Abstract
Many Endocrinologists believe that a single determination of eucortisolism or a single demonstration of appropriate suppression to dexamethasone excluded Cushing’s syndrome, except in what was previously thought to be the rare patient with episodic or periodic Cushing’s syndrome. We hypothesize that episodic Cushing’s syndrome is relatively common and a single test assessing hypercortisolism may not be sufficient to accurately rule out or diagnose Cushing’s syndrome and retrospectively examined the number of normal and abnormal tests assessing hypercortisolism performed on multiple occasions in 66 patients found to have mild and/or episodic Cushing’s syndrome compared to a similar group of 54 patients evaluated for, but determined not to have Cushing’s syndrome. We found that 65 of the 66 patients with Cushing’s syndrome had at least one normal test of cortisol status and most patients had several normal tests. The probability of having Cushing’s syndrome when one test was negative was 92 % for 23:00 h salivary cortisol, 88 % for 24-h UFC, 86 % for 24-h 17OHS, and 54 % for nighttime plasma cortisol. These results demonstrated that episodic hypercortisolism is highly prevalent in subjects with mild Cushing’s syndrome and no single test was effective in conclusively diagnosing or excluding the condition. Rather, the paradigm for the diagnosis should be a careful history and physical examination and in those patients in whom mild Cushing’s syndrome/disease is strongly suspected, multiple tests assessing hypercortisolism should be performed on subsequent occasions, especially when the patient is experiencing signs and symptoms of short-term hypercortisolism.
Keywords: Cushing’s syndrome, episodic, periodic, urinary free cortisol, salivary cortisol, cortisol-binding globulin, 17-hydroxycorticosteroids
Introduction
Cushing’s syndrome is a relatively rare disorder with potential adverse consequences if the patient is incorrectly diagnosed as either having or not having the syndrome. As such, tests with high sensitivity and specificity are needed to suggest the diagnosis of hypercortisolism, particularly in light of the fact that Cushing’s syndrome is much less common than other mimicking conditions, such as polycystic ovarian syndrome, depression, alcoholism, the metabolic syndrome, and obesity.
Most published articles on Cushing’s syndrome have examined patients with sustained and severe hypercortisolism. However, because of increased recognition of Cushing’s syndrome among both patients and physicians, some patients may have mild cases of Cushing’s syndrome when they seek medical attention [1, 2]. Additionally, although multiple case reports have described patients with Cushing’s syndrome having periodic episodes of hypercortisolemia (reviewed in [3–5]), no published series has documented the prevalence of episodic hypercortisolemia in consecutive patients being evaluated for hypercortisolism. Periodic or cyclical Cushing’s syndrome refers to elevated cortisol levels present at regular intervals, while episodic Cushing’s syndrome refers to elevated cortisol levels occurring without any temporal pattern. A recent review article suggested that cyclic cortisol production is present in about 20–40 % of patients with Cushing’s syndrome [5]. The diagnosis of episodic Cushing’s syndrome is an important issue in clinical practice as patients with episodic hypercortisolemia would be incorrectly excluded from having Cushing’s syndrome if they underwent single tests when in a nonhypercortisolemic phase. In fact, the recent Endocrine Society Clinical Practice Guideline on the diagnosis of Cushing’s syndrome recognized the importance of episodic Cushing’s syndrome and suggested that at least 2 tests be performed on 2 occasions and that any value above the normal range for the laboratory be considered abnormal [6].
Standard tests for diagnosing Cushing’s syndrome have classically included 24-h urinary free cortisol (UFC), 24-h urinary 17-hydroxycorticosteroids (17OHS), overnight and low-dose dexamethasone suppression tests, and nighttime plasma and salivary cortisol assays [1, 7–14]. These tests have been found to be effective in diagnosing Cushing’s syndrome in patients with sustained and pronounced hypercortisolemia. However, their utility in patients with mild and/or episodic hypercortisolemia has not been evaluated.
Therefore, our hypotheses were: 1) tests used to diagnose Cushing’s syndrome such as UFC and nighttime salivary and plasma cortisol, may not, on all occasions, detect a mild increase in cortisol production; 2) the episodic nature of Cushing’s syndrome often may lead to normal measurements of cortisol status when a patient is tested in a quiescent phase; and 3) episodic hypercortisolism can be diagnosed using an array of biochemical tests performed at different times. This study determined the usefulness of several tests for hypercortisolism when performed on multiple occasions in a group of consecutive patients with signs and symptoms of glucocorticoid excess who eventually were confirmed to have Cushing’s disease. For comparison, we performed similar testing in a group of patients suspected of having mild Cushing’s syndrome, but determined not to have the diagnosis.
Patients and Methods
Patients: Clinical evaluation
The initial, clinical determination was on patients who were seen in an endocrinology clinic and were evaluated for Cushing’s syndrome by T.C.F. as part of their clinical care over a period of 4 years (January 2004 to December 2007). Many patients had seen other endocrinologists and either the patient or the endocrinologist suspected Cushing’s syndrome and often episodic Cushing’s syndrome. Patients were considered for Cushing’s syndrome if they had rapid, unexplained weight gain and associated symptoms of hypercortisolism including adult-onset hirsutism and acne, menstrual irregularities, and proximal muscle weakness. Although some patients had mild dysthymic or anxiolytic symptoms, no patient had major depression, alcoholism or other causes of pseudo-Cushing’s syndrome [15]. Only 2 patients were taking antidepressants at the time of diagnosis. Similarly, the diagnosis of PCOS was considered unlikely by the absence of elevated total/bioavailable testosterone [16] and lack of anovulation.
