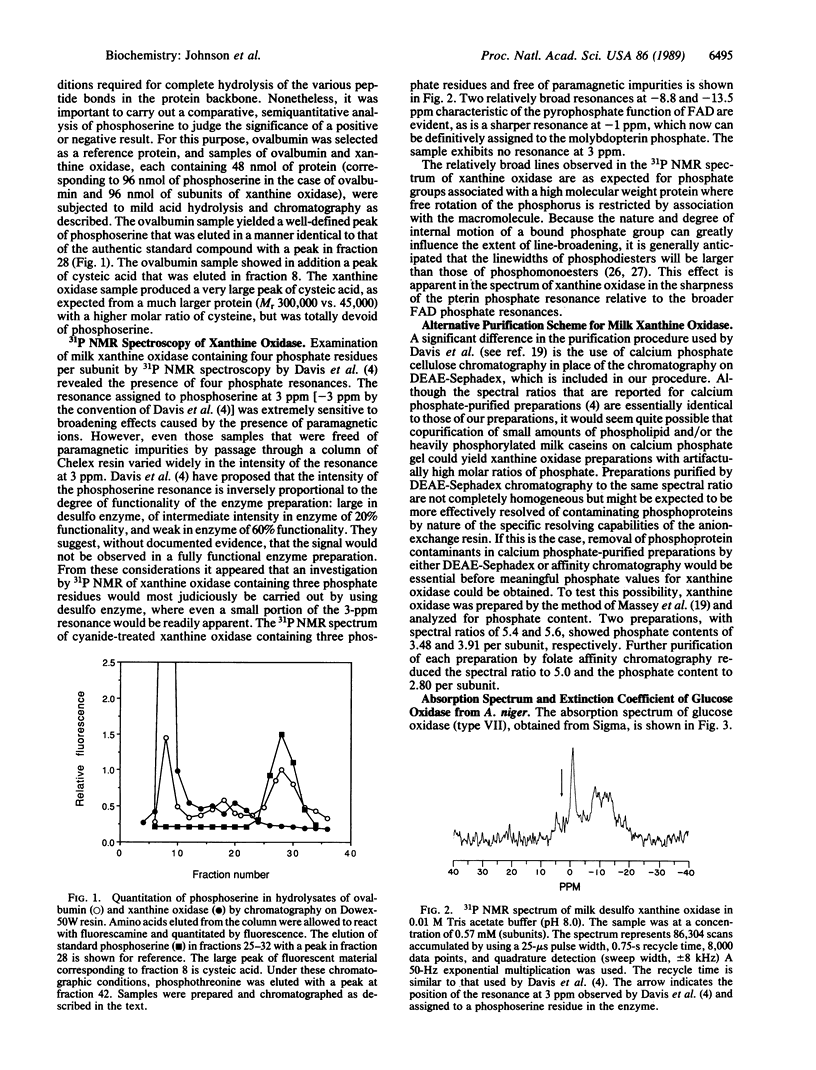
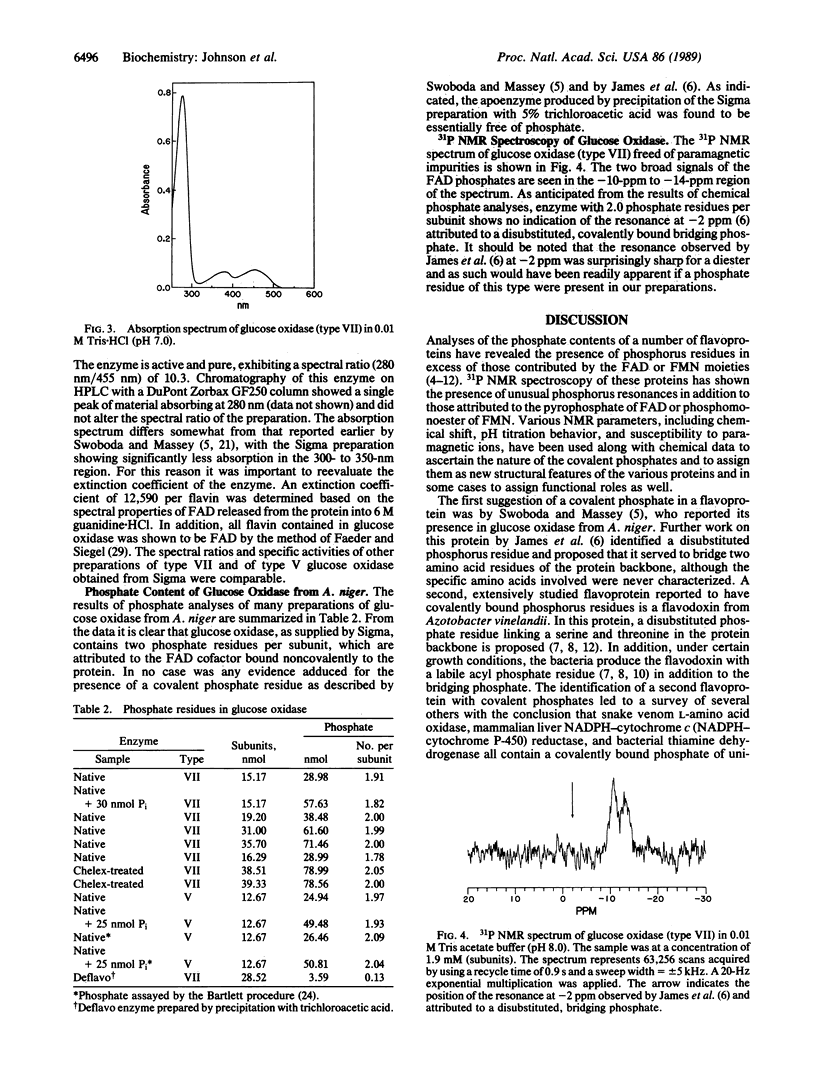
Abstract
The reported presence of covalently bound phosphate residues in flavoproteins has significant implications with regard to the catalytic mechanisms and structural stability of the specific enzymes themselves and in terms of general cellular metabolic regulation. These considerations have led to a reevaluation of the presence of covalently bound phosphorus in the flavoproteins xanthine oxidase (xanthine: oxygen oxidoreductase, EC 1.1.3.22) and glucose oxidase (beta-D-glucose: oxygen 1-oxidoreductase, EC 1.1.3.4). Milk xanthine oxidase purified by a procedure that includes anion-exchange chromatography is shown to contain three phosphate residues. All three are noncovalently associated with the protein, two with the FAD cofactor, and one with the molybdenum cofactor. Results of chemical analysis and 31P NMR spectroscopy indicate that enzyme purified by this method contains no phosphoserine residues. Xanthine oxidase preparations purified by chromatography on calcium phosphate gel in place of DEAE-Sephadex yielded higher phosphate-to-protein ratios, which could be reduced to the expected values by additional purification on a folate affinity column. Highly active, highly purified preparations of glucose oxidase are shown to contain only the two phosphate residues of the FAD cofactor. The covalently bound bridging phosphate reported by others may arise in aged or degraded preparations of the enzyme but appears not to be a constituent of functional glucose oxidase. These results suggest that the presence of covalent phosphate residues in other flavoproteins should be rigorously reevaluated as well.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Davis M. D., Edmondson D. E., Müller F. 31P nuclear magnetic resonance and chemical studies of the phosphorus residues in bovine milk xanthine oxidase. Eur J Biochem. 1984 Dec 3;145(2):237–243. doi: 10.1111/j.1432-1033.1984.tb08544.x. [DOI] [PubMed] [Google Scholar]
