Abstract
Most targeted anticancer drugs are inhibitors of kinases that are aberrantly activated in cancer cells. However, the mechanisms by which kinase inhibitors suppress tumor growth remain unclear. In this study, we found that UCN-01, a staurosporine analogue and broad-range kinase inhibitor used in clinical trials, inhibits colon cancer cell growth by inducing apoptosis via PUMA, a BH3-only Bcl-2 family member and a p53 target. PUMA expression was markedly elevated in a p53-independent fashion following UCN-01 treatment. The induction of PUMA by UCN-01 was mediated by direct binding of FoxO3a to the PUMA promoter following inhibition of AKT signaling. Deficiency in PUMA abrogated UCN-01-induced apoptosis, caspase activation, and mitochondrial dysfunction, and rendered UCN-01 resistance in a clonogenic assay, whereas elevated PUMA expression or a BH3 mimetic sensitized UCN-01-induced apoptosis. Chemosensitization by UCN-01 appeared to involve simultaneous PUMA induction through both p53-dependent and -independent mechanisms. Furthermore, deficiency in PUMA suppressed the anti-tumor effects of UCN-01 in a xenograft model, concurrent with reduced apoptosis and caspase activation in vivo. These results suggest that PUMA-mediated apoptosis is pivotal for the anticancer activities of UCN-01, and possibly other clinically used kinase inhibitor drugs, and that PUMA manipulation may be useful for improving their anticancer activities.
Keywords: PUMA, FoxO3a, UCN-01, apoptosis, mitochondria, AKT, colon cancer
Introduction
Inhibition of aberrant kinase signaling in cancer cells represents one of the most effective approaches for anticancer therapy. Most recently approved anticancer drugs are inhibitors of kinase signaling pathways required for tumor cell growth. However, there is little understanding of how kinase inhibition leads to therapeutic response. Virtually all kinase inhibitor drugs inhibit multiple kinases, and their anticancer activities are often attributed to off-target effects. One critical anticancer mechanism of kinase inhibitors is their ability to induce apoptosis (1). For example, clinical response to epidermal growth factor receptor (EGFR) targeted anticancer therapies is correlated with induction of apoptosis in tumor cells (2).
It is well known that several broad-range kinase inhibitors, such as staurosporine (STS) and its more selective derivative UCN-01 (7-hydroxystaurosporine), have anticancer activities. UCN-01 is being evaluated in a number of clinical trials as a single agent or chemosensitizer (http://clinicaltrials.gov). It can potentiate cell cycle arrest and apoptosis induced by a variety of chemotherapeutic agents, such as cisplatin, topotecan, and 5-Fluorouracil (5-FU) (3). UCN-01 inhibits a variety of kinases involved in regulating cell cycle progression and apoptosis, such as cyclin-dependent kinases (CDKs), checkpoint kinase 1 (CHK1), protein kinase C (PKC), phosphoinositide-dependent protein kinase 1 (PDK1), and AKT (4). Although the effects of UCN-01 on cell cycle checkpoints have been well characterized, the mechanism by which UCN-01 promotes apoptosis remains unclear. Recent studies suggest that UCN-01 can modulate Bcl-2 family members to potentiate apoptosis in cancer cells (5, 6).
PUMA, p53-upregulated modulator of apoptosis, is a BH3-only Bcl-2 family member and a potent inducer of apoptosis. Transcription of PUMA is activated by p53 in response to DNA damaging agents such as γ-irradiation and common chemotherapeutic drugs (7). PUMA binds to all five anti-apoptotic Bcl-2 family members, such as Bcl-2 and Bcl-XL, which relieves their inhibition of Bax and Bak, leading to mitochondrial membrane permeabilization, and subsequently caspase cascade activation (7). PUMA knockout renders resistance to p53-dependent apoptosis induced by genotoxic agents in human cancer cells and mice (8–10). Nevertheless, p53-dependent regulation of PUMA is dysfunctional in most cancer cells due to p53 abnormalities, causing survival of tumor cells and therapeutic resistance. PUMA also mediates p53-independent apoptosis induced by a variety of non-genotoxic stimuli, such as TNF-α (11), serum starvation (12), cytokine withdrawal (13), STS (10, 14), glucocorticoids (9, 10), and ischemia/reperfusion (15). Several transcription factors, including p65, p73, and Forkhead Box O3a (FoxO3a), have been implicated in p53-independent PUMA induction. For example, PUMA is induced in response to cytokine deprivation by FoxO3a (13, 16), whose activity is negatively regulated by phosphorylation via AKT (17).
In this study, we investigated how PUMA is induced by the kinase inhibitors UCN-01 and STS, and its role in UCN-01-induced apoptosis and chemosensitization. We found FoxO3a-mediated PUMA induction is pivotal for the anticancer effects of UCN-01. The results provide novel mechanistic insight into therapeutic response to kinase inhibitors, and may have broad implications for their future applications.
Materials and Methods
Cell culture and treatment
The human colorectal cancer cell lines, including HCT116, RKO, Lim2405, LOVO, (all WT p53), and HT29 and DLD1, (both mutant p53), were obtained from American Type Culture Collection (Manassas, VA) before 2002. The isogenic cell lines included the previously described p53-knockout (KO), p21-KO, PUMA-KO, p21-KO/PUMA-KO (from Dr. Bert Vogelstein at Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins in 2002) (8), p21-KO/p53 binding site-KO (BS-KO) HCT116 cells, and PUMA-KO DLD1 cells created in our laboratory in 2007 (14). Cell lines were last tested and authenticated for absence of mycoplasma, genotypes, drug response, and morphology in our laboratory in April, 2010.
All cell lines were cultured in McCoy’s 5A modified media (Invitrogen, Carlsbad, CA) supplemented with 10% defined FBS (HyClone, Logan, UT), 100 units/ml penicillin, and 100 μg/ml streptomycin (Invitrogen). Cells were maintained in a 37°C incubator at 5% CO2. For drug treatment, cells were plated in 12-well plates at 20–30% density 24 hr prior to treatment. The DMSO (Sigma, St. Louis, MO) stocks of the agents used, including UCN-01, STS, Wortmannin, LY294002 (Sigma), and triciribine (Enzo Life Sciences AG, Lausen, Switzerland), and MK-2206 (MediMol, Centereach, NY, USA), were diluted into appropriate concentrations with the cell culture medium. Cisplatin (Sigma) was diluted with 0.9% NaCl.
Western blotting and treatments
Western blotting was performed as previously described (11). The following antibodies were used: PUMA (8), FoxO3a (07702MI, Millipore, Billerica, MA), phospho-FoxO3a (9464, Cell Signaling, Beverly, MA), AKT (9272, Cell Signaling), phospho-AKT (S473) (4058, Cell Signaling), active caspase 3 (9661, Cell Signaling), CoxIV (A21348, Invitrogen), cytochrome c (7159, Santa Cruz Biotechnology, Santa Cruz, CA), β-actin (A5441, Sigma), and α-tubulin (CP06, Oncogene Science, Cambridge, MA).
