Abstract
A common procedure for studying the effects on cognition of repetitive transcranial magnetic stimulation (rTMS) is to deliver rTMS concurrent with task performance, and to compare task performance on these trials versus on trials without rTMS. Recent evidence that TMS can have effects on neural activity that persist longer than the experimental session itself, however, raises questions about the assumption of the transient nature of rTMS that underlies many concurrent (or “online”) rTMS designs. To our knowledge, there have been no studies in the cognitive domain examining whether the application of brief trains of rTMS during specific epochs of a complex task may have effects that spill over into subsequent task epochs, and perhaps into subsequent trials. We looked for possible immediate spill-over and longer-term cumulative effects of rTMS in data from two studies of visual short-term delayed recognition. In 54 subjects, 10-Hz rTMS trains were applied to five different brain regions during the 3-second delay period of a spatial task, and in a second group of 15 subjects, electroencephalography (EEG) was recorded while 10-Hz rTMS was applied to two brain areas during the 3-sec delay period of both spatial and object tasks. No evidence for immediate effects was found in the comparison of the memory probe-evoked response on trials that were vs. were not preceded by delay-period rTMS. No evidence for cumulative effects was found in analyses of behavioral performance, and of EEG signal, as a function of task block. The implications of these findings, and their relation to the broader literature on acute vs. long-lasting effects of rTMS, are considered.
Keywords: repetitive transcranial magnetic stimulation, long-term effects, electroencephalography, working memory
INTRODUCTION
TMS is a powerful tool that uses the principle of electromagnetic induction to alter neural activity in a localized brain area. The safe, noninvasive nature of this technique allows for study of normal brain function in humans (e.g., Pascual-Leone et al. 1999; Sack et al. 2007). TMS complements such physiological measurement techniques as functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) by supporting stronger conclusions than these inherently correlational methods about brain-behavior relationships. And because the effects of TMS on brain function and behavior can differ considerably as a function of how it is delivered, it is also a very flexible technique. For example, when delivered for an extended period of time at a relatively low frequency (≤ 1 Hz), or in “theta-burst” mode (brief 50 Hz trains delivered at 5 Hz), it has the effect of decreasing overall activity in the targeted region, thereby taking the region temporarily “offline” and enabling a “virtual lesion” procedure (e.g., Gerschlager et al. 2001; Romero et al. 2002; Huang et al. 2005). When delivered as single pulses that are carefully timed relative to stimulus delivery (with up to millisecond precision), it can serve as a tool for mental chronometry (Walsh and Pascual-Leone 2003). These are just two examples. The focus of this report is a third type of procedure that takes advantage of the ability to precisely control the timing of TMS: When delivered concurrent with performance of a cognitive task, high-frequency repetitive TMS (rTMS; ≥ 5 Hz) can target discrete components of multicomponent cognitive tasks (e.g., Pascual-Leone et al. 2000; Robertson et al. 2003).
Often intended in this mode as a tool for “virtual neuropsychology” (Walsh and Pascual-Leone 2003), the concurrent rTMS approach is touted as having the advantage over lesion studies of isolating the experimental intervention to the trial component of interest, leaving the other components of the trial unaffected. For example, by comparing the effects of rTMS delivered during the delay period versus the response period of a spatial delayed-recognition task, we were able, in previous work, to demonstrate selective sensitivity of the former to rTMS of the superior parietal lobule (SPL) and of the latter to rTMS of the prefrontal cortex (PFC). We interpreted these results as evidence for a selective role for these two regions in short-term retention vs. probe discrimination and response processes, respectively (Hamidi et al. 2008; Hamidi et al. 2009). Note that this interpretation depended on the assumption that the effects of rTMS on brain function were limited to the periods of time during which it was being delivered. We will refer to this as the transience assumption. Although this assumption is of central importance to the concurrent rTMS approach, it has not, to our knowledge, been thoroughly evaluated. The purpose of the analyses described here is to evaluate the transience assumption using two types of measure – behavior and electroencephalography (EEG) -- and from the standpoint of two different time scales -- immediate (i.e., that a train of rTMS may have an effect that persists immediately after it’s offset -- a “residual” or “spillover” effect), and cumulative (i.e., that later portions of an experimental session may differ from early portions as a function of the number of trains of rTMS delivered over the course of the experiment).
The importance of these two time scales can be illustrated by considering the Hamidi et al. (2008) study in which delivery of a 3-sec train of 10 Hz rTMS during the (3 sec-long) delay period had the selective effect for SPL, but not other cortical areas, (including PFC and the leg area of primary somatosensory cortex (S1) of the postcentral gyrus, a control area), of speeding the response time (RT) to the memory probe. The interpretation of this result was that the SPL, but not the other regions, contributed importantly to the delay-period retention of spatial information. A failure of the transience assumption on the immediate time scale, however, would mean that our results might actually be due to a residual effect of delay-period rTMS on SPL contribution to one or more probe-related functions (e.g., probe perception, comparison to the probe to the memory trace, generation and/or execution of the response). A failure of the transience assumption on the cumulative time scale, in contrast, would complicate our interpretation for a different reason. It would be possible that, if, for example, PFC was more susceptible to cumulative effects of rTMS than was SPL, the cumulative effect of rTMS on rTMSabsent trials, which were interleaved with rTMSpresent trials, might minimize the difference between the two, thus rendering ambiguous whether delay-period rTMS had no effect on PFC (as we concluded in the Hamidi et al. (2008) study) or whether it had an effect on the control trials (i.e., rTMSabsent trials) that was comparable in magnitude to its effect on the experimental trials (i.e., rTMSpresent trials).
