Abstract
Purpose
Tumor infiltrating lymphocytes (TIL) and interleukin (IL)-2 administered following lymphodepletion can cause the durable complete regression of bulky metastatic melanoma in patients refractory to approved treatments. However, the generation of a unique tumor-reactive TIL culture for each patient may be prohibitively difficult. We therefore investigated the clinical and immunological impact of unscreened, CD8+ enriched “young” TIL.
Experimental Design
Methods were developed for generating TIL that minimized the time in culture and eliminated the individualized tumor-reactivity screening step. Thirty-three patients were treated with these CD8+ enriched young TIL and IL-2 following non-myeloablative lymphodepletion (NMA). Twenty-three additional patients were treated with CD8+ enriched young TIL and IL-2 after lymphodepletion with NMA and 6Gy of total body irradiation (TBI).
Results
Young TIL cultures for therapy were successfully established from 83% of 122 consecutive melanoma patients. Nineteen of 33 patients (58%) treated with CD8+ enriched young TIL and NMA had an objective response (RECIST) including three complete responders. Eleven of 23 patients (48%) treated with TIL and 6Gy TBI had an objective response including two complete responders. At one month after TIL infusion the absolute CD8+ cell numbers in the periphery were highly correlated with response.
Conclusion
This study shows that a rapid and simplified method can be used to reliably generate CD8+ enriched young TIL for administration as an individualized therapy for advanced melanoma, and may allow this potentially effective treatment to be applied at other institutions and to reach additional patients.
Keywords: adoptive immunotherapy, interleukin-2, lymphodepletion, personalized medicine, homeostasis
Introduction
Patients with metastatic melanoma have limited treatment options and only two have received U.S. Food and Drug Administration approval. Interleukin (IL)-2 can mediate durable complete responses in 3-5% of treated patients, with an overall response rate of about 13-16%(1); decarbazine results in a 15% response rate with rare durable responses(2). Other promising experimental treatments being evaluated in randomized trials for efficacy and toxicity include inhibitors of the dominant BRAFV600E mutation(3) and an anti-CTLA-4 antibody(4;5). Despite these recent advances, a pressing need remains for effective treatments.
Adoptive cell therapy (ACT) combines lymphodepletion with a patient's own tumor reactive tumor infiltrating lymphocytes (TIL) to generate an individualized therapy (6). A total of 93 refractory melanoma patients were treated at our institution with TIL selected for tumor recognition following one of three different lymphodepleting regimens(7). Forty-three patients received non-myeloablative chemotherapy alone (NMA), 25 patients received NMA with 2Gy of total body irradiation (TBI) and 25 patients received NMA plus 12Gy TBI. The objective response rates (RECIST) for these sequential trials was 48%, 52%, and 72%, respectively, including 15 patients who had complete responses, all but one of which are ongoing at 31 to 89 months. Despite these promising clinical results, the extensive effort, cost and time required to generate the individual TIL cultures limited the application of this promising therapy to only a few institutions.
Tumor reactive TIL used in prior studies required an extended duration of multiple microcultures and an individualized assay to identify tumor recognition. Analysis of demographic and biological factors that correlated with TIL function indicated that the size of the lesion and the degree of lymphocyte infiltration impacted TIL generation (8). However, many patients underwent procurement of metastatic tissue for TIL generation but were not ultimately eligible for treatment because their TIL failed the test for tumor recognition, or rapid disease progression occurred during TIL establishment (8). Thus, the complex TIL production process was a substantial limitation to patient therapy. We have now simplified and standardized the TIL production process by generating “young” TIL for therapy(9). These minimally manipulated young TIL cultures consisted of bulk lymphocytes rather than microcultures, and the batch-specific assay for tumor recognition was eliminated. Young TIL had attributes associated with improved persistence and response in vivo including long telomeres and high expression of CD27 and CD28(10-13). In a small clinical trial, young TIL were shown to be capable of mediating objective clinical responses in some patients(14;15). We have now developed a simplified procedure to enrich for tumor reactive CD8+ cells, eliminate non-specific CD4+ T cells, and deplete T regulatory cells(16).
We report here the first clinical trial with CD8+ enriched young TIL administered to patients following lymphodepletion. CD8+ enriched young TIL were effective for the treatment of some patients with metastatic melanoma. The simplified methods of TIL production allowed rapid accrual to this clinical trial using methods more easily adaptable to laboratories in multiple centers. Here we analyze the clinical and immunological features of this individualized patient therapy.
Methods
Patients, clinical samples and trial design
Patients were eligible for this study who were 18 years or older with measurable metastatic melanoma, at least one lesion resectable for TIL, good clinical performance, adequate liver and kidney function tests, blood counts near the normal range, free from active systemic infections without coagulation disorders or cardiovascular disease or immunodeficiency, negative for HIV antibody and hepatitis B and C, and a life expectancy of greater than three months. All patients signed an informed consent approved by the Institutional Review Board of the National Cancer Institute.