Real-time Reverse Transcriptase (RT) PCR
Total RNA was isolated from UCN-01-or STS-treated cells using the Mini RNA Isolation II Kit (Zymo Research, Orange, CA) according to the manufacturer’s protocol. One μg of total RNA was used to generate cDNA using SuperScript II reverse transcriptase (Invitrogen). Real-time PCR was carried out as before for PUMA and GAPDH (11).
Transfection and siRNA knockdown
Cells were transfected with Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. pCMV, FoxO3a triple mutant (FoxO3a TM; Addgene, Cambridge, MA), WT and constitutively active AKT vectors were used in the transfection. siRNA knockdown was performed 24 hr before UCN-01 or STS treatment using 400 pmoles of FoxO3a (ON-TARGETplus J-003007-10) or the control scrambled siRNA (Dharmacon, Chicago, IL).
Luciferase assays
PUMA luciferase reporter construct was generated by cloning a genomic fragment (WT fragment: −500 to +739 bp) containing two FoxO3a sites located within the first intron of PUMA into the pBV-Luc plasmid as previously described (12). Mutations were introduced into the FoxO3a binding sites using QuickChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA). For reporter assays, cells were transfected with the WT or mutant PUMA reporter along with the transfection control β-galactosidase reporter pCMVβ (Promega, Madison, WI, USA). Cell lysates were collected and luciferase activities were measured as previously described (12). All reporter experiments were performed in triplicate and repeated three times.
Chromatin immunoprecipitation (ChIP)
ChIP was performed using the Chromatin Immunoprecipitation Assay kit (Upstate Biotechnology, Lake Placid, NY, USA) as previously described (14), with FoxO3a antibody for chromatin precipitation. The precipitates were analyzed by PCR using primers 5′GCGCACAGGTGCCTCGGC 3′ and 5′ TGGGTGTGGCCGCCCCT 3′.
Stable knockdown of FoxO3a
The sequence of FoxO3a siRNA used for transient knockdown was cloned into the pSUPER vector (OligoEngine, Seattle, WA). The shRNA construct was transfected into HCT116 cells, and cells were plated in 96-well plates in the presence of puromycin (2 μg/ml, Invitrogen). After puromycin-resistant clones were isolated, Western blotting was used to identify clones with reduced FoxO3a levels.
Analysis of apoptosis
Analysis of apoptosis by nuclear staining with Hoechst 33258 (Invitrogen) was performed as previously described (18). Annexin V/propidium iodide (PI) staining was performed using annexin Alexa 488 (Invitrogen) and PI as described (14). For analysis of cytochrome c release, mitochondrial and cytosolic fractions were isolated by the differential centrifugation method previously described (19), and probed by Western blotting for cytochrome c. For colony formation assays, the treated cells were plated in 12-well plates at appropriate dilutions, and allowed to grow for 10–14 days before staining with crystal violet (Sigma). For detection of mitochondrial membrane potential change, the treated cells were stained by MitoTracker Red CMXRos (Invitrogen) for 15 min at room temperature, and then analyzed by flow cytometry.
Xenograft studies
All animal experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Female 5–6 week-old Nu/Nu mice (Charles River, Wilmington, MA) were housed in a sterile environment with micro isolator cages and allowed access to water and chow ad libitum. Mice were injected subcutaneously in both flanks with 4×106 p21-KO or p21-KO/PUMA-KO HCT116 cells. Following tumor growth for 7 days, mice were injected intraperitoneally (i.p.) for 5 consecutive days with 9 mg/kg UCN-01 (20) diluted in 20 mM sodium citrate buffer, pH 6. UCN-01 used for the animal study was obtained from Kwoya Hakko Kirin, Co. Ltd. (Tokyo, Japan) through the Developmental Therapeutics Program at the National Cancer Institute (NCI). Mice were euthanized when tumors reached ~ 1 cm3 in size. Tumors were dissected and fixed in 10% formalin before paraffin embedding.
Immunostaining
Immunostaining was carried out on 5 μM paraffin-embedded tumor sections. TUNEL and active caspase 3 immunofluorescence was performed as previously described (21), with an AlexaFluor 594-conjugated secondary antibody (Invitrogen) for detection. p-AKT and p-FoxO3a immunohistochemistry was performed using the same antibodies used for Western blotting, and the ABC/DAB method for detection.
Statistical Analysis
Statistical analyses were carried out using GraphPad Prism IV software. p values were calculated by the student’s t-test and were considered significant if p <0.05. The means ± one standard deviation (s.d.) were displayed in the figures.
Results
PUMA is induced by UCN-01 in a p53-indepdendent manner
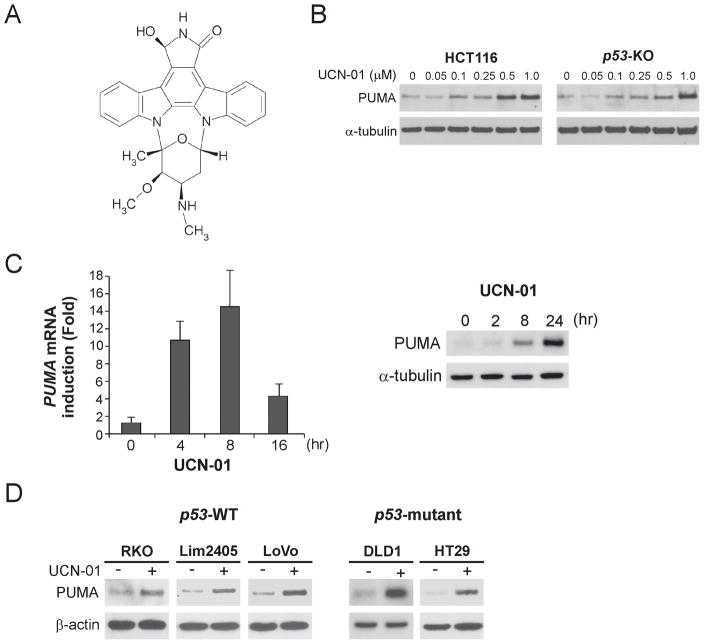
The requirement for PUMA in STS-induced apoptosis (10, 14) prompted us to investigate whether it contributes to apoptosis induced by UCN-01, a STS derivative and more selective kinase inhibitor that has been tested in clinical trials (Fig. 1A). PUMA was found to be induced by 0.1–1.0 μM of UCN-01 in a dose-dependent manner in both WT and p53-KO HCT116 colon cancer cells (Fig. 1B). Both PUMA mRNA and protein were induced by UCN-01 within several hours, with the peak level of PUMA mRNA induction at 8 hr (Fig. 1C, left panel), and that of protein at 24 hr (Fig. 1C, right panel). This induction time course was similar to that of STS (Fig. S1A and B), which also induced PUMA independently of p53 (Fig. S1C). The induction of PUMA by UCN-01 or STS could be blocked by the transcription inhibitor actinomycin D (data not shown). Furthermore, UCN-01 treatment resulted in PUMA induction in five additional colon cancer cell lines regardless of their p53 status (Fig. 1D). Taken together, these data indicate that PUMA can be induced by the broad-spectrum kinase inhibitor UCN-01 through p53-independent transcription.