The biochemical evaluation for Cushing’s syndrome consisted of multiple measurements for 24-h UFC and 17OHS, and nighttime salivary and serum cortisol measurements. Nighttime (23:00±01:00 h) plasma cortisol determination was measured in ambulatory patients following venipuncture during the patients’ initial clinic visit; therefore, this test was generally performed once and not necessarily when the patient had signs/symptoms of short-term hypercortisolism. This test was not performed in women taking oral contraceptives or oral estrogens due to the effect of these drugs on raising cortisol-binding globulin (CBG) [17, 18]. Subjects from different time zones had their blood drawn at 23:00±01:00 h of their local time. Subjects were fasting for at least 3 h prior to venipuncture. Patients collected nighttime salivary cortisol levels and urine (simultaneously measured for both UFC and 17OHS) on different days; these tests were done in the patient’s own area and were sent to Esoterix Laboratories (see below) for analysis. Most patients reported that their symptoms were more severe at certain times. Therefore, subjects were instructed to collect saliva for nighttime salivary cortisol levels and urine (for both UFC and 17OHS) when they had signs/symptoms of short-term hypercortisolism, including sleep disturbances, acne, increased appetite, and home self-monitoring for hypertension and hyperglycemia (in those patients with hypertension and diabetes, respectively).
In general, patients were diagnosed with Cushing’s syndrome, if they had convincing and progressive signs and symptoms of hypercortisolism coupled with two or more separate abnormal values on tests of hypercortisolism (UFC, 17OHS, or 17OHS/Cr ratio, nighttime salivary cortisol measurement or night time serum cortisol measurement). The number of tests performed was done on a clinical basis and was stopped if either the patient had convincing biochemical evidence of hypercortisolism to diagnose them with Cushing’s syndrome or when Cushing’s syndrome had been excluded. The latter was determined by finding a) another diagnosis, b) lack of progression of symptoms and signs over a 1-year period, or 3) lack of hypercortisolism of two or more different types of tests after a minimum of 3 tests in each category (UFC, 17OHS, or 17OHS/Cr ratio, night-time salivary cortisol measurement) for most subjects. Biochemical testing was only included before their initial pituitary surgery, even if subjects underwent further testing after an unsuccessful initial surgery.
The type of Cushing’s syndrome was then determined by finding a nonsuppressed ACTH level (excluding adrenal adenomas) after which we performed biochemical testing including dexamethasone suppression, magnetic resonance imaging (in general, 1.5T magnet with thin slices through the sella and dynamic post-contrast sequences), and if needed, bilateral inferior petrosal sinus sampling with CRH [19]. In patients found to have Cushing’s disease, pituitary surgery was performed by experienced skull-base surgeons or neurosurgeons, including H.K.S in Los Angeles, I.E.M. in Houston, and other experienced surgeons in Los Angeles, San Francisco, and Pittsburgh. An endonasal transsphenoidal approach was performed in all patients. The gland was exposed from cavernous sinus to cavernous sinus in all patients and a thorough surface examination performed. Abnormal areas in the pituitary suggestive of tumor were visualized and selectively excised. No total or hemi-hypophysectomies of the anterior lobe were performed in this series. All patients were placed on 20–25 mg/day of hydrocortisone post-operatively and remained on cortisol replacement for a period of at least 3 months. Cortisol replacement was tapered off between 3 and 9 months following surgery and was based on morning serum cortisol levels and symptoms.
Patients: Retrospective evaluation
The second part of the evaluation was a retrospective review of charts of all patients seen in the clinic of T.C.F. for hypercortisolism and was performed by D.E.G., L.Z., and S.S. The Institutional Review Board at Charles Drew University deemed that retrospective review of the collected data was exempt from formal Institutional Review Board review in accordance with federal regulations [45CFR 46.101 (b) (4)]. Records were retrospectively reviewed on 191 patients, 74 of whom were determined to have Cushing’s syndrome by biochemical testing, 54 patients were determined to not have Cushing’s syndrome (Cushing’s excluded group) and 63 patients either did not undergo enough tests to have Cushing’s syndrome diagnosed or excluded, or were lost to follow-up. To be included in the Cushing’s syndrome group, subjects needed to have resolution of their clinical signs and symptoms as assessed at an in person or phone appointment between 3 months to 1 year after definitive surgery (pituitary surgery or bilateral adrenalectomy following unsuccessful pituitary surgery). All patients in this group were found to have pituitary Cushing’s disease (no patients had either ectopic or adrenal Cushing’s syndrome).
Because most patients exhibited episodic hypercortisolism and it was not known if their tumor was secreting ACTH at the time of surgery, traditional criteria of cure of Cushing’s disease including positive staining of the tumor for ACTH and post-operative hypocortisolism were deemed unlikely to be helpful. Rather, to determine cure from Cushing’s disease, patients had multiple UFC, 17-OHS, and 23:00 h salivary cortisol measurements in the 3–9-month post-operative period that were all normal, including normalization of the elevated pre-operative test(s). Patients were also surveyed either in person, by phone or by email to determine if they thought they had experienced a cure by their definitive surgery (pituitary surgery or bilateral adrenalectomy). 66 of the 74 patients responded that their surgery was definitely or probably successful and they had resolution of their signs and symptoms and were included in the Cushing’s syndrome group. In 8 patients, we could not confirm postoperative resolution of hypercortisolism with clinical remission. One of these patients underwent radiation therapy and it is too early to tell if she is cured. In the other 7 subjects, we could not ascertain if the patient was cured or not, either because surgery was relatively recent or the patient was lost to follow-up post-operatively. As we do not have evidence that these 8 patients improved following surgery, we do not feel justified in including them in the group with Cushing’s syndrome.
Our control group consisted of the 54 patients who were evaluated for Cushing’s syndrome in the same clinic and had similar testing but determined thereby not to have the condition. Cushing’s syndrome was excluded by lack of progression of symptoms (for at least 12 months following evaluation) and less than 2 different positive biochemical tests [6]. Often the patient was diagnosed with another condition.
