Abstract
Prion diseases are transmissible fatal neurodegenerative disorders affecting humans and animals. A central step in disease progression is the accumulation of a misfolded form (PrPSc) of the host encoded prion protein (PrPC) in neuronal and non-neuronal tissues. The involvement of peripheral tissues in preclinical states increases the risk of accidental transmission. On the other hand, detection of PrPSc in non-neuronal easy-accessible compartments such as muscle may offer a novel diagnostic tool. Primate models have proven invaluable to investigate prion diseases. We have studied the deposition of PrPSc in muscle and central nervous system of rhesus monkeys challenged with sporadic Creutzfeldt-Jakob disease (sCJD), variant CJD (vCJD) and bovine spongiform encephalopathy (BSE) in preclinical and clinical stage using biochemical and morphological methods. Here, we show the preclinical presence of PrPSc in muscle and central nervous system of rhesus monkeys experimentally infected with vCJD.
Introduction
Prion diseases are transmissible neurodegenerative disorders characterized by neuronal loss, astrocytosis and deposition of the pathogenic isoform (PrPSc) of the cellular prion protein (PrPC). Conversion of physiological PrPC into pathogenic PrPSc plays a major role in disease pathophysiology and PrPSc is a principal component of prion infectivity [1], [2], [3]. PrPSc differs from PrPC in its increased ß-sheet content, which renders it relatively resistant to proteolytic digestion [4], [5].
Prion diseases affect humans and animals alike and both human-to-human and animal-to-human transmission may occur [6]. Human prion diseases include sporadic, genetic and acquired forms [7], [8]. Different prion-isolates show specific clinical and biochemical traits which are referred to as prion strains [9]. Sporadic Creutzfeldt-Jakob disease (sCJD) has unknown aetiology and may result from spontaneous conversion of PrPC to PrPSc [10]. Genetic Creutzfeldt-Jakob disease (gCJD) co-segregates with mutations in the gene encoding the prion protein and is inherited autosomal dominantly [11], whereas acquired forms are caused by exposure to infectious human prions during medical or neurosurgical procedures (iatrogenic Creutzfeldt-Jakob disease, iCJD) or a non-human prion source such as bovine spongiform encephalopathy (BSE)-prions (variant Creutzfeldt-Jakob disease, vCJD) [12]. Interhuman transmission of vCJD through blood transfusions has occurred in several instances [6], [13]. In contrast, no such transmissions have been reported with other human prion diseases such as sCJD. This demonstrates that peripheral tissues of vCJD infected individuals harbouring relatively low prion titer may lead to prion transmission following host-adaption.
Non-human primate studies using macaques as model organism have been valuable in elucidating pathophysiology of prion diseases [14], [15], [16], [17]. Primate studies routinely assess animals in clinical disease states, thus distribution of PrPSc during subclinical disease has not been investigated in primates [16]. Detailed knowledge on the distribution of PrPSc in model organisms closely resembling the human situation is important because it allows evidence based decisions aimed at limiting the spread of vCJD through iatrogenic procedures [18], [19], [20]. Furthermore, knowledge on the temporal kinetics of PrPSc accumulation in tissue compartments, which are easily accessible such as muscle may help in devising novel approaches for diagnostic tests, aimed at detecting prion diseases at subclinical stage.
Muscle biopsy has been proposed as a novel tool for diagnosing human prion diseases [21]. PrPSc is detectable in muscle of a wide range of human prion diseases and data from rodent studies show presence of muscular PrPSc in preclinical stages [22], [23], [24], [25].
We therefore systematically investigated the deposition of PrPSc in muscle and central nervous system in rhesus monkeys challenged with sCJD, vCJD and BSE-prions in preclinical and clinically diseased animals using ultrasensitive western blotting, ELISA and PET-blot. Our results show subclinical deposition of PrPSc in muscle of vCJD infected primates. These results should be taken into consideration when devising strategies aimed at limiting iatrogenic transmission of vCJD. The variability of detection of PrPSc in muscle of clinical and subclinical primates suggests inhomogeneous distribution and challenges the idea of employing muscle biopsy as a routine diagnostic tool in prion diseases.
Results
Clinical prion disease following intraperitoneal application of vCJD and BSE
To investigate if intraperitoneal prion inoculation leads to the development of clinical prion disease, we infected rhesus monkeys with vCJD, sCJD and BSE-prions or saline intraperitoneally. Animals were monitored until signs of prion disease were present and the experiment was terminated 335 weeks post inoculation (wpi). Incubation times until clinical signs of prion disease were 172 wpi for the vCJD infected animal and 212 wpi for the BSE infected primate, whereas the sCJD infected primate did not show any signs of prion disease until the experiment was terminated (table 1).