One group of 33 patients received non-myeloablative chemotherapy (NMA) consisting of 60mg/kg/day cyclophosphamide for two days followed by five days of 25mg/m2/day fludarabine. A second cohort of 23 patients received two days of 60mg/kg cyclophosphamide overlapping the first two of five days of 25mg/m2/day fludarabine. On the final day of fludarabine, patients received two fractions of 2Gy TBI separated by at least 6hr, and the following day they received one fraction of 2Gy TBI. On the day following chemotherapy or radiation all patients received a bolus intravenous infusion of CD8+ enriched young TIL and started high dose IL-2 therapy (720,000IU/kg intravenously every 8hr to tolerance). One day after TIL infusion, patients who received 6Gy TBI received a minimum of 2 × 106/kg autologous purified (Miltenyi) CD34+ hematopoietic stem cells from a G-CSF±plerixafor mobilized pheresis.
Patients received trimethoprim, sulfamethoxazole, and fungal prophylaxis following therapy; herpes virus seropositive patients also received valacyclovir. Platelets and packed red blood cells were administered as needed during hematopoietic recovery, and empiric antibiotics were initiated for neutropenic fevers (38.3°C once or two temperatures of 38.0°C at least one hour apart and absolute neutrophil count <500). Patient response was assessed using standard radiographic studies and physical examination at approximately four weeks following TIL administration and at regular intervals thereafter. The Response Evaluation Criteria In Solid Tumors (RECIST) guidelines were followed and patients were categorized into complete, partial, or non-responding categories. Complete blood counts (CBC) were obtained at least once per day while patients were in the hospital and differential counts were obtained when CBC was over 200 cells per microliter.
Generation and characterization of CD8+ enriched young TIL
Patients with metastatic melanoma underwent biopsy and as much of the sample as possible was processed to a single cell suspension for generation of young TIL as previously described (9;17) and detailed in Supplemental Methods. The single cell suspension was evaluated on a hemacytometer with lymphocytes and tumor cells determined based on size and morphology and viability determined by trypan blue staining. After minimum time in culture, successfully initiated “bulk” young TIL were CD8+ enriched (Miltenyi CliniMACS)(16) and rapidly expanded to clinical cell numbers (18;19). Aliquots of infused samples were evaluated by FACS and cytokine release assays to determine lymphocyte phenotype and antigen specificity respectively using standard techniques(18).
Statistical analysis
Standard statistical methods are cited in the text, and all p values are two tailed assuming unequal variance with p<0.05 considered significant.
Results
CD8+ enriched young TIL mediated anti-tumor responses
The safety and efficacy of therapy using CD8+ enriched young TIL following lymphodepletion was investigated in two cohorts of patients with metastatic melanoma. Thirty-three patients received CD8+ enriched TIL with NMA, and 23 patients received CD8+ enriched young TIL following NMA plus 6Gy of total body irradiation (TBI). The demographic characteristics of these patients and the treatments administered are shown in Table 1. 64% of patients had received prior IL-2. Median follow up as of April 1, 2010 in the NMA and 6Gy TBI cohorts was 14 months and 7 months, respectively.
Table 1.
Demographics of patients and characteristics of treatments administered
| NMA | 6Gy TBI | |
|---|---|---|
| Patients | 33 | 23 |
| Sex | ||
| Male | 14 | 13 |
| Female | 19 | 10 |
| Age (years) | ||
| ≤30 | 3 | 3 |
| 30-39 | 6 | 5 |
| 40-49 | 12 | 4 |
| 50-59 | 10 | 11 |
| ≥60 | 2 | 0 |
| HLA-A2+ | ||
| Yes | 11 | 10 |
| No | 22 | 13 |
| Prior IL-2 | ||
| Yes | 25 | 11 |
| No | 8 | 12 |
| Stage of disease | ||
| M1a | 2 | 1 |
| M1b | 11 | 10 |
| M1c | 20 | 12 |
| *Cell number (×109) | 47.7 (±3.3) | 43.1 (±7.5) |
| IL-2 (doses) | 6.3 (±0.3) | 7.5 (±0.5) |
| Age of cells at Infusion (days) | 32.7 (±0.7) | 35.4 (±1.3) |
| CD4+ cells (%) | ||
| Prior to CD8+ enrichment | 21.8 (±3.2) | 22.7 (±3.6) |
| Infused | 0.6 (±0.3) | 2.1 (±0.8) |
| CD8 + cells (%) | ||
| Prior to CD8+ enrichment | 57.2 (±4.3) | 49.0 (±4.7) |
| Infused | 96.0 (±0.6) | 97.3 (±0.8) |
| Tissue of TIL origin† | ||
| Lymph node | 16 | 6 |
| Subcutaneous | 10 | 7 |
| Liver | 4 | 3 |
| Lung | 3 | 5 |
| Large bowel | 0 | 1 |
| Intramuscular | 0 | 2 |
| Other visceral site | 2 | 1 |
Average (±Standard Error)
Some patients were treated with TIL from multiple tissues of origin
Nineteen of the 33 patients (58%) in the NMA cohort exhibited an objective tumor regression by RECIST criteria, including 16 partial responders (48%), and 3 complete responders (9%). Eleven of 23 patients (48%) who in the 6Gy TBI cohort achieved an objective response, including two complete responders (9%). Illustrative examples of clinical tumor regression are shown in Figure 1. Fifteen of 24 patients with M1a or M1b melanoma and 15 out of 32 patients with M1c disease responded to therapy. As reported previously (7;18)all patients experienced transient hematological toxicities from the lymphodepleting conditioning and received platelet and red blood cell transfusions as medically indicated. Patients were also treated for symptoms associated with high dose IL-2 therapy. All toxicities typically returned to baseline within a few days. All non-hematological grade 3 and 4 toxicities not attributable to IL-2 are listed in Table 2. There were no Grade 3 or 4 toxicities directly attributable to the infused cells. There were two treatment related mortalities, one in each cohort that resulted from acute sepsis during the neutropenic period associated with lymphodepletion about five days after TIL infusion.