Figure 1. p53-independent PUMA induction by UCN-01 in colon cancer cells.
A. Chemical structure of UCN-01. B. WT and p53-knockout (p53-KO) HCT116 colon cancer cells were treated with increasing doses of UCN-01 for 24 hr. PUMA expression was analyzed by Western blotting. C. Left: p53-KO HCT116 cells were treated with 1 μM UCN-01 and mRNA extracted at indicated time points. Reverse transcription followed by real-time PCR was completed as described in Materials and Methods. Right: Following treatment of p53-KO HCT116 cells with 1 μM UCN-01, PUMA protein expression at the indicated times was analyzed by Western blotting. D. Five colon cancer cell lines with different p53 status were treated with UCN-01 for 24 hr. PUMA expression was analyzed by Western blotting.
FOXO3a directly activates PUMA transcription following UCN-01 treatment
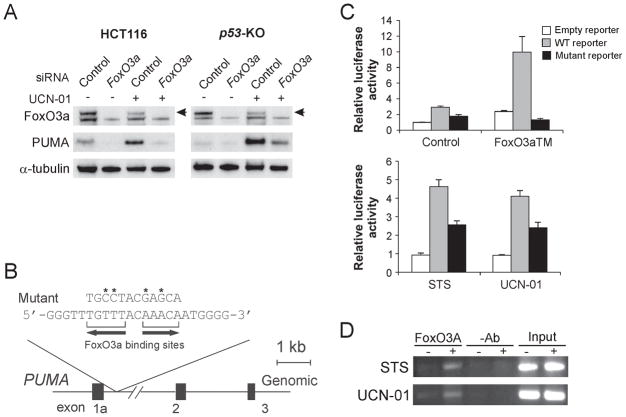
PUMA can be induced by the transcription factor FoxO3a in T and mast cells following cytokine deprivation (13, 16). Since UCN-01 was shown to inhibit AKT (22), which phosphorylates FoxO3a and prevents its nuclear localization (17), we determined whether FoxO3a and AKT are involved in PUMA induction by UCN-01. Expression of a constitutively active FoxO3a mutant (FoxO3aTM) induced PUMA in p53-KO cells (Fig. S2A). siRNA depletion of FoxO3a in WT and p53-KO HCT116 cells abrogated PUMA induction by UCN-01 or STS (Fig. 2A and data not shown), indicating the requirement of FoxO3a for the induction of PUMA following kinase inhibition.
Figure 2. FoxO3a directly activates PUMA transcription following UCN-01 treatment.
A. p53-KO HCT116 cells were transfected with either a control scrambled siRNA or a FoxO3a siRNA for 24 hr, and then treated with 1 μM UCN-01 for 24 hr. FoxO3a and PUMA expression were probed by Western blotting. Arrow denotes FoxO3a-specific band. B. Schematic representation of the genomic structure of PUMA highlighting the two FoxO3a binding sites (AAACA) within the first intron. Asterisks indicate binding site mutations. C. Upper: p53-KO HCT116 cells were transfected with a reporter plasmid containing the WT or mutant FoxO3a binding sites in the PUMA promoter, along with a FoxO3a triple mutant (FoxO3aTM) expression construct or the control empty vector. The reporter activities were measured by luciferase assay on the next day. Lower: p53-KO HCT116 cells were transfected overnight with the reporter containing the WT or mutant FoxO3a binding sites, and then treated with 60 nM staurosporine (STS) or 0.5 μM UCN-01 for 16 hr. Luciferase activity was quantified. D. Chromatin immunoprecipitation (ChIP) was performed on fixed p53-KO HCT116 cells following 8 hr 60 nM STS or 1 μM UCN-01 treatment. An antibody specific for FoxO3a or no antibody was used to show specificity. PCR was carried out using primers surrounding the FoxO3a binding sites in the PUMA promoter.
A conserved FoxO binding element (AAACA) was previously identified in the human and mouse PUMA promoters (13). We noted that in addition to this site, there is another FoxO3a binding element 2 bases apart within the first intron of PUMA, but in the reverse orientation (Fig. 2B). To determine the role of these putative FoxO3a binding sites in PUMA induction, luciferase reporters containing a 1.1-kb fragment including these two sites were constructed and analyzed. Both FoxO3a binding sites were also mutated to dissect out their specific role in PUMA induction (Fig. 2B). Transfection of FoxO3aTM or treating cells with UCN-01 or STS markedly activated the WT PUMA reporter. In contrast, the binding site mutations abolished the responsiveness of the PUMA reporter to FoxO3aTM, and also abrogated the effects of STS and UCN-01 (Fig. 2C). To determine whether FoxO3a directly activates the PUMA promoter, chromatin immunoprecipitation (ChIP) was performed on lysates from STS- or UCN-01-treated cells using the FoxO3a antibody. The binding of FoxO3a to the PUMA promoter was significantly enhanced after treatment with either compound (Fig. 2C). Taken together, these data suggest that FoxO3a can directly bind to the PUMA promoter to activate its transcription following STS or UCN-01 treatment.
AKT inhibition drives PUMA induction by UCN-01
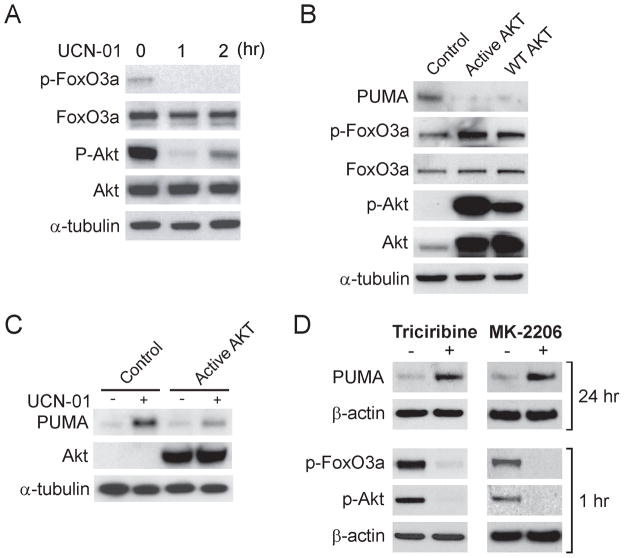
We then determined whether AKT, an antiapoptotic kinase often activated aberrantly in cancer cells, is involved in the effect of UCN-01 on PUMA expression. UCN-01 treatment markedly inhibited AKT phosphorylation at S473, concurrent with reduced FoxO3a phosphorylation (Fig. 3A), which is known to prevent its nuclear translocation and ensuing transactivation (17). On the other hand, transfection with WT or constitutively active AKT induced FoxO3a phosphorylation, suppressed basal PUMA expression (Fig. 3B), and overcame the induction of PUMA by UCN-01 (Fig. 3C). Furthermore, treating cells with the AKT inhibitor triciribine or MK-2206, an AKT inhibitor in clinical trials, was sufficient to induce PUMA, subsequent to diminished AKT and FoxO3a phosphorylation (Fig. 3D). In addition, PUMA could also be induced similarly by the phosphatidylinositol 3-kinase (PI3K) inhibitors wortmannin and LY294002 in p53-KO cells (Fig. S2B). Therefore, the induction of PUMA by FoxO3a following UCN-01 treatment seemed to be mediated through AKT inhibition.