The specifics of the situation summarized here (and of the analyses to be presented in this report) are somewhat idiosyncratic to the task procedure and rTMS protocol that we have employed in several of our studies of working memory function (i.e., Postle et al. 2006; Feredoes et al. 2007; Hamidi et al. 2008; Hamidi et al. 2009; Hamidi et al. 2010). For example, accepted safety standards dictate that trials containing a seconds-long train of delay period-spanning rTMS be followed by a lengthy intertrain interval (Wassermann 1998; Rossi et al. 2010), which is implemented in our studies by intertrial intervals (ITI) of > 10 sec. More common in the literature are rTMS studies that employ shorter stimulation trains and, consequently, shorter intertrain intervals (e.g., see the extensive review in Rossi et al. 2010). Nonetheless, understanding the conditions under which the transience assumption does and does not hold is of general interest for the design and interpretation of rTMS experiments, and the analyses presented here will contribute to this effort. For example, one can see how a failure of the assumption of transience, at either of the two time scales considered up to this point, would also be problematic for concurrent rTMS designs in which rTMS delivery is blocked, and its effects compared against blocks featuring no rTMS, low intensity rTMS, or sham rTMS. There are many examples of such designs in the literature, with some that have been used to study working memory discussed in Hamidi et al. (2008).
The importance of these considerations is heightened by the fact that there is a third time scale over which it is well documented that the assumption of transience is decidedly not valid: A recent review of the results from more than 50 published reports makes clear that most types of rTMS protocols can be expected to produce effects that endure after the experimental session is over (Thut and Pascual-Leone 2010), what we will refer to as postsession effects1. Indeed, regardless of whether the procedure entailed low-frequency, theta-burst, or high-frequency rTMS, the vast majority of studies reported postsession effects that endured beyond the experimental session by an average of 35 min. What varied as a function of procedure was the sign of these long-lasting effects, with low-frequency and theta-burst most often producing negative (or suppressive) aftereffects and high-frequency rTMS most often producing positive (or facilitative) aftereffects.
Unlike postsession effects, direct evidence pertaining to immediate and cumulative effects of rTMS is sparse. In a very literal sense, we know that TMS does have immediate effects, in that there is a TMS-evoked response that plays out over the few hundred milliseconds after the discharge of the TMS coil, whether TMS is delivered as single pulses (e.g., Komssi et al. 2002; Ferrarelli et al. 2008; Rosanova et al. 2009) or in high-frequency trains (Hamidi et al. 2010). In the context of the transience assumption, however, we are concerned with whether the effects of rTMS delivered during one epoch of a trial may “spill over” to influence task-related neuronal processing in the subsequent epoch. Just such an effect has been described in the anaesthetized cat, for which a 4 sec-long train of 4 Hz rTMS has a suppressive effect on visually evoked spiking, an effect that lasts between 100 and 200 sec (Pasley et al. 2009). In the human, we have seen that the magnitude of several components of the response evoked by each pulse within a 30-pulse train of 10 Hz rTMS varies quadratically as a function of position within the train (Hamidi et al. 2010). What we do not yet know, however, and what we will investigate here, is whether this train of 10 Hz rTMS, which is delivered during the delay period of a delayed-recognition task, also influences the processing of the memory probe that follows the delay period. With regard to the cumulative time scale, there exists even less relevant data of which we are aware. Indeed, all we know at present is that the within-train quadratic trends described above did not vary as a function of task block (Hamidi et al. 2010). (That is, there seems to be no cumulative change in the within-train immediate effects of TMS.) For the present report we reanalyzed data from two recent studies (Hamidi et al. 2008; Hamidi et al. 2009) in which rTMSpresent trials were randomly interleaved with rTMSabsent trials, with the reasoning that if exposure to multiple trains of rTMS leads to functionally-relevant changes in brain activity, then behavioral and/or EEG measures on later trials in the experimental session would be affected to a greater extent than on earlier trials.
METHODS
Detailed descriptions of the experimental procedures are presented in Hamidi et al. (2008; which we will refer to as the rTMS-only study) and Hamidi et al. (2009; which we will refer to as the rTMS-EEG study). We briefly describe them below.
Subjects
In the rTMS-only study 54 young adults (28 male, mean age = 22.7 [S.D. = 4.4]) volunteered. In the rTMS-EEG study, 15 young adults (12 male, mean age = 22.5 [S.D. = 3.8]) participated. In both studies, subjects did not have any psychiatric or neurological conditions, as determined by a psychiatrist or clinical psychologist who administered a structured psychiatric diagnostic interview (MINI, Sheehan et al. 1998) and mood assessment (HAM-D, Hamilton 1960). The study protocols were approved by the local ethics committee.
Behavioral Task
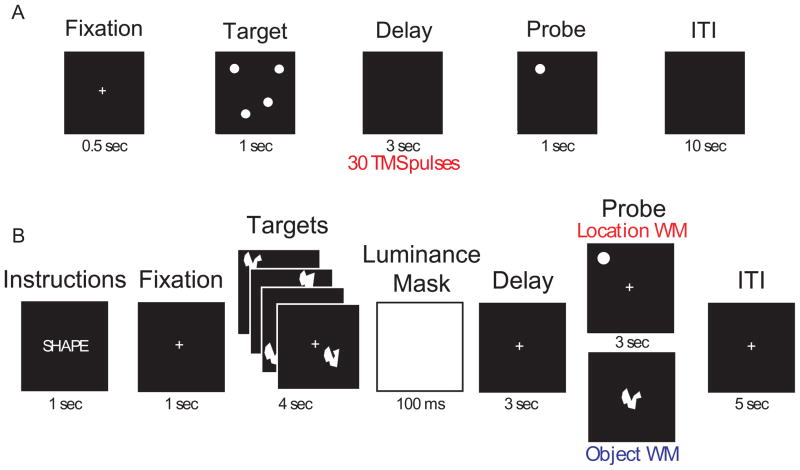
In both studies, subjects performed a delayed-recognition spatial working memory task. In the rTMS-only study, only location memory trials were performed (see Fig. 1A). Prior to data collection, each subject practiced the task(s) until achieving a criterion level of performance of ≥ 75% correct. In the rTMS-EEG study, trials of location memory and object visual memory were interleaved (See Fig. 1B). In both studies, subjects were presented with four targets in random locations on the screen. After a 3-sec delay a probe stimulus was presented for 1 sec. Subjects were required to make a yes/no response with a button box, indicating whether the probe stimulus matched either the location or the identity, depending on the trial type, of any of the target stimuli. They were told that only responses registered within a 3-sec response window would be scored as correct, and instructed to respond as quickly as possible while prioritizing accuracy over RT.
Figure 1.
