Abstract
The first essential step in protein photoreception is the capture and storage of energy from a photon. We have recently identified and isolated, from the purple photoautotrophic bacterium, Ectothiorhodospira halophila, a 13,000-dalton photoactive yellow protein (PYP) that has a photocycle with kinetics similar to sensory rhodopsin and a very high quantum efficiency. To study the structural chemistry of protein photoreception, we determined, refined, and analyzed the crystallographic structure of PYP at 2.4 A resolution and report here that it is composed of two perpendicular antiparallel beta-sheets that enclose the chromophore. Each of the 10 beta-strands of PYP is connected directly to its nearest neighbor with +1 topology. Globally, an asymmetric distribution of side chains places aromatic and acidic side chains in an ellipsoidal band around the chromophore with a cluster of basic side chains on one side. Locally, the electron density maps place an internal lysine and the chromophore in an apparent Schiff base linkage stabilized by a buried glutamate and a tyrosine side chain. To our knowledge, the atomic resolution structure of a protein with a reversible photoisomerization has not been reported previously. Furthermore, PYP may also represent a class of proteins that bind conjugated molecules and interact with a secondary receptor system.
Full text
PDF
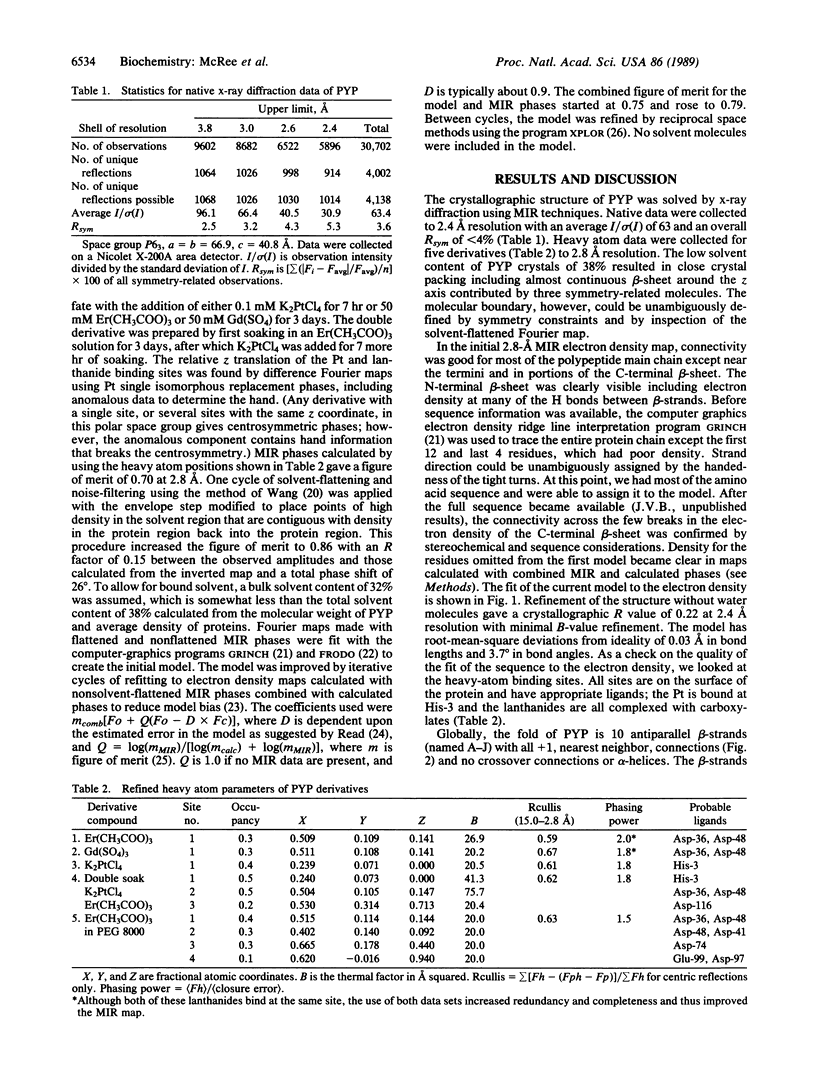
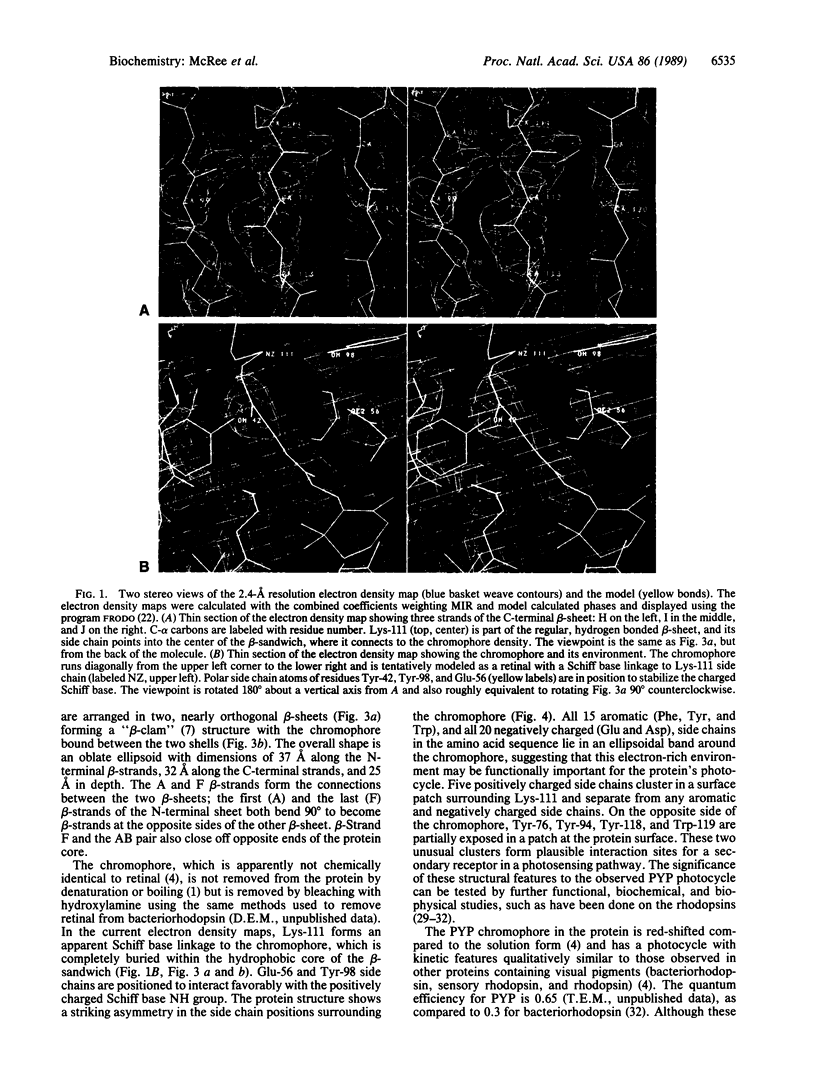


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bignetti E., Cavaggioni A., Pelosi P., Persaud K. C., Sorbi R. T., Tirindelli R. Purification and characterisation of an odorant-binding protein from cow nasal tissue. Eur J Biochem. 1985 Jun 3;149(2):227–231. doi: 10.1111/j.1432-1033.1985.tb08916.x. [DOI] [PubMed] [Google Scholar]
- Brooks D. E., Means A. R., Wright E. J., Singh S. P., Tiver K. K. Molecular cloning of the cDNA for two major androgen-dependent secretory proteins of 18.5 kilodaltons synthesized by the rat epididymis. J Biol Chem. 1986 Apr 15;261(11):4956–4961. [PubMed] [Google Scholar]
- Brünger A. T., Kuriyan J., Karplus M. Crystallographic R factor refinement by molecular dynamics. Science. 1987 Jan 23;235(4787):458–460. doi: 10.1126/science.235.4787.458. [DOI] [PubMed] [Google Scholar]