Table 1. Primates included in the study.
| Group | wpi | Clinical disease | Muscular PrPSc | CNS PrPSc | |||||||
| Tongue | Arm | Heart | |||||||||
| ELISA | WB | ELISA | WB | ELISA | WB | ELISA | WB | PET | |||
| vCJD | 132 | no | − | − | − | − | − | − | + | + | + |
| 144 | no | + | + | − | − | + | + | + | + | + | |
| 158 | no | + | + | + | + | − | − | + | + | + | |
| 172 | yes | + | + | − | + | + | − | + | + | + | |
| sCJD | 243 | no | − | − | − | − | − | − | − | − | − |
| 335 | no | n.d. | − | n.d. | − | n.d. | − | n.d. | − | − | |
| BSE | 107 | no | − | − | − | − | − | − | − | − | − |
| 212 | yes | + | + | − | − | − | − | n.d. | + | + | |
| Mock | 106 | no | n.d. | − | n.d. | − | n.d. | − | n.d. | − | − |
| 222 | no | − | − | − | − | − | − | n.d. | − | − | |
Exponential increase of PrPSc in brains of vCJD infected primates
To investigate pattern and amounts of PrPSc deposition in preclinical primates, we performed a time course study in rhesus monkeys infected with vCJD, sCJD and BSE or saline intraperitoneally. For vCJD, animals were sacrificed at three preclinical time points (132 wpi (n = 1), 144 wpi (n = 1), 158 wpi (n = 1)), and one clinical time point (172 wpi (n = 1)). For sCJD one monkey was taken at 243 wpi. The other sCJD challenged monkey was sacrificed at 335 wpi and both failed to develop clinical prion disease within the observation period. For BSE, one animal was taken preclinically (107 wpi) and one animal clinically (212 wpi) (table 1). In order to assess PrPSc deposition in the central nervous system (CNS) we performed standard and ultrasensitive NaPTA-enhanced Western blot analysis of cerebellum and frontal cortex.
PrPSc could be detected in all preclinical and clinical vCJD infected macaques. In contrast, PrPSc could only be detected in the clinical BSE diseased animal, whereas no PrPSc deposition was detectable in a preclinical BSE monkey as well as in both preclinical sCJD infected animals (figure 1A, B).
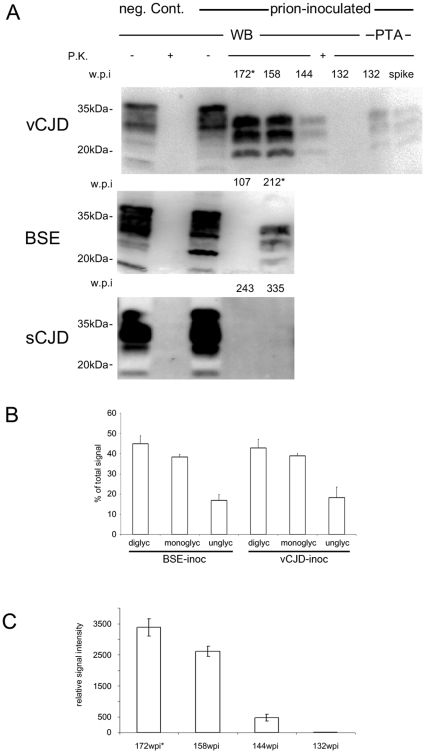
Figure 1. Biochemical analysis of PrPSc in the CNS.
A) Western Blot analysis for PrPSc (frontal cortex) of vCJD, BSE and sCJD infected animals. PrPSc could be demonstrated in the brains of preclinical vCJD and prion-diseased vCJD and BSE inoculated animals. In the vCJD cohort, PrPSc is detectable (using NaPTA) in subclinical state 40 weeks before onset of symptoms. sCJD inoculated macaques did not show PrPSc at any time point in cerebellum (data not shown) and frontal cortex in conventional as well as NaPTA enhanced Western blot. (* indicates prion-diseased animals). B) PrPSc-glycotype analysis demonstrates comparable glycotypes of vCJD and BSE when transmitted to primates. Densitometric measurement of relative band intensities for di-, mono- and unglycosylated form of PrPSc is shown in % of total signal. C) Quantification of PrPSc-signal shows initial exponential increase of PrPSc until 158 wpi when PrPSc levels off. Relative amounts of PrPSc are shown in arbitrary units as quantified in three independent experiments.
The setup of the study allowed us to assess temporal development of PrPSc accumulation in the CNS of vCJD-infected monkeys. PrPSc could be detected as early as 132 wpi in vCJD inoculated primates and the amount of PrPSc increased until clinical disease (figure 1A). Densitometric analysis of western blots showed exponential increase of PrPSc until 158 wpi, whereas PrPSc amounts only increased moderately from 158 to 172 wpi (figure 1C). Strain properties of BSE and vCJD as reflected by the glycoprofile and running behaviour of the unglycosylated core fragment of PrPSc remained unchanged (figure 1A, B). vCJD and BSE-inoculated primates showed comparable sizes of unglycosylated PrPSc core fragments and compatible glycosylation profiles indicating congruent PrPSc-types (figure 1A, B).