Figure 3. Induction of PUMA by UCN-01 is mediated through AKT inhibition.
A. p53-KO HCT116 cells were treated with 1 μM UCN-01 for 1 or 2 hr. Western blotting was used to analyze phospho-FoxO3a (p-FoxO3a), total FoxO3a, phospho-AKT (p-AKT), and total AKT. B. p53-KO cells were transfected for 24 hr with WT, constitutively active mutant AKT, or control empty vector. Indicated proteins were probed by Western blotting. C. p53-KO cells were transfected for 24 hr with constitutively active mutant AKT or control empty vector, and then were treated with 1 μM UCN-01 for 24 hr. PUMA and AKT expression were probed by Western blotting. D. p53-KO cells were treated with the AKT inhibitor triciribine (1 μM) or MK-2206 (25 μM) for 1 hr (for analysis of p-FoxO3a and p-AKT) or 24 hr (for analysis of PUMA), and indicated proteins were analyzed by Western blotting.
PUMA plays an essential role in UCN-01-induced apoptosis
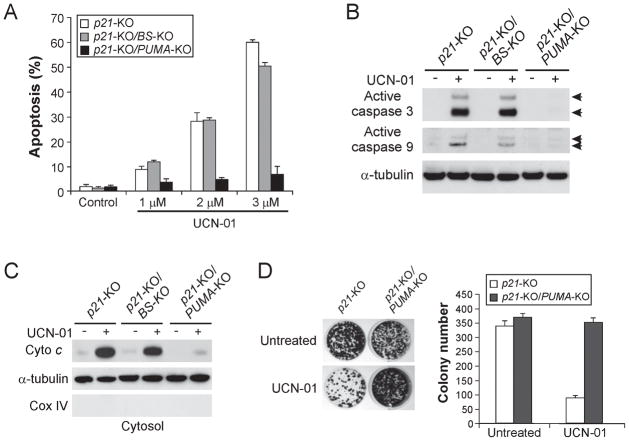
In order to investigate the role of PUMA in UCN-01-induced apoptosis, we analyzed p21-KO cells with different PUMA status because they harbor a more pronounced apoptotic phenotype due to defects in cell cycle checkpoints (23). UCN-01 treatment induced dose-dependent apoptosis in p21-KO cells, with over 50% apoptotic cells detected following treatment with 3 μM UCN-01 (Fig. 4A). Remarkably, UCN-01-induced apoptosis was almost completely abolished in PUMA-deficient p21-KO cells (Fig. 4A). The requirement of PUMA in UCN-01-induced apoptosis is not cell line specific, as the apoptosis was also abolished in the previously described PUMA-knockout DLD1 colon cancer cells (14) (Fig. S3A). Analysis of apoptosis by Annexin V/PI staining confirmed reduced apoptosis in PUMA-deficient cells (Fig. S3B). PUMA deficiency abrogated UCN-01-induced caspase 9 and caspase 3 activation (Fig. 4B), cytosolic release of cytochrome c (Fig. 4C), as well as mitochondrial membrane potential change (Fig. S3C). PUMA-deficient cells were also found to be significantly more resistant to UCN-01 than WT cells in a long-term clonogenic assay (Fig. 4D). As a control, we also analyzed p21-KO cells with a deletion of the p53 binding sites in the PUMA promoter (p21-KO/BS-KO), which abolishes apoptosis induced by p53 and DNA damaging agents (14). The apoptotic effect of UCN-01 remained intact in these cells, confirming the dispensability of p53. Furthermore, transient knockdown of FoxO3a by a siRNA or stable depletion of FoxO3a with a shRNA suppressed PUMA transcription and UCN-01-induced apoptosis (Fig. S3D and data not shown). Collectively, these results demonstrate that PUMA is essential for UCN-01-induced apoptosis in colon cancer cells.
Figure 4. PUMA mediates the apoptotic and anticancer activities of UCN-01 through the mitochondrial pathway.
HCT116 cells with different genotypes, including p21-KO, p21-KO/p53 binding site-KO (BS-KO), and p21-KO/PUMA-KO, were treated with 0–3 μM UCN-01 for 48 hr. Apoptosis was analyzed using several methods. A. Apoptosis was measured by nuclear fragmentation assay following staining with Hoechst 33258. B. Activation of caspase 3 and caspase 9 in the cells treated with 3 μM UCN-01 was analyzed by Western blotting. C. Cytosolic fractions were isolated from the cells treated with 3 μM UCN-01 and probed for cytochrome c by Western blotting. Cytochrome oxidase subunit IV (Cox IV), which is a mitochondrial marker, was analyzed as the control for fractionation. α-tubulin was used as a control for loading. D. Colony formation assay was performed by seeding a equal number of UCN-01 (2 μM)-treated p21-KO and p21-KO/PUMA-KO cells in 12-well plates, and then staining attached cells with crystal violet 14 days later. Left: Representative pictures of colonies. Right: Quantification of colony numbers. The results were averages of 3 experiments ± standard deviation.
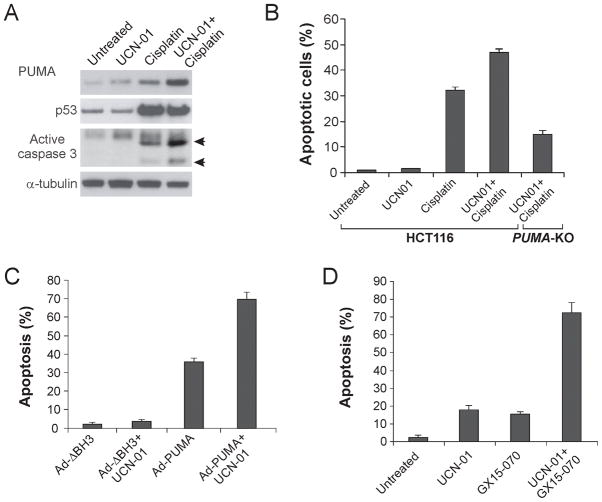
PUMA mediates chemosensitization by UCN-01 and enhances its anticancer activity
UCN-01 has mostly been used as a chemosensitizer and can potentiate the effects of common chemotherapeutic drugs such as cisplatin (3). Since both UCN-01 and cisplatin induce PUMA-dependent apoptosis (Fig. 4, S4A and S4B), we asked whether chemosensitization by UCN-01 involves PUMA induction. A combination of UCN-01 and cisplatin induced PUMA at a much higher level in HCT116 cells compared with UCN-01 or cisplatin alone (Fig. 5A), consistent with two distinct p53-dependent and -independent mechanisms of PUMA induction by these agents. Apoptosis and caspase 3 activation was also markedly enhanced by the combination treatment in HCT116 cells (Fig. 5A and 5B). However, the enhanced apoptosis and caspase activation by the combination treatment was greatly reduced in the PUMA-KO cells (Fig. 5B and S4C), suggesting that p53-independent induction of PUMA by UCN-01 mediates its chemosensitization effect. We also found that an adenovirus expressing PUMA (Ad-PUMA), but not a BH3 domain-deleted mutant (Ad-ΔBH3), enhanced UCN-01-induced apoptosis in HCT116 cells (Fig. 5C). Furthermore, GX15-070, a PUMA-like BH3 mimetic compound capable of binding to all five antiapoptotic Bcl-2 family members, significantly enhanced UCN-01 induced apoptosis (Fig. 5D). These observations suggest that manipulating apoptosis regulators such as PUMA can potentiate the anticancer effects of kinase inhibitors.
