletion of the HO-1 gene exacerbates neointima formation following wire injury of mouse femoral arteries [63] and following the creation of an arteriovenous fistula in mice [65]. Importantly, HO-1 may also influence the remodeling response following arterial injury in humans. Patients with the long (GT)n repeat in the HO-1 promoter exhibit a significantly enhanced risk of restenosis following peripheral percutaneous transluminal angioplasty [66,67]. The presence of long (GT)n repeats is also associated with stenosis-related arteriovenous fistula failure in a cohort of Chinese hemodialysis patients [68]. However, conflicting data has been presented relating this (GT)n length polymorphism to the risk of restenosis following coronary stenting [69,70].
The beneficial actions of HO-1 following arterial injury are mediated, in part, by its ability to suppress vascular smooth muscle cell proliferation. Induction or gene delivery of HO-1 in cultured vascular smooth muscle cells blocks cell growth and DNA synthesis [63,71]. In addition, vascular smooth muscle cells derived from HO-1-null mice display enhanced growth and DNA synthesis compared to cells obtained from wild-type animals. Moreover, greater smooth muscle cell proliferation is observed in arterial lesions from HO-1-deficient mice compared to wild-type animals, confirming the anti-proliferative effect of HO-1 in vivo [63]. The anti-proliferative action of HO-1 is mediated through the soluble guanylate cyclase/cGMP pathway since inhibition of soluble guanylate cyclase or protein kinase G restores cell growth [63]. Flow cytometry experiments indicate that HO-1 arrests smooth muscle cells in the G0/G1 phase of the cell cycle and this is associated with a pronounced increase in p21 expression [65]. The exogenous administration of CO or bilirubin also inhibits vascular smooth muscle cell proliferation, cell cycle progression, DNA synthesis, and the expression of cell cycle regulatory proteins, suggesting a role for both these products in the anti-proliferative action of HO-1 [71–74]. More recently, HO-1 has also been demonstrated to inhibit vascular smooth muscle cell migration via the CO-mediated inhibition of Nox1 enzyme activity and downstream redox-sensitive pro-migratory pathways [75]. The ability of HO-1 to inhibit the migration of vascular smooth muscle cells from the media to the intima may provide another possibility by which HO-1 limits neointima formation.
Interestingly, HO-1 stimulates cell cycle progression and proliferation in vascular endothelium. Transduction of the HO-1 gene into endothelial cells promotes their growth and the development of capillary-like tube structures while inhibition or deletion of HO-1 blocks endothelial cell growth [76]. HO-1 also facilitates endothelial cell proliferation, migration and capillary sprout formation in response to specific angiogenic factors [77,78]. In addition, HO-1 promotes the re-endothelialization of injured arteries. Pharmacological induction of HO-1 or systemic administration of an HO-1 adenovirus accelerates the re-endothelialization of denuded blood vessels while HO-1 deletion impairs this process [79–81]. Aside from stimulating the proliferation and migration of endothelial cells from the injured border zone or from branching vessels adjacent to the site of injury, HO-1 enhances re-endothelialization by promoting the mobilization and homing of endothelial progenitor cells to sites of injury [79–81]. HO-1 increases circulating levels of endothelial progenitor cells and elevates the number of endothelial progenitor cells detected in cultures of mononuclear cells. Moreover, the expression of HO-1 by endothelial progenitor cells is required for their incorporation into blood vessels. Notably, endothelial progenitor cells lacking HO-1 are unable to re-endothelialize the retinal vasculature following ischemic injury [77]. The HO-1-mediated increase in endothelial regrowth following arterial injury is dependent on the induction of stromal cell-derived factor-1 and vascular endothelial growth factor, and is mimicked by the inhalation of CO, suggesting a key role for this gas in repairing endothelium-denuded areas of blood vessels [80,81]. The ability of HO-1 to restore endothelial cells at sites of arterial injury provides another mechanism to blunt intimal expansion since the re-endothelialization of the vessel wall assists in maintaining the underlying smooth muscle in a quiescent, non-proliferative, and non-migratory state.
HO-1 may also preserve blood vessel patency by inhibiting thrombosis. HO-1 deficiency accelerates arterial thrombus formation following photochemical injury and increases thrombus size in a murine model of deep vein thrombosis [11,82]. The absence of HO-1 in mice also leads to arterial thrombosis following allogeneic aortic transplantation [83]. On the contrary, induction of HO-1 retards micro and macrovascular thrombus formation following endothelial injury [84,85]. Interestingly, there are no significant differences in bleeding time, platelet counts, or prothrombin times between HO-1-knockout and wild-type mice, suggesting that abnormalities in primary or secondary hemostasis are not responsible for the accelerated thrombosis [12]. The anti-thrombotic actions of HO-1 are likely mediated by CO and biliverdin since the exogenous administration of either compound rescues HO-1-deficient animals from thrombosis [11,83]. Both these HO-1 products may prevent intravascular thrombosis by ameliorating endothelial cell damage, which is a crucial factor in thrombus formation. In addition, HO-1-derived CO may block thrombosis by inhibiting platelet aggregation and the expression of plasminogen activator inhibitor-1 and tissue factor [11,87].
HO-1 and Myocardial Infarction
Substantial data support a protective role for HO-1 against myocardial ischemia/reperfusion-induced injury. Pharmacological induction of HO-1 significantly attenuates infarct size and the incidence of reperfusion arrythmias following ischemia/reperfusion, while HO inhibition aggravates cardiac tissue damage [88–90]. Studies in transgenic animals also confirm the importance of HO-1 in ischemic cardiac injury. Hearts from heterozygous HO-1 knockout mice are more prone to ischemia-reperfusion injury whereas cardiac-specific HO-1 overexpressing animals suffer less damage [91–93]. A maladaptive response consisting of increased ventricular dilation, infarction, and thrombosis has also been reported in HO-1-deficient mice during chronic hypoxia [94]. Significantly, human HO-1 gene transfer using adeno-associated virus several weeks prior to acute coronary artery ligation and release results in sustained myocardial protection from ischemia-reperfusion injury in rats [95]. In addition, overexpression of the transgene prevents long-term pathologic tissue remodeling and normalizes tissue function. Moreover, the improvement in cardiac function is accompanied by decreases in oxidative stress, inflammation, and interstitial fibrosis. These findings support the utility of using a pre-emptive HO-1 gene transfer approach to protect tissues from future episodes of injury and may provide a potential preventative therapy for individuals at risk for developing coronary ischemic events.
Both bilirubin and CO contribute to the protective actions of HO-1 in the heart. Exogenously administered bilirubin significantly improves cardiac function and decreases myocardial infarct size and mitochondrial damage upon reperfusion insult [96]. Similarly, treatment of isolated cardiac cells or hearts with a CO-releasing molecule preserves cell viability and myocardial performance against hyperoxia-reoxygenation damage [97]. In addition, administration of a CO-releasing agent at the time of reperfusion reduces infarct size in a murine model of coronary occlusion [98]. Interestingly, brief exposure to CO produces sustained, long-term cardioprotection that mimics the late phase of ischemic preconditioning [99]. Inhalation of CO gas prior to myocardial ischemia-reperfusion injury likewise reduces infarct area in mice and this is associated with diminished migration of macrophages and monocytes into the infarcted zone, and with reduced expression of tumor necrosis factor-α [100]. Inhalation of CO also improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs, and attenuates ischemia-reperfusion injury following cardiac transplantation in mice [101,102]. Indeed, protective actions of CO against an ischemic insult have been reported following the transplantation of other organs [see 103].
HO-1 and Hypertension
HO-1 is an important modulator of blood pressure and alterations in the expression or activity of this enzyme has been linked to the pathogenesis of hypertension. However, divergent regulatory effects on blood pressure have been reported in different experimental models of hypertension. Chemical induction of HO-1 or the administration of HO substrates attenuates hypertension in spontaneously hypertensive rats (SHR) and this effect is abolished when HO inhibitors are introduced prior to HO-1 induction [104–106]. In addition, gene transfer of HO-1 in SHR results in a significant decline in blood pressure [107]. More recently, Wang et al [108] demonstrated that administration of hemin for 3 consecutive weeks to 12-week old SHR rats normalizes systolic blood pressure. Importantly, this normalization persists for 9 months after the discontinuation of hemin. The mechanism underlying this dramatic and sustained reversal in hypertension is not known but may be related to the persistent elevation in vascular HO-1 expression and the reversal of eutrophic inward remodeling of small resistance arteries. Notably, the antihypertensive effect of hemin is not associated with any changes in body weight or hepatotoxicity, raising the possibility that hemin administration may be a viable treatment option in humans.
The antihypertensive effect is likely mediated by CO, since it is the only HO-1 product able to decrease blood pressure in SHR [104]. There are several potential mechanisms by which CO may lower blood pressure. In particular, CO can reduce peripheral resistance by directly dilating blood vessels via the activation of soluble guanylate cyclase and/or calcium-activated potassium channels [109,110]. Beyond its direct vasodilating effect, CO may also decrease vascular tone by regulating the production of vasoactive molecules. In this regard, CO blocks the synthesis of the potent vasoconstrictor endothelin-1 and the cytochrome P450-mediated generation of vasoconstrictors [105,106,111]. CO may also exert an antihypertensive effect by stimulating the release of NO from intracellular stores, by depressing central sympathetic outflow, and by promoting sodium excretion by the kidney [112–114].