Figure 1.
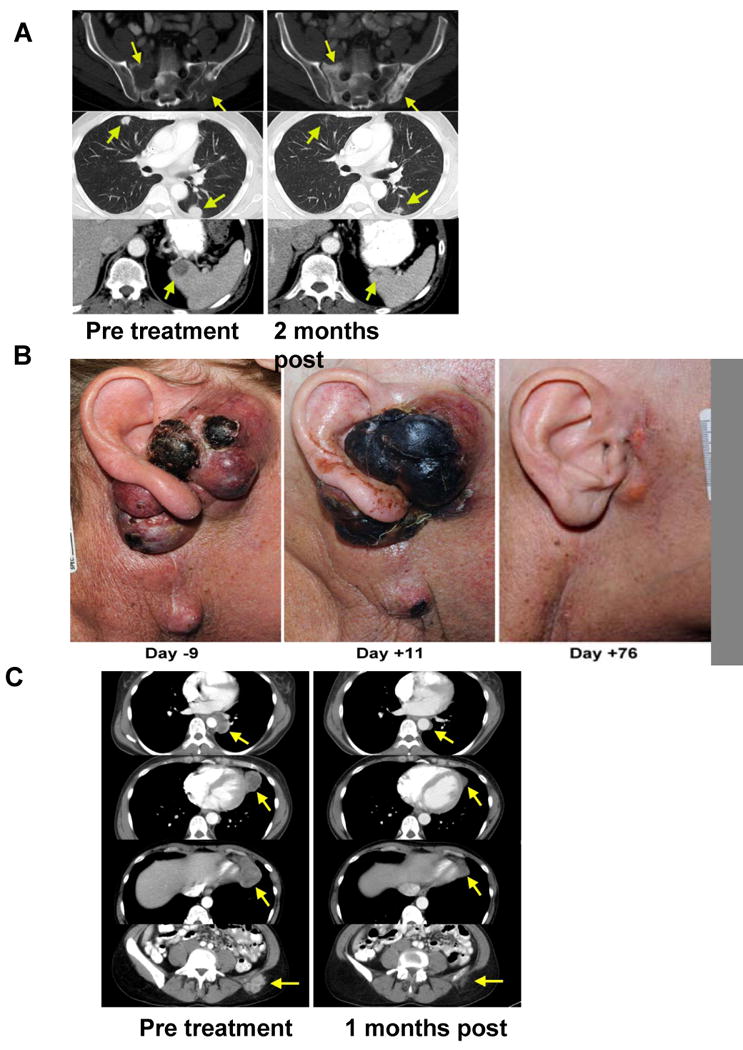
CD8+ enriched young TIL caused regression of bulky melanoma lesions at multiple sites. A) CT scans of metastatic melanoma lesions (arrows) before CD8+ enriched young TIL therapy in the sacrum and ilium (top left), lung (middle left) and spleen (lower left). All lesions showed regression at two months (right) with visible signs of recalcification of prior metastatic sites in the sacrum and ilium. B) (Left) Subcutaneous melanoma around the ear and in the auditory canal caused complete hearing loss. (Middle) Eleven days after CD8+ enriched young TIL infusion, gross necrosis of the melanoma was visible. (Right) At 76 days after treatment the patient experienced a partial response at all sites including liver and subcutaneous lesions. Tumor was absent from the auditory canal and the patient's hearing returned to normal. C) CT scans show mediastinal, lung, nodal and subcutaneous metastatic deposits before (Left) and one month after (Right) treatment with CD8+ enriched young TIL, demonstrating the rapid initial pace of tumor regression in a patient who eventually achieved a complete response.
Table 2.
Responses and toxicities to CD8+ enriched young TIL therapy. *
| NMA | 6Gy TBI | Total | |
|---|---|---|---|
| Total Patients | 33 | 23 | 56 |
| Clinical Responses (RECIST) | |||
| Non-response | 14 (42%) | 12 (52%) | 26 (46%) |
| Partial Response | 16 (48%) | 9 (39%) | 25 (45%) |
| Durations (months) | 16+, 15+, 14+, 14, 13+, 13, 12, 10, 9, 8, 8, 7, 6, 5, 3, 2 | 9+, 8+, 6, 5+, 4+, 4+, 4, 3, 2 | |
| Complete Response | 3 (9%) | 2 (9%) | 5 (9%) |
| Durations (months) | 18+,15+,12+ | 5+, 5+ | |
| Toxicities† | |||
| Positive blood culture | 8 | 4 | 12 (21%) |
| Febrile neutropenia (Grade 3) | 17 | 11 | 28 (50%) |
| Intubation | 2 | 2 | 4 (7%) |
| Treatment related death | 1 | 1 | 2 (4%) |
Updated as of April 1, 2010
Listed once for each patient at the highest grade. Usual IL-2 related toxicities not listed.