A. Schematic diagram of the task used in Study 1. In half the trials, rTMS (10 Hz, 110% MT, corrected) was delivered throughout the entire 3-sec delay period (marked by the red bar). Task procedures were the same in both Experiment 1 and Experiment 2. B. Diagram of the task used in the rTMS-EEG study. For each brain area targeted (SPL and S1), subjects performed 192 memory trials (96 location memory and 96 object memory, randomly interleaved). On half the trials, orthogonal to the factor of memory task and randomly distributed, a 3-sec train of 10-Hz rTMS (30 pulses) coincided with the onset of the delay period.
In both tasks, rTMS was blocked by brain area, with order of brain area counterbalanced across subjects. In the rTMS-only study, the task was administered in 4 runs of 12 trials each per target brain area, for a total of 48 trials per brain area targeted, with rTMS delivered unpredictably on half of these trials. In the rTMS-EEG study, the task was administered in 8 blocks of 24 trials, for a total of 192 trials per brain area targeted, 96 of which were location memory trials, with rTMS delivered unpredictably on half of these trials. Thus, the rTMS-only study administered rTMS on a total of 24 trials per brain area, and the rTMS-EEG study administered rTMS on a total of 48 location-memory trials and 48 object-memory trials per brain area.
rTMS
In both studies, during trials with rTMS, subjects received a 3-sec, 10-Hz (110% MT2) rTMS train coinciding with the 3-sec delay period. TMS was delivered with a Magstim Standard Rapid magnetic stimulator fit with a 70-mm figure-8 stimulating coil (Magstim Co., Whitland, UK). Accurate targeting of each brain areas was achieved by obtaining a high resolution anatomical MRI of each subject with a 3-T scanner (GE Signa VH/I, 256 saggital slices, 0.5mm × 0.5mm × 0.8mm) and using an infrared-based frameless stereotaxy system (eXimia Navigated Brain Stimulation, NexStim, Helsinki, Finland).
In the rTMS-only study participants were divided into two groups. In Group 1, the dlPFC and SPL were experimental targets, whereas S1 served as a cortical control area. In Group 2, the FEF and IPS were targeted, in addition to S1. For Group 1, the dlPFC target was identified as the middle frontal gyrus on the ventral bank of the superior frontal sulcus at the level of the sulcus frontalis medius (intended to target the border of Brodmann areas 9 and 46), and the SPL target as gyral tissue dorsal and medial to the intraparietal sulcus and posterior to the postcentral sulcus (intended to target Brodmann area 7). The left hemisphere was targeted in 18 subjects, the right in 12. For Group 2, the FEF target was defined as the rostroventral portion of the intersection of the superior frontal and precentral sulci, and the IPS as the medial bank of the IPS at the level of the parieto-occipital fissure. In Group 2 the left hemisphere was targeted in 12 subjects and the right in 12. In both groups, S1 served as a control stimulation site, as was identified (in the same hemisphere as the experimental targets for each subject) as an area immediately posterior to the central sulcus and close to the midline. Each trial lasted 15.5 sec, and with 12 trials each, a task block lasted 3 min 6 sec with a three-to-five minute break between blocks. Stimulation was blocked by region, with order counterbalanced across subjects. After 4 blocks, the TMS coil was repositioned over the second target (which typically took approximately 10 to 15 min) and another 4 blocks of task was performed. Finally, another 4 blocks of task was similarly performed for the third brain area. For each rTMSpresent trial, 30 TMS pulses were delivered during the delay period of the task, resulting in 180 pulses per block, 720 pulses per brain area targeted, and 2160 pulses overall.
In the rTMS-EEG study, the left SPL and left S1 were targeted in all subjects, identified as in the rTMS-only study, with each region stimulated on a separate day, and order or region counterbalanced across subjects. For each rTMSpresent trial, 30 TMS pulses were delivered during the delay period of the task, resulting in 360 pulses per block, and 2880 pulses per brain area targeted.
EEG
Details of the EEG recording and analysis techniques are described in Hamidi et al. (2009; 2010). In summary, EEG was recorded with a 60-channel carbon cap and TMS-compatible amplifier (NexStim, Helsinki, Finland). To reduce residual TMS-related artifacts, the impedance at each electrode was kept below 3 kU. The right mastoid was used as the reference and eye movements were recorded using two additional electrodes. Data were acquired at 1450 Hz sampling rate with 16-bit resolution. Data were processed offline using the EEGLAB toolbox (Delorme and Makeig 2004) running in a MATLAB environment (Mathworks, Natick Massachusetts, USA). The data were first down-sampled to 500 Hz and then bandpass filtered between 0.1 and 500 Hz. After this, the data were cleaned of large movement-related artifacts, and channels with excessive noise were reinterpolated by using spherical spline interpolation. Prior to analysis, the data were rereferenced to an average of all 60 channels. Independent components analysis (ICA) was used to identify nonphysiological TMS-related artifacts, which were removed prior to partitioning data by behavioral condition.
Analysis strategy
The original analyses of the behavioral data from the rTMS-only and rTMS-EEG experiments aggregated across trials and averaged across subjects. Here, the results are organized by time scale over which the transience assumption is being analyzed.
Immediate effects
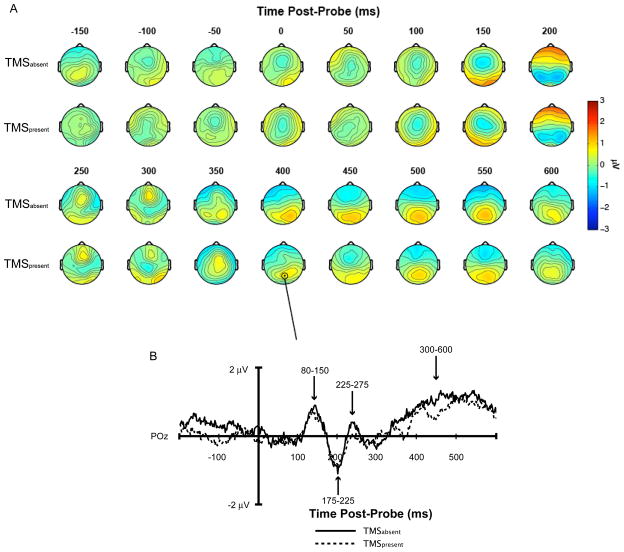
For the delayed-recognition task, immediate effects of rTMS can only be studied with a physiological measure. This is because the button press only occurs several hundred msec after the onset of the memory probe, and, therefore, one cannot know whether a change in behavior associated with delay-period rTMS was due to the intervention’s influence on neural processing that occurred concurrent with the rTMS, that occurred shortly after the offset of the rTMS, or to some combination of the two. Therefore, the analyses related to immediate effects were restricted to analyzing the probe-evoked event-related potential (ERP) from the rTMS-EEG study (Hamidi et al. 2009). Specifically, for rTMSpresent and rTMSabsent conditions, we compared the mean amplitude of the ERP recorded at posterior electrode POz across four different temporal windows corresponding to each of the major deflections in the probe-evoked ERP (see Fig. 2).