Western blot findings could be confirmed by PET-blot of cerebellum showing abundant deposits of PrPSc in monkeys challenged with vCJD and BSE-prions at terminal prion disease and faint PrPSc deposits in a subclinical, vCJD challenged animal (figure 2). No PrPSc could be detected in cerebellum in either of the sCJD-infected monkeys as well as the preclinical BSE-challenged primate.
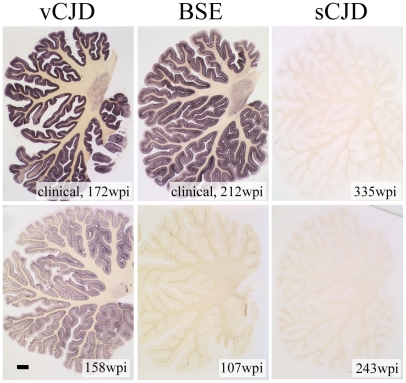
Figure 2. PET blots of cerebellum.
PET blots show prominent deposition of PrPSc in granular and molecular cell layers in clinically diseased vCJD and BSE inoculated animals whereas the subclinical vCJD inoculated primate only shows faint PrPSc in granular and molecular cell layers (scale bar in lower left image 1 mm).
Preclinical detection of PrPSc in muscle of vCJD inoculated primates
To assess whether and to what extent PrPSc can be found in muscle we determined PrPSc contents using NaPTA-enhanced Western blot analysis and PrPSc specific ELISA (summarized in table 1). At 132 wpi, the earliest time point assessed for vCJD, no PrPSc could be detected in either western blot or ELISA of arm, tongue and heart (figure 3, western blot data for heart not shown). In contrast, at 144 wpi, PrPSc appeared in heart and tongue muscle but not in arm muscle with ELISA and western blot (figure 3, western blot data for heart not shown). At 158 wpi, PrPSc was demonstrated in arm and tongue by ELISA and western blot (figure 3A, B), whereas PrPSc was below detection limit by either method in heart (figure 3A, B). In the clinical diseased animal from the vCJD cohort, peripheral PrPSc deposits were abundant in tongue tissue when measured by western blot and ELISA, yet PrPSc in heart could only be detected by ELISA and PrPSc in arm tissue only by western blot. In the BSE cohort, no PrPSc could be found preclinically in arm, tongue or heart muscle using either method. The clinically affected BSE challenged primate showed unambiguous deposition of PrPSc in tongue tissue only (figure 3A, B). No PrPSc was observable in arm or heart. Both sCJD infected primates did not show any PrPSc in the investigated muscle tissue when assessed by western blot or ELISA (figure 3A, B, table 1).
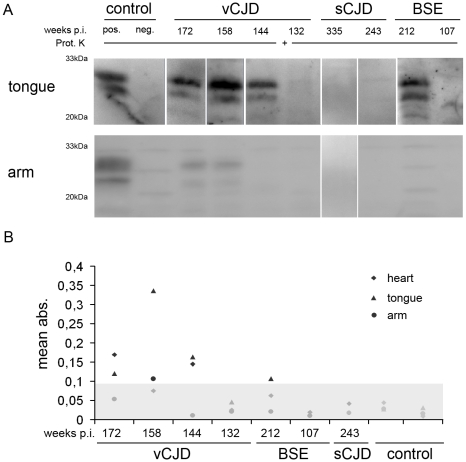
Figure 3. Preclinical detection of PrPSc in muscle of vCJD inoculated primates.
A) Western Blot analysis of PrPSc from NaPTA precipitated arm and tongue tissue of sCJD, vCJD and BSE infected primates and controls show PrPSc in arm and tongue of subclinical vCJD challenged monkeys and in clinically affected vCJD and BSE inoculated animals. sCJD infected macaques did not show any detectable PrPSc in arm or tongue. B) Detection of PrPSc in arm, tongue and heart tissue from sCJD, vCJD and BSE infected primates and controls by ELISA. The scatter graph shows values (mean of two independent experiments) for arm (circle), tongue (triangle) and heart (diamond). The cut-off is indicated by grey shade and values below were considered as negative. PrPSc could be detected in tongue, arm and heart of healthy and clinically diseased vCJD inoculated animals. In BSE infected animals PrPSc could only be detected in tongue tissue and in sCJD infected animals no PrPSc could be detected.