Generation of young TIL for treatment was reliable and rapid
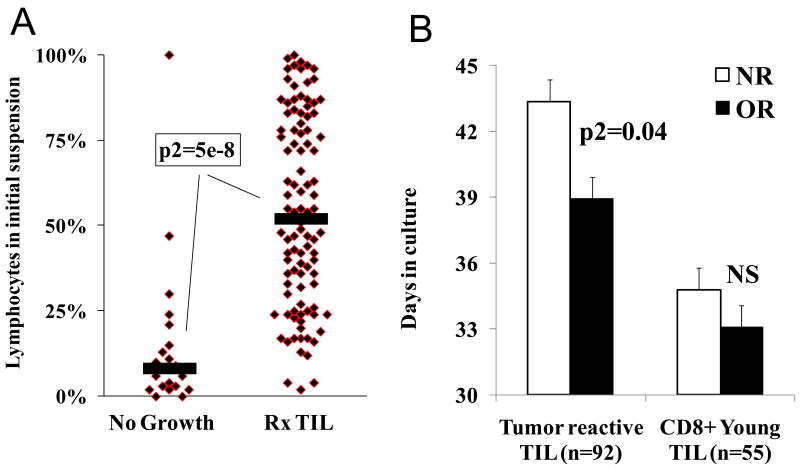
During the period of this study (August, 2008 through September, 2009) 176 tumors from 122 patients were processed to establish young TIL cultures. TIL were successfully grown (>50×106 cells within five weeks)from 124 of the 176 lesions (70%), comprising 101 of the 122 patients (83%). A striking correlation was observed between the success of establishing TIL and the initial proportion of lymphocytes in the single cell suspension (Figure 2A). Tumors that successfully yielded TIL had an initial median of 52% lymphocytes while tumors that failed to grow in vitro had a median of 8% lymphocytes (p2=5×10-8). Among these 122 patients, 53 patients were treated (three additional patients received cryopreserved TIL from prior resections), 21 patients had samples that failed to grow TIL cultures, 20 patients developed rapidly progressive disease that prevented treatment, 13 patients were resected free of evaluable disease (although nine patients recurred and received TIL treatment subsequently), nine patients received other treatments including one complete responder to high dose IL-2 therapy, and six patients experienced individual laboratory or clinical issues, including exactly one patient whose CD8+ TIL failed to expand during the REP.
Figure 2.
Process improvements for the young TIL protocol resulted in predictable, simpler TIL generation. A) The percent of lymphocytes in the initial single cell suspension correlated with TIL growth. Specimens from 122 sequential patients who were eligible for TIL therapy were processed to single cell suspensions. The fraction of lymphocytes among viable cells was determined by morphological criteria after trypan blue staining. The samples were plotted based on whether sufficient TIL grew to use for treatment (>5×107 cells in 28 days, Rx TIL) or whether growth was insufficient for treatment (No growth). Black bars indicate median values of the populations. B) Process improvements in TIL generation led to significantly younger cells administered to patients. Prior protocols required all TIL to undergo individualized testing for tumor reactivity (Specific TIL, n=92). TIL from non-responding patients (n=40, NR) spent significantly longer time in culture prior to administration than TIL from objective responders (n=52, OR). There was no difference (NS) between the time spent in culture of CD8+ enriched young TIL (Young TIL) administered to non-responding patients (n=26) or responders (n=30). Standard error bars are shown.
The CD8+ enrichment was highly effective for reducing the fraction of CD4+ cells in the infused TIL (Table 1) and Supplemental Table 1. The CD4 cell component comprised an average of 22% of the cells in bulk young TIL prior to CD8 enrichment, and NK cells comprised 21% (Supplemental Table 1). TIL from prior protocols administered without CD8 enrichment after NMA conditioning also contained about 21% CD4+ cells. Following CD8+ enrichment and expansion, CD4+ cells were reduced to an average of 2% of the infused young TIL.
The age of TIL was compared for patients who received CD8+ enriched TIL and patients on prior TIL protocols at our institution (Figure 2B). Microculture generated, tumor-selected TIL administered to responding patients were significantly younger than TIL given to patients who did not respond. In the cohorts treated in this report, there was no difference between the age of CD8+ enriched young TIL cultures for responding and non-responding patients but these cultures were significantly younger than TIL cultures administered on prior protocols (p2=3×10-7).