Figure 5. PUMA is required for the chemosensitization effects of UCN-01 and enhances its anticancer activity.
A. WT HCT116 cells were treated with 0.5 μM UCN-01, 50 μM cisplatin, or their combination for 24 hr. PUMA, p53, and active caspase 3 were analyzed by Western blotting. B. WT and PUMA-KO HCT116 cells were treated with 0.5 μM UCN-01, 50μM cisplatin, or their combination for 48 hr. Apoptosis was determined by nuclear staining with Hoechst 33258. C. p21-KO HCT116 cells were treated with 0.5 μM UCN-01, alone or in combination with an adenovirus (10 MOI) expressing WT PUMA (Ad-PUMA) or mutant PUMA with BH3 domain deletion (Ad-ΔBH3). Apoptosis was determined by nuclear staining 48 hr after treatment. D. p21-KO HCT116 cells were treated with 2μM UCN-01, alone or in combination with 1μM of the BH3 mimetic GX15-070. Apoptosis was determined by nuclear staining 48 hr after treatment. The results were averages of three independent experiments ± standard deviation.
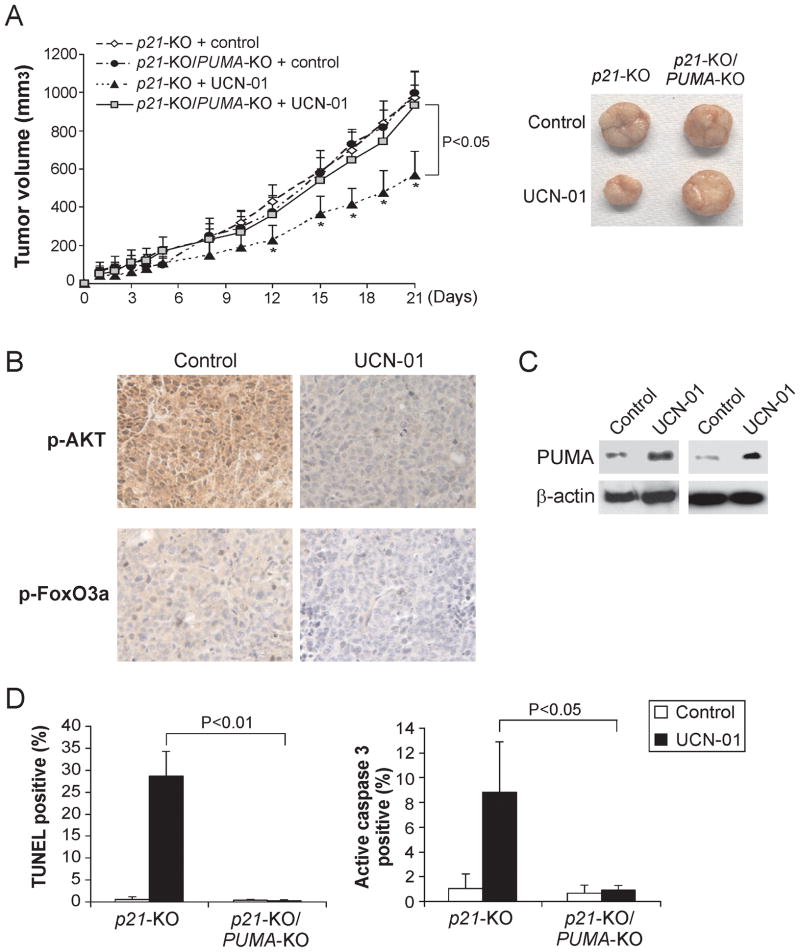
PUMA contributes to the anti-tumor activity of UCN-01 in a xenograft model
To determine whether the PUMA-dependent apoptotic effect of UCN-01 contributes to its anti-tumor activity in vivo, p21-KO and p21-KO/PUMA-KO HCT116 cells were injected into nude mice to establish xenograft tumors. Tumor-bearing mice were treated with 9 mg/kg of UCN-01 for 5 days as previously described (20), and tumor volumes were measured every 2–3 days for 3 weeks. The p21-KO tumors responded to the UCN-01 treatment with slower growth, and were roughly half the size of the untreated tumors on day 12 following treatment (Fig. 6A). In contrast, the p21-KO/PUMA-KO tumors were indistinguishable from the untreated mice, and did not respond to UCN-01 treatment (Fig. 6A). UCN-01 treatment suppressed AKT and FoxO3a phosphorylation (Fig. 6B), and induced PUMA expression in the p21-KO tumors (Fig. 6C). TUNEL and active caspase 3 staining revealed significant apoptosis induction in the p21-KO tumors, which was almost completely abolished in the p21-KO/PUMA-KO tumors (Fig. 6D and S5). These data clearly demonstrate the necessity of PUMA for the in vivo anti-tumor and apoptotic effects of UCN-01.
Figure 6. PUMA mediates the anti-tumor effect of UCN-01 in a xenograft model.
A. Nude mice were injected s.c. with 4×106 p21-KO or p21-KO/PUMA-KO HCT116 cells. After one week, mice were treated with 9 mg/kg of UCN-01 or the control sodium citrate buffer by i.p. injection for 5 consecutive days. Left: Tumor volume was calculated and plotted. Right: Representative tumors at the end of experiment. B. Paraffin-embedded tumor sections were stained for p-AKT and p-FoxO3a according to Materials and Methods. C. PUMA expression in representative tumors with or without UCN-01 treatment was analyzed by Western blotting. D. Left: Following staining tumor sections for TUNEL according to Materials and Methods, TUNEL-positive cells were counted and plotted. Right: Tumor sections were stained for active caspase 3 according to Materials and Methods. Active caspase 3-positive cells were counted and plotted.