Figure 2.
A. Topography of probe-evoked ERP in SPL rTMSpresent versus rTMSabsent trials for the location-memory condition. B. rTMSpresent versus rTMSabsent probe-evoked ERPs recorded from electrode POz.
Cumulative effects
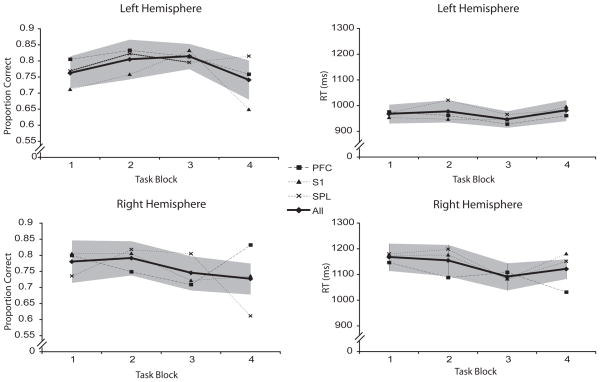
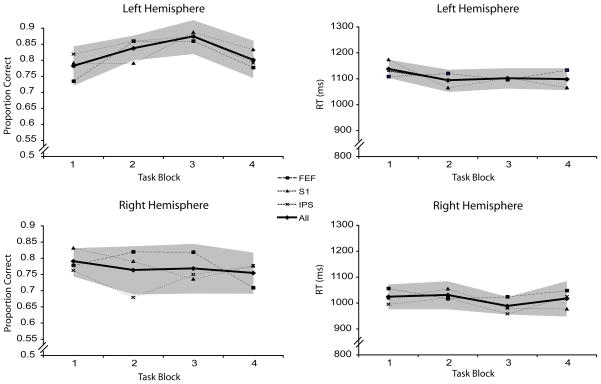
The existence of cumulative effects can be assessed with both behavioral and physiological measures. The two studies that we reanalyzed for this report were each designed with rTMSpresent and rTMSabsent trials occurring unpredictably, and in equal numbers, in each block. Thus, cumulative effects on behavior were assessed by averaging performance on rTMSabsent trials by block, and testing for systematic changes as a function of block. This was done separately for the TMS-only, Group 1 (SPL and dlPFC; Fig. 3), TMS-only, Group 2 (IPL and FEF; Fig. 4), and TMS-EEG (Fig. 5) datasets.
Figure 3.
Accuracy and RT during rTMSabsent trials for TMS-only, Group 1 (SPL and dlPFC). Dark line indicates performance averaged across all three brain areas targeted. Shaded areas represent the within-subject 95% confidence interval for the averaged performance.
Figure 4.
Accuracy and RT during rTMSabsent trials for TMS-only, Group 2 (IPS and FEF). Dark line indicates performance averaged across all three brain areas targeted. Shaded areas represent the within-subject 95% confidence interval for the averaged performance.
Figure 5.
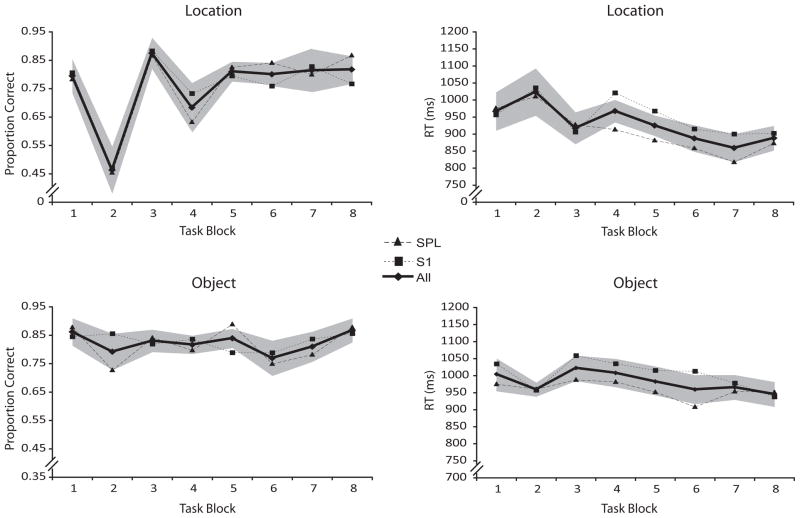
Accuracy and RT during rTMSabsent trials in location- and object-memory trials of the rTMS-EEG study. Dark line indicates performance averaged across both brain areas targeted. Shaded areas represent the within-subject 95% confidence interval for the averaged performance.
Analogously, cumulative effects on the EEG could be assessed by selectively averaging the signal by block. For rTMSabsent trials, we calculated the mean global field power (GFP, Lehmann and Skrandies 1980) across the central 2000 msec of the delay period (beginning 500 msec after the onset of the delay period), separately for location and object trials, and for brain area, as a function of task block (Fig. 6 and Fig. 7)3. This allowed us to assess changes across blocks in the mean amplitude of delay-period potentials without making any assumptions about their temporal or topographic distribution. For rTMSpresent trials, we examined changes in the TMS-evoked ERP across task blocks. Specifically, we calculated GFP time-locked to the onset of each TMS pulse4. The response elicited by each TMS pulse in the 30-pulse train is stereotypical and contains five major deflections within the first 100 ms after the pulse (Hamidi et al. 2010). To determine the long-term effects of rTMS on the immediate neurophysiological effects of individual TMS pulses, therefore, we assessed changes in the amplitude of each ERP deflection across the 8 task blocks. Because the significant behavioral effects of rTMS were restricted to SPL rTMS, the analysis was restricted to trials in which the SPL was targeted.