PrPSc deposits are associated to nerve fibers as well as to myocytes in tongue tissue
To investigate the distribution of PrPSc in tongue tissue of vCJD infected primates we used PET blotting. Of the four investigated animals only one showed detectable PrPSc. Tongue tissue from this clinically diseased animal (172 wpi) was shown to contain PrPSc by western blotting and ELISA (figure 3A, B). PrPSc labelling occurred in linear fashion in addition to dot like deposits. By directly correlating PET blot data with information from consecutive sections processed for histology we were able to identify an association of PrPSc with nerve fibers (linear deposits, figure 4B, C) and myocytes (dot like deposits, figure 4B, C).
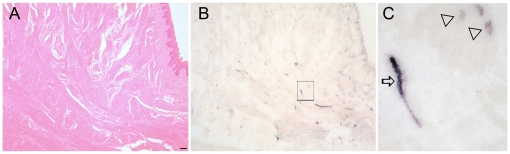
Figure 4. Morphological analysis of tongue reveals PrPSc deposition in nerve fibers and myocytes.
A) Histological analysis of tongue from the clinical, vCJD infected macaque shows muscle fibers intermingled with peripheral nerves (Haematoxilin and Eosin staining, scale bar 50 µm). B, C) PET blot (consecutive section from A) showing labelling of PrPSc. Higher magnification (C) of the insert shown in (B) revealed PrPSc staining of longitudinal nerve fibers (arrow) and adjacent myocytes (triangles).
Discussion
The occurrence of human-to-human transmission of vCJD prions trough medical interventions has lead to concerns regarding the further development of the vCJD epidemic [26], [27]. Although a wealth of studies have provided us with detailed information on the temporal and spatial kinetics of prion diseases in rodents, there is an ongoing controversy on the preclinical distribution of prion infectivity and PrPSc in human prion diseases [28], [29]. Furthermore, the detailed analysis of PrPSc distribution in preclinical prion disease in an animal model closely resembling the human situation is a prerequisite for the validation of novel diagnostic tools such as muscle biopsy [16].
Non-human primate models have been used to determine transmission efficiency of sCJD, iCJD, vCJD or BSE [14], [15], [16]. Infectious brain homogenates have been applied intracerebrally, intravenously, orally or intratonsillary. The transmissibility of human prion diseases to non-human primates could be proven in any case [14], [15]. With our study we show that the intraperitoneal route, which is widely used in rodent models of prion diseases, is also effective in transmitting prion diseases in primates. Incubation time to clinical prion disease after intraperitoneal inoculation of BSE was comparable with oral infection [16], [30] with 48 months versus 47, 51 and 63 months respectively. For vCJD, no data are available for oral prion application. Incubation times were 25 to 37 month after intratonsillar and/or intracerebral inoculation [30]. In our study, one animal developed clinical disease after 40 months. Three other animals were taken preclinically 30, 32 and 34 months after prion challenge and PrPSc deposition could be readily shown in brain tissue of all four monkeys. PrPSc could not be detected in any of the investigated tissues using various methods in two primates intraperitoneally inoculated with sCJD, 56 and 77 month post inoculation. In contrast, Herzog [30] could show efficient transmission of sCJD with clinical prion disease 56 month post infection. Besides disparity in the administration of prions, these differences may be attributed to variations in prion titers of the inocula.
The shortening of incubation time in our experiment between BSE and vCJD inoculated macaques could be due to vCJD-strain adaptation or differences of titers of infectious prions present in inocula. Since prion titers were not determined in samples used in this study, we cannot rule out that these differences are due to prion titer variations. Nonetheless, other studies attribute shorting of incubation times between BSE and vCJD to strain adaptation effects using either intravenous or intracerebral routes of inoculation [14].
However, none of the former studies addressed the question of preclinical PrPSc distribution and the temporal development of PrPSc deposition. In our study we could show a drastic increase of PrPSc in the CNS of vCJD inoculated macaques over time. Interestingly, PrPSc accumulation seems to saturate the end of the incubation time. This fact is also known from rodent models of prion infection [31], but had never been demonstrated in primates before.
The peripheral distribution of PrPSc has been well characterized in rodent models of prion diseases [32], [33]. Deposition of PrPSc in muscle tissue had been shown in terminally sick hamsters experimentally infected with scrapie [25], mice infected with mouse adapted BSE and vCJD [24] as well as in sheep with naturally occurring scrapie [34], [35]. Preclinical PrPSc deposition in muscle tissue could be demonstrated in experimentally infected hamsters [36]. No preclinical PrPSc could be detected in heart muscle [36]. PrPSc had also been detected in muscle of patients suffering from sCJD, vCJD and iCJD [20], [23]. Moreover, the tissue distribution of PrPSc had been studied intensively in clinically diseased vCJD patients where PrPSc could be detected in limb, but not in tongue muscle [37], [38]
Here we show the deposition of PrPSc in muscle tissue of subclinical vCJD challenged primates. In agreement with other studies, PrPSc deposits within the musculoskeletal system showed patterns that are consistent with nerve fibers, yet we could also observe PrPSc in structures resembling myocytes [23]. The fact that highly innervated tongue muscle harbours more PrPSc than arm muscle points to prominent involvement of nerve fibers in the tongue not only in prion neuroinvasion [39] but also in PrPSc accumulation. Quantification of PrPSc in muscle using Western blotting or ELISA is variable. This may be due to inhomogeneous distribution of PrPSc in the muscular compartment as visualized by in situ detection methods for PrPSc such as PET blotting.