Hormone analyses
Nighttime plasma cortisol level were measured by direct RIA in diluted serum at Esoterix Endocrinology (Calabasas Hills, CA, USA) [20, 21]. A positive test for nighttime plasma cortisol level was defined as a 23:00 h plasma cortisol greater than 7.5 mg/dl (207 nmol/l) similar to the cutoff of Papinicolaou et al. [22] for a 00:00 h plasma cortisol. Saliva was collected by having the patient chew a cylindrical cotton swab (Salivette, Sarstedt, Germany) for 3 min after rinsing the mouth with water. Salivary cortisol was measured by enzyme immunoassay (EIA) [23] by ACL Laboratories (Milwaukee, WI, USA) after collection in a saliva collection device at 23:00±00:30 h [24]. A positive test was defined as a salivary cortisol greater than 4.3 nmol/l [23]
24-h UFC, assayed by HPLC Tandem Mass Spectrometry, was measured by Esoterix Endocrinology [25, 26]. The 95 % tolerance interval (normal range) for women was determined by measurement in 20 healthy adult females and found to be 10–34 µg/d (28–94 nmol/d) in females and 11–84 µg/d (28–94 nmol/d) in males [25]. A positive test was therefore defined as a 24-h UFC greater than 34 µg/d (94 nmol/d) in females and 84 µg/d (224 nmol/d) in males. No subjects were taking carbamazapine or fenofibrate that can give a falsely elevated UFC [27]. 24-h urine for 17OHS analyzed on the same urine sample as the 24-h UFC was measured as Porter-Silber chromogens following enzymatic hydrolysis and purification by solvent extraction and column chromatography on silica gel (Esoterix Endocrinology) [28, 29] and expressed as both mg/d and mg/g/creatinine/d. A positive test was defined as a 24-h 17OHS greater than 6 mg/d (16.6 µmol/d) or 3.6 mg/g/creatinine/d (9.9 µmol/g creatinine/d) as defined by the normal range of the laboratory for women. The upper limit of the normal range for males is 10 mg/d (27.6 µmol/d) or 4.3 mg/g/creatinine/d (11.8 µmol/g creatinine/d) and therefore a positive test for males was defined as having a value greater than the upper limit of the normal range for these tests. 24-h UFC measurement on 3 patients and 24-h 17OHS on 5 patients were performed with similar methodology in outside laboratories with different normal ranges; the reported values were corrected using a correction factor to reflect the different normal range.
24-h UFC measurements were performed between 1 and 6 times on all 66 patients with Cushing’s syndrome and 51 of 55 patients in whom Cushing’s syndrome was excluded. The 23:00 h salivary cortisol measurements were performed between 1 and 6 times on 64 of the 66 patients with Cushing’s syndrome and 53 of 54 patients in whom Cushing’s syndrome was excluded. Urinary 17OHS measurements (with and without correction for urinary creatinine) were performed between 1 and 6 times on 63 of the 66 patients with Cushing’s syndrome and 50 of 54 patients in whom Cushing’s syndrome was excluded. Nighttime serum cortisol measurements were performed on 57 of the 66 patients with Cushing’s syndrome and 44 of 54 patients in whom Cushing’s syndrome was excluded.
Statistical analyses
Group differences in quantitative outcomes were evaluated by Student’s t-test and a p-value of <0.05 was considered significant. 95 % exact confidence intervals for the probability of patients with Cushing’s syndrome having at least one negative test or the probability of patients with Cushing’s syndrome excluded having at least one negative test were determined using the Clopper–Pearson method based on the inversion of the exact binomial probability calculation [30]. The frequency of a positive result pairing each of the biochemical tests was performed using McNemar’s test for paired comparisons (with the p-value calculated based on the exact binomial distribution) [31].
Results
Of the 66 patients found to be diagnosed with Cushing’s syndrome, 60 were females (55 Caucasians, 3 Hispanic, 1 Black, 1 Pacific Islander) and 6 were males (5 Caucasians, 1 Hispanic). The age range was 16–61 years (median 39 years) and BMI was 35.9±8.5 kg/m2 (mean±SD). The mean weight gain was 30.8±18.3 kg (mean±SD), which occurred between one and 10 years prior to their visit. All patients presented with signs and symptoms of hypercortisolism. Most interestingly, a large percentage of patients reported cognitive impairment, sleep disturbances (including both trouble falling asleep and frequent awakenings) and severe debilitating fatigue. 40 patients were cured after their first pituitary surgery, 11 patients were cured after their second pituitary surgery, 3 patients were cured after their third pituitary surgery, 7 patients were cured by bilateral adrenalectomy after their first unsuccessful pituitary surgery, and 6 patients were cured by bilateral adrenalectomy after their second unsuccessful pituitary surgery.
Of the 54 patients who were evaluated for Cushing’s syndrome and determined not to have Cushing’s syndrome, 52 were females and 2 were males. All were Caucasians except for one Hispanic male and one Hispanic female. The age range of the non-Cushing’s patients was 15–61 years (median 35 years, p=0.33 compared to Cushing’s syndrome) and BMI was 32.9±8.4 kg/m2 (mean±SD, p=0.052). Non-Cushing’s patients had some signs and symptoms of hypercortisolism prompting the workup, however, their clinical stigmata were less impressive, including less weight gain [22.0±15.8 kg (mean±SD, p<0.006, compared to Cushing’s syndrome group)].
Most patients with Cushing’s syndrome had two or more abnormal cortisol measurements. The exceptions (n=6) who were diagnosed with Cushing’s syndrome with only one type of positive test of hypercortisolism are patient #17 who had two very high night-time salivary cortisol levels, patient #33 who had two very high 24-h UFC levels, patient #38 who had 6 high UFC levels, patient #39 who had multiple high midnight serum cortisol levels and patients #56 and 64 who had 4 high 24-h 17OHS levels.