- Cavaggioni A., Sorbi R. T., Keen J. N., Pappin D. J., Findlay J. B. Homology between the pyrazine-binding protein from nasal mucosa and major urinary proteins. FEBS Lett. 1987 Feb 23;212(2):225–228. doi: 10.1016/0014-5793(87)81349-2. [DOI] [PubMed] [Google Scholar]
- Connolly M. L. Solvent-accessible surfaces of proteins and nucleic acids. Science. 1983 Aug 19;221(4612):709–713. doi: 10.1126/science.6879170. [DOI] [PubMed] [Google Scholar]
- Drayna D., Fielding C., McLean J., Baer B., Castro G., Chen E., Comstock L., Henzel W., Kohr W., Rhee L. Cloning and expression of human apolipoprotein D cDNA. J Biol Chem. 1986 Dec 15;261(35):16535–16539. [PubMed] [Google Scholar]
- Engelhard M., Gerwert K., Hess B., Kreutz W., Siebert F. Light-driven protonation changes of internal aspartic acids of bacteriorhodopsin: an investigation by static and time-resolved infrared difference spectroscopy using [4-13C]aspartic acid labeled purple membrane. Biochemistry. 1985 Jan 15;24(2):400–407. doi: 10.1021/bi00323a024. [DOI] [PubMed] [Google Scholar]
- Hayward S. B., Stroud R. M. Projected structure of purple membrane determined to 3.7 A resolution by low temperature electron microscopy. J Mol Biol. 1981 Sep 25;151(3):491–517. doi: 10.1016/0022-2836(81)90007-3. [DOI] [PubMed] [Google Scholar]
- Holden H. M., Rypniewski W. R., Law J. H., Rayment I. The molecular structure of insecticyanin from the tobacco hornworm Manduca sexta L. at 2.6 A resolution. EMBO J. 1987 Jun;6(6):1565–1570. doi: 10.1002/j.1460-2075.1987.tb02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Schneider M., Epp O., Mayr I., Messerschmidt A., Pflugrath J., Kayser H. Crystallization, crystal structure analysis and preliminary molecular model of the bilin binding protein from the insect Pieris brassicae. J Mol Biol. 1987 May 20;195(2):423–434. doi: 10.1016/0022-2836(87)90661-9. [DOI] [PubMed] [Google Scholar]
- Huber R., Schneider M., Mayr I., Müller R., Deutzmann R., Suter F., Zuber H., Falk H., Kayser H. Molecular structure of the bilin binding protein (BBP) from Pieris brassicae after refinement at 2.0 A resolution. J Mol Biol. 1987 Dec 5;198(3):499–513. doi: 10.1016/0022-2836(87)90296-8. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Bergfors T., Sedzik J., Unge T. The three-dimensional structure of P2 myelin protein. EMBO J. 1988 Jun;7(6):1597–1604. doi: 10.1002/j.1460-2075.1988.tb02985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. H., Wells R. G., Reed R. R. Isolation of an olfactory cDNA: similarity to retinol-binding protein suggests a role in olfaction. Science. 1987 Feb 27;235(4792):1053–1056. doi: 10.1126/science.3493528. [DOI] [PubMed] [Google Scholar]