A beneficial role for HO-1 has also been demonstrated in other experimental models of hypertension. Systemic induction of HO-1 by cobalt protoporphyrin-IX lowers blood pressure in angiotensin II-treated hypertensive mice and this is associated with a pronounced decline in renal superoxide production [115]. Similarly, a single intraventricular injection of a retroviral vector containing human HO-1 results in widespread transgene expression and blunts the angiotensin II-mediated pressor response in rats [116]. Surprisingly, kidney-specific induction of HO-1 is sufficient to attenuate angiotensin II-dependent hypertension, suggesting a critical role for this organ in mediating the antihypertensive effect of HO-1 in this experimental model [117]. HO-1 also affords protection in the one kidney-one clip model of renovascular hypertension [118]. In this model, HO-1-deficient mice exhibit more severe hypertension and renal injury compared to wild-type mice. Furthermore, HO-1-null mice exhibit a sustained and significant elevation in systemic arterial pressure when subjected to deoxycorticosterone acetate (DOCA)-salt whereas wild-type animals remain normotensive [119]. Significantly, the antihypertensive action of HO-1 is not limited to the systemic circulation. Overexpression of HO-1 or inhalation of CO inhibits the development of pulmonary hypertension in response to hypoxia or monocrotaline [120–123]. In addition, injection of an adeno-associated virus expressing HO-1 through the portal vein markedly diminishes portal hypertension in carbon tetrachloride-treated rats [124].
Surprisingly, HO-1 promotes hypertension in some animal models. Dahl salt-sensitive rats placed on high-salt diet for 4 weeks develop severe systemic hypertension that is associated with a significant increase in vascular HO-1 protein and CO production [125,126]. In addition, administration of the HO inhibitor, zinc deuteroporphryin 2,4-bis glycol, selectively lowers blood pressure in hypertensive Dahl rats but not in control animals fed a low-salt diet. Obese Zucker rats, a well established genetic model of metabolic syndrome, also develop hypertension that is coupled to an increase in respiratory CO production [127]. However, treatment with a HO inhibitor lowers CO excretion and normalizes blood pressure in these obese animals. Interestingly, endothelium-dependent vasodilation is impaired in both obese and salt-sensitive rats, and acute treatment of blood vessels with a HO inhibitor fully restores endothelial function in these animals. Furthermore, the co-application of CO prevents the restoration of endothelial function by HO inhibition. Taken together, these findings suggest that elevated CO production contributes to endothelial dysfunction and hypertension in these animals. Consistent with this notion, exogenously applied or endogenously derived CO inhibits endothelial NO release and vasodilation in several vascular beds [112,128,129]. Moreover, transgenic mice that selectively overexpress HO-1 in vascular smooth muscle cells are also hypertensive and display impaired nitrovasodilator-mediated vasodilation and cGMP production [130].
In summary, HO-1 lowers blood pressure in most experimental forms of hypertension but pro-hypertensive effects have also been reported in some animal models. Although reasons for the divergent effects of HO-1 on blood pressure are not known, the complex interaction between the HO-1 and NO synthase enzymes can lead to alterations in gaseous monoxide production and competition between CO and NO for specific target proteins that impact blood pressure regulation (see 6).
Therapeutic Strategies Targeting HO-1 in Vascular Disease
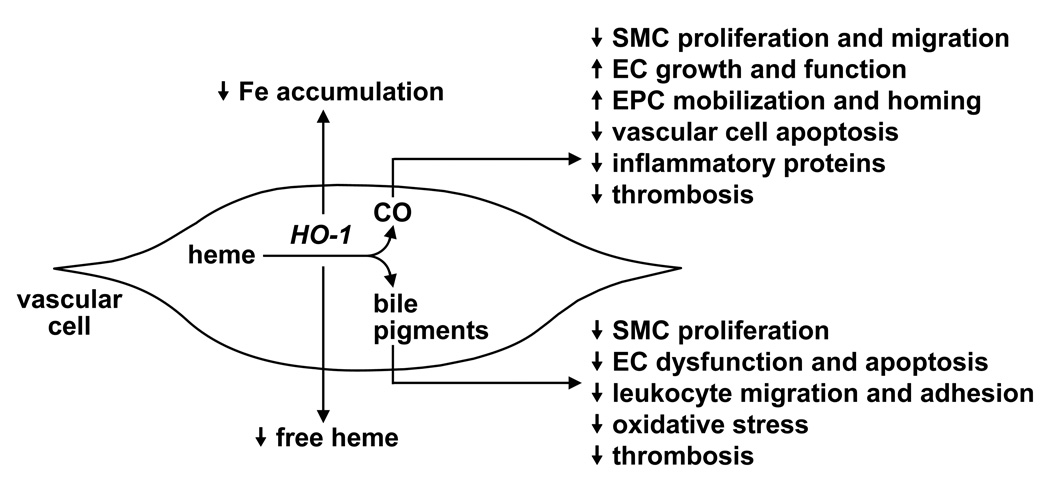
HO-1 has recently emerged as a promising therapeutic target in the treatment of vascular disease. Numerous mechanisms contribute to the vasoprotective action of HO-1 (Figure 2). In particular, HO-1 exerts a direct antioxidant effect by degrading pro-oxidant heme and preventing intracellular iron accumulation. In addition, the HO-1 end products CO and bile pigments possess potent anti-inflammatory, antioxidant, anti-apoptotic, and anti-thrombotic actions. Moreover, CO and bile pigments act to preserve vascular homeostasis at sites of arterial injury by influencing the proliferation, migration, and adhesion of vascular smooth muscle cells, endothelial cells, endothelial progenitor cells, or leukocytes.
Figure 2.
Vasoprotective effects of heme oxygenase (HO-1) and its end products. HO-1 exerts a direct antioxidant effect by degrading pro-oxidant heme and by preventing intracellular iron (Fe) accumulation. In addition, the HO-1 end products, CO and the bile pigments biliverdin and bilirubin, possess potent anti-inflammatory, antioxidant, anti-apoptotic, and anti-thrombotic actions. Moreover, CO and bile pigments act to preserve vascular homeostasis at sites of arterial injury by influencing the proliferation, migration, and adhesion of vascular smooth muscle cells (SMC), endothelial cells (EC), endothelial progenitor cells (EPC), or leukocytes.
Several strategies can be employed to target HO-1 in vascular disease (Table 1). One promising approach involves the use of pharmacological inducers. Heme and its synthetic analogues are potent inducers of HO-1 and have been shown to protect against the development of vascular disease in numerous animal models. In addition to upregulating HO-1 expression, heme is a substrate for HO-1 and this may serve to further enhance the synthesis of CO and bilirubin since HO-1 activity may be substrate-limited in vascular cells [131]. Hemin is already approved by the United States Food and Drug Administration for the treatment of acute porphyria and heme compounds have been used to treat thalassemia intermedia, myelodysplastic syndrome, and liver allograft failure following erythropoietic protoporphyria [132–135]. Interestingly, a recent small clinical study affirmed the efficacy of a single intravenous infusion of hemin to stimulate plasma HO-1 protein expression and activity in healthy subjects without any adverse effects [136], illustrating the potential of using heme derivatives to elevate HO-1 expression. Clearly, further clinical studies employing larger subject populations are needed to establish optimal safe and effective dosing regimens for these compounds.
Table 1.
Therapeutic Strategies Targeting HO-1 in Vascular Disease
|
Since free heme possess pro-oxidant and pro-inflammatory properties [137], a potentially less toxic approach to inducing HO-1 gene expression may involve the use of dietary antioxidants. The dietary antioxidants tert-butylhydroquinone, quercetin, caffeic acid phenethyl ester, and curcumin are all potent inducers of HO-1 [138,139]. Similarly, catechins, the major polyphenolic ingredient in green tea, and α-lipoic acid, a thiol-containing antioxidant found in certain vegetables, are strong inducers of HO-1 [140,141]. Furthermore, resveratrol, a phytoalexin found in high concentration in red wine, stimulates the expression of HO-1 in vascular cells raising the possibility that HO-1 contributes to the cardioprotection associated with the consumption of red wine [142]. In addition, a host of other dietary antioxidants have been found to enhance HO-1 expression, including the coffee diterpenes cafestol and kahwoel, carnosol, and sulphoraphane [138]. Interestingly, phenolic antioxidants which fail to stimulate HO-1 do not confer protection against vascular disease, underscoring the crucial role of HO-1 in mediating the atheroprotective effects of these compounds [79]. Aside from antioxidants, amino acids such as methionine, alanine, and glutamine have also been reported to induce HO-1 expression [143–145]. Thus, a variety of dietary approaches may be used to stimulate HO-1 expression. However, careful clinical studies are needed to establish the efficacy and safety of any nutritional approach.
There is a growing appreciation that many frontline drugs used in the treatment of cardiovascular disease exert their therapeutic effect, at least in part, through the induction of HO-1. Statins are widely prescribed lipid lowering agents that decrease mortality in patients with coronary artery disease. Several statins are capable of elevating HO-1 expression in cultured vascular cells [146,147]. In addition, oral administration of statins results in statin- and tissue-specific increases in HO-1 that is associated with increased resistance to oxidative stress and inhibition of smooth muscle cell proliferation, suggesting that HO-1 may contribute to the pleiotropic and anti-atherogenic actions of statins [148]. However, the induction of HO-1 by statins is not universally observed and it remains unclear whether statins at concentrations pharmacologically relevant to humans elevate HO-1 expression, and whether this induction contributes to protection against coronary artery disease [149].
