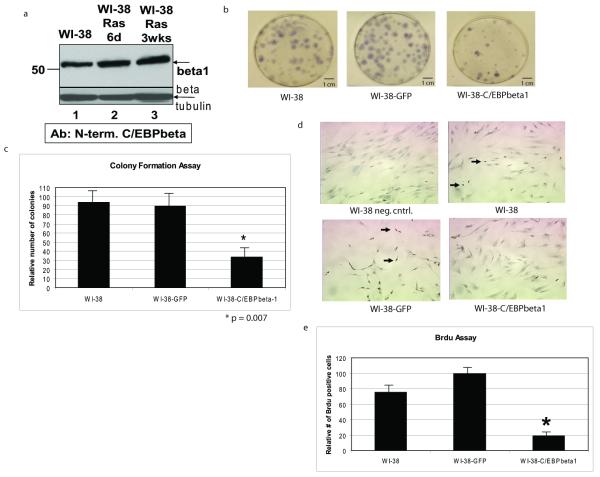
Figure 4.
C/EBPbeta1 is not degraded by introduction of activated Ras in WI-38 normal fibroblasts and exogenous expression of C/EBPbeta1 inhibits growth in WI-38 cells a. WI-38 cells were infected with pBABE-Ras(V12)-puromycin and selected with puromycin for 6 days or 3 weeks. Evidence for Ras(V12) expression in these cells includes their senescent phenotype and induction of IL6 (Figures 5 and 6 and data not shown). Lane 1 is uninfected WI-38 cells, lane 2 is WI-38-Ras(V12) cells after 6 days, and lane 3 is WI-38-Ras(V12) cells after 3 weeks. Equal amounts of total protein were loaded in each lane of a 10% SDS-PAGE. Immunoblot analysis was performed with an antibody specific to C/EBPbeta1 as described in Eaton et al. (2001). The arrow indicates endogenous p52-C/EBPbeta1. Immunoblot analysis for beta-tubulin was performed as a loading control (Sigma T7816). (beta = C/EBPbeta, d = days, wks = weeks) b. WI-38 human diploid fibroblasts were infected with LZRS-GFP or LZRS-T7-C/EBPbeta1-IRES-eGFP and sorted by FACS using GFP as a marker. 800 cells were plated and after 10 days the cells were fixed with 95% ethanol and stained with Gills hematoxylin (Sigma). The number of stained colonies were counted. T7-C/EBPbeta1 expression was verified by immunoblot (data not shown). c. Quantitative comparison of the colony formation assay. This experiment was repeated three times with the standard deviation represented by error bars. Quantitative comparison using the student t test indicates that there is a statistically significant difference in colony number in the T7-C/EBPbeta1-expressing WI-38 cells versus uninfected or GFP only. *p = 0.007. d. WI-38 human diploid fibroblasts were infected with LZRS-GFP or LZRS-T7-C/EBPbeta1-IRES-eGFP and sorted by FACS using GFP as a marker. 13000 cells were seeded per well in an 8-well chamber slide 6 days post-infection. 24 hours post-plating the cells were pulsed with 10ug/mL Brdu (Sigma) for 24 hours. Cells were then fixed in formalin for 5 minutes. The Invitrogen streptavidin-biotin system for Brdu staining kit was then followed per manufacturer’s instructions. The dark nuclear stain indicates replicating cells in which Brdu was incorporated (examples indicated by arrows). Hematoxylin was used as the counterstain. T7-C/EBPbeta1 expression was verified via immunoblot (data not shown). e. Quantitative comparison of the Brdu assay. Quantitative comparison using the student t test indicates that there is a statistically significant difference in Brdu staining in the T7-C/EBPbeta1-expressing WI-38 cells versus uninfected or GFP only. *p = 0.027. Standard deviation is represented by error bars.