CD8+ enriched young TIL frequently exhibited tumor recognition
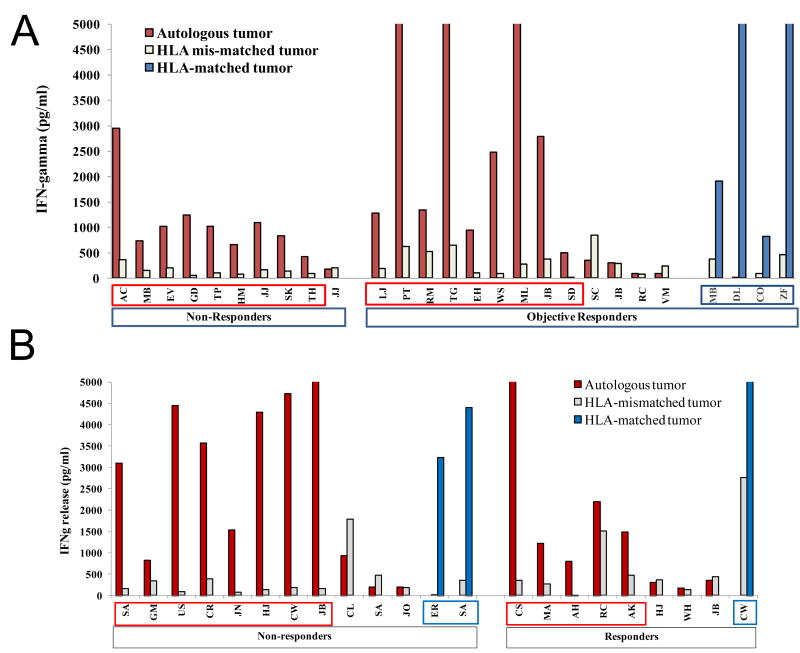
We retrospectively evaluated the ability of the administered CD8+ enriched TIL to recognize tumor and looked for any correlation with clinical efficacy. Recognition of autologous or HLA- matched tumor was evaluated by cytokine release assay (Figure 3). Twenty-three of the 33 NMA patients had autologous tumor available (18 with cryopreserved tumor digest and 5 with a tumor cell line). Eight of 10 non-responding patients and 8 of 13 objective responders demonstrated autologous tumor recognition. Four additional objective responding patients recognized HLA-A matched tumor cell lines Nineteen of 23 patients treated with 6Gy TBI had autologous tumor available (15 with fresh frozen tumor, and four with a cell line). 8 of 11 non-responding patients and 5 of 8 responding patients demonstrated specific tumor recognition. In addition, three patients' TIL recognized HLA-matched tumor cell lines, including one non-responding patient (SA) whose TIL failed to recognize autologous tumor. In total, 29 of 42 evaluable CD8+ enriched young TIL samples (69%) demonstrated specific autologous tumor recognition and 36 of 56 (64%) patients demonstrated specific recognition at all. Strikingly, 11of 30 objective responses were mediated by CD8+ enriched young TIL with no evidence of specific tumor recognition as defined in prior TIL clinical protocols.
Figure 3.
CD8+ enriched young TIL administered to patients were highly tumor reactive. TIL from cryopreserved aliquots of each infused treatment was thawed and rested overnight in IL-2, then washed and incubated at a 1:1 ratio with autologous, HLA-matched, or HLA-mismatched tumors. Interferon (IFN)-gamma secreted in the coculture supernatant was quantified by ELISA. The data from each separate coculture assay was aggregated and plotted. Patients with specific tumor recognition (greater than 200 pg/ml IFN-gamma and 2X HLA-mis-matched tumor) are boxed. Offscale >5000 pg/ml. A) CD8+ enriched young TIL administered to patients after NMA conditioning. B) CD8+ enriched young TIL administered to patients after 6Gy TBI conditioning
CD8+ enriched young TIL influenced lymphocyte reconstitution of the host
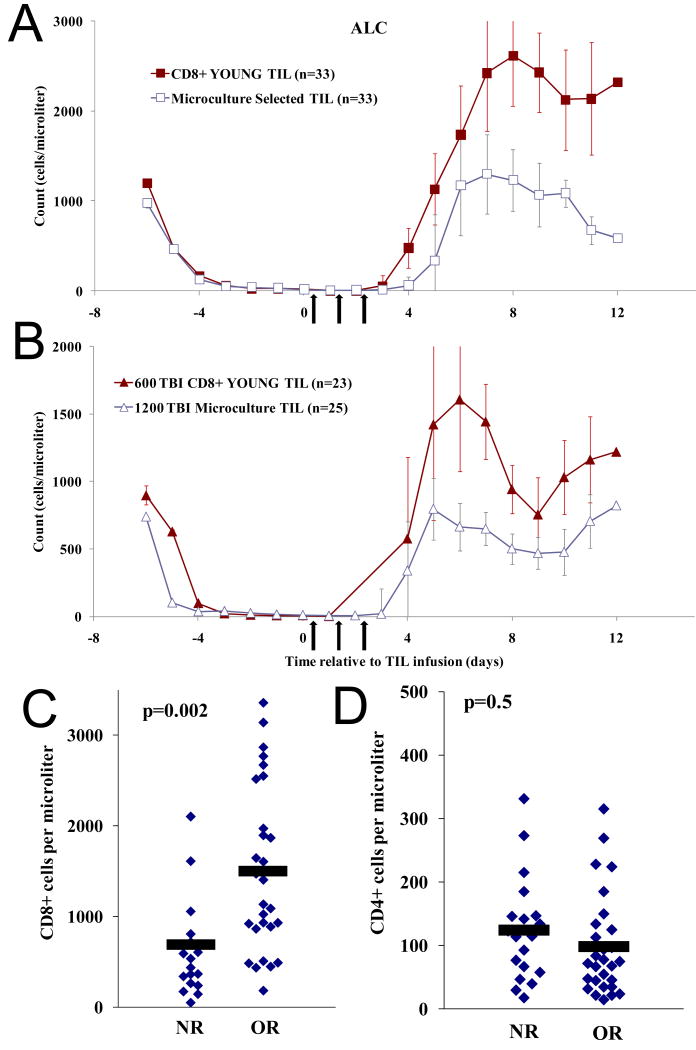
The average absolute lymphocyte count (ALC) for all patients who received CD8+ enriched young TIL after NMA or 6Gy TBI lymphodepletion is plotted in Figure 4A and 4B, respectively. For comparison, lymphocyte reconstitution from patients who received antigen selected TIL derived from microculture expansions after NMA or 12Gy TBI(7) lymphodepletion are also shown. Interestingly, patients who received CD8+ enriched young TIL demonstrated higher peak ALC's suggesting that CD8+ enriched young TIL have increased capacity for in vivo expansion compared to selected TIL
Figure 4.