In summary, we could show that (i) PrPSc builds up exponentially in the CNS of vCJD infected primates, (ii) PrPSc is detectable in both CNS and the muscular compartment preclinically in vCJD challenged rhesus monkeys, (iii) PrPSc distribution in muscle tissue of clinical and subclinically infected primates is inhomogeneous.
These data should be taken into consideration when devising appropriate measures against iatrogenic transmission of prion diseases or when employing muscle biopsy in diagnosing human prion disease [15], [21], [23], [26]
Materials and Methods
Animals
All procedures involving animals were performed in accordance with European and German legal and ethical regulations (which are in line with the recommended practices of the use of non-human primates in research) and approved by the responsible boards and authorities. Captive-bred rhesus macaques (Macacca mulatta) were checked for absence of common primate pathogens and were inoculated intraperitoneally with 10 ml of a 10% homogenate in PBS of either BSE (mixture of cerebral cortex specimen from five clinically BSE affected cattle) sCJD (prion protein gene codon 129 MM, PrPSc type 2 according to [40]) and vCJD (prion protein gene codon 129 MM, PrPSc type 4 according to [40]; kindly provided by John Collinge, London, both cerebral cortex). Control animals received saline only. Animals were sacrificed at defined time points post inoculation and a subgroup of animals were allowed to progress to clinical prion disease (see table 1). These animals were sacrificed 4 weeks after onset of first clinical symptoms of prion disease (slowness, weight loss, trembling). The study was terminated 335 weeks post inoculation.
Tissues were either fixed in 4% formalin and prepared for histological examination or snap-frozen in liquid nitrogen and stored at −80°C for biochemical analyses.
Western Blot analysis
For Western blot analysis, brain samples (Cerebellum, frontal cortex) were homogenised (FastPrep FP120, Qbiogene, Cedex, France) at 10% (weight/volume, w/v) in buffer (150 mM NaCl, 1 % NP-40, 0.5 % DOC, 0.1 % SDS, 50 mM Tris-HCl pH 8.0) and 0.1 mg wet brain was digested with proteinase K (PK) (20 µg/ml) for 45 min at 37°C. Digestion was stopped by adding 10× sample buffer and boiling for 10 min. Samples were loaded on a 12% SDS-PAGE gel transferred to PVDF membranes, blocked for 1 hour at room temperature in protein-free blocking buffer (Thermo Scientific, Rockford, USA) and incubated overnight at 4°C with monoclonal anti-PrP antibody 6H4 raised against bovine recombinant PrP, recognizing amino acids 144–152 of human PrP (1∶1000 in blocking buffer, Prionics, Schlieren, Switzerland). After incubation for 1 hour at room temperature with an HRP-conjugated anti-mouse secondary antibody (1∶5000 in blocking buffer), signal was detected using ECL femto reagent (Thermo Scientific, Rockford, USA) and visualized with a BioRad ChemiDoc imaging station and glycotpyes were evaluated using published protocols [41].
Sodium phosphotungstic acid (NaPTA) precipitation
NaPTA precipitation was done according to published protocols [21], [42]) with minor changes. Muscle tissue (100 mg) was dissociated in 900 µl buffer (25 mM HEPES (pH 7.2), 0.3 M sucrose and 53,6 µg Liberase Blendzyme 2 (Roche, Mannheim, Germany)). Samples were incubated for 30 min at 37°C and homogenised (FastPrep FP120, Qbiogene, Cedex, France). 500 µl of 10% (w/v) tissue homogenates and 500 µl of 4% (w/v) sarkosyl-PBS were vortexed and incubated for 10 min at 37°C with constant agitation. Benzonase (Novagen, Merck, Nottingham, UK (50 U/ml)) and 1 mM MgCl2 were added and incubated at 37°C for 30 min with vigorous shaking. 81.3 µl of a pre-warmed (to 37°C) 4% (w/v) NaPTA/170 mM MgCl2 solution (pH 7.4) was added, vortexed and incubated with vigorous agitation for 30 min at 37°C. Samples were centrifuged at 25,000 g for 30 min; pellets were resuspended in 22.5 µl of 0.1% sarkosyl-PBS, followed by proteinase K digestion (Roche, Mannheim, Germany) with a final concentration of 20 µg/ml for 1 hour at 37°C and processed for western blotting.