Discussion
Our results provide novel mechanistic insight into the anticancer mechanism of the broad-range kinase inhibitor UCN-01. Most of the previous studies have focused on the effect of UCN-01 on cell cycle checkpoints, which is widely believed to be its major mode of action in anticancer therapy. Several reports suggest UCN-01 can potentiate apoptosis via the mitochondrial pathway (24–26). However, the exact mechanism underlying this activity is unclear, and the role of apoptosis in the therapeutic response to UCN-01 remains conjecture. Our results demonstrate for the first time that UCN-01 treatment leads to PUMA induction by FoxO3a following AKT inhibition. Induction of PUMA accounts for most, if not all, of in vitro and in vivo activities of UCN-01 against colon cancer cells, and such a requirement for PUMA was not affected by the genetic background and p53 status. In addition to PUMA, other BH3-only proteins, such as Bim, may also contribute to the effect of UCN-01 on other tumor types (6). It is notable that UCN-01 induces apoptosis in solid tumor cells typically at micromolar concentrations, which are significantly higher than those required for checkpoint inhibition (27, 28). The UCN-01 dose used in our study (1.0 μM) is similar to those used in the previous apoptosis studies (5, 29, 30). Pharmacokinetic analyses showed that UCN-01 plasma concentrations can be as high as 30 μM (31, 32), suggesting that apoptosis-inducing concentrations are achievable.
UCN-01 is commonly used as a chemosensitizer in combination with other anticancer agents. It has additive or even synergistic effects on apoptosis induced by a variety of commonly used chemotherapeutic drugs, such as 5-fluorouracil (33), topotecan (34), tamoxifen (35), and mitomycin C (36). UCN-01 can also potentiate a number of non-genotoxic agents, such as the CDK inhibitor roscovitine (37), the mTOR inhibitor rapamycin (38), the PKC activator PMA (25), and the NF-κB inhibitor BAY 11-7082 (39). Research into its mechanism of action revealed activation of the ERK pathway by UCN-01 (40), supporting a rationale of inhibiting the proliferative ERK signal to enhance the activity of UCN-01. It is exciting that a number of ERK inhibiting agents were found to potentiate UCN-01-induced apoptosis, such as the MEK inhibitor PD184352 (41), the farnesyltransferase inhibitor L744832 (42), and the Hsp90 antagonist 17-AAG (43). These studies highlight the multitudinous ways UCN-01 can be used in chemotherapy, as well as the importance of investigating UCN-01 modes of action to design rational combinations. Our results suggest enhanced PUMA induction may be a novel mechanism of chemosensitization by UCN-01. The expression of PUMA is controlled by several proapoptotic transcription factors in a cell type- and stimuli-dependent manner (7). Combinations of different classes of PUMA-inducing agents may allow simultaneous PUMA induction via multiple pathways, thereby lowering the threshold proapoptotic activity required for apoptosis induction.
Targeting cancer-specific molecular changes has brought new hopes to therapeutic intervention of cancer. Most of the targeted drugs proven to be clinically useful are inhibitors of the kinase signaling pathways that are required for tumor cell growth. In addition to STS and UCN-01, PUMA can also be induced by other kinase inhibitors, such as the MEK inhibitor U0126 (44), phosphoinositide-3 kinase (PI3K) inhibitors wortmanin and LY294002 (12, 45), the human epidermal growth factor receptor (HER)/vascular endothelial growth factor receptor (VEGFR) inhibitor BMS-690514 (46), and the farnesyltransferase inhibitor BMS-214662 (47). It has recently been shown that PUMA can be induced by the clinically used EGFR inhibitors, gefitinib and erlotinib, in head and neck cancer cells, and its expression correlated with therapeutic response to the EGFR inhibitors (48). Therefore, PUMA may play a broad functional role in determining response to targeted therapies, and the induction of PUMA may serve as a marker for predicting therapeutic response to these agents.
Maintenance of tumor phenotypes is dependent upon the suppression of apoptosis by certain pro-survival proteins, because neoplastic transformation would normally trigger an apoptotic response (49). The ability to reinstate the induction of apoptosis in cancer cells is an attractive approach for anticancer therapy. It is perhaps not surprising that apoptosis has emerged as a critical therapeutic endpoint of kinase inhibitors (1). The induction of PUMA by kinase inhibitors primarily occurs via p53-independent mechanisms. Such induction would bypass the p53 network that is required for the efficacy of common chemotherapeutics. Another way of circumventing the p53 pathway would be to use compounds that directly target the apoptotic machinery. For example, agents that mimic the BH3 domains of the proapoptotic Bcl-2 family members, such as ABT-263 and GX15-070, are currently being tested in clinical trials (50). The effectiveness of Ad-PUMA and GX15-070 in enhancing UCN-01-induced apoptosis supports a rationale for combining kinase inhibitors and apoptosis targeting agents.
In conclusion, we demonstrated that PUMA is a critical mediator of therapeutic response to UCN-01, and possibly other kinase inhibitors, in colon cancer cells. Our study may serve as a proof-of-principle for targeting PUMA to improve therapeutic effects of kinase inhibitors, for using PUMA expression as a biomarker for predicting therapeutic response, and for rational design of new therapeutic regimens.
Supplementary Material
Acknowledgments
The authors would like to thank our lab members for helpful discussion. This work is supported in part by the NIH grant CA106348, CA121105, American Cancer Society Grant RSG-07-156-01-CNE (L.Z.), the Flight Attendant Medical Research Institute (FAMRI), the Alliance for Cancer Gene Therapy (ACGT), the NIH grant CA129829 (J.Y.), PhRMA Foundation postdoctoral fellowship, NIH NRSA postdoctoral fellowship F32CA139882 (C.D.), and China Scholarship Council (R.P.). L.Z. is a scholar of the V Foundation for Cancer Research.
Grant Support: NIH grant CA106348, CA121105, American Cancer Society Grant RSG-07-156-01-CNE (L.Z.), the Flight Attendant Medical Research Institute (FAMRI), the Alliance for Cancer Gene Therapy (ACGT), the NIH grant CA129829 (J.Y.), PhRMA Foundation postdoctoral fellowship, NIH NRSA postdoctoral fellowship F32CA139882 (C.D.), and China Scholarship Council (R.P.).