CD8+ enriched young TIL impacted lymphocyte reconstitution in the peripheral blood of reconstituting patients. Arrows: IL-2 therapy A) CD8+ enriched young TIL quickly repopulated patient peripheral blood to high levels after NMA conditioning. Patient absolute lymphocyte counts (ALC) were determined daily and average ALC is plotted. Filled square: average of all patients who received CD8+ enriched young TIL with NMA (n=33). Open square: (historic control) average of all patients who received extensively expanded, tumor selected TIL with NMA as their first treatment (n=33). Not all patients had ALC determined every day. B) CD8+ enriched young TIL quickly repopulated patient peripheral blood to high levels after 6Gy TBI conditioning. Filled triangles: average of all patients who received CD8+ enriched young TIL with 6Gy TBI (n=23). Open triangles: (historic control) average of all patients who received extensively expanded, tumor selected TIL with 12Gy TBI (n=25). Vertical bars: standard error. C and D) CD8+ ALC one month after TIL infusion correlated with response. CD4+ and CD8+ ALC was determined in a blinded manner by the Clinical Center Core Immunology Laboratory for 47 of 56 patients treated with CD8+ enriched young TIL at approximately one month after TIL infusion. C: CD8+ ALC plotted for each patient. D: CD4+ ALC plotted for each patient. NR: non-responder, OR: objective responder. Black bars: mean ALC for the population.
PBL from 47 of the 56 patients who received CD8+ enriched young TIL were sampled at approximately one month after cell infusion. ALC and absolute CD3+CD8+ and CD3+CD4+ cell numbers were assessed by FACS analysis in a blinded manner. The treatment resulted in low CD4 counts at one month in most patients (average: 109 CD4+ cells/ul), with no difference between responding and non-responding patients (p2=0.5; Figure 4D). Patients in prior clinical trials who received TIL that contained CD4+ lymphocytes had higher peripheral CD4+ cell counts at one month after TIL infusion (average: 187 cells/ul). In contrast to CD4+ cells, CD8+ ALC for most patients treated with CD8+ enriched young TIL was in the normal range at one month, and there was a significant increase in CD8+ ALC in responding patient compared to non-responders (p2=.002; Figure 4C). Patients who responded had CD8+ ALC about two fold higher than non-responders (1504 vs. 696 cells/ul). In prior clinical trials the average CD8+ cell count was 652 CD8+ cells/ul at one month after TIL infusion. Infused TIL were examined by FACS for expression of additional markers including CD27, CD28, CD62L, CCR7, but none correlated with clinical response.
Discussion
Adoptive cell therapy can mediate durable objective regression (including durable complete responses) in patients with metastatic melanoma refractory to other treatments. The complexity of preparation of cell products unique for each patient has limited the widespread use of this method. In the current study we developed a simplified method for cell preparation that may allow wider application of this effective treatment.
Previously, ninety-three patients were treated over 84 months using highly expanded TIL selected for specific tumor recognition(7)Corresponding to about one treatment per month and about 27% of resected patients who finally received a TIL product(8). In the current report 56 additional patients were treated with CD8+ enriched young TIL over 14 months, corresponding to about 3 to 4 patients treated per month. with 53% of eligible patients who underwent resection able to receive TIL therapy. With both methods, clinical response rates were about 55%, but strikingly 11 objective responders out of 30 in the current study had TIL that would have been ineligible for the prior study. While comparisons of sequential clinical trials should be made with caution, the changes in the CD8+ enriched young TIL methods were apparently effective for improving delivery of individualized TIL therapy to eligible patients without compromising therapeutic efficacy.