ELISA
ELISA measurements were performed using a modified version of the Bio-Rad test (Platelia®BSE, TeSeE®) that is also used for the post mortem diagnosis of BSE in cattle according to published protocols [34]. This kit uses two different monoclonal antibodies allowing a sensitive detection of denatured PrP. PrPSc was purified and concentrated from 50 mg fresh wet tissue homogenate using the TeSeE® purification kit following the manufacturer's instructions. This procedure included a PK treatment (0.4 µg/mg of tissue) and a centrifugation step to concentrate PrPSc. The corresponding pellet was denatured at 100°C in the presence of a mixture of a chaotropic agent and a detergent. After fivefold dilution in an appropriate buffer, denatured PrPSc was successively reacted with capture antibody (Sha-31) and tracer antibody (Pri-308) that were specific for the detection of primate prion protein.
Paraffin-embedded tissue (PET)-Blot
PET blots were performed according to published protocol [43]. Briefly, formalin-fixed and decontaminated tissue was embedded in paraffin, sectioned (5 µm), collected on a prewetted nitrocellulose membrane (Bio-Rad, Hercules, USA) and completely dried at 55°C. After deparaffinization and rehydration, the membrane was washed in Tween 20 (0.1%) and dried for PrP detection.
Prewetted membranes were digested with proteinase K (250 µg/ml) for 8 hours at 55°C. After washing, proteins were denatured with 4 mol/l guanidine isothiocyanate for 15 minutes, washed again and immunodetection was performed after preincubation in blocking solution. Monoclonal antibody 12F10 recognizing amino acid 142–160 of human PrP (kindly provided by J. Grassi; [44], commercially available through SPIbio, Montigny, France) was used as primary antibody for at least 1 hour. Samples were washed again, incubated with alkaline-phosphatase-coupled rabbit anti-mouse antibody (Dako, Glostrup, Denmark) and detected using NBT/BCIP. Blots were evaluated with an Olympus dissecting microscope (Olympus, Hamburg, Germany).
Morphological analyses
Brain (cerebellum) or muscle tissue (tongue) was fixed with 4% buffered formalin, inactivated by 98% formic acid for one hour and embedded in paraffin. Sections were cut (3 µm) deparaffinised and stained with haematoxylin and eosin according to standard protocols [21].
Acknowledgments
We thank J. Grassi (CEA, France) for providing high-quality antibodies and support with PrPSc-ELISA. Thanks to Petra Kiesel, Andreas Kues and Stephanie Wurr for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was financed by the European Union grant EU BMH4 CT 98 7026, the Deutsche Forschungs Gemeinschaft (DFG) grants KA 864/2-1 and FOR885. S.K. was supported by the Deutsches Primaten Zentrum Foerderpreis, M.N. was supported by DFG grant Gl 589/2-1. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
References
- 1.Cohen FE, Pan KM, Huang Z, Baldwin M, Fletterick RJ, et al. Structural clues to prion replication. Science. 1994;264:530–531. doi: 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- 2.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aguzzi A. Understanding the diversity of prions. Nat Cell Biol. 2004;6:290–292. doi: 10.1038/ncb0404-290. [DOI] [PubMed] [Google Scholar]
- 4.Horiuchi M, Caughey B. Specific binding of normal prion protein to the scrapie form via a localized domain initiates its conversion to the protease-resistant state. Embo J. 1999;18:3193–3203. doi: 10.1093/emboj/18.12.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caughey B, Raymond GJ, Ernst D, Race RE. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J Virol. 1991;65:6597–6603. doi: 10.1128/jvi.65.12.6597-6603.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peden A, McCardle L, Head MW, Love S, Ward HJ, et al. Variant CJD infection in the spleen of a neurologically asymptomatic UK adult patient with haemophilia. Haemophilia. 2010 doi: 10.1111/j.1365-2516.2009.02181.x. [DOI] [PubMed] [Google Scholar]
- 7.Ironside JW. Prion diseases in man. J Pathol. 1998;186:227–234. doi: 10.1002/(SICI)1096-9896(1998110)186:3<227::AID-PATH174>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.Belay ED. Transmissible spongiform encephalopathies in humans. Annu Rev Microbiol. 1999;53:283–314. doi: 10.1146/annurev.micro.53.1.283. [DOI] [PubMed] [Google Scholar]