Abbreviations
- CDK
cyclin-dependent kinases
- ChIP
Chromatin immunoprecipitation
- CHK1
checkpoint kinase 1
- EGFR
epidermal growth factor receptor
- FoxO3a
Forkhead Box O3a
- 5-FU
5-Fluorouracil
- KO
knockout
- PDK1
phosphoinositide-dependent protein kinase 1
- PI3K
phosphoinositide-3 kinase
- PKC
protein kinase C
- RT-PCR
reverse transcriptase-PCR
- shRNA
small hairpin RNA
- siRNA
small interfering RNA
- STS
staurosporine
- VEGFR
vascular endothelial growth factor receptor
References
- 1.Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight? J Clin Invest. 2005;115:2610–7. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science. 2004;305:1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 3.Tse AN, Carvajal R, Schwartz GK. Targeting checkpoint kinase 1 in cancer therapeutics. Clin Cancer Res. 2007;13:1955–60. doi: 10.1158/1078-0432.CCR-06-2793. [DOI] [PubMed] [Google Scholar]
- 4.Swanton C. Cell-cycle targeted therapies. Lancet Oncol. 2004;5:27–36. doi: 10.1016/s1470-2045(03)01321-4. [DOI] [PubMed] [Google Scholar]
- 5.Bhonde MR, Hanski ML, Magrini R, et al. The broad-range cyclin-dependent kinase inhibitor UCN-01 induces apoptosis in colon carcinoma cells through transcriptional suppression of the Bcl-x(L) protein. Oncogene. 2005;24:148–56. doi: 10.1038/sj.onc.1207842. [DOI] [PubMed] [Google Scholar]
- 6.Pei XY, Dai Y, Tenorio S, et al. MEK1/2 inhibitors potentiate UCN-01 lethality in human multiple myeloma cells through a Bim-dependent mechanism. Blood. 2007;110:2092–101. doi: 10.1182/blood-2007-04-083204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Zhang L. PUMA, a potent killer with or without p53. Oncogene. 2008;27 (Suppl 1):S71–83. doi: 10.1038/onc.2009.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci U S A. 2003;100:1931–6. doi: 10.1073/pnas.2627984100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeffers JR, Parganas E, Lee Y, et al. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell. 2003;4:321–8. doi: 10.1016/s1535-6108(03)00244-7. [DOI] [PubMed] [Google Scholar]
- 10.Villunger A, Michalak EM, Coultas L, et al. p53- and Drug-Induced Apoptotic Responses Mediated by BH3-Only Proteins Puma and Noxa. Science. 2003;302:1036–8. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 11.Wang P, Qiu W, Dudgeon C, et al. PUMA is directly activated by NF-kappaB and contributes to TNF-alpha-induced apoptosis. Cell Death Differ. 2009;16:1192–202. doi: 10.1038/cdd.2009.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ming L, Sakaida T, Yue W, Jha A, Zhang L, Yu J. Sp1 and p73 Activate PUMA Following Serum Starvation. Carcinogenesis. 2008;29:1878–84. doi: 10.1093/carcin/bgn150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.You H, Pellegrini M, Tsuchihara K, et al. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–63. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P, Yu J, Zhang L. The nuclear function of p53 is required for PUMA-mediated apoptosis induced by DNA damage. Proc Natl Acad Sci U S A. 2007;104:4054–9. doi: 10.1073/pnas.0700020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu B, Qiu W, Wang P, et al. p53 independent induction of PUMA mediates intestinal apoptosis in response to ischaemia-reperfusion. Gut. 2007;56:645–54. doi: 10.1136/gut.2006.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekoff M, Kaufmann T, Engstrom M, et al. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood. 2007;110:3209–17. doi: 10.1182/blood-2007-02-073957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–87. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 18.Kohli M, Yu J, Seaman C, et al. SMAC/Diablo-dependent apoptosis induced by nonsteroidal antiinflammatory drugs (NSAIDs) in colon cancer cells. Proc Natl Acad Sci U S A. 2004;101:16897–902. doi: 10.1073/pnas.0403405101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Wang P, Ming L, Wood MA, Zhang L. SMAC/Diablo mediates the proapoptotic function of PUMA by regulating PUMA-induced mitochondrial events. Oncogene. 2007;26:4189–98. doi: 10.1038/sj.onc.1210196. [DOI] [PubMed] [Google Scholar]
- 20.Kurata N, Kuwabara T, Tanii H, et al. Pharmacokinetics and pharmacodynamics of a novel protein kinase inhibitor, UCN-01. Cancer Chemother Pharmacol. 1999;44:12–8. doi: 10.1007/s002800050939. [DOI] [PubMed] [Google Scholar]
- 21.Qiu W, Carson-Walter EB, Liu H, et al. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–83. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato S, Fujita N, Tsuruo T. Interference with PDK1-Akt survival signaling pathway by UCN-01 (7-hydroxystaurosporine) Oncogene. 2002;21:1727–38. doi: 10.1038/sj.onc.1205225. [DOI] [PubMed] [Google Scholar]
- 23.Facchinetti MM, De Siervi A, Toskos D, Senderowicz AM. UCN-01-induced cell cycle arrest requires the transcriptional induction of p21(waf1/cip1) by activation of mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase pathway. Cancer Res. 2004;64:3629–37. doi: 10.1158/0008-5472.CAN-03-3741. [DOI] [PubMed] [Google Scholar]
- 24.Dai Y, Dent P, Grant S. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) promotes mitochondrial dysfunction and apoptosis induced by 7-hydroxystaurosporine and mitogen-activated protein kinase kinase inhibitors in human leukemia cells that ectopically express Bcl-2 and Bcl-xL. Mol Pharmacol. 2003;64:1402–9. doi: 10.1124/mol.64.6.1402. [DOI] [PubMed] [Google Scholar]
- 25.Rahmani M, Grant S. UCN-01 (7-hydroxystauorsporine) blocks PMA-induced maturation and reciprocally promotes apoptosis in human myelomonocytic leukemia cells (U937) Cell Cycle. 2002;1:273–81. doi: 10.4161/cc.1.4.137. [DOI] [PubMed] [Google Scholar]
- 26.McKinstry R, Qiao L, Yacoub A, et al. Inhibitors of MEK1/2 interact with UCN-01 to induce apoptosis and reduce colony formation in mammary and prostate carcinoma cells. Cancer Biol Ther. 2002;1:243–53. doi: 10.4161/cbt.75. [DOI] [PubMed] [Google Scholar]
- 27.Kohn EA, Ruth ND, Brown MK, Livingstone M, Eastman A. Abrogation of the S phase DNA damage checkpoint results in S phase progression or premature mitosis depending on the concentration of 7-hydroxystaurosporine and the kinetics of Cdc25C activation. J Biol Chem. 2002;277:26553–64. doi: 10.1074/jbc.M202040200. [DOI] [PubMed] [Google Scholar]