Figure 6.
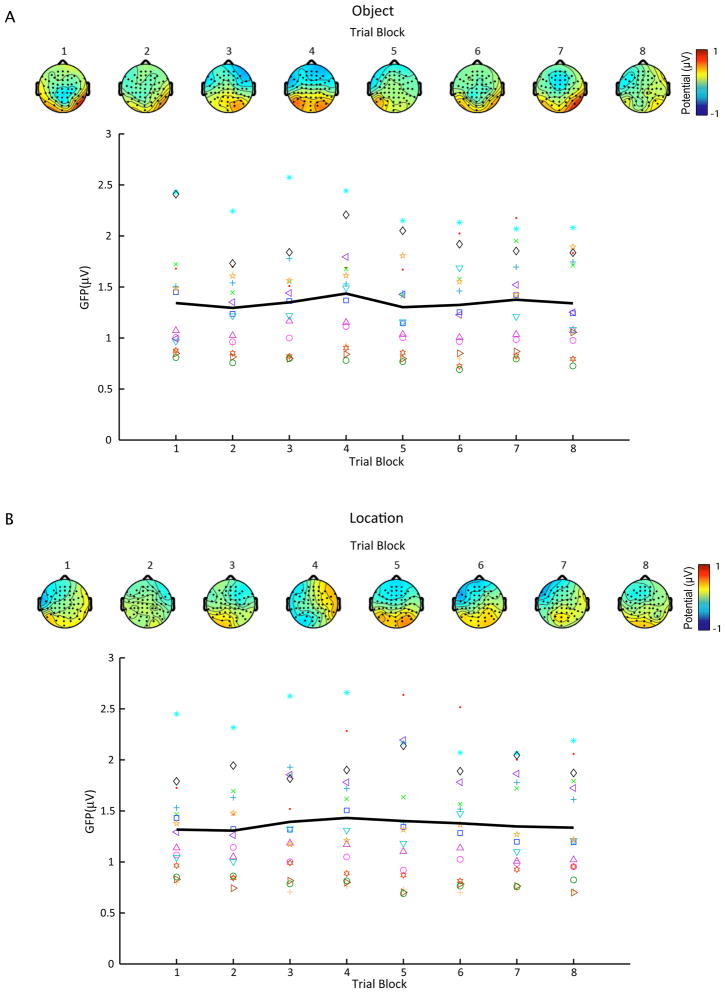
Mean delay-period GFP for S1/rTMSabsent trials as a function of task block for both object- (A) and location-memory (B) conditions. Different marker types indicate mean GFP across blocks for each subject, whereas the black line represents average GFP across subjects. Scalp maps show the topography of group-averaged delay-period potentials for each block.
Figure 7.
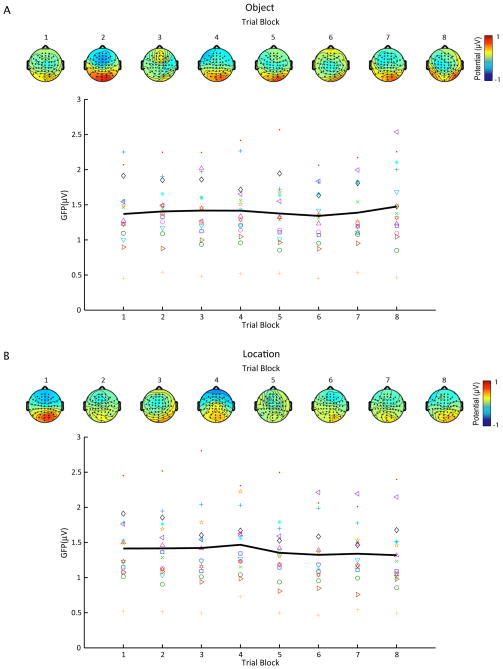
Mean delay-period GFP for SPL/rTMSabsent trials as a function of task block for both object- (A) and location-memory (B) conditions. Different marker types show mean GFP across blocks for each subject, whereas the black line represents average GFP across subjects. Scalp maps show the topography of group-averaged delay-period potentials for each block.
RESULTS
Immediate effects
The original analyses of the rTMS-EEG study found two sets of results that are of interest here. First, the aggregated results revealed that delay-period rTMS had the effect of improving accuracy on the location-memory task, but not the object-memory task. Second, an individual-differences analysis revealed a region-by-task specificity of the effects of delay-period rTMS, such that only when SPL was targeted, and only during location trials, did the magnitude and direction of the effect of rTMS on delay-period oscillatory power in the upper alpha band (roughly 10–14 Hz) predict the magnitude and direction of the effect of rTMS on accuracy (increased power corresponded to lower accuracy, and decreased power to higher accuracy, Hamidi et al. 2009). The question being tested here is whether the pattern in the aggregated data may have been due, in part, to immediate effects of rTMS on probe-related processing, in addition to the effects on the delay-period EEG that were previously documented. (Because neither of the original analyses revealed any theoretically interesting effects of rTMS on object memory trials (Hamidi et al. 2009), we restricted the present analyses to location trials.)
As illustrated in Figure 2, the probe-evoked response measured at electrode POz yielded three distinct peaks followed by a gradually building, sustained positivity that emerged beginning at approximately 350 msec following the onset of the probe. For rTMS delivered to SPL, as well as to S1, visual inspection suggested no substantial differences for either this ERP or for whole-scalp voltage maps. To verify this quantitatively, we ran 2-way ANOVAs (targeted region (SPL, S1) × trial type (rTMSpresent, rTMSabsent)) on the scalp topography data for the time periods encompassing the four prominent features of the ERP: Peak I (80–150 ms); Peak II (175–225 ms); Peak III (225–275 ms); and the late positivity (300–600 ms). No significant effects were seen for any of the three peaks (all Fs < 1), and for the late positivity only the main effect of trial type approached significance [F(1,14)=3.94, p = .07], reflecting the fact that this increase in positivity was smoother on rTMSabsent than rTMSpresent trials. Together, these null results suggest that, for this data set, the transience assumption held up over the immediate time scale.