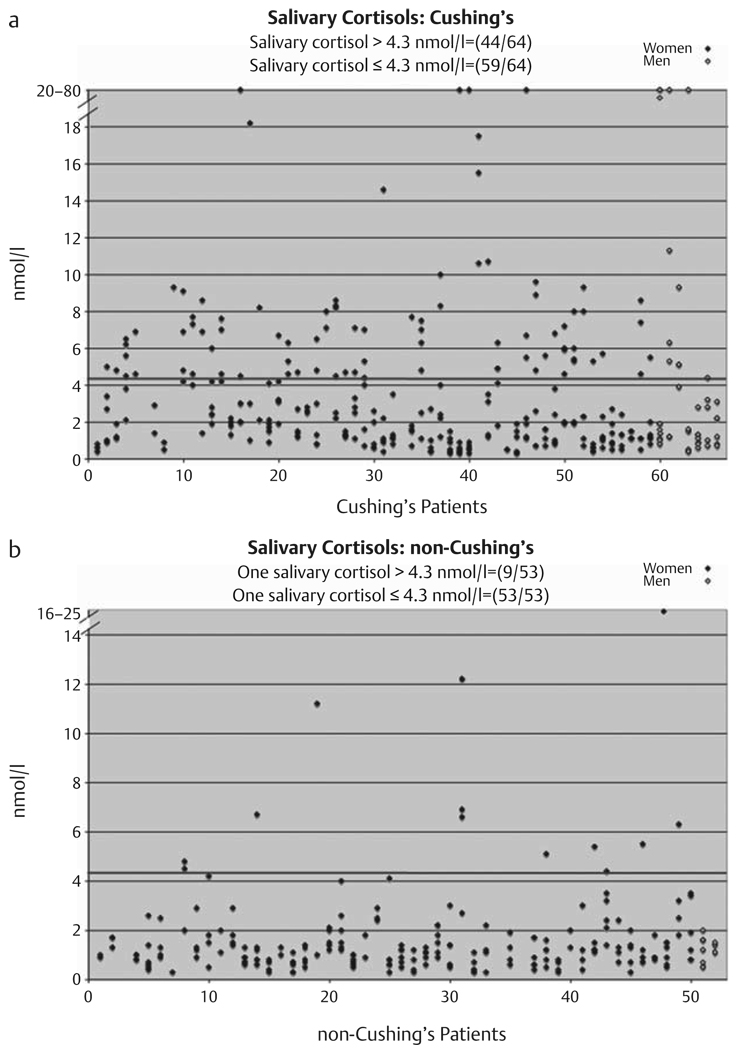
As shown in Fig. 1a, Table 1, 59 of 64 (0.92, 95 % CI: 0.83–0.97) patients with Cushing’s syndrome had at least one normal (≤4.3 mmol/l) nighttime salivary cortisol. 44 of 64 patients with Cushing’s syndrome had at least one elevated nighttime salivary cortisol (Fig. 1a). As shown in Fig. 1b, Table 1, 9 of 53 (0.17, 95 % CI: 0.08–0.30) patients in whom Cushing’s syndrome was excluded had at least one elevated (>4.3 mmol/l) nighttime salivary cortisol and 53 of 53 patients had at least one normal nighttime salivary cortisol.
Fig. 1.
The 23:00 h salivary cortisol levels performed 1–6 times on patients with Cushing’s syndrome (a) and patients determined not to have Cushing’s syndrome (b). Red line indicates upper limit of normal of 4.3 nmol/l [23]. Male subjects are in open diamonds and appear on the right.
Table 1.
Probability of a patient with Cushing’s syndrome having at least one negative test and probability of patient without Cushing’s syndrome having at least one positive testa
| Test | Probability of patients with Cushing’s syndrome having at least one negative test (95 % confidence interval) | Probability having one positive test in a patient without Cushing’s syndrome (95 % confidence interval) |