Probucol is another cholesterol lowering drug that reduces the risk of postangioplasty restenosis. The protective effect of probucol depends not only on its ability to suppress lipid peroxidation, but also on the induction of HO-1 [79]. Probucol inhibits macrophage accumulation and smooth muscle cell proliferation, and stimulates endothelial cell regrowth following arterial injury in a HO-1-dependent fashion. Aspirin is another drug used in the treatment of cardiovascular disease that induces HO-1 expression. Indeed, pharmacologically relevant concentrations of aspirin stimulate HO-1 expression and this may contribute to the drugs antioxidant and anti-thrombotic profile [150]. Interestingly, the induction of HO-1 is unique for aspirin and is not mimicked by other non-steroidal anti-inflammatory agents. The organic nitrate ester, pentaesithrityl tetranitrate (PETN) has also been observed to stimulate vascular HO-1 expression and this may, in part, mediate its antioxidant and anti-atherogenic actions [151]. Moreover, the induction of HO-1 by PETN prevents the development of tolerance by eliminating nitrate-induced generation of reactive oxygen species [152]. Similarly, sildenafil, the phosphodiesterase-5 inhibitor used for the treatment of erectile dysfunction, upregulates HO-1 expression both in cultured endothelial cells and in vivo, and this may contribute to its biological actions in cavernous tissue [153,154]. Finally, presently employed drug eluting stents may prevent coronary artery restenosis through the induction of HO-1. Both rapamycin and paclitaxel stimulate HO-1 expression in vascular smooth muscle cells and HO-1 contributes to the anti-proliferative action of these compounds [155–157].
Collectively, the above results validate the use of pharmacological approaches in targeting HO-1 in vascular disease. However, there are some challenges associated with the deployment of pharmacological inducers. One concern relates to possible non-specific effects of HO-1 inducers that can potentially negate the beneficial effects associated with the upregulation of HO-1 [137,158). Furthermore, the induction and duration of HO-1 expression must be carefully titrated since prolonged, high level HO-1 expression can adversely affect cell viability [159,160]. In addition, the presence of polymorphisms in the promoter that restrains the induction of HO-1 may limit the efficacy of HO-1 inducers in certain patient populations. Thus, assessment of patient genotype prior to pharmacological intervention may be necessary. Increasing HO-1 gene expression via viral-mediated delivery obviates this problem and allows for the specific induction of this gene in all patients. Gene therapy approaches with HO-1 have proven highly effective in animal studies and the recent development of inducible and cell-specific vectors allows for selective and temporal patterns of gene expression [161,162]. However, current limitations in human gene therapy are well recognized and will require additional improvement and documentation of clinical safety and efficacy.
As an alternative to direct targeting of HO-1, products of the HO-1 reaction can be administered to treat vascular disease. As outlined above, CO is effective in several preclinical models of cardiovascular disease. Moreover, a growing number of studies suggest that acute, periodic inhalation of low concentrations of CO (50–500 parts per million) is sufficient to elicit protection in animal models of pulmonary hypertension, intimal hyperplasia, sickle cell disease, ischemia-reperfusion injury, and postoperative ileus [72, 122,163–165]. Significantly, no deleterious effects on heart rate, blood chemistry, serum electrolytes, and arterial oxygen saturation are noted with this low-dose CO inhalation strategy (158,159). A number of clinical trials are currently exploring the pharmacokinetics, safety, and efficacy of acute, episodic CO inhalation regimens in human subjects for the treatment of lung inflammation, chronic obstructive pulmonary disease, and renal transplantation. Initial reports indicate that inhalation of CO (95–500 parts per million) for one or two hours are well-tolerated, safe, feasible, and of potential benefit to patients with chronic obstructive pulmonary disease [166,167].
The use of CO-saturated solutions and prodrugs provides another vehicle for the administration of CO. In this respect, we recently reported that brief local delivery of a saturated solution of CO blocks the pathophysiological response to arterial injury [168]. Alternatively, intraperitoneal injection of CO-saturated solutions provides a simple procedure for the systemic delivery of CO. Indeed, a single intraperitoneal dose of CO–saturated Ringer’s lactate solution ameliorates post-operative ileus in mice [169]. Systemic delivery of CO can also be achieved using the prodrug dichloromethane which is readily metabolized in the liver by cytochrome P450 isozymes to CO. Oral ingestion of dichloromethane results in a marked increase in CO production that is associated with a significant decrease in aortic intimal thickening in rodent model of chronic allogeneic rejection, demonstrating the ability of this approach to generate biologically relevant concentrations of CO [170]. However, there are serious concerns with toxicity related to the use of this compound [171). More promising, are the recently developed CO-releasing compounds (CORMs) that liberate CO under physiologic conditions. Several CORMs have been synthesized with various solubility and release kinetics and their biological activity verified both in vitro and in vivo [172]. These compounds may allow for a more controlled and targeted delivery of CO and are good candidates for therapeutic development.
As a complementary approach, CO-sensitizing compounds such as 3-(−5’-hydroxymethyl-2’-furyl)-1-benzyl indazole, or YC-1, can be utilized to potentiate the biological activity of exogenously administered CO. This benzyl indazole derivative augments CO-mediated activation of soluble guanylate cyclase and markedly enhances the anti-aggregatory and vasodilator potency of CO [173,174]. Moreover, we recently demonstrated that YC-1 also induces the expression of HO-1 in vascular cells (175). The ability of YC-1 to sensitize soluble guanylate cyclase to CO and simultaneously stimulate the production of CO through the induction of HO-1 may provide an important mechanism by which YC-1 amplifies the vascular actions of CO. Thus, co-administration of YC-1 may increase the efficacy of CO and lessen the risk of CO-mediated toxicity by reducing CO dosing regimens.
Systemic or local application of the bile pigments biliverdin and bilirubin may provide another approach in treating vascular disease. The administration of biliverdin has salutary effects following transplantation or ischemia-reperfusion in several organs, including the heart [96]. In addition, the delivery of biliverdin prevents intimal thickening following arterial injury in rodents [73,74]. Surprisingly, the protective effects associated with the administration of bile pigments in animals occur with modest increases in the levels of circulating bilirubin. This finding is in-line with clinical studies showing that mild increases in serum bilirubin are sufficient to reduce the risk of coronary artery disease [14], suggesting that a suitable therapeutic window for these compounds exist. In this respect, a preliminary clinical report found that traditional biliverdin-containing Chinese medicines are safe and effective in treating chronic liver disease [176]. However, given the potential neurotoxicity associated with bile pigments [177], further pharmacological studies are needed to establish safe and effective treatment regimens. Since the cytoprotective properties of HO-1 may be mediated through the synergistic effects of its reaction products, dual therapy with CO and biliverdin may confer greater benefit than the singular application of either HO-1 product [178].
Circulating levels of bilirubin may also be elevated by blocking the conjugation and excretion of bilirubin into bile salts. The hepatic enzyme uridine-diphosphate-glucuronosyltransferase 1A1 (UGT1A1) is responsible for conjugating bilirubin and genetic mutations in the UGT1A1 gene in humans (Crigler-Najjar or Gilbert’s syndrome) that reduce the activity of the enzyme lead to increased levels of unconjugated bilirubin. Significantly, individuals with this syndrome exhibit a diminished risk for coronary artery disease [see 179]. Similarly, the homozygous Gunn rat which lacks the counterpart enzyme has elevated serum bilirubin levels and is also protected against vascular disease [73,119]. Thus, development of specific pharmacological inhibitors that target UGT1A1 may provide a unique approach to raise endogenous bilirubin levels. However, inhibition of UGTA1A must be carefully calibrated to avoid the development of liver dysfunction.
In some instances inhibition of HO-1 may be therapeutically desirable. Metalloporphyrins such as tin protoporphyrin, zinc protoporphyrin, and chromium mesoporphyrin are well-recognized and widely used non-selective inhibitors that block both HO-1 and HO-2 activity. They reversibly compete with heme for binding to HO and block HO activity both acutely and chronically. However, metalloporphyrins are not selective for HO and can regulate the activity and expression of numerous other proteins (180,181). Paradoxically, metalloporphyrins can induce HO-1 expression further confounding interpretations when using these inhibitors. Recently, several non-porphyrin imidazole-dioxalane derivatives have been isolated that are more selective for HO (see 182). Their inhibitory activity has been validated both in isolated organs and rodents, and certain compounds exhibit isoform selectivity for HO-1. However, additional pharmacokinetic and pharmacodynamic studies are needed to establish the therapeutic potential of these second generation HO-1 inhibitors. Finally, molecular approaches using antisense or small interference RNA (siRNA) technology have been successfully employed to knockdown HO-1 expression both in vitro and in vivo (183–185). Although siRNA shows much promise, current difficulties in delivery and potential off-target effects limit the clinical utility of this approach.
Conclusion
Studies in the past decade indicate that HO-1 is a promising candidate for the development of therapies to treat vascular disease. Several approaches can be used to target HO-1. The use of pharmacological inducers represents an attractive near-term strategy. Numerous inducers of HO-1 have been identified and quite a few are already in clinical use. Indeed, several vanguard drugs used to treat cardiovascular disease may exert their clinical effects, in part, via the induction of HO-1. The use of dietary supplements, either alone or in combination with HO-inducing drugs, provides another viable avenue to target HO-1. However, the occurrence of polymorphisms in the promoter that minimizes the induction of HO-1 may limit the effectiveness of pharmacological and nutritional approaches in some patients. In this case, HO-1 gene therapy or direct application of HO-1 end products may be more efficacious. Inhalation of CO has proven highly effective in preclinical animal models of vascular disease and has recently been demonstrated to be well tolerated in phase I clinical trials. Furthermore, recently developed CO-releasing and CO-sensitizing molecules offer an appealing alternative or complement to inhalational gas therapy. Given recent experimental and epidemiological studies showing beneficial effects of biliverdin and bilirubin, the delivery of bile pigments may also be clinically useful. Since HO-1 and its end products are potentially toxic, a major challenge will be to develop clinically effective therapeutic regimes that target HO-1 without causing any detrimental effects.
Acknowledgement
The author acknowledges the support of grants from the National Institutes of Health HL59976 and HL74966, and the American Heart Association Midwest Affiliate.