Cumulative effects
Behavioral. TMS-Only, Group 1 (SPL and dlPFC)
Analysis of the accuracy data (Fig. 3) revealed a marginal main effect of task block [F(3,84) = 2.70; p=0.05]. However, we found no evidence of a linear or quadratic trend across blocks (all Fs < 1.67), suggesting that there was no systematic, cumulative change across blocks. The only other significant contrast in the ANOVA was a Brain Area × Hemisphere × Task Block interaction [F(6,168) = 2.59; p<0.05]. Visual inspection of the data from each cell of the design, combined with post hoc contrasts, revealed only a quadratic trend [F(1,11) = 4.45; p=0.06] when the right SPL was targeted (Fig. 3). For RT there was main effect of task block [F(3,84) = 4.70; p<0.005], and post hoc polynomial testing revealed a marginal linear trend when the PFC was targeted [F(1,29) = 3.98; p=0.06; Fig. 3]. This was driven by a decrease in RT in later blocks of the task. There were no other significant interactions involving task block (all Fs < 1.73).
Behavioral. TMS-Only, Group 2 (IPS and FEF)
For these brain areas, we found no significant main effects or interaction involving task block with accuracy or RT (all Fs < 1.93, Fig. 4).
Behavioral. TMS-EEG
Analyses were run separately for rTMSabsent trials for each type of memory and for both dependent measures (Fig. 5). The only reliable effects were the main effect of blocks for both the location/accuracy [F(7,98)=13.64, p<.001] and the location/RT [F(7,98)=4.83, p<.001] ANOVAs. Both of these seem to be due to anomalously poor performance on block 2 that was observed regardless of brain area targeted. (This was an artifact of the experimental construction – although the order in which trials occurred in each block was randomly determined,
rTMS-evoked ERP across blocks
For rTMSabsent trials, figures 6 and 7 show mean delay-period GFP and topography for each task block in the object- and spatial-memory conditions, with S1 (Fig. 6) and SPL (Fig. 7) plotted separately. Topographically, the delay-period activity in rTMSabsent trials was characterized by a positive deflection around the posterior electrodes (centered on electrode PO4) and a slight negative deflection in the frontal electrodes. This pattern was stable across all task blocks. To test for changes in mean potential during the delay-period across task blocks, we performed a repeated-measures ANOVA with task block as a within-subject variable on rTMSabsent trials on data from electrode PO4 (which showed the greatest change in potential). This analysis revealed no significant effect of task block [F(7, 105) = 1.29; n.s.; Fig. 5].
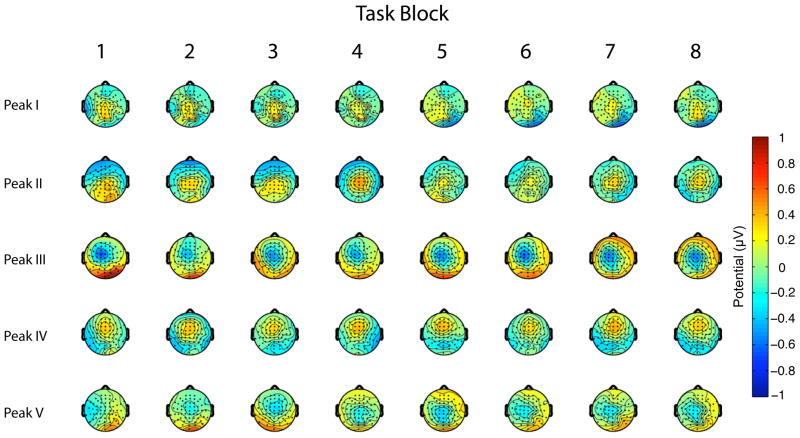
As discussed previously, an analysis of rTMSpresent trials has revealed a complex response to each pulse within the train, with most peaks of this response varying quadratically in magnitude across the train. Of relevance to the consideration of cumulative effects, this within-train pattern did not vary as a function of task block (Hamidi et al. 2010). For this study we performed an additional, confirmatory analysis with a 2-Way (Electrode × Task Block) ANOVA comparing vector-normalized potentials across blocks (McCarthy and Wood 1985), and this analysis revealed no significant topographic changes across task blocks (Fig. 8).
Figure 8.
Topography of each peak of the TMS-related ERP across the eight task blocks.
DISCUSSION
The past two decades have witnessed rapid technical and methodological developments with TMS, such that its use has become commonplace in all corners of human neuroscience research. Nonetheless, several fundamental questions about the physiological effects of TMS and rTMS remain unknown. In the present report we have addressed an assumption that, implicitly or explicitly, underlies many applications of rTMS: the assumption of transience. The validity of this assumption, at least under certain conditions, is critical if one is to exploit the temporal precision with which rTMS can be delivered.
The tasks whose data we reanalyzed here are delayed-recognition tasks, in which theoretically dissociable cognitive processes are engaged serially, and in a temporally dependent manner (i.e., stimulus perception and encoding precedes retention, which precedes probe perception and evaluation, which precedes the motor response). In our experiments we delivered high-frequency (10 Hz) rTMS during the length of the 3 sec-long delay period, intending to influence retention-related processes while leaving unperturbed those that preceded and followed them. The results of the present analyses suggest that the transience assumption held up in these experiments, and that we were therefore justified in interpreting our results as reflecting the influence of rTMS on retention-related processes. More specifically, analysis of the probe-evoked ERP revealed no differences when the probe was preceded by rTMS than when it was not, indicating no immediate effects of the delay-period rTMS train “spilling over” into the memory probe portion of the trial. This outcome is particularly intriguing in view of the fact that immediate effects have been observed in extracellular responses evoked by visual stimuli that followed a train of rTMS (Pasley et al. 2009), as well as in EEG responses to individual pulses embedded within the very same trains of rTMS that were reanalyzed in this report (Hamidi et al. 2010). Thus, with regard to immediate effects of rTMS, the role of such factors as stimulation parameters and type of neural processes that follow the rTMS train are clearly in need of further study.