- 9.Geissen M, Krasemann S, Matschke J, Glatzel M. Understanding the natural variability of prion diseases. Vaccine. 2007. [DOI] [PubMed]
- 10.Brandner S, Isenmann S, Raeber A, Fischer M, Sailer A, et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature. 1996;379:339–343. doi: 10.1038/379339a0. [DOI] [PubMed] [Google Scholar]
- 11.Prusiner SB, Scott MR. Genetics of prions. Annu Rev Genet. 1997;31:139–175. doi: 10.1146/annurev.genet.31.1.139. [DOI] [PubMed] [Google Scholar]
- 12.Collinge J, Sidle KC, Meads J, Ironside J, Hill AF. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature. 1996;383:685–690. doi: 10.1038/383685a0. [DOI] [PubMed] [Google Scholar]
- 13.Zou S, Fang CT, Schonberger LB. Transfusion transmission of human prion diseases. Transfus Med Rev. 2008;22:58–69. doi: 10.1016/j.tmrv.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Lasmezas CI, Fournier JG, Nouvel V, Boe H, Marce D, et al. Adaptation of the bovine spongiform encephalopathy agent to primates and comparison with Creutzfeldt-- Jakob disease: implications for human health. Proc Natl Acad Sci U S A. 2001;98:4142–4147. doi: 10.1073/pnas.041490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lasmezas CI, Comoy E, Hawkins S, Herzog C, Mouthon F, et al. Risk of oral infection with bovine spongiform encephalopathy agent in primates. Lancet. 2005;365:781–783. doi: 10.1016/S0140-6736(05)17985-9. [DOI] [PubMed] [Google Scholar]
- 16.Herzog C, Sales N, Etchegaray N, Charbonnier A, Freire S, et al. Tissue distribution of bovine spongiform encephalopathy agent in primates after intravenous or oral infection. Lancet. 2004;363:422–428. doi: 10.1016/S0140-6736(04)15487-1. [DOI] [PubMed] [Google Scholar]
- 17.Comoy EE, Casalone C, Lescoutra-Etchegaray N, Zanusso G, Freire S, et al. Atypical BSE (BASE) transmitted from asymptomatic aging cattle to a primate. PLoS One. 2008;3:e3017. doi: 10.1371/journal.pone.0003017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bishop MT, Hart P, Aitchison L, Baybutt HN, Plinston C, et al. Predicting susceptibility and incubation time of human-to-human transmission of vCJD. Lancet Neurol. 2006;5:393–398. doi: 10.1016/S1474-4422(06)70413-6. [DOI] [PubMed] [Google Scholar]
- 19.Dietz K, Raddatz G, Wallis J, Muller N, Zerr I, et al. Blood transfusion and spread of variant Creutzfeldt-Jakob disease. Emerg Infect Dis. 2007;13:89–96. doi: 10.3201/eid1301.060396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glatzel M, Ott PM, Linder T, Gebbers JO, Gmur A, et al. Human prion diseases: epidemiology and integrated risk assessment. Lancet Neurol. 2003;2:757–763. doi: 10.1016/s1474-4422(03)00588-x. [DOI] [PubMed] [Google Scholar]
- 21.Glatzel M, Abela E, Maissen M, Aguzzi A. Extraneural pathologic prion protein in sporadic Creutzfeldt-Jakob disease. N Engl J Med. 2003;349:1812–1820. doi: 10.1056/NEJMoa030351. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs GG, Lindeck-Pozza E, Chimelli L, Araujo AQ, Gabbai AA, et al. Creutzfeldt-Jakob disease and inclusion body myositis: abundant disease-associated prion protein in muscle. Ann Neurol. 2004;55:121–125. doi: 10.1002/ana.10813. [DOI] [PubMed] [Google Scholar]
- 23.Peden AH, Ritchie DL, Head MW, Ironside JW. Detection and localization of PrPSc in the skeletal muscle of patients with variant, iatrogenic, and sporadic forms of Creutzfeldt-Jakob disease. Am J Pathol. 2006;168:927–935. doi: 10.2353/ajpath.2006.050788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomzig A, Cardone F, Kruger D, Pocchiari M, Brown P, et al. Pathological prion protein in muscles of hamsters and mice infected with rodent-adapted BSE or vCJD. J Gen Virol. 2006;87:251–254. doi: 10.1099/vir.0.81277-0. [DOI] [PubMed] [Google Scholar]
- 25.Thomzig A, Kratzel C, Lenz G, Kruger D, Beekes M. Widespread PrP(Sc) accumulation in muscles of hamsters orally infected with scrapie. EMBO Rep. 2003;4:1–4. doi: 10.1038/sj.embor.embor827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lefrere JJ, Hewitt P. From mad cows to sensible blood transfusion: the risk of prion transmission by labile blood components in the United Kingdom and in France. Transfusion. 2009;49:797–812. doi: 10.1111/j.1537-2995.2008.02044.x. [DOI] [PubMed] [Google Scholar]