|---|---|---|
| Salivary | 59/64=0.92 | 9/53=0.17 |
| cortisol | (0.83, 0.97) | (0.08, 0.30) |
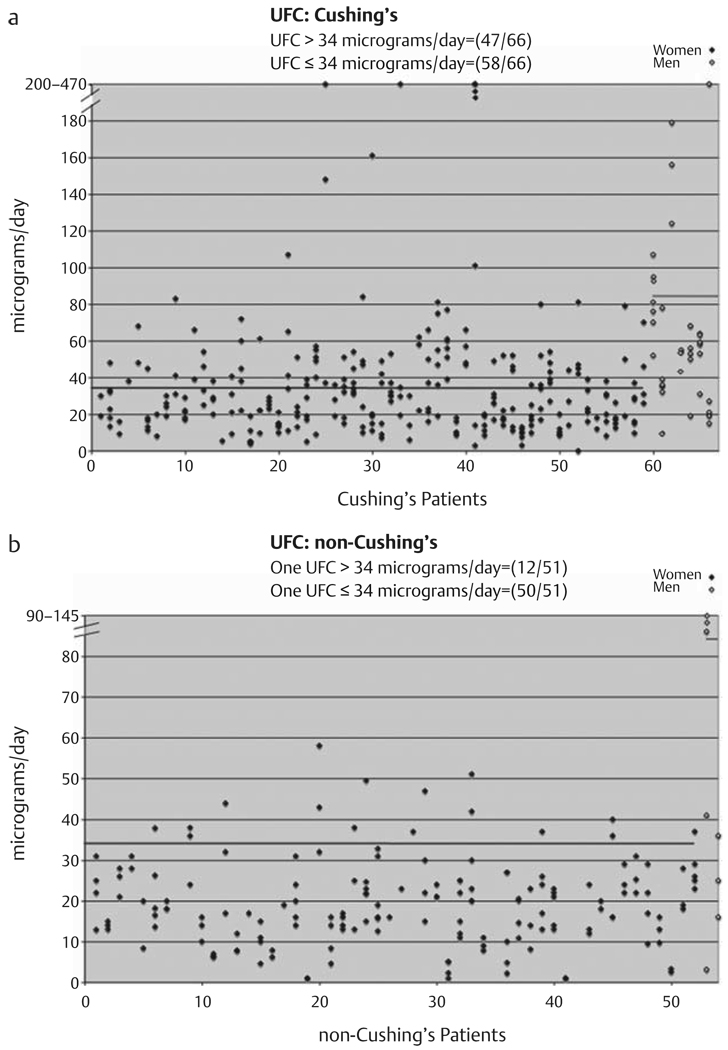
| UFC | 58/66=0.88 | 12/51=0.24 |
| (0.78, 0.95) | (0.13, 0.37) | |
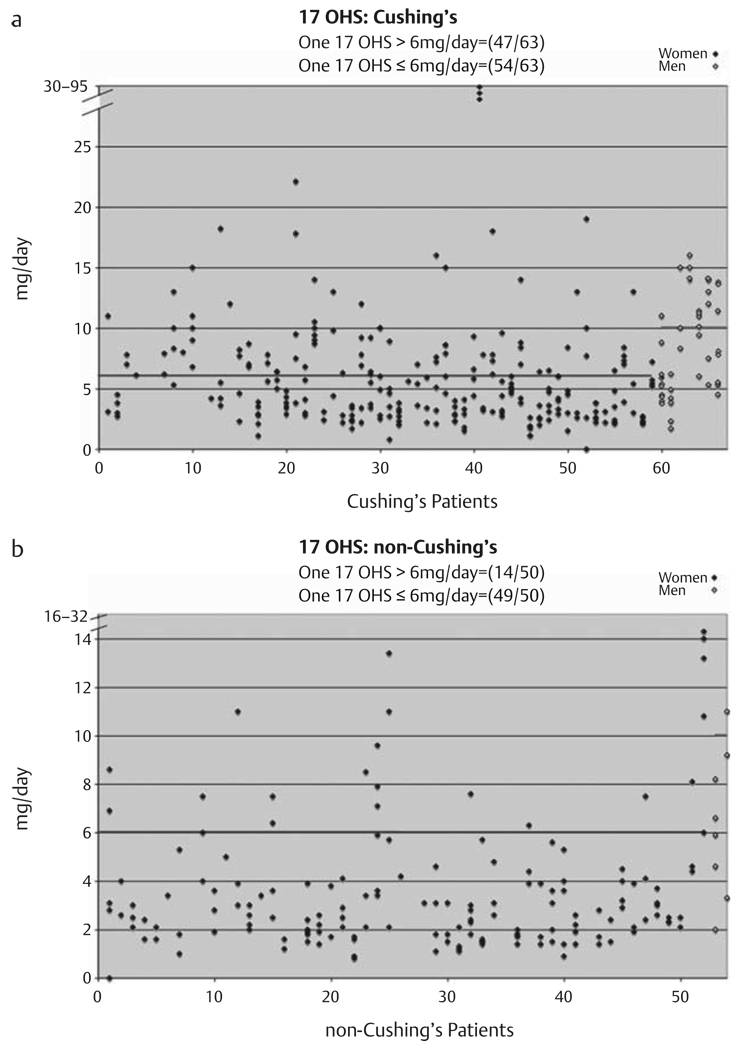
| 17OHS | 54/63=0.86 | 14/50=0.28 |
| (0.75, 0.93) | (0.16, 0.42) | |
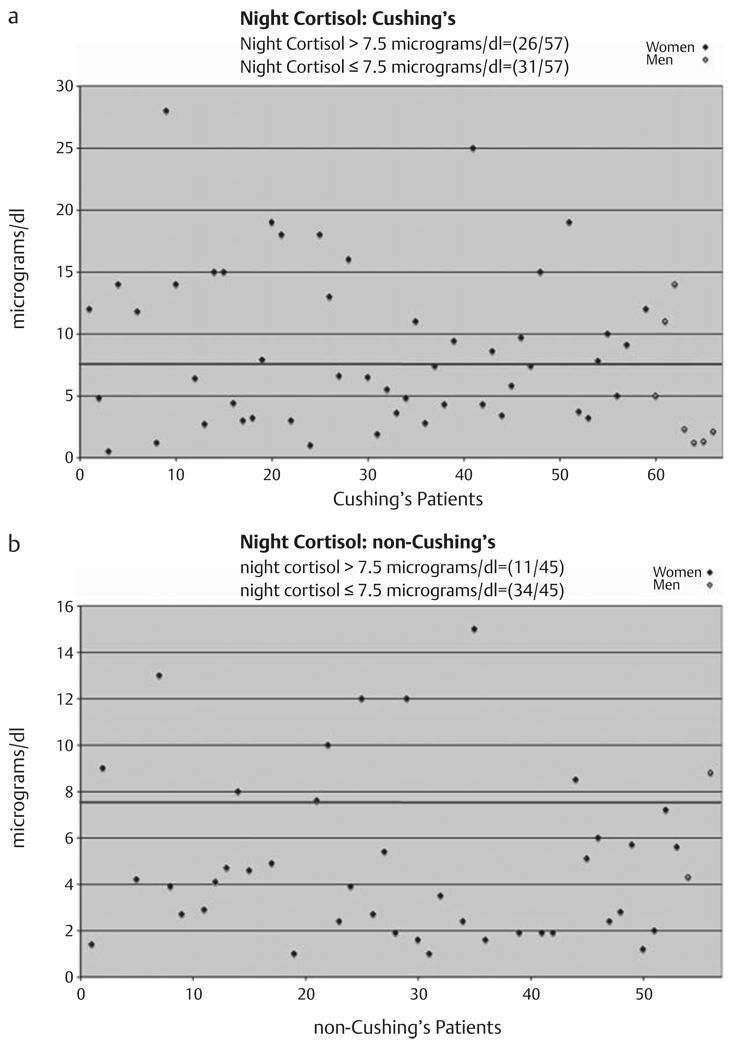
| Nighttime | 31/57=0.54 | 11/45=0.24 |
| cortisol | (0.41, 0.68) | (0.14, 0.39) |
A 95 % confidence interval is also given
As shown in Fig. 2a, Table 1, 58 of 66 (0.88, 95 % CI: 0.78–0.95) patients with Cushing’s syndrome had at least one normal 24-h UFC [females ≤34 µg/d (94 nmol/d) and males ≤81 µg/d (224 nmol/d)], while 47 of 66 patients had at least one elevated 24-h UFC. 60 of 66 patients with Cushing’s syndrome had mild hypercortisolemia as made evident by maximum UFC determinations that were less than 2.5-fold of the upper-normal laboratory range. As shown in Fig. 2b, Table 1, 12 of 51 (0.24, 95 % CI: 0.13–0.37) patients in whom Cushing’s syndrome was excluded had at least one elevated 24-h UFC, while 50 of 51 patients had at least one normal 24-h UFC.