The second time scale over which we assessed the transience assumption was that of the experimental session, and the possibility of a cumulative effect that might build across the experimental session as a result of experiencing an increasing number of pulses and/or trains of pulses over time. For these analyses, too, the transience assumption was supported: Across 69 subjects and five brain areas tested, our analyses yielded little evidence of cumulative effects of rTMS on behavior, and across 15 subjects and two brain areas tested, we found no evidence of cumulative effects of rTMS on task-related EEG. These findings are from sessions that delivered a total of either 720 or 2880 pulses per brain area in a single session, depending on the study.
There are several caveats that must be considered along with our conclusions. The first that we will consider is the fact that, to a first order of approximation, our results amount to a series of null findings. With regard to the null findings of the immediate-effect analysis of the rTMS-EEG data, one might question whether, with 15 subjects, we had the sensitivity to detect possible subtle differences. Although we cannot rule out Type II error, it bears noting that this same data set produced clear evidence of immediate effects on the response evoked by individual pulses within each rTMS train, in that the magnitude of the response evoked by a given pulse within each 30-pulse train was dependent on that pulse’s position in the train (Hamidi et al. 2010). With regard to the lack of significant cumulative effects of rTMS on behavior, there were nonetheless some nonspecific trends suggesting that, though weak, some cumulative effects may in fact be present. For example, when the right SPL was targeted, there was a greater decrease in accuracy in later blocks of the study. Furthermore, when the right dlPFC was targeted, there was a decrease in RT in later blocks. The regional specificity of these effects precludes fatigue, learning, strategy, or other global effects as the factor behind the trends. Another possibility could be accumulating discomfort with regionally-specific scalp sensations or muscle contractions. This possibility would be most prominent in prefrontal areas, because they underlie the frontalis muscle. Indeed, we see evidence of long-term effects when the dlPFC is targeted. However, that these effects were limited to the right dlPFC, and that the possibility of long-term effects was also observed when the SPL was targeted, argues against discomfort being an important factor. Additionally, discomfort would most likely result in slowing down of the response, whereas the trend we observed with right dlPFC rTMS was towards speeding up. Thus, the origin of these effects is unclear.
A broader caveat has to do with the generalizeability of the findings reported here to other rTMS protocols. As noted in the Introduction, the procedure that we used in these studies features longer rTMS trains, and consequently longer intertrain intervals, than is typical of designs using concurrent rTMS. And independent of how “typical” the design might be, the results presented here were derived from just one type of task (spatial delayed recognition) during which rTMS was delivered at just one frequency (10 Hz). With regard to the factor of stimulation frequency, for example, it is known that the behavioral effects of rTMS can be highly dependent on the frequency at which it is delivered (Klimesch et al. 2003; Luber et al. 2007). Additionally, long periods of low-frequency rTMS are well known to have long-term effects post-stimulation (Gerschlager et al. 2001; Romero et al. 2002), and even some high-frequency rTMS protocols have produced effects (Esser et al. 2006; Brignani et al. 2008; Fuggetta et al. 2008) that endure beyond the period of stimulation.
A final question that we will consider is the seeming discrepancy between the apparent validity of the transience assumption in the analyses that we have presented, and the fact that the vast majority of studies that have looked for them have found EEG evidence for post-session aftereffects. Indeed, even more striking is that our rTMS-EEG study delivered a relatively large number of pulses in each session (2880), and a recent comprehensive review of TMS aftereffects concluded that the magnitude of the aftereffect size is positively related to the number of pulses delivered during the session in question (Thut and Pascual-Leone 2010). One possible explanation is the procedural differences between our study and the majority of those reviewed by Thut and Pascual-Leone (2010). A second is that, as demonstrated in our analyses of immediate effects, it can happen that the same rTMS protocol can demonstrate aftereffects with one measure (e.g., the sensitivity of the magnitude of the pulse-evoked response to that pulses position within a high-frequency train) but no aftereffects with another (e.g., the probe-evoked response). Thus, it may be that there are, indeed, cumulative effects in our data, but we simply have not used measures that are sensitive to these effects.
In conclusion, although our analyses have produced some support for the transience assumption across two different time scales, they have also highlighted many questions about the physiological consequences of rTMS that remain unresolved. If nothing else, we hope that this report conveys the importance of explicitly questioning and, when possible, testing assumptions about the physiological consequences of one’s rTMS protocols.
Footnotes
For procedures that are intended to have long-lasting clinical effects, such as the treatment of depression, achieving long-lasting effects is, of course, an important goal of the intervention.
Except for 12 subjects in experiment 1, for all subjects the stimulation intensity was corrected for scalp-cortex distance (Stokes et al. 2005). For the remaining 12 subjects, rTMS was applied at 110% MT at all brain areas targeted.
Similar analyses have been used to assess delay-period EEG activity as a function of, for example, visual short-term memory capacity (Vogel and Machizawa 2004) or the number of items held in visual short-term memory (Vogel et al. 2005).
To avoid contamination by neurophysiological signals reflecting either the initial orienting response seen at the beginning of the delay, or overlap with probe-related activity following the delay, our analyses focused on the 5th through 29th pulses in the 30-pulse train.