References
- 1.Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci USA. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam J, Cook JL. Transcriptional regulation of the heme oxygenase-1 gene via the stress response pathway. Curr Pharm Des. 2003;9:2499–2511. doi: 10.2174/1381612033453730. [DOI] [PubMed] [Google Scholar]
- 3.Ferrandiz ML, Devesa I. Inducers of heme oxygenase-1. Curr Pharm Des. 2008;14:473–486. doi: 10.2174/138161208783597399. [DOI] [PubMed] [Google Scholar]
- 4.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide from basic science to therapeutic implications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 5.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 6.Durante W, Johnson FK, Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poss KD, Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yet SF, Layne MD, Liu X, Chen YH, Ith B, Sibinga NE, Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerosis lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 9.Yachie A, Niida Y, Wada T, Igarashi N, Kaneda H, Toma T, Ohta K, Kasahara Y, Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawashima A, Oda T, Yachie A, Koizumi S, Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 11.True AL, Olive M, Boehm M, San H, Westrick RJ, Raghavachari N, Xu X, Lynn EG, Sack MN, Munson PJ, Gladwin MT, Nabel EG. Heme oxygenase-1 deficiency accelerates formation of arterial thrombosis through oxidative damage to the endothelium, which is rescued by inhaled carbon monoxide. Circ Res. 2007;101:893–901. doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 12.Juan SH, Lee TS, Tseng KW, Liou JY, Shyue SK, Wu KK, Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 13.Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Schwertner HA, Jackson WG, Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clin Chem. 1994;40:18–23. [PubMed] [Google Scholar]
- 15.Perlstein TS, Pande RL, Beckman JA, Creager MA. Serum total bilirubin level and prevalent lower-extremity peripheral artery disease. Arterioscler Thromb Vasc Biol. 2008;28:166–172. doi: 10.1161/ATVBAHA.107.153262. [DOI] [PubMed] [Google Scholar]
- 16.Mayer M. Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 2000;46:1723–1727. [PubMed] [Google Scholar]
- 17.Wang LJ, Lee TS, Lee FY, Pai RC, Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1988;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng C, Noordeloos AM, Jeney V, Soares MP, Moll F, Pasterkamp G, Serruys PW, Duckers HJ. Heme oxygenase-1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119:3017–3027. doi: 10.1161/CIRCULATIONAHA.108.808618. [DOI] [PubMed] [Google Scholar]
- 19.Song J, Sumiyoshi S, Nakashima Y, Doi Y, Iida M, Kiyohara Y, Sueishi K. Overexpression of heme oxygenase-1 in coronary atherosclerosis of Japanese autopsies with diabetes mellitus: Hisayama study. Atherosclerosis. 2009;202:573–581. doi: 10.1016/j.atherosclerosis.2008.05.057. [DOI] [PubMed] [Google Scholar]
- 20.Ameriso SF, Villamil AR, Zedda C, Parodi JC, Garrido S, Sarchi MI, Shultz M, Bocskowski J, Sevlever GE. Heme oxygenase-1 is expressed in carotid atherosclerotic plaque infected by Helicobacter pylori and is more prevalent in asymptomatic patients. Stroke. 2005;36 doi: 10.1161/01.STR.0000177494.43587.9e. 1896-1890. [DOI] [PubMed] [Google Scholar]
- 21.Chen YH, Lin SY, Lin MW, Tsai HL, Kuo SS, Chen JW, Charng MJ, Wu TC, Chen LC, Ding YA, Pan WH, Jou YS, Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type II diabetic patients. Hum Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 22.Kaneda H, Ohno M, Taguchi J, Hashimoto H, Ogasawura T, Aizawa T, Ishizaka N, Nagai R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol. 2002;22:1680–1685. doi: 10.1161/01.atv.0000033515.96747.6f. [DOI] [PubMed] [Google Scholar]
- 23.Endler G, Exner M, Schillinger M, Marculescu R, Sunder-Plassmann R, Raith M, Jordanova N, Wojita J, Mannhalter C, Wagner OF, Huber K. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb Haemostasis. 2004;91:155–161. doi: 10.1160/TH03-05-0291. [DOI] [PubMed] [Google Scholar]
- 24.Ono K, Goto Y, Takagi S, Baba S, Tago N, Nonogi H, Iwai N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173:315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Ishikawa K, Sugawara D, Goto J, Watanabe K, Kawamura S, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 26.Ishikawa K, Sugawara D, Wang XP, Suzuki K, Itabe H, Maruyama Y, Lusis AJ. Heme oxygenase-1 inhibits atherosclerosis lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa K, Navab M, Leitinger N, Fogelman AM, Lusis AJ. Induction of heme oxygenase-1 inhibits monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foresti R, Sarathchandra P, Clark JE, Green CJ, Motterlini R. Peroxynitrite induces heme oxygenase-1 in vascular endothelial cells: a link to apoptosis. Biochem J. 1999;339:729–736. [PMC free article] [PubMed] [Google Scholar]
- 29.Liu XM, Peyton KJ, Ensenat D, Wang H, Schafer AI, Alam J, Durante W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle: role in cell survival. J Biol Chem. 2005;280:872–877. doi: 10.1074/jbc.M410413200. [DOI] [PubMed] [Google Scholar]
- 30.Durante W, Kroll MH, Christodoulides N, Peyton KJ, Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 31.Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwal A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-β in human renal epithelial cells. J Biol Chem. 2000;275:40904–40909. doi: 10.1074/jbc.M006621200. [DOI] [PubMed] [Google Scholar]
- 32.Durante W, Peyton KJ, Schafer AI. Platelet-derived growth factor stimulates heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:2666–2672. doi: 10.1161/01.atv.19.11.2666. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Liu XM, Peyton KJ, Wang H, Johnson FK, Johnson RA, Durante W. Hypochlorous acid-induced heme oxygenase-1 gene expression promotes human endothelial cell survival. Am J Physiol Cell Physiol. 2009;297:C907–C915. doi: 10.1152/ajpcell.00536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishikawa K, Sugawara D, Goto J, Watanabe K, Kawamura S, Shiomi M, Itabe H, Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 35.Bouche D, Chauveau BD, Roussel JC, Mathieu P, Bradeau C, Tesson L, Soulillou JP, Iyer S, Buelow R, Anegon I. Inhibition of graft arteriosclerosis development in rat aortas following heme oxygenase-1 gene transfer. Transpl Immunol. 2002;9:235–238. doi: 10.1016/s0966-3274(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 36.Du D, Chang S, Chen B, Zhou H, Chen ZK. Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transpl Proc. 2007;39:3446–3448. doi: 10.1016/j.transproceed.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 37.Tsui TY, Wu X, Lau CK, Ho DW, Xu T, Siu YT, Fan ST. Prevention of chronic deterioration of heart allograft by recombinant adeno-associated virus-mediated heme oxygenase-1 gene transfer. Circulation. 2003;107:2623–2629. doi: 10.1161/01.CIR.0000066911.03770.8D. [DOI] [PubMed] [Google Scholar]
- 38.Orozco LD, Kapturczak MH, Barajas B, Wang X, Weinstein MM, Wong J, Deshane J, Bolisetty S, Shaposhnik Z, Shih D, Agarwal A, Lusis AJ, Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100:1703–1711. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 39.Lee TS, Shiao MS, Pan CC, Chau LY. Iron-deficient diet reduces atherosclerotic lesions in apoE-deficient mice. Circulation. 1999;99:1222–1229. doi: 10.1161/01.cir.99.9.1222. [DOI] [PubMed] [Google Scholar]
- 40.Ferris CD, Jaffrey SR, Sawa A, Takahashi M, Brady SD, Barrow RK, Tysoe SA, Wolosker H, Baranano DE, Dore S, Poss KD, Snyder SH. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 41.Nakayama M, Takahashi K, Komaru T, Fukuchi M, Shioiri H, Sato K, Kitamuro T, Shirato K, Yamaguchi T, Suematsu M, Shibahara S. Increased expression of heme oxygenase-1 and bilirubin accumulation in foam cells of rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2001;21:1373–1377. doi: 10.1161/hq0801.093592. [DOI] [PubMed] [Google Scholar]
- 42.Piantadosi CA, Carraway MS, Suliman HB. Carbon monoxide, oxidative stress, and mitochondrial permeability pore transition. Free Radic Biol Med. 2006;15:1332–1339. doi: 10.1016/j.freeradbiomed.2005.11.020. 40. [DOI] [PubMed] [Google Scholar]
- 43.Taille C, El-Benna J, Lanone S, Boczkowski Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 44.Turkseven AM, Mingone CJ, Gupte SA, Wolin MS, Abraham NG. Heme oxygenase-1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell Mol Biol. 2005;51:371–376. [PubMed] [Google Scholar]
- 45.Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, Itabe H, Kodama T, Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- 46.Di Pascoli M, Rodella L, Sacerdoti D, Bolognesi M, Turkseven S, Abraham NG. Chronic CO levels have a beneficial effect on vascular relaxation seen in diabetes. Biochem Biophys Res Commun. 2006;340:935–943. doi: 10.1016/j.bbrc.2005.12.082. [DOI] [PubMed] [Google Scholar]