- Edmondson D. E., James T. L. Covalently bound non-coenzyme phosphorus residues in flavoproteins: 31P nuclear magnetic resonance studies of Azotobacter flavodoxin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3786–3789. doi: 10.1073/pnas.76.8.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D., Massey V., Palmer G., Beacham L. M., 3rd, Elion G. B. The resolution of active and inactive xanthine oxidase by affinity chromatography. J Biol Chem. 1972 Mar 10;247(5):1597–1604. [PubMed] [Google Scholar]
- Faeder E. J., Siegel L. M. A rapid micromethod for determination of FMN and FAD in mixtures. Anal Biochem. 1973 May;53(1):332–336. doi: 10.1016/0003-2697(73)90442-9. [DOI] [PubMed] [Google Scholar]
- Holt J. C., Creeth J. M. Studies of the denaturation and partial renaturation of ovalbumin. Biochem J. 1972 Sep;129(3):665–676. doi: 10.1042/bj1290665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James T. L., Edmondson D. E., Husain M. Glucose oxidase contains a disubstituted phosphorus residue. Phosphorus-31 nuclear magnetic resonance studies of the flavin and nonflavin phosphate residues. Biochemistry. 1981 Feb 3;20(3):617–621. doi: 10.1021/bi00506a026. [DOI] [PubMed] [Google Scholar]
- Johnson J. L., Hainline B. E., Rajagopalan K. V., Arison B. H. The pterin component of the molybdenum cofactor. Structural characterization of two fluorescent derivatives. J Biol Chem. 1984 May 10;259(9):5414–5422. [PubMed] [Google Scholar]
- Klugkist J., Voorberg J., Haaker H., Veeger C. Characterization of three different flavodoxins from Azotobacter vinelandii. Eur J Biochem. 1986 Feb 17;155(1):33–40. doi: 10.1111/j.1432-1033.1986.tb09455.x. [DOI] [PubMed] [Google Scholar]