Fig. 2.
24-h urinary free cortisol (UFC) performed 1–6 times on patients with Cushing’s syndrome (a) and patients determined not to have Cushing’s syndrome (b). Blue lines indicates upper limit of normal for females [34 µg/d (94 nmol/d)] and green lines indicates upper limit of normal for males [84 µg/d (224 nmol/d)] (established by laboratory). Male subjects are in open diamonds and appear on the right.
Additionally, as shown in Fig. 3a, Table 1, 54 of 63 (0.86, 95 % CI: 0.75–0.93) patients with Cushing’s syndrome had at least one normal [females ≤6 mg/d (16.6 µmol/d) and males ≤10 mg/d (27.6 µmol/d] 24-h 17OHS, while 47 of 63 patients with Cushing’s syndrome had at least one elevated 24-h 17OHS. 12 subjects determined to have Cushing’s syndrome had an elevated 24-h 17OHS with all 24-h UFC measurements that were normal. As shown in Fig. 3b, Table 1, 14 of 50 (0.28, 95 % CI: 0.16–0.42) patients in whom Cushing’s syndrome was excluded had at least one elevated 24-h 17OHS. Furthermore, 49 of 50 patients in whom Cushing’s syndrome was excluded had at least one normal 24-h 17OHS (Fig. 3b). Results expressed as 24-h 17OHS/Cr measurements were similar (data not shown).
Fig. 3.
24-h urinary 17-hydroxycorticosteroids/g creatinine performed 1–6 times on patients with Cushing’s syndrome (a) and patients determined not to have Cushing’s syndrome (b). Blue lines indicate upper limit of normal for females [3.6 mg/g creatinine/d (9.9 µmol/g creatinine/d)] and green lines indicates upper limit of normal for males [4.3 mg/g creatinine/d (11.8 µmol/g creatinine/d)] (established by laboratory). Male subjects are in open diamonds and appear on the right.
As shown in Fig. 4a, Table 1, 31 of 57 (0.54, 95 % CI: 0.41–0.68) patients with Cushing’s syndrome had a normal [≤7.5 µg/dl (207 nmol/d)] plasma night-time cortisol on the initial measurement. 26 of 57 patients with Cushing’s syndrome had an elevated night-time plasma cortisol on the initial measurement. 11 of 45 patients (0.24, 95 % CI: 0.14–0.39) in whom Cushing’s syndrome was excluded had one elevated [greater than 7.5 µg/dl (207 nmol/d)] plasma night-time cortisol, while 34 of 45 patients had normal values only (Fig. 4b).
Fig. 4.
Night-time plasma cortisol performed one time on patients with Cushing’s syndrome (a) and patients determined not to have Cushing’s syndrome (b). Blue line indicates upper limit of normal of 7.5 µg/dl (207 nmol/d) [22]. Male subjects are in open diamonds and appear on the right.
Discussion
We have demonstrated that the great majority of patients in whom Cushing’s syndrome was biochemically confirmed at a referral endocrinology clinic had episodic Cushing’s syndrome, as indicated by normal testing on one or more occasions. A large majority of patients (60 of 66) had mild hypercortisolemia as shown by maximum UFC determinations that were at most 2.5-fold of the upper-normal laboratory range. Most interesting, the salivary cortisol level was the test found to be normal on at least one occasion in the greatest number of patients with Cushing’s syndrome, as it was normal in 59 of 64 patients (2 patients did not have the test done). However, salivary cortisol levels were found to be elevated in the least number of non-Cushing’s syndrome patients as they were elevated in 9 of 53 patients found not to have Cushing’s syndrome. 12 subjects with Cushing’s syndrome had normal 24-h UFC, but elevated 24-h 17OHS, while 13 subjects had elevated 24-h UFC, but normal 24-h 17OHS, suggesting that both urinary tests are complementary and should be performed together to correctly diagnose patients with mild Cushing’s syndrome. A manuscript comparing sensitivities and specificities of the various tests assessing cortisol status is in preparation.
Although case reports of episodic or periodic Cushing’s syndrome have been well-described, series on this condition have been limited. McCance et al. [32] found episodically evaluated serum cortisol levels in 7 of 41 patients with Cushing’s disease following transsphenoidal surgery. Streeten and colleagues [33] demonstrated intermittent hypercortisolism in 6 of 31 patients with Cushing’s disease after transsphenoidal surgery. Atkinson et al. [34] found cyclic hormonogenesis as assessed by frequent cortisol measurements in 5 of 14 patients with Cushing’s syndrome during a 2-year interval.
A few recent papers have described patients with mild Cushing’s syndrome. Kidambi et al. reported 11 patients with Cushing’s syndrome whose cortisol tests were sometimes elevated and sometimes normal [2]. Nunes et al. reported 12 patients with mild recurrent Cushing’s disease [35]. However, neither of these reports described a consecutive series and it is unclear whether these patients were representative of those seen at these medical centers, and what percent of the overall Cushing’s syndrome cohort do these mild cases represent. In contrast, our series depicts consecutive patients evaluated in one clinic and demonstrates that the vast majority of patients have one or more normal determinations of cortisol status.