- 47.Tricot O, Mallat Z, Heymes C, Belmin J, Leseche G, Tedgui A. Relation between endothelial cell apoptosis and blood direction in human atherosclerotic plaques. Circulation. 2000;101:2450–2453. doi: 10.1161/01.cir.101.21.2450. [DOI] [PubMed] [Google Scholar]
- 48.Morse D, Lin L, Choi AM, Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hancock WW, Buelow R, Sayegh MH, Turka LA. Antibody-induced transplant arteriosclerosis is prevented by graft expression of anti-oxidant and anti-apoptotic genes. Nat Med. 1998;4:1392–1396. doi: 10.1038/3982. [DOI] [PubMed] [Google Scholar]
- 50.Brouard S, Otterbein LE, Anrather J, Tobiasch E, Bach FH, Choi AM, Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1029. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovascular Res. 2002;55:396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 52.Liu XM, Chapman GB, Peyton KJ, Schafer AI, Durante W. Antiapoptotic action of carbon monoxide in cultured vascular smooth muscle cells. Exp Biol Med (Maywood) 2003;228:572–575. doi: 10.1177/15353702-0322805-30. [DOI] [PubMed] [Google Scholar]
- 53.Clarke MC, Figg N, Maguire JJ, Davenport AP, Goddard M, Littlewood TD. Apoptosis of vascular smooth muscle cells induces features of plaque vulnerability in atherosclerosis. Nat Med. 2006;12:1075–1080. doi: 10.1038/nm1459. [DOI] [PubMed] [Google Scholar]
- 54.Soares MP, Seldon MP, Gregoire IP, Vassilevskaia T, Berberat PO, Yu J, Tsui T-Y, Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 55.Otterbein LE, Bach FH, Alam J, Soares MP, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 56.Sowle P, Foresti R, Mann BE, Johnson TR, Green CJ, Motterlini R. Carbon monoxide releasing molecules (CO-RMs) attenuate the inflammatory response elicited by lipopolysaccharide in RAW264.7 murine macrophages. Br J Pharmacol. 2005;145:800–810. doi: 10.1038/sj.bjp.0706241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song R, Ning W, Liu F, Ameredes BT, Calhoun WJ, Otterbein LE, Choi AMK. Regulation of IL-1β-induced GM-CSF production in human airway smooth muscle cells by carbon monoxide. Am J Physiol Lung Cell Mol Physiol. 2002;27:L50–L56. doi: 10.1152/ajplung.00212.2002. [DOI] [PubMed] [Google Scholar]
- 58.Sarady-Andrews JK, Liu F, Gallo D, Nakao A, Overhaus M, Ollinger R, Choi AM, Otterbein LE. Biliverdin administration protects against endotoxin-induced acute lung injury in rats. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1131–L1137. doi: 10.1152/ajplung.00458.2004. [DOI] [PubMed] [Google Scholar]
- 59.Vachharajan TJ, Work J, Issekutz AC, Granger DN. Heme oxygenase modulates selectin expression in different vascular beds. Am J Physiol Heart Circ Physiol. 2000;278:H1613–H1617. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- 60.Aizawa T, Ishizaka N, Taguchi J, Kimura S, Kurokawa K, Ohno M. Balloon injury does not induce heme oxygenase-1 gene expression, but administration of hemin inhibits neointimal formation in balloon injured rat carotid arteries. Biochem Biophys Res Commun. 1999;261:302–307. doi: 10.1006/bbrc.1999.1020. [DOI] [PubMed] [Google Scholar]
- 61.Togane Y, Toshisuki M, Suematsu M, Ishimura Y, Yamazaki J, Katayama S. Protective roles of endogenous carbon monoxide in neointimal development elicited by arterial injury. Am J Physiol Heart Circ Physiol. 2000;278:H623–H632. doi: 10.1152/ajpheart.2000.278.2.H623. [DOI] [PubMed] [Google Scholar]
- 62.Tulis DA, Durante W, Peyton KJ, Evans AJ, Schafer AI. Heme oxygenase-1 attenuates vascular remodeling following balloon injury in rat carotid arteries. Atherosclerosis. 2001;155:113–122. doi: 10.1016/s0021-9150(00)00552-9. [DOI] [PubMed] [Google Scholar]
- 63.Duckers HJ, Boehm M, True AL, Yet S-F, Park JL, Webb RC, Lee M-E, Nabel GJ, Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 64.Tulis DA, Durante W, Liu X, Evan AJ, Peyton KJ, Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 65.Juncos JP, Tracz MJ, Croatt AJ, Grande JP, Ackerman AW, Katusic ZS, Nath KA. Genetic deficiency of heme oxygenase-1 impairs functionality and form of arteriovenous fistula in the mouse. Kidney Int. 2008;74:47–51. doi: 10.1038/ki.2008.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Exner M, Schillinger M, Minar E, Mlekusch W, Schlerka G, Haumer M, Mannhalter C, Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther. 2001;8:433–440. doi: 10.1177/152660280100800501. [DOI] [PubMed] [Google Scholar]
- 67.Schillinger M, Exner M, Minar E, Mlekusch W, Mullner M, Mannhalter C, Bach FH, Wagner O. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: a novel vascular protective factor. J Am Coll Cardiol. 2004;43:950–957. doi: 10.1016/j.jacc.2003.09.058. [DOI] [PubMed] [Google Scholar]
- 68.Lin CC, Yang WC, Lin SJ, Chen TW, Lee WS, Chang CF, Lee PC, Lee SD, Su TS, Fann CS, Chung MY. Length polymorphism in heme oxygenase-1 is associated with arteriovenous fistula patency in hemodialysis patients. Kidney Int. 2006;69:165–172. doi: 10.1038/sj.ki.5000019. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y-H, Chau L-Y, Lin M-W, Chen L-C, Yo M-H, Chen J-W, Lin S-J. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur Heart J. 2004;25:39–47. doi: 10.1016/j.ehj.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 70.Tiroch K, Koch W, von Beckerath N, Kastrati A, Schomig A. Heme oxygenase-1 gene promoter polymorphism and restenosis following coronary stenting. Eur. Heart J. 2007;28:968–973. doi: 10.1093/eurheartj/ehm036. [DOI] [PubMed] [Google Scholar]
- 71.Peyton KJ, Reyna SV, Chapman GB, Ensenat D, Liu X, Wang H, Schafer AI, Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;51:441–446. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 72.Otterbein LE, Zuckerbraun BS, Haga M, Liu F, Song R, Usheva A, Stachulak C, Bodyak N, Neil Smith R, Czismadia E, Tyagi S, Akamatsu Y, Flavell RJ, Billiar TR, Tzeng E, Bach FH, Choi AMK, Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 73.Ollinger R, Bilban M, Erat A, Froio A, McDaid J, Tyagi S, Csizmadia E, Graca-Souza AV, Liloia A, Soares MP, Otterbein LE, Usheva A, Yamashita K, Bach FH. Bilirubin: a natural inhibitor of vascular smooth muscle cell proliferation. Circulation. 2005;112:1030–1039. doi: 10.1161/CIRCULATIONAHA.104.528802. [DOI] [PubMed] [Google Scholar]
- 74.Nakao A, Murase N, Ho C, Toyokawa H, Billiar TR, Kanno S. Biliverdin administration prevents the formation of intimal hyperplasia induced by vascular injury. Circulation. 2005;112:587–591. doi: 10.1161/CIRCULATIONAHA.104.509778. [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez AI, Gangopadhyay A, Kelley EE, Pagano PJ, Zuckerbraun BS, Bauer PM. HO-1 and CO decrease platelet-derived growth factor-induced vascular smooth muscle cell migration via inhibition of Nox1. Arterioscler Thromb Vasc Biol. 2010;30:98–104. doi: 10.1161/ATVBAHA.109.197822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Deramaudt BM, Braunstein S, Remy P, Abraham NG. Gene transfer of human heme oxygenase-1 into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68:121–127. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 77.Deshane J, Chen S, Caballero S, Grochot-Przeczek A, Was H, Li Calzi S, Lach R, Hock TD, Chen B, Hill-Kapturczak N, Siegal GP, Dulak J, Jozkowicz A, Grant MB, Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bussolati B, Ahmed A, Pemberton H, Landis RC, Di Carlo F, Haskard DO, Mason JC. Bifunctional role of VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocyte infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 79.Wu BJ, Kathir K, Witting PK, Beck K, Choy K, Li C, Croft KD, Mori TA, Tanous D, Adams MR, Lau AK, Stocker R. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin H-H, Chen Y-H, Yet S-F, Chau L-Y. After vascular injury, heme oxygenase-1/carbon monoxide enhances re-endothelialization via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost. 2009;7:1401–1408. doi: 10.1111/j.1538-7836.2009.03478.x. [DOI] [PubMed] [Google Scholar]
- 81.Wu BJ, Midwinter RG, Cossano C, Beck K, Wang Y, Changsiri D, Gamble JR, Stocker R. Heme oxygenase-1 increases endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2009;29:1537–1542. doi: 10.1161/ATVBAHA.109.184713. [DOI] [PubMed] [Google Scholar]