References
- Brignani D, Manganotti P, Rossini PM, Miniussi C. Modulation of cortical oscillatory activity during transcranial magnetic stimulation. Hum Brain Mapp. 2008;29(5):603–612. doi: 10.1002/hbm.20423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Esser SK, Huber R, Massimini M, Peterson MJ, Ferrarelli F, Tononi G. A direct demonstration of cortical LTP in humans: A combined TMS/EEG study. Brain Res Bull. 2006;69(1):86–94. doi: 10.1016/j.brainresbull.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Feredoes E, Tononi G, Postle BR. The neural bases of the short-term storage of verbal information are anatomically variable across individuals. The Journal of Neuroscience. 2007;27:11003–11008. doi: 10.1523/JNEUROSCI.1573-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrarelli F, Massimini M, Peterson MJ, et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: a TMS/EEG study. Am J Psychiatry. 2008;165(8):996–1005. doi: 10.1176/appi.ajp.2008.07111733. [DOI] [PubMed] [Google Scholar]
- Fuggetta G, Pavone EF, Fiaschi A, Manganotti P. Acute modulation of cortical oscillatory activities during short trains of high-frequency repetitive transcranial magnetic stimulation of the human motor cortex: A combined EEG and TMS study. Hum Brain Mapp. 2008;29(1):1–13. doi: 10.1002/hbm.20371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerschlager W, Siebner HR, Rothwell JC. Decreased corticospinal excitability after subthreshold 1 Hz rTMS over lateral premotor cortex. Neurology. 2001;57(3):449–455. doi: 10.1212/wnl.57.3.449. [DOI] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Repetitive transcranial magnetic stimulation affects behavior by biasing endogenous cortical oscillations. Frontiers in Integrative Neuroscience. 2009;3 doi: 10.3389/neuro.07.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Brain responses evoked by high-frequency repetitive TMS: An ERP study. Brain Stimulation. 2010;3:2–14. doi: 10.1016/j.brs.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Slagter HA, Tononi G, Postle BR. Brain responses evoked by high-frequency repetitive TMS: An ERP study. Brain Stimulation. 2010;3:2–24. doi: 10.1016/j.brs.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Res. 2008;1230:202–210. doi: 10.1016/j.brainres.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating frontal and parietal contributions to spatial working memory with repetitive transcranial magnetic stimulation. Brain Research. 2008;1230:202–210. doi: 10.1016/j.brainres.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi M, Tononi G, Postle BR. Evaluating the role of prefrontal and parietal cortices in memory-guided response with repetitive transcranial magnetic stimulation. Neuropsychologia. 2009;47(2):295–302. doi: 10.1016/j.neuropsychologia.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatr. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Gerloff C. Enhancing cognitive performance with repetitive transcranial magnetic stimulation at human individual alpha frequency. Eur J Neurosci. 2003;17(5):1129–1133. doi: 10.1046/j.1460-9568.2003.02517.x. [DOI] [PubMed] [Google Scholar]
- Komssi S, Aronen H, Huttunen J, et al. Ipsi- and contralateral EEG reactions to transcranial magnetic stimulation. Clinical Neurophysiology. 2002;113(2):175–184. doi: 10.1016/s1388-2457(01)00721-0. [DOI] [PubMed] [Google Scholar]
- Lehmann D, Skrandies W. Reference-free identification of components of checkerboard-evoked multichannel potential fields. Electroencephalography and Clinical Neurophysiology. 1980;48:609–621. doi: 10.1016/0013-4694(80)90419-8. [DOI] [PubMed] [Google Scholar]
- Luber B, Kinnunen LH, Rakitin BC, Ellsasser R, Stern Y, Lisanby SH. Facilitation of performance in a working memory task with rTMS stimulation of the precuneus: Frequency- and time-dependent effects. Brain Res. 2007;1128(1):120–129. doi: 10.1016/j.brainres.2006.10.011. [DOI] [PubMed] [Google Scholar]
- McCarthy G, Wood CC. Scalp distributions of event-related potentials: an ambiguity associated with analysis of varance models. Electroencephalogr Clin Neurophysiol. 1985;62:203–208. doi: 10.1016/0168-5597(85)90015-2. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Bartres-Faz D, Keenan J. Transcranial magnetic stimulation: studying the brain-behavior relationship by induction of ‘virtual lesions’. Philosophical Transactions of the Royal Society of London, B Biological Sciences. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Leone A, Walsh V, Rothwell J. Transcranial magnetic stimulation in cognitive neuroscience--virtual lesion, chronometry, and functional connectivity. Curr Opin Neurobiol. 2000;10(2):232–7. doi: 10.1016/s0959-4388(00)00081-7. [DOI] [PubMed] [Google Scholar]
- Pasley BN, Allen EA, Freeman RD. State-dependent variability of neuronal responses to transcranial magnetic stimulation of the visual cortex. Neuron. 2009;62:291–303. doi: 10.1016/j.neuron.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle BR, Ferrarelli F, Hamidi M, Feredoes E, Massimini M, Peterson MJ, Alexander A, Tononi G. Repetitive transcranial magnetic stimulation dissociates working memory manipulation from retention functions in prefrontal, but not posterior parietal, cortex. Journal of Cognitive Neuroscience. 2006;18:1712–1722. doi: 10.1162/jocn.2006.18.10.1712. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Theoret H, Pascual-Leone A. Studies in Cognition: The Problems Solved and Created by Transcranial Magnetic Stimulation. J Cogn Neurosci. 2003;15(7):948–960. doi: 10.1162/089892903770007344. [DOI] [PubMed] [Google Scholar]
- Romero JR, Anschel D, Sparing R, Gangitano M, Pascual-Leone A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin Neurophysiol. 2002;113(1):101–107. doi: 10.1016/s1388-2457(01)00693-9. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. The Journal of Neuroscience. 2009;29:7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A The Safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2010;120:2008–2039. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack AT, Kohler A, Bestmann S, Linden DEJ, Dechent P, Goebel R, Baudewig J. Imaging the brain activity changes underlying impaired visuospatial judgments: Similar fMRI, TMS, and behavioral studies. Cerebral Cortex. 2007;17:2841–2852. doi: 10.1093/cercor/bhm013. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(S20):22–33. [PubMed] [Google Scholar]
- Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB. A simple metric for scaling motor threshold based on scalp-cortex distance: Application to studies using transcranial magnetic stimulation. J Neurophysiol. 2005;94(6):4520–4527. doi: 10.1152/jn.00067.2005. [DOI] [PubMed] [Google Scholar]
- Thut G, Pascual-Leone A. A Review of Combined TMS-EEG Studies to Characterize Lasting Effects of Repetitive TMS and Assess Their Usefulness in Cognitive and Clinical Neuroscience. Brain Topography. 2010;22:219–232. doi: 10.1007/s10548-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428:748–751. doi: 10.1038/nature02447. [DOI] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:368–387. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Walsh V, Pascual-Leone A. Transcranial Magnetic Stimulation: A Neurochronometrics of Mind. Cambridge, MA: MIT Press; 2003. [Google Scholar]
- Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–6, 1996. Electroencephalography and Clinical Neurophysiology. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]