Do patients with some elevated and some normal free cortisol measurements have mild hypercortisolism or episodic hypercortisolism? We postulate that these 2 conditions occur together. When cortisol production is near the level of the cortisol-binding threshold of CBG, only a few measurements that are based on detecting elevated plasma free cortisol will be above the normal range; thus the patient will appear to have episodic hypercortisolism if assays are performed on multiple occasions. Similarly, if excess cortisol production is quite infrequent (as in a patient with episodic hypercortisolism on rare occasions), then fewer assays will be abnormal. As cortisol production increases to levels well above the cortisol-binding threshold of CBG, or as excess cortisol production becomes more frequent, then most assays will show hypercortisolism more frequently when performed on multiple occasions. Thus, mild and episodic hypercortisolism are expected to occur together.
It was reported that UFC was better able to distinguish Cushing’s syndrome from normal individuals than could 17OHS [36]. The subjects in that study, however, had sustained and substantial hypercortisolemia (UFC range 140–7 800 µg/d; 390–21 850 nmol/d). We suspect that in high cortisol production states, the UFC assay would be a better assay to distinguish patients with Cushing’s syndrome from those without Cushing’s syndrome. However, at lower cortisol production rates, the 17OHS assay may be able to detect mild hypercortisolism in some patients with normal 24-h UFC levels as the UFC measurement will increase only mildly until a point of saturation of cortisol binding to CBG is exceeded, while the 17OHS measurements, which are not dependent on cortisol exceeding the threshold of binding to CBG, are more likely to be linear with cortisol production.
Our finding of normal assessments of cortisol in some patients could be explained by factors besides the patient being eucortisolemic at the time of testing. For example, the urine collections may have been incomplete or the salivary tests not collected properly. These conditions are unlikely as all subjects were carefully instructed on proper collection techniques. Additionally, interassay variation may sometimes lead to a normal value in a patient who has a mildly elevated value. However, such variation was minimized here by consistency of techniques applied to all specimens collected; and due to the rigor of our collection methods and the conformation of complete collection by urinary creatinine measurement, we do not believe that preanalytical factors contributed to the high degree of normal cortisol measurements seen in our subjects.
Our data demonstrate that if only one test to detect hypercortisolism were performed on only one occasion, the majority of patients would be excluded from the correct diagnosis. In the past, many such patients would have been told by their physician that they do not have Cushing’s syndrome due to normal results of just one test. We have recently described poor sensitivity of a single overnight dexamethasone test in detecting hypercortisolism in patients with mild or episodic Cushing’s syndrome [37]. Thus, our data suggest that current screening tests performed once are inadequate to detect or exclude hypercortisolism in patients with mild or episodic Cushing’s disease. The major limitation of our study is that it is a retrospective analysis of patients in whom the decision to undergo surgery was made on clinical grounds along with biochemical tests documenting hypercortisolemia. This resulted in an uneven number of tests per patient. In general, the number of tests was determined based on clinical suspicion and on the results of initial testing. If initial testing showed sustained hypercortisolism, then testing stopped and the patient was sent to surgery. Conversely, if there was a high degree of clinical suspicion for Cushing’s syndrome but initial tests were negative, then testing continued until either sustained eucortisolism was found, the patient received another diagnosis explaining their symptoms, or the patient was lost to follow-up or declined further testing. Therefore, the more times a test was done, the more likely it would be for a Cushing’s syndrome patient to have a normal test or for a normal individual to have a positive test. Additionally, in the initial determination of hypercortisolism, more weight was placed in substantially elevated cortisol measurements than on mildly elevated values. Thus, the determination of who had Cushing’s syndrome was done on clinical and biochemical grounds, as it is done in a nonresearch setting.
The second caveat is the lack of pathological confirmation of Cushing’s disease. Although positive ACTH immunostaining is considered the gold-standard for pathological confirmation of Cushing’s disease, it is becoming increasingly recognized that many patients with confirmed Cushing’s disease do not have positive pathology demonstrating an ACTH-staining tumor. For example, Kruse et al. found that in only 58 % of patients with Cushing’s disease who were cured by surgery could an adenoma be verified [38]. Lack of pathological confirmation is thought to be due to either the small size of the tumor that is missed by sectioning or that some tumors may be lost during surgery (for example, in suction) prior to sending to pathology. Tumors as small as 0.25 mm in diameter have been demonstrated by serial sectioning of hypophysectomy specimens [39]. These tumors are often missed at pathological examination. Additionally, we postulate that if the tumor is quiescent at the time of surgery in a patient with episodic Cushing’s disease, the immunostaining for intact ACTH might be negative. While further pathological studies would be helpful on these excised specimens, it would be difficult as the tumors were collected at 5 medical centers and were reviewed by different pathologists.
While pituitary pathology was variable, all 13 patients with Cushing’s syndrome who underwent bilateral adrenalectomy following unsuccessful pituitary surgery had enlarged bilateral adrenals with a pathological diagnosis of adrenal hyperplasia. These patients who underwent bilateral adrenalectomy are typical of the patients in the series with Cushing’s syndrome and were unlikely to have ectopic ACTH syndrome, These adrenal findings gives further credence to our belief that the patients all had pituitary Cushing’s disease in spite of the variable pituitary pathology.
The subjects seen were either referred or self-referred to our endocrinology clinic, known to have expertise in evaluating patients with mild and episodic Cushing’s syndrome. Thus, our series likely has a higher number of patients with episodic Cushing’s syndrome than most endocrinology clinics due to referral bias. We do not have a definitive explanation on the lack of adrenal adenoma or ectopic ACTH syndrome patients in our series as these conditions have been reported to be cyclical or episodic [5]. It is possible that patients with adrenal adenoma or ectopic ACTH syndrome have more severe hypercortisolism and are more readily detected by referring endocrinologists, while episodic pituitary Cushing’s disease may manifest with lower cortisol values necessitating referral to a specialized center. Following the December 2007 cutoff for data analysis for this paper, we did confirm Cushing’s syndrome due to an adrenal adenoma in 2 patients, one with sustained hypercortisolism and the other with episodic hypercortisolism.