- 82.Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Katusic ZS, Nath KA. Induction of heme oxygenase-1 is a beneficial response in a murine model of venous thrombosis. Am J Pathol. 2008;173:1882–1890. doi: 10.2353/ajpath.2008.080556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen B, Guo L, Fan C, Bolisetty S, Joseph R, Wright MW, Agarwal A, George JF. Carbon monoxide rescues heme oxygenase-1-deficient mice from arterial thrombosis in allogeneic aortic transplantation. Am J Pathol. 2009;175:422–429. doi: 10.2353/ajpath.2009.081033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peng L, Mundada L, Stomel JM, Liu JJ, Sun J, Yet SF, Fay WP. Induction of heme oxygenase-1 expression inhibits platelet-dependent thrombosis. Antioxid Redox Signal. 2004;6:729–735. doi: 10.1089/1523086041361677. [DOI] [PubMed] [Google Scholar]
- 85.Lindenblatt N, Bordel R, Schareck W, Menger MD, Vollmar B. Vascular heme oxygenase-1 induction suppresses microvascular thrombus formation in vivo. Arterioscler Thromb Vasc Biol. 2004;24:1–6. doi: 10.1161/01.ATV.0000118279.74056.8a. [DOI] [PubMed] [Google Scholar]
- 86.Wagner CT, Durante W, Christodoulides N, Hellums JD, Schafer AI. Hemodynamic forces induce the expression of heme oxygenase in cultured vascular smooth muscle cells. J Clin Invest. 1997;100:589–596. doi: 10.1172/JCI119569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujita T, Toda K, Karimova A, Yan S-F, Naka Y, Yet S-F, Pinsky DJ. Paradoxical rescue from ischemic lung injury by inhaled carbon monoxide driven by derepression of fibrinolysis. Nat Med. 2001;7:598–604. doi: 10.1038/87929. [DOI] [PubMed] [Google Scholar]
- 88.Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol Heart Circ Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 89.Hangaishi M, Ishizaka N, Aizawa T, Kurihara Y, Taguchi J, Nagai R, Kimura S, Ohno M. Induction of heme oxygenase-1 can act protectively against cardiac ischemia/reperfusion in vivo. Biochem Biophys Res Commun. 2000;279:582–588. doi: 10.1006/bbrc.2000.3973. [DOI] [PubMed] [Google Scholar]
- 90.Masini E, Vannacci A, Marzocca C, Pierpaoli S, Giannini L, Fantappie O, Mazzanti R, Mannaioni PF. Heme oxygenase-1 and the ischemia-reperfusion injury in the rat heart. Exp Biol Med. 2003;228:546–549. doi: 10.1177/15353702-0322805-25. [DOI] [PubMed] [Google Scholar]
- 91.Yoshida T, Maulik N, Ho Y-S, Alam J, Das DK. Hmox-1 constitutes an adaptive response to effect antioxidant cardioprotection: a study with transgenic mice heterozygous for targeted disruption of the heme oxygenase-1 gene. Circulation. 2001;103:1695–1701. doi: 10.1161/01.cir.103.12.1695. [DOI] [PubMed] [Google Scholar]
- 92.Yet S-F, Tiang R, Layne MD, Wang ZY, Maemura K, Solovyeva M, Ith B, Melo LG, Zhang L, Ingwall JS, Dzau VJ, Lee M-U, Perrella MA. Cardiac-specific expression of heme oxygenase-1 protects against ischemia and reperfusion injury in transgenic mice. Circ Res. 2001;89:168–173. doi: 10.1161/hh1401.093314. [DOI] [PubMed] [Google Scholar]
- 93.Vulapalli RS, Chen Z, Chua BHL, Wang T, Liang C-S. Cardioselective overexpression of HO-1 prevents cardiac dysfunction and apoptosis. Am J Physiol Heart Circ Physiol. 2002;283:H688–H694. doi: 10.1152/ajpheart.00133.2002. [DOI] [PubMed] [Google Scholar]
- 94.Yet S-F, Perrella MA, Layne MD, Hsieh C-M, Maemura K, Kobzik L, Wiesel P, Christou H, Kourembanas S, Lee M-E. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Melo LG, Agrawal R, Zhang L, Rezvani M, Mangi AA, Ehsan A, Griese DP, Dell’Acqua G, Mann MJ, Oyama J, Yet S-F, Layne MD, Perrella MA, Dzau VJ. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 96.Clark JE, Foresti R, Sarathchandra P, Kaur H, Green CJ, Motterlini R. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am J Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 97.Clark JE, Naughton P, Shurey S, Green CJ, Johnson TR, Mann BE, Foresti R, Motterlini R. Cardioprotective actions by a water-soluble carbon monoxide-releasing molecule. Circ Res. 2003;93:e2–e8. doi: 10.1161/01.RES.0000084381.86567.08. [DOI] [PubMed] [Google Scholar]
- 98.Guo Y, Stein AB, Wu WJ, Tan W, Zhu X, Li QH, Dawn B, Motterlini R, Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286 doi: 10.1152/ajpheart.00971.2003. H1649-H1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stein AB, Guo Y, Tan W, Wu WJ, Zhu X, Li Q, Luo C, Dawn B, Johnson TR, Motterlini R, Bolli R. Administration of CO-releasing molecule induces late preconditioning against myocardial infarction. J Mol Cell Cardiol. 2005;38:127–134. doi: 10.1016/j.yjmcc.2004.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, Yoshida K, Nagai R. Carbon monoxide protects against cardiac ischemia-reperfusion injury in vivo via MAPK and Akt-eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1–7. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- 101.Lavitrano M, Smolesnki RT, Musumeci A, Maccherini M, Slominska E, Di Florio E, Bracco A, Mancini A, Stassi G, Patti M, Giovannoni R, Froio A, Simeone F, Forni M, Bacci ML, D’Alise G, Cozzi E, Otterbein LE, Yacoub MH, Bach FH, Calise F. Carbon monoxide improves cardiac energetics and safeguards the heart during reperfusion after cardiopulmonary bypass in pigs. FASEB J. 2004;18:1093–1095. doi: 10.1096/fj.03-0996fje. [DOI] [PubMed] [Google Scholar]
- 102.Akamatsu Y, Haga M, Tyagi S, Yamashita K, Graca-Souza AV, Ollinger R, Czismadia E, May GA, Ifedigbo E, Otterbein LE, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide protects hearts from transplant associated ischemic reperfusion injury. FASEB J. 2004;18:771–772. doi: 10.1096/fj.03-0921fje. [DOI] [PubMed] [Google Scholar]
- 103.Nakao A, Choi AM, Murase N. Protective effect of carbon monoxide in transplantation. J Cell Mol Med. 2006;10:650–671. doi: 10.1111/j.1582-4934.2006.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johnson RA, Lavesa M, Askari B, Abraham NG, Nasjletti A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension. 1996;25:166–169. doi: 10.1161/01.hyp.25.2.166. [DOI] [PubMed] [Google Scholar]
- 105.Sacerdoti D, Escalante B, Abraham NG, McGiff JC, Levere RD, Schwartzman ML. Treatment with tin prevents the development of hypertension in spontaneously hypertensive rats. Science. 1989;243:388–390. doi: 10.1126/science.2492116. [DOI] [PubMed] [Google Scholar]
- 106.Levere RD, Martasek P, Escalante B, Schwartzman ML, Abraham NG. Effect of heme arginate administration on blood pressure in spontaneously hypertensive rats. J Clin Invest. 1990;17:776–779. doi: 10.1172/JCI114686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sabaawy HE, Zhang F, Nguyen X, ElHosseiny A, Nasjletti A, Schwartzman M, Dennery P, Kappas A, Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 108.Wang R, Shamloul R, Wang X, Meng Q, Wu L. Sustained normalization of high blood pressure in spontaneously hypertensive rats by implanted hemin pump. Hypertension. 2006;48:685–692. doi: 10.1161/01.HYP.0000239673.80332.2f. [DOI] [PubMed] [Google Scholar]
- 109.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide, and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 110.Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- 111.Morita T, Kourembanas S. Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide. J Clin Invest. 1995;96:2676–2682. doi: 10.1172/JCI118334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Thorup C, Jones CL, Gross SS, Moore LC, Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol Heart Circ Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 113.Johnson RA, Colombari E, Columbari DS, Lavesa M, Talman WT. Role of endogenous carbon monoxide in central regulation of arterial pressure. Hypertension. 1997;30:962–967. doi: 10.1161/01.hyp.30.4.962. [DOI] [PubMed] [Google Scholar]
- 114.Rodriguez F, Kemp R, Balazy M, Nasjletti A. Effects of exogenous heme on renal function: role of heme oxygenase and cyclooxygenase. Hypertension. 2003;42:680–684. doi: 10.1161/01.HYP.0000085785.40581.1A. [DOI] [PubMed] [Google Scholar]
- 115.Vera T, Kelsen S, Yanes LL, Reckelhoff JF, Stec DE. HO-1 induction lowers blood pressure and superoxide production in the renal medulla of angiotensin II hypertensive mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1472–R1478. doi: 10.1152/ajpregu.00601.2006. [DOI] [PubMed] [Google Scholar]
- 116.Yang L, Quan S, Nasjletti A, Laniado-Schwartzman M, Abraham NG. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension. 2004;43:1–6. doi: 10.1161/01.HYP.0000126287.62060.e6. [DOI] [PubMed] [Google Scholar]
- 117.Vera T, Kelsen S, Stec DE. Kidney-specific induction of heme oxygenase-1 prevents angiotensin II hypertension. Hypertension. 2008;52:660–665. doi: 10.1161/HYPERTENSIONAHA.108.114884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wiesel P, Patel AP, Carvajal IM, Wang ZY, Pellacani A, Maemura K, DiFonzo N, Rennke HG, Layne MD, Yet S-F, Lee M-E, Perrella MA. Exacerbation of chronic renovascular hypertension and acute renal failure in heme oxygenase-1-deficient mice. Circ Res. 2001;88:1088–1094. doi: 10.1161/hh1001.091521. [DOI] [PubMed] [Google Scholar]