- 28.Levesque AA, Fanous AA, Poh A, Eastman A. Defective p53 signaling in p53 wild-type tumors attenuates p21waf1 induction and cyclin B repression rendering them sensitive to Chk1 inhibitors that abrogate DNA damage-induced S and G2 arrest. Mol Cancer Ther. 2008;7:252–62. doi: 10.1158/1535-7163.MCT-07-2066. [DOI] [PubMed] [Google Scholar]
- 29.Patel V, Lahusen T, Leethanakul C, et al. Antitumor activity of UCN-01 in carcinomas of the head and neck is associated with altered expression of cyclin D3 and p27(KIP1) Clin Cancer Res. 2002;8:3549–60. [PubMed] [Google Scholar]
- 30.Bredel M, Pollack IF, Freund JM, Rusnak J, Lazo JS. Protein kinase C inhibition by UCN-01 induces apoptosis in human glioma cells in a time-dependent fashion. J Neurooncol. 1999;41:9–20. doi: 10.1023/a:1006047025425. [DOI] [PubMed] [Google Scholar]
- 31.Perez RP, Lewis LD, Beelen AP, et al. Modulation of cell cycle progression in human tumors: a pharmacokinetic and tumor molecular pharmacodynamic study of cisplatin plus the Chk1 inhibitor UCN-01 (NSC 638850) Clin Cancer Res. 2006;12:7079–85. doi: 10.1158/1078-0432.CCR-06-0197. [DOI] [PubMed] [Google Scholar]
- 32.Lara PN, Jr, Mack PC, Synold T, et al. The cyclin-dependent kinase inhibitor UCN-01 plus cisplatin in advanced solid tumors: a California cancer consortium phase I pharmacokinetic and molecular correlative trial. Clin Cancer Res. 2005;11:4444–50. doi: 10.1158/1078-0432.CCR-04-2602. [DOI] [PubMed] [Google Scholar]
- 33.Sigmond J, Comijn EM, Kamphuis JA, Peters GJ. Combinations of 5-fluorouracil with UCN-01 or staurosporine. Nucleosides Nucleotides Nucleic Acids. 2004;23:1503–6. doi: 10.1081/NCN-200027718. [DOI] [PubMed] [Google Scholar]
- 34.Redkar A, Mixter P, Daoud SS. Implications of p53 in growth arrest and apoptosis on combined treatment of human Mammary epithelial cells with topotecan and UCN-01. J Exp Ther Oncol. 2004;4:213–22. [PubMed] [Google Scholar]
- 35.Koh J, Kubota T, Koyama T, et al. Combined antitumor activity of 7-hydroxystaurosporine (UCN-01) and tamoxifen against human breast carcinoma in vitro and in vivo. Breast Cancer. 2003;10:260–7. doi: 10.1007/BF02966727. [DOI] [PubMed] [Google Scholar]
- 36.Sugiyama K, Shimizu M, Akiyama T, et al. UCN-01 selectively enhances mitomycin C cytotoxicity in p53 defective cells which is mediated through S and/or G(2) checkpoint abrogation. Int J Cancer. 2000;85:703–9. doi: 10.1002/(sici)1097-0215(20000301)85:5<703::aid-ijc17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Cory AH, Somerville L, Cory JG. Blockage of cyclin cdk’s, PKC and phosphoinositol 3-kinase pathways leads to augmentation of apoptosis in drug-resistant leukemia cells: evidence for interactive effects of flavopiridol, LY 294002, roscovitine, wortmannin and UCN-01. Anticancer Res. 2005;25:101–6. [PubMed] [Google Scholar]
- 38.Hahn M, Li W, Yu C, Rahmani M, Dent P, Grant S. Rapamycin and UCN-01 synergistically induce apoptosis in human leukemia cells through a process that is regulated by the Raf-1/MEK/ERK, Akt, and JNK signal transduction pathways. Mol Cancer Ther. 2005;4:457–70. doi: 10.1158/1535-7163.MCT-04-0137. [DOI] [PubMed] [Google Scholar]
- 39.Dai Y, Pei XY, Rahmani M, Conrad DH, Dent P, Grant S. Interruption of the NF-kappaB pathway by Bay 11-7082 promotes UCN-01-mediated mitochondrial dysfunction and apoptosis in human multiple myeloma cells. Blood. 2004;103:2761–70. doi: 10.1182/blood-2003-09-3037. [DOI] [PubMed] [Google Scholar]
- 40.Dai Y, Yu C, Singh V, et al. Pharmacological inhibitors of the mitogen-activated protein kinase (MAPK) kinase/MAPK cascade interact synergistically with UCN-01 to induce mitochondrial dysfunction and apoptosis in human leukemia cells. Cancer Res. 2001;61:5106–15. [PubMed] [Google Scholar]
- 41.Dai Y, Landowski TH, Rosen ST, Dent P, Grant S. Combined treatment with the checkpoint abrogator UCN-01 and MEK1/2 inhibitors potently induces apoptosis in drug-sensitive and -resistant myeloma cells through an IL-6-independent mechanism. Blood. 2002;100:3333–43. doi: 10.1182/blood-2002-03-0940. [DOI] [PubMed] [Google Scholar]
- 42.Pei XY, Dai Y, Rahmani M, Li W, Dent P, Grant S. The farnesyltransferase inhibitor L744832 potentiates UCN-01-induced apoptosis in human multiple myeloma cells. Clin Cancer Res. 2005;11:4589–600. doi: 10.1158/1078-0432.CCR-04-2346. [DOI] [PubMed] [Google Scholar]
- 43.Jia W, Yu C, Rahmani M, et al. Synergistic antileukemic interactions between 17-AAG and UCN-01 involve interruption of RAF/MEK- and AKT-related pathways. Blood. 2003;102:1824–32. doi: 10.1182/blood-2002-12-3785. [DOI] [PubMed] [Google Scholar]
- 44.Wang YF, Jiang CC, Kiejda KA, Gillespie S, Zhang XD, Hersey P. Apoptosis induction in human melanoma cells by inhibition of MEK is caspase-independent and mediated by the Bcl-2 family members PUMA, Bim, and Mcl-1. Clin Cancer Res. 2007;13:4934–42. doi: 10.1158/1078-0432.CCR-07-0665. [DOI] [PubMed] [Google Scholar]
- 45.Han J, Flemington C, Houghton AB, et al. Expression of bbc3, a pro-apoptotic BH3-only gene, is regulated by diverse cell death and survival signals. Proc Natl Acad Sci U S A. 2001;98:11318–23. doi: 10.1073/pnas.201208798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de La Motte Rouge T, Galluzzi L, Olaussen KA, et al. A novel epidermal growth factor receptor inhibitor promotes apoptosis in non-small cell lung cancer cells resistant to erlotinib. Cancer Res. 2007;67:6253–62. doi: 10.1158/0008-5472.CAN-07-0538. [DOI] [PubMed] [Google Scholar]
- 47.Gomez-Benito M, Marzo I, Anel A, Naval J. Farnesyltransferase inhibitor BMS-214662 induces apoptosis in myeloma cells through PUMA up-regulation, Bax and Bak activation, and Mcl-1 elimination. Mol Pharmacol. 2005;67:1991–8. doi: 10.1124/mol.104.007021. [DOI] [PubMed] [Google Scholar]
- 48.Sun Q, Ming L, Thomas SM, et al. PUMA mediates EGFR tyrosine kinase inhibitor-induced apoptosis in head and neck cancer cells. Oncogene. 2009;18:2348–57. doi: 10.1038/onc.2009.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 50.Labi V, Erlacher M, Kiessling S, Villunger A. BH3-only proteins in cell death initiation, malignant disease and anticancer therapy. Cell Death Differ. 2006;13:1325–38. doi: 10.1038/sj.cdd.4401940. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.