- McRee D. E., Meyer T. E., Cusanovich M. A., Parge H. E., Getzoff E. D. Crystallographic characterization of a photoactive yellow protein with photochemistry similar to sensory rhodopsin. J Biol Chem. 1986 Oct 15;261(29):13850–13851. [PubMed] [Google Scholar]
- Meyer T. E. Isolation and characterization of soluble cytochromes, ferredoxins and other chromophoric proteins from the halophilic phototrophic bacterium Ectothiorhodospira halophila. Biochim Biophys Acta. 1985 Jan 23;806(1):175–183. doi: 10.1016/0005-2728(85)90094-5. [DOI] [PubMed] [Google Scholar]
- Meyer T. E., Yakali E., Cusanovich M. A., Tollin G. Properties of a water-soluble, yellow protein isolated from a halophilic phototrophic bacterium that has photochemical activity analogous to sensory rhodopsin. Biochemistry. 1987 Jan 27;26(2):418–423. doi: 10.1021/bi00376a012. [DOI] [PubMed] [Google Scholar]
- Monaco H. L., Zanotti G., Spadon P., Bolognesi M., Sawyer L., Eliopoulos E. E. Crystal structure of the trigonal form of bovine beta-lactoglobulin and of its complex with retinol at 2.5 A resolution. J Mol Biol. 1987 Oct 20;197(4):695–706. doi: 10.1016/0022-2836(87)90476-1. [DOI] [PubMed] [Google Scholar]
- Newcomer M. E., Jones T. A., Aqvist J., Sundelin J., Eriksson U., Rask L., Peterson P. A. The three-dimensional structure of retinol-binding protein. EMBO J. 1984 Jul;3(7):1451–1454. doi: 10.1002/j.1460-2075.1984.tb01995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papiz M. Z., Sawyer L., Eliopoulos E. E., North A. C., Findlay J. B., Sivaprasadarao R., Jones T. A., Newcomer M. E., Kraulis P. J. The structure of beta-lactoglobulin and its similarity to plasma retinol-binding protein. 1986 Nov 27-Dec 3Nature. 324(6095):383–385. doi: 10.1038/324383a0. [DOI] [PubMed] [Google Scholar]
- Pervaiz S., Brew K. Homology of beta-lactoglobulin, serum retinol-binding protein, and protein HC. Science. 1985 Apr 19;228(4697):335–337. doi: 10.1126/science.2580349. [DOI] [PubMed] [Google Scholar]