Prior clinical trials with TIL in our institution(7)as well as preclinical mouse models (20-22)suggested that increased lymphodepletion improves ACT. The current study comparing two cohorts of patients treated with different conditioning regimens found similar objective response rates and toxicity profiles. This outcome could result from relatively small patient numbers, sequential cohort enrolment, and short follow up. Alternately, 6Gy of TBI delivered in fractionated doses may only minimally impact host mechanisms that support the transferred CD8+ cells compared to 12Gy TBI(23).
Despite methodological improvements, 21of the 123 patients (17%) had TIL that failed to grow in culture. On average, these melanoma lesions started with a median of only 8% lymphocytes (Figure 2A). In the future, changing the lymphocyte:tumor ratio in vitro could improve the generation of TIL for some tumors. Additionally, some studies have noted a correlation between good prognosis and T cell infiltration for non-melanoma cancers including colon cancer(24), breast cancer(25), ovarian cancer(26), and non-small cell lung cancer(27), suggesting that CD8+ enriched young TIL from metastatic lesions from non-melanoma histologies may be therapeutically active in ACT treatments.
The enrichment of CD8+ cells prior to rapid expansion has practical benefits for TIL generation(16) but the enrichment process is expensive, and depletion of CD4+ cells raises questions about lymphocyte persistence, tumor regression and treatment toxicity. CD4+ T helper cells are involved in generating and maintaining CD8+ T memory cells in mice (28;29) and a CD4+ clone was associated with melanoma regression in a patient(30) However, CD4+ T regulatory cells may block CD8+ cell function in tumor immunity(20;31). The impact of CD4+ cells on the persistence and function of CD8 TIL in vivo is not known. In the current study, the CD8+ enriched young TIL repopulated peripheral blood and persisted at high levels for over a month. Furthermore, the objective response rate of 54% reported here is comparable to the 56% objective response rate observed in prior trials with CD4+ replete TIL. This data suggests that elimination of the CD4+ cells was beneficial to the therapy. More study is needed to understand the role of CD4+ CD25+ FoxP3+ cells in the regulation of TIL responses after infusion and CD4+ T helper cells in the duration of responses. To directly address the impact of CD4+ cells in young TIL therapy, we have initiated a randomized clinical trial where one arm receives unselected young TIL that contain both CD4+ and CD8+ cells, while a second arm receives CD8+ enriched young TIL.
The current study presents data from two cohorts of patients treated with autologous TIL following lymphodepletion for metastatic melanoma. The production process for CD8+ young TIL was relatively rapid and reliable, and the manufactured product was capable of generating tumor regression and objective responses in patients. The minimally manipulated CD8+ cell product had a high frequency of autologous tumor reactivity, and repopulated the host PBL compartment rapidly. Persistently high CD8+ cell numbers were correlated with tumor regression in treated patients. This method for the generation of CD8+ enriched young TIL represents a major simplification in the application of therapeutically effective TIL because of the ease of cell production (one culture vs. many microcultures) and the elimination of testing for anti-tumor specificity. These simplifications can serve to extend the ability to apply this treatment approach to additional institutions.
Supplementary Material
Footnotes
Statement of Translational Relevance: Cell-based immunotherapy using autologous, tumor-reactive lymphocytes can mediate the regression of bulky, established cancer in animal models and human patients. Tumor infiltrating lymphocytes (TIL) selected for tumor recognition and highly expanded in vitro have been especially effective for treating melanoma patients, but there are logistic and technical difficulties associated with generating an individualized tumor-reactive therapy for each patient. In this report we use simple, reliable methods of generating individual, autologous TIL products, and test them for the first time in a two-arm, Phase II clinical trial. Our results support a wider application of individualized cell therapies based on TIL, and define correlates of clinical treatment and response for improvement of future therapies.