- 119.Nath KA, d’Uscio LV, Juncos JP, Croatt AJ, Manriquez MC, Pittock ST, Katusic ZS. An analysis of the DOCA-salt model of hypertension in HO-1−/− mice and the Gunn rat. Am J Physiol Heart Circ Physiol. 2007;293:H333–H342. doi: 10.1152/ajpheart.00870.2006. [DOI] [PubMed] [Google Scholar]
- 120.Christou H, Morita T, Hsieh C-M, Koike H, Arkonac B, Perrella MA, Kourembanas S. Prevention of hypoxia-induced pulmonary hypertension by enhancement of endogenous heme oxygenase-1 in the rat. Circ Res. 2000;86:1224–1229. doi: 10.1161/01.res.86.12.1224. [DOI] [PubMed] [Google Scholar]
- 121.Minamino T, Christou H, Hsieh CM, Liu Y, Dhawan V, Abraham NG, Perrella MA, Mitsialis SA, Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zuckerbraun BS, Chin BY, Wegiel B, Billiar TR, Czsimadia E, Rao J, Shimoda L, Ifedigbo E, Kanno S, Otterbein LE. Carbon monoxide reverses established pulmonary hypertension. J Exp Med. 2006;203:2109–2119. doi: 10.1084/jem.20052267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shimzu K, Takahashi T, Iwasaki T, Shimizu H, Inoue K, Morimatsu H, Omori E, Matsumi M, Akagi R, Morita K. Hemin treatment abrogates monocrotaline-induced pulmonary hypertension. Med Chem. 2008;4:572–576. doi: 10.2174/157340608786241972. [DOI] [PubMed] [Google Scholar]
- 124.Tsui TY, Lau CK, Ma J, Glockzin G, Obed A, Schlitt HJ, Fan ST. Adeno-associated virus-mediated heme oxygenase-1 gene transfer suppresses the progression of micronodular cirrhosis in rats. World J Gastroenterol. 2006;12:2016–2023. doi: 10.3748/wjg.v12.i13.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Johnson FK, Durante W, Peyton KJ, Johnson RA. Heme oxygenase inhibitor restores arteriolar nitric oxide function in DAHL rats. Hypertension. 2003;41:149–155. doi: 10.1161/01.hyp.0000046923.52222.58. [DOI] [PubMed] [Google Scholar]
- 126.Teran FJ, Johnson RA, Stevenson BK, Peyton KJ, Jackson KE, Appleton SD, Durante W, Johnson FK. Heme oxygenase-derived carbon monoxide promotes arteriolar endothelial dysfunction and contributes to salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp. Physiol. 2005;288:R615–R622. doi: 10.1152/ajpregu.00123.2004. [DOI] [PubMed] [Google Scholar]
- 127.Johnson FK, Johnson RA, Durante W, Jackson KE, Stevenson BK, Peyton KJ. Metabolic syndrome increases endogenous carbon monoxide production to promote hypertension and endothelial dysfunction. Am J Physiol Regul Integr Comp Physiol. 2006;290:R601–R608. doi: 10.1152/ajpregu.00308.2005. [DOI] [PubMed] [Google Scholar]
- 128.Imai T, Morita T, Shindo T, Nagai R, Yazaki Y, Kurihara H, Suematsu M, Katayama S. Vascular smooth muscle cell-directed overexpression of heme oxygenase-1 elevates blood pressure through attenuation of nitric oxide-induced vasodilation in mice. Cir Res. 2001;89:55–62. doi: 10.1161/hh1301.092679. [DOI] [PubMed] [Google Scholar]
- 129.Johnson FK, Johnson RA. Carbon monoxide promotes endothelium-dependent constriction of isolated gracilis muscle arterioles. Am J Physiol Heart Circ Physiol. 2003;285:R536–R541. doi: 10.1152/ajpregu.00624.2002. [DOI] [PubMed] [Google Scholar]
- 130.Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuka E, Suematsu M. Carbon monoxide from heme oxygenase-2 is a tonic regulator against NO-dependent vasodilation in the adult rat cerebral microcirculation. Circ Res. 2005;97:e104–e114. doi: 10.1161/01.RES.0000196681.34485.ec. [DOI] [PubMed] [Google Scholar]
- 131.Morimoto Y, Durante W, Lancaster DG, Klattenhoff J, Tittel FK. Real-time measurements of endogenous CO production from vascular cells using an ultrasensitive laser sensor. Am J Physiol Heart Circ Physiol. 2001;280:H483–H488. doi: 10.1152/ajpheart.2001.280.1.H483. [DOI] [PubMed] [Google Scholar]
- 132.Rank JM, Straka JG, Weimer MK, Boissenmaier, Taddeini BL, Bloomer JR. Hematin therapy in late onset congenital erythropoietic porphyria. Br J Haematol. 1990;75:617–618. doi: 10.1111/j.1365-2141.1990.tb07809.x. [DOI] [PubMed] [Google Scholar]
- 133.Ruutu T, Volin L, Tenhunen R. Haeme arginate as a treatment for myelodysplastic syndromes. Br J Haematol. 1987;65:425–428. doi: 10.1111/j.1365-2141.1987.tb04144.x. [DOI] [PubMed] [Google Scholar]
- 134.Dellon ES, Szczepiorkowski ZM, Dzik WH, Graeme-Cook F, Ades A, Bloomer JR, Cosimi AB, Chung RT. Treatment of recurrent allograft dysfunction with intravenous hematin after liver transplantation for erythropoietic protoporphyria. Transplantation. 2002;73:911–915. doi: 10.1097/00007890-200203270-00014. [DOI] [PubMed] [Google Scholar]
- 135.Rund D, Rachmilewitz E. New trends in the treatment of beta-thalasemmia. Crit Rev Oncol Hematol. 2000;33:105–118. doi: 10.1016/s1040-8428(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 136.Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, Gibbons SJ, Zinsmeister AR, Farrugia G. First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther. 2009:221. doi: 10.1038/clpt.2009.221. doi 10:1038/clpt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wagener FA, Eggert A, Boerman OC, Oyen WJ, Verhofstad A, Abraham NG, Adema G, van Kooyk Y, de Witte T, Figdor CG. Heme is a potent inducer of inflammation in mice and is counteracted by heme oxygenase. Blood. 2001;98:1802–1811. doi: 10.1182/blood.v98.6.1802. [DOI] [PubMed] [Google Scholar]
- 138.Ogborne RM, Rushworth SA, Charalambos CA, O’Connell MA. Haem oxygenase-1: a target for dietary antioxidants. Biochem Soc Trans. 2004;32:1003–1005. doi: 10.1042/BST0321003. [DOI] [PubMed] [Google Scholar]
- 139.Scapagnini G, Foresti R, Calabrese V, Giuffrida Stella AM, Green CJ, Motterlini R. Caffeic acid phenethyl ester and curcumin: a novel class of heme oxygenase-1 inducers. Mol Pharmacol. 2002;61:554–561. doi: 10.1124/mol.61.3.554. [DOI] [PubMed] [Google Scholar]
- 140.Wu CC, Hsu MC, Hsieh CW, Lin JB, Lai PH, Wung BS. Upregulation of heme oxygenase-1 by epigallocatechin-3-gallate via the phosphatidylinositol 3-kinase/Akt and ERK pathways. Life Sci. 2006;78:2889–2897. doi: 10.1016/j.lfs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 141.Penumathsa SV, Maulik N. Resveratrol: a promising agent in promoting cardioprotection against coronary heart disease. Can J Physiol Pharmacol. 2009;87:275–286. doi: 10.1139/Y09-013. [DOI] [PubMed] [Google Scholar]
- 142.Cheng PY, Lee YM, Shih NL, Chen YC, Yen MH. Heme oxygenase-1 contributes to the cytoprotection of alpha-lipoic acid via the activation of p44/42 mitogen-activated protein kinase in vascular smooth muscle cells. Free Radic Biol Med. 2006;40:1313–1322. doi: 10.1016/j.freeradbiomed.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 143.Erdmann K, Grosser N, Schroder H. L-methionine reduces oxidant stress in endothelial cells: role of heme oxygenase-1, ferritin, and nitric oxide. AAPS J. 2005;7:E195–E200. doi: 10.1208/aapsj070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Grosser N, Oberle S, Berndt G, Erdmann K, Hemmerle A, Schroder H. Antioxidant action of L-alanine: heme oxygenase-1 and ferritin as possible mediators. Biochem Biophys Res Commun. 2004;314:351–355. doi: 10.1016/j.bbrc.2003.12.089. [DOI] [PubMed] [Google Scholar]
- 145.Uehara K, Takahashi T, Fujii H, Shimizu H, Omori E, Matsumi M, Yokoyama M, Morita K, Akagi T, Sassa S. The lower intestinal tract-specific induction of heme oxygenase-1 by glutamine protects against endotoxemic intestinal injury. Crit Care Med. 2005;33:381–390. doi: 10.1097/01.ccm.0000153407.14237.7f. [DOI] [PubMed] [Google Scholar]
- 146.Grosser N, Hemmerle A, Berndt G, Erdmann K, Hinkelmann U, Schurger S, Wijayanti N, Immenschuh S, Schroder H. The antioxidant defense protein heme oxygenase-1 is a novel target for statins in endothelial cells. Free Radic Biol Med. 2004;37:2061–2071. doi: 10.1016/j.freeradbiomed.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 147.Lee TS, Chang CC, Zhu Y, Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 148.Hsu M, Muchova L, Moriaka I, Wong JR, Schroder H, Stevenson DK. Tissue-specific effects of statins on the expression of heme oxygenase-1 in vivo. Biochem Biophys Res Commun. 2006;343:738–744. doi: 10.1016/j.bbrc.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 149.Loboda A, Jazwa A, Jozkowicz, Dorosz J, Balla J, Molema G, Dulak J. Atorvastatin prevents hypoxia-induced inhibition of endothelial nitric oxide synthase expression but does not affect heme oxygenase-1 in human microvascular endothelial cells. Atherosclerosis. 2006;187:26–30. doi: 10.1016/j.atherosclerosis.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Grosser N, Abate A, Oberle S, Vreman HJ, Dennery PA, Becker JC, Pohle T, Seidman DS, Schroder H. Heme oxygenase-1 induction may explain the antioxidant profile of aspirin. Biochem Biophys Res Commun. 2003;308:956–960. doi: 10.1016/s0006-291x(03)01504-3. [DOI] [PubMed] [Google Scholar]