- Pevsner J., Reed R. R., Feinstein P. G., Snyder S. H. Molecular cloning of odorant-binding protein: member of a ligand carrier family. Science. 1988 Jul 15;241(4863):336–339. doi: 10.1126/science.3388043. [DOI] [PubMed] [Google Scholar]
- Raymond J. C., Sistrom W. R. ctothiorhodospira halophila: a new species ofthe genus Ectothiorhodospira. Arch Mikrobiol. 1969;69(2):121–126. doi: 10.1007/BF00409756. [DOI] [PubMed] [Google Scholar]
- Remington S., Wiegand G., Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J Mol Biol. 1982 Jun 15;158(1):111–152. doi: 10.1016/0022-2836(82)90452-1. [DOI] [PubMed] [Google Scholar]
- Sacchettini J. C., Gordon J. I., Banaszak L. J. The structure of crystalline Escherichia coli-derived rat intestinal fatty acid-binding protein at 2.5-A resolution. J Biol Chem. 1988 Apr 25;263(12):5815–5819. [PubMed] [Google Scholar]
- Sawyer L. Protein structure. One fold among many. 1987 Jun 25-Jul 1Nature. 327(6124):659–659. doi: 10.1038/327659a0. [DOI] [PubMed] [Google Scholar]
- Simondsen R. P., Tollin G. Transient kinetics of redox reactions of flavodoxin: effects of chemical modification of the flavin mononucleotide prosthetic group on the dynamics of intermediate complex formation and electron transfer. Biochemistry. 1983 Jun 7;22(12):3008–3016. doi: 10.1021/bi00281a034. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Tronrud D. E., Schmid M. F., Matthews B. W. Structure and X-ray amino acid sequence of a bacteriochlorophyll A protein from Prosthecochloris aestuarii refined at 1.9 A resolution. J Mol Biol. 1986 Apr 5;188(3):443–454. doi: 10.1016/0022-2836(86)90167-1. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Henderson R. Molecular structure determination by electron microscopy of unstained crystalline specimens. J Mol Biol. 1975 May 25;94(3):425–440. doi: 10.1016/0022-2836(75)90212-0. [DOI] [PubMed] [Google Scholar]
- Wang B. C. Resolution of phase ambiguity in macromolecular crystallography. Methods Enzymol. 1985;115:90–112. doi: 10.1016/0076-6879(85)15009-3. [DOI] [PubMed] [Google Scholar]
- Wolff E. K., Bogomolni R. A., Scherrer P., Hess B., Stoeckenius W. Color discrimination in halobacteria: spectroscopic characterization of a second sensory receptor covering the blue-green region of the spectrum. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7272–7276. doi: 10.1073/pnas.83.19.7272. [DOI] [PMC free article] [PubMed] [Google Scholar]