Additionally, we measured cortisol by venipuncture and it is possible that the anticipation of the venipuncture may lead to higher cortisol levels. This is unlikely as Meeran et al. [40] found that serum cortisol did not rise until 4 min after an 00:00 h venipuncture in volunteers subjects awaken from sleep who had blood drawn by venipuncture. In none of our patients was blood collected in a second venipuncture following an unsuccessful first venipuncture.
An alternative approach to the patient with mild/episodic Cushing’s syndrome would be to wait until full-blown hypercortisolism occurs prior to surgery. We reported that a patient with documented Cushing’s disease for 26 years exhibited signs/symptoms suggestive of periodic hypercortisolism and had a 4-mm ACTH-positive pituitary tumor found at surgery [41]. While there is no clear evidence that patients with mild/episodic Cushing’s syndrome all progress to sustained/severe hypercortisolism, mild hypercortisolism may be quite devastating to the patient. Thus, we cannot advocate a “tincture of time” approach.
More complex and expensive tests (such as DEX-CRH) are likely to suffer the same barriers in detecting hypercortisolism in patients with episodic Cushing’s syndrome. The DEX-CRH test needs to be performed in a large number of subjects with mild/episodic Cushing’s syndrome before it can be recommended; in this study, it was positive in only one of 3 patients.
Most interestingly, our finding of a large number of patients with mild hypercortisolism support those of a paper published more than 40 years ago by Gabrilove [42] who postulated that adrenocortical hyperfunction is a continuum from normality to the florid clinical state and that its manifestation will vary widely among individuals. Gabrilove also proposed, without experimental data, that many tests to diagnosis adrenocortical hyperfunction will fail in the mild patient, a postulate proven by the data here.
We conclude that no single test can be used to diagnose or exclude mild Cushing’s disease and that the approach of obtaining a single screening test to diagnose/exclude hypercortisolism will result in the incorrect exclusion of many patients with mild Cushing’s syndrome, and incorrect inclusion of some patients without the syndrome. Our results do not apply to those with more severe Cushing’s disease and likely do not apply to those patients with ectopic ACTH syndrome or ACTH-independent Cushings’s syndrome. Our recommendations for mild Cushing’s disease are similar to those of the Endocrine Society Clinical Practice Guideline [6] in that the diagnosis needs to be made by a careful physical exam and history to identify patients with multiple and progressive features compatible with Cushing’s syndrome who would have a high pre-test likelihood of having the condition. In subjects who are suspected of having episodic hypercortisolism, multiple tests should be performed, ideally when the patient is in a suspected phase of short-term hypercortisolism. The exact paradigm for testing for hypercortisolism in those patients with mild and/or episodic hypercortisolism remains to be elucidated by prospective, multi-site studies. Additionally, investigation into the etiology of episodic Cushing’s syndrome should be pursued.
Acknowledgements
We thank John Tayek, M.D. (UCLA-Harbor, Los Angeles, CA) and Karen L. Herbst, M.D. (VA San Diego Healthcare System, San Diego, CA) for their helpful comments. This work was supported in part by the following NIH grants: U54 RR14616, S06 GM068510, U54 HD41748, and R25 RR019488.
References
- 1.Newell-Price J, Trainer P, Besser M, Grossman A. The diagnosis and differential diagnosis of Cushing’s syndrome and pseudo-Cushing’s states. Endocr Rev. 1998;19:647–672. doi: 10.1210/edrv.19.5.0346. [DOI] [PubMed] [Google Scholar]
- 2.Kidambi S, Raff H, Findling JW. Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing’s syndrome. Eur J Endocrinol. 2007;157:725–731. doi: 10.1530/EJE-07-0424. [DOI] [PubMed] [Google Scholar]
- 3.Mantero F, Scaroni CM, Albiger NM. Cyclic Cushing’s syndrome: an overview. Pituitary. 2004;7:203–207. doi: 10.1007/s11102-005-4025-5. [DOI] [PubMed] [Google Scholar]
- 4.Velez DA, Mayberg MR, Ludlam WH. Cyclic Cushing syndrome: definitions and treatment implications. Neurosurg Focus. 2007;23 doi: 10.3171/foc.2007.23.3.5. E4; discussion E4a. [DOI] [PubMed] [Google Scholar]
- 5.Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing’s syndrome: a clinical challenge. Eur J Endocrinol. 2007;157:245–254. doi: 10.1530/EJE-07-0262. [DOI] [PubMed] [Google Scholar]
- 6.Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab. 2003;88:5593–5602. doi: 10.1210/jc.2003-030871. [DOI] [PubMed] [Google Scholar]
- 8.Crapo LM. Cushing’s syndrome: a review of diagnostic tests. Metab. 1979;28:955–977. doi: 10.1016/0026-0495(79)90097-0. [DOI] [PubMed] [Google Scholar]
- 9.Findling JW, Raff H. Diagnosis and differential diagnosis of Cushing’s syndrome. Endocrinol Metab Clin North Am. 2001;30:729–747. doi: 10.1016/s0889-8529(05)70209-7. [DOI] [PubMed] [Google Scholar]
- 10.Kaye TB, Crapo L. The Cushing syndrome: An update on diagnostic tests. Ann Intern Med. 1990;112:434–444. doi: 10.7326/0003-4819-76-3-112-6-434. [DOI] [PubMed] [Google Scholar]
- 11.Meier CA, Biller BM. Clinical and biochemical evaluation of Cushing’s syndrome. Endocrinol Metab Clin North Am. 1997;26:741–762. doi: 10.1016/s0889-8529(05)70280-2. [DOI] [PubMed] [Google Scholar