- Kramer S. P., Johnson J. L., Ribeiro A. A., Millington D. S., Rajagopalan K. V. The structure of the molybdenum cofactor. Characterization of di-(carboxamidomethyl)molybdopterin from sulfite oxidase and xanthine oxidase. J Biol Chem. 1987 Dec 5;262(34):16357–16363. [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- Meyer O., Rajagopalan K. V. Molybdopterin in carbon monoxide oxidase from carboxydotrophic bacteria. J Bacteriol. 1984 Feb;157(2):643–648. doi: 10.1128/jb.157.2.643-648.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T., Nishino T., Tsushima K. Purification of highly active milk xanthine oxidase by affinity chromatography on Sepharose 4B/folate gel. FEBS Lett. 1981 Aug 31;131(2):369–372. doi: 10.1016/0014-5793(81)80406-1. [DOI] [PubMed] [Google Scholar]
- Otvos J. D., Krum D. P., Masters B. S. Localization of the free radical on the flavin mononucleotide of the air-stable semiquinone state of NADPH-cytochrome P-450 reductase using 31P NMR spectroscopy. Biochemistry. 1986 Nov 4;25(22):7220–7228. doi: 10.1021/bi00370a068. [DOI] [PubMed] [Google Scholar]
- SWOBODA B. E., MASSEY V. PURIFICATION AND PROPERTIES OF THE GLUCOSE OXIDASE FROM ASPERGILLUS NIGER. J Biol Chem. 1965 May;240:2209–2215. [PubMed] [Google Scholar]
- Vogel H. J., Bridger W. A., Sykes B. D. Frequency-dependent phosphorus-31 nuclear magnetic resonance studies of the phosphohistidine residue to succinyl-CoA synthetase and the phosphoserine residue of glycogen phosphorylase a. Biochemistry. 1982 Mar 16;21(6):1126–1132. doi: 10.1021/bi00535a003. [DOI] [PubMed] [Google Scholar]
- WHITBY L. G. A new method for preparing flavin-adenine dinucleotide. Biochem J. 1953 Jun;54(3):437–442. doi: 10.1042/bj0540437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl R. C., Rajagopalan K. V. Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J Biol Chem. 1982 Feb 10;257(3):1354–1359. [PubMed] [Google Scholar]
- Waud W. R., Brady F. O., Wiley R. D., Rajagopalan K. V. A new purification procedure for bovine milk xanthine oxidase: effect of proteolysis on the subunit structure. Arch Biochem Biophys. 1975 Aug;169(2):695–701. doi: 10.1016/0003-9861(75)90214-3. [DOI] [PubMed] [Google Scholar]