- 151.Oberle S, Oberle S, Abate A, Grosser N, Hemmerle A, Vreman HJ, Dennery PA, Schneider HT, Stalleicken D, Schröder H. Endothelial protection by pentaerithrityl trinitrate: bilirubin and carbon monoxide as possible mediators. Exp Biol Med (Maywood) 2003;228:529–534. doi: 10.1177/15353702-0322805-21. [DOI] [PubMed] [Google Scholar]
- 152.Wenzel P, Oelze M, Coldewey M, Hortmann M, Seeling A, Hink U, Mollnau H, Stalleicken D, Weiner H, Lehmann J, Li H, Fostermann U, Munzel T, Daiber A. Heme oxygenase-1: a novel key player in the development of tolerance in response to organic nitrates. Arterioscler Thromb Vasc Biol. 2007;27:1729–1735. doi: 10.1161/ATVBAHA.107.143909. [DOI] [PubMed] [Google Scholar]
- 153.Vidavalur R, Penumathsa SV, Zhan L, Thirunavukkarasu M, Maulik N. Sildenafil induces angiogenic response in human coronary arteriolar endothelial cells through the expression of thioredoxin, hemeoxygenase and vascular endothelial growth factor. Vascu Pharmacol. 2006;45:91–95. doi: 10.1016/j.vph.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 154.Abdel Aziz MT, El-Asmer MF, Mostafa T, Mostafa S, Atta H, Aziz Wassef MA, Fouad H, Rashed L, Sabry D, Mahfouz S. Heme oxygenase vs nitric oxide synthase in signaling mediating sildenafil citrate action. J Sex Med. 2006;4:1098–1107. doi: 10.1111/j.1743-6109.2007.00533.x. [DOI] [PubMed] [Google Scholar]
- 155.Visner GA, Lu F, Zhou H, Liu J, Kazemfar K, Agarwal A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: implications in the antiproliferative response to rapamycin. Circulation. 2003;107:911–916. doi: 10.1161/01.cir.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- 156.Zhou H, Liu H, Porvasnik SL, Terada N, Agarwal A, Cheng Y, Visner GA. Heme oxygenase-1 mediates the protective effects of rapamycin in monocrotaline-induced pulmonary hypertension. Lab Invest. 2006;86:62–71. doi: 10.1038/labinvest.3700361. [DOI] [PubMed] [Google Scholar]
- 157.Choi BM, Kim YM, Jeong YR, Pae HO, Song CE, Park JE, Ahn YK, Chung HT. Induction of heme oxygenase-1 is involved in the antiproliferative effects of paclitaxel in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2004;25:39–47. doi: 10.1016/j.bbrc.2004.06.120. [DOI] [PubMed] [Google Scholar]
- 158.Schmidt R. Cobalt protoporphyrin as a potential therapeutic agent? FASEB J. 2007;21:2639. doi: 10.1096/fj.07-0904ltr. [DOI] [PubMed] [Google Scholar]
- 159.Liu XM, Chapman GB, Wang H, Durante W. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- 160.Suttner DM, Dennery PA. Reversal of HO-1 related cytoprotection with increased expression due to reactive iron. FASEB J. 1999;13:1800–1809. doi: 10.1096/fasebj.13.13.1800. [DOI] [PubMed] [Google Scholar]
- 161.Abraham NG, Asija A, Drummond G, Peterson S. Heme oxygenase-1 gene therapy: recent advances and therapeutic applications. Curr Gene Ther. 2007;7:89–108. doi: 10.2174/156652307780363134. [DOI] [PubMed] [Google Scholar]
- 162.Tang YL, Qian K, Zhang YC, Shen L, Phillips MI. A vigilant, hypoxia-regulatable heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J. Cardiovasc Pharmacol Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 163.Moore BA, Overhaus M, Whitcomb J, Ifedigbo E, Choi AM, Otterbein LE, Bauer AJ. Brief inhalation of low-dose carbon monoxide prevents graft-induced intimal hyperplasia in swine. J Surg. Res. 2007;138:121–127. doi: 10.1016/j.jss.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 164.Belcher JD, Mahaseth H, Welch TE, Otterbein LE, Hebbel RP, Vercelotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle cell mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ott MC, Scott JR, Bihari A, Badhwar A, Otterbein LE, Gray DK, Harris KA, Potter RF. Inhalation of carbon monoxide prevents liver injury and inflammation following hindlimb ischemia/reperfusion. FASEB J. 2005;19:106–108. doi: 10.1096/fj.04-2514fje. [DOI] [PubMed] [Google Scholar]
- 166.Mayr FB, Spiel A, Leitner J, Marsik C, Germann P, Ullrich R, Wagner O, Jilma B. Effect of carbon monoxide inhalation during experimental endotoxemia in humans. Am J Respir Crit Care Med. 2005;171:354–360. doi: 10.1164/rccm.200404-446OC. [DOI] [PubMed] [Google Scholar]
- 167.Bathoorn E, Slebos D-J, Postma DS, Koeter GH, van Oosterhout AJM, van der Toorn M, Boezen HM, Kerstjens HAM. Anti-inflammatory effects of inhaled carbon monoxide in patients with COPD: a pilot study. Eur Respir J. 2007;30:1131–1137. doi: 10.1183/09031936.00163206. [DOI] [PubMed] [Google Scholar]
- 168.Tulis DA, Keswani AN, Peyton KJ, Wang H, Schafer AI, Durante W. Local administration of carbon monoxide inhibits neointima formation in balloon injured rat carotid arteries. Cell Mol Biol. 2005;51:441–446. [PMC free article] [PubMed] [Google Scholar]
- 169.Nakao A, Schmidt J, Harada T, Tsung A, Stoffels B, Cruz RJ, Jr, Kohmoto J, Peng X, Tomiyama K, Murase N, Bauer AJ, Fink MP. A single intraperitoneal dose of carbon monoxide-saturated Ringer’s lactate solution ameliorates post-operative ileus in mice. J Pharmacol Exp Ther. 2006;319:1265–1275. doi: 10.1124/jpet.106.108654. [DOI] [PubMed] [Google Scholar]
- 170.Chauveau C, Bouchet D, Roussel JC, Mathieu P, Bradeau C, Renaudin K, Tesson L, Soulillou JP, Iyer S, Buelow R, Anegon I. Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am J Transplant. 2002;2:581–592. doi: 10.1034/j.1600-6143.2002.20702.x. [DOI] [PubMed] [Google Scholar]
- 171.Rioux JP, Myers RA. Methylene chloride poisoning: a paradigmatic review. J Emerg Med. 1988;6:227–238. doi: 10.1016/0736-4679(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 172.Foresti R, Shurey C, Ansari T, Sibbons P, Mann BE, Johnson TR, Green CJ, Motterlini R. Reviewing the use of carbon monoxide-releasing molecules (CO-RMs) in biology: implications in endotoxin-mediated vascular dysfunction. Cell Mol Biol (Noisy-le-grand) 2005;51:409–423. [PubMed] [Google Scholar]
- 173.Friebe A, Schultz G, Koesling D. Sensitizing soluble guanylate cyclase to become a highly CO-sensitive enzyme. EMBO J. 1996;15:6863–6868. [PMC free article] [PubMed] [Google Scholar]
- 174.McLaughlin BE, Chretien ML, Choi C, Brien JF, Nakatsu K, Marks GS. Potentiation of carbon monoxide-induced relaxation of rat aorta by YC-1 [3-(5’-hydroxymethyl-2’-furyl)-1-benzylindazole] Can J Physiol Pharmacol. 2000;78:343–349. [PubMed] [Google Scholar]
- 175.Liu XM, Peyton KJ, Mendelev NN, Wang H, Tulis DA, Durante W. YC-1 stimulates the expression of gaseous monoxides in vascular smooth muscle cells. Mol Pharmacol. 2009;75:208–217. doi: 10.1124/mol.108.048314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Matsumoto N, Nakashima T, Kashima K. Effectiveness of bovine gallstone (Goou) and bear gall powder (Yutan) on chronic liver diseases: a preliminary report. Tokai J Exp Ther. 1995;20:9–16. [PubMed] [Google Scholar]
- 177.Kapitulnik J. Bilirubin: an endogenous product of heme degradation with both cytotoxic and cytoprotective properties. Mol Pharmacol. 2004;66:773–779. doi: 10.1124/mol.104.002832. [DOI] [PubMed] [Google Scholar]
- 178.Nakao A, Neto JS, Kanno S, Stolz DB, Kimizuka K, Liu F, Bach FH, Billiar TR, Choi AM, Otterbein LE, Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 179.Schwertner HA, Vitek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects and therapeutic applications of bilirubin. Atherosclerosis. 2008;198:1–11. doi: 10.1016/j.atherosclerosis.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 180.Cary SP, Marletta MA. The case of CO signaling: why the jury is still out. J Clin Invest. 2001;107:1071–1073. doi: 10.1172/JCI12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Grundemar L, Ny L. Pitfalls using metalloprophyrins in carbon monoxide research. Trends Pharmacol Sci. 1997;18:193–195. doi: 10.1016/s0165-6147(97)01065-1. [DOI] [PubMed] [Google Scholar]
- 182.Kinobe RT, Dercho RA, Nakatsu K. Inhibitors of the heme oxygenase-carbon monoxide system: on the doorstep of the clinic? Can J Physiol Pharmacol. 2008;86:577–599. doi: 10.1139/y08-066. [DOI] [PubMed] [Google Scholar]
- 183.Quan S, Yang L, Abraham NG, Kappas A. Regulation of human heme oxygenase in endothelial cells by using sense and antisense retroviral constructs. Proc Natl Acad Sci USA. 2001;98:12203–12208. doi: 10.1073/pnas.211399398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lin CC, Liu XM, Peyton KJ, Wang H, Yang WC, Lin SJ, Durante W. Far infrared therapy inhibits vascular endothelial inflammation via the induction of heme oxygenase-1. Arterioscler Thromb Vasc Biol. 28:739–745. doi: 10.1161/ATVBAHA.107.160085. 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang X, Shan P, Jiang D, Noble PW, Abraham NG, Kappas A, Lee PJ. Small interference RNA targeting heme oxygenase-1 enhances ischemia-reperfusion-induced lung apoptosis. J Biol Chem. 2003;279:10677–10684. doi: 10.1074/jbc.M312941200. [DOI] [PubMed] [Google Scholar]