Reference List
- 1.Smith FO, Downey SG, Klapper JA, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Restifo NP, Levy CL, et al. Treatment of metastatic melanoma using interleukin-2 alone or in conjunction with vaccines. Clin Cancer Res. 2008 Sep 1;14(17):5610–8. doi: 10.1158/1078-0432.CCR-08-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tawbi HA, Kirkwood JM. Management of metastatic melanoma. Semin Oncol. 2007 Dec;34(6):532–45. doi: 10.1053/j.seminoncol.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Poulikakos PI, Zhang C, Bollag G, Shokat KM, Rosen N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature. 2010 Mar 18;464(7287):427–30. doi: 10.1038/nature08902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, Restifo NP, Haworth LR, Seipp CA, Freezer LJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003 Jul 8;100(14):8372–7. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N Engl J Med. 2010 Jun 14; doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008 Apr;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008 Nov 10;26(32):5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goff SL, Smith FO, Klapper JA, Yang JC, Sherry R, Wunderlich JR, Steinberg SM, White DE, Rosenberg SA, Dudley ME. Tumor Infiltrating Lymphocyte (TIL) Therapy for Metastatic Melanoma: Analysis of Tumors Resected for TIL. 2010 doi: 10.1097/CJI.0b013e3181f05b91. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tran KQ, Zhou J, Durflinger KH, Langhan MM, Shelton TE, Wunderlich JR, Robbins PF, Rosenberg SA, Dudley ME. Minimally cultured tumor-infiltrating lymphocytes display optimal characteristics for adoptive cell therapy. J Immunother. 2008 Oct;31(8):742–51. doi: 10.1097/CJI.0b013e31818403d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou J, Shen X, Huang J, Hodes RJ, Rosenberg SA, Robbins PF. Telomere length of transferred lymphocytes correlates with in vivo persistence and tumor regression in melanoma patients receiving cell transfer therapy. J Immunol. 2005 Nov 15;175(10):7046–52. doi: 10.4049/jimmunol.175.10.7046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Khong HT, Dudley ME, el Gamil M, Li YF, Rosenberg SA, Robbins PF. Survival, persistence, and progressive differentiation of adoptively transferred tumor-reactive T cells associated with tumor regression. J Immunother. 2005 May;28(3):258–67. doi: 10.1097/01.cji.0000158855.92792.7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang J, Kerstann KW, Ahmadzadeh M, Li YF, el Gamil M, Rosenberg SA, Robbins PF. Modulation by IL-2 of CD70 and CD27 expression on CD8+ T cells: importance for the therapeutic effectiveness of cell transfer immunotherapy. J Immunol. 2006 Jun 15;176(12):7726–35. doi: 10.4049/jimmunol.176.12.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou J, Dudley ME, Rosenberg SA, Robbins PF. Persistence of multiple tumor-specific T-cell clones is associated with complete tumor regression in a melanoma patient receiving adoptive cell transfer therapy. J Immunother. 2005 Jan;28(1):53–62. doi: 10.1097/00002371-200501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Schallmach E, Kubi A, Shalmon B, Hardan I, Catane R, et al. Minimally cultured or selected autologous tumor-infiltrating lymphocytes after a lympho-depleting chemotherapy regimen in metastatic melanoma patients. J Immunother. 2009 May;32(4):415–23. doi: 10.1097/CJI.0b013e31819c8bda. [DOI] [PubMed] [Google Scholar]
- 15.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D, Kubi A, Hovav E, Chermoshniuk N, et al. Clinical Responses in a Phase II Study Using Adoptive Transfer of Short-term Cultured Tumor Infiltration Lymphocytes in Metastatic Melanoma Patients. Clin Cancer Res. 2010 Apr 20; doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 16.Prieto PA, Durflinger KH, Wunderlich JR, Rosenberg SA, Dudley ME. Enrichment of CD8+ cells from melanoma tumor infiltrating lymphocyte cultures reveals tumor reactivity for use in adoptive cell therapy. J Immunother. 2010 Jun;33(5):547–556. doi: 10.1097/CJI.0b013e3181d367bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003 Jul;26(4):332–42. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005 Apr 1;23(10):2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riddell SR, Greenberg PD. The use of anti-CD3 and anti-CD28 monoclonal antibodies to clone and expand human antigen-specific T cells. JImmunol Methods. 1990 Apr 17;128(2):189–201. doi: 10.1016/0022-1759(90)90210-m. [DOI] [PubMed] [Google Scholar]
- 20.Antony PA, Piccirillo CA, Akpinarli A, Finkelstein SE, Speiss PJ, Surman DR, Palmer DC, Chan CC, Klebanoff CA, Overwijk WW, et al. CD8+ T cell immunity against a tumor/self-antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005 Mar 1;174(5):2591–601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gattinoni L, Finkelstein SE, Klebanoff CA, Antony PA, Palmer DC, Spiess PJ, Hwang LN, Yu Z, Wrzesinski C, Heimann DM, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005 Oct 3;202(7):907–12. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulos CM, Wrzesinski C, Kaiser A, Hinrichs CS, Chieppa M, Cassard L, Palmer DC, Boni A, Muranski P, Yu Z, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007 Aug;117(8):2197–204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wrzesinski C, Paulos CM, Kaiser A, Muranski P, Palmer DC, Gattinoni L, Yu Z, Rosenberg SA, Restifo NP. Increased intensity lymphodepletion enhances tumor treatment efficacy of adoptively transferred tumor-specific T cells. J Immunother. 2010 Jan;33(1):1–7. doi: 10.1097/CJI.0b013e3181b88ffc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005 Dec 22;353(25):2654–66. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 25.Denkert C, Loibl S, Noske A, Roller M, Muller BM, Komor M, Budczies J, rb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010 Jan 1;28(1):105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 26.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003 Jan 16;348(3):203–13. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 27.Kawai O, Ishii G, Kubota K, Murata Y, Naito Y, Mizuno T, Aokage K, Saijo N, Nishiwaki Y, Gemma A, et al. Predominant infiltration of macrophages and CD8(+) T Cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer. 2008 Sep 15;113(6):1387–95. doi: 10.1002/cncr.23712. [DOI] [PubMed] [Google Scholar]
- 28.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003 Apr 11;300(5617):337–9. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 29.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003 Apr 11;300(5617):339–42. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008 Jun 19;358(25):2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999 Nov 15;163(10):5211–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.