- 27.Glatzel M, Aguzzi A. Prion infections, blood and transfusions. Nature Clin Prac Neurol. 2006;2:321–329. doi: 10.1038/ncpneuro0214. [DOI] [PubMed] [Google Scholar]
- 28.Aguzzi A. Prion diseases of humans and farm animals: epidemiology, genetics, and pathogenesis. J Neurochem. 2006;97:1726–1739. doi: 10.1111/j.1471-4159.2006.03909.x. [DOI] [PubMed] [Google Scholar]
- 29.Glatzel M, Stoeck K, Seeger H, Luhrs T, Aguzzi A. Human prion diseases: molecular and clinical aspects. Arch Neurol. 2005;62:545–552. doi: 10.1001/archneur.62.4.545. [DOI] [PubMed] [Google Scholar]
- 30.Herzog C, Riviere J, Lescoutra-Etchegaray N, Charbonnier A, Leblanc V, et al. PrPTSE distribution in a primate model of variant, sporadic, and iatrogenic Creutzfeldt-Jakob disease. J Virol. 2005;79:14339–14345. doi: 10.1128/JVI.79.22.14339-14345.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bueler H, Raeber A, Sailer A, Fischer M, Aguzzi A, et al. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol Med. 1994;1:19–30. [PMC free article] [PubMed] [Google Scholar]
- 32.Aguzzi A, Heikenwalder M. Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol. 2006;4:765–775. doi: 10.1038/nrmicro1492. [DOI] [PubMed] [Google Scholar]
- 33.Glatzel M, Heppner FL, Albers KM, Aguzzi A. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron. 2001;31:25–34. doi: 10.1016/s0896-6273(01)00331-2. [DOI] [PubMed] [Google Scholar]
- 34.Andreoletti O, Simon S, Lacroux C, Morel N, Tabouret G, et al. PrPSc accumulation in myocytes from sheep incubating natural scrapie. Nat Med. 2004;10:591–593. doi: 10.1038/nm1055. [DOI] [PubMed] [Google Scholar]
- 35.Casalone C, Corona C, Crescio MI, Martucci F, Mazza M, et al. Pathological prion protein in the tongues of sheep infected with naturally occurring scrapie. J Virol. 2005;79:5847–5849. doi: 10.1128/JVI.79.9.5847-5849.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomzig A, Schulz-Schaeffer W, Kratzel C, Mai J, Beekes M. Preclinical deposition of pathological prion protein PrPSc in muscles of hamsters orally exposed to scrapie. J Clin Invest. 2004;113:1465–1472. doi: 10.1172/JCI21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Head MW, Ritchie D, McLoughlin V, Ironside JW. Investigation of PrPres in dental tissues in variant CJD. Br Dent J . 2003;195:339–343; discussion 331. doi: 10.1038/sj.bdj.4810536. [DOI] [PubMed] [Google Scholar]
- 38.Notari S, Moleres FJ, Hunter SB, Belay ED, Schonberger LB, et al. Multiorgan detection and characterization of protease-resistant prion protein in a case of variant CJD examined in the United States. PLoS ONE. 2010;5:e8765. doi: 10.1371/journal.pone.0008765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartz JC, Kincaid AE, Bessen RA. Rapid prion neuroinvasion following tongue infection. J Virol. 2003;77:583–591. doi: 10.1128/JVI.77.1.583-591.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hill AF, Joiner S, Wadsworth JD, Sidle KC, Bell JE, et al. Molecular classification of sporadic Creutzfeldt-Jakob disease. Brain. 2003;126:1333–1346. doi: 10.1093/brain/awg125. [DOI] [PubMed] [Google Scholar]
- 41.Glatzel M, Rogivue C, Ghani A, Streffer JR, Amsler L, et al. Incidence of Creutzfeldt-Jakob disease in Switzerland. Lancet. 2002;360:139–141. doi: 10.1016/S0140-6736(02)09384-4. [DOI] [PubMed] [Google Scholar]
- 42.Wadsworth JDF, Joiner S, Hill AF, Campbell TA, Desbruslais M, et al. Tissue distribution of protease resistant prion protein in variant CJD using a highly sensitive immuno-blotting assay. Lancet. 2001;358:171–180. doi: 10.1016/s0140-6736(01)05403-4. [DOI] [PubMed] [Google Scholar]
- 43.Schulz-Schaeffer WJ, Tschoke S, Kranefuss N, Drose W, Hause-Reitner D, et al. The paraffin-embedded tissue blot detects PrP(Sc) early in the incubation time in prion diseases. Am J Pathol. 2000;156:51–56. doi: 10.1016/S0002-9440(10)64705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krasemann S, Groschup MH, Harmeyer S, Hunsmann G, Bodemer W. Generation of Monoclonal Antibodies Against Human Prion Proteins in Prp0/0 Mice. Molecular Medicine. 1996;2:725–734. [PMC free article] [PubMed] [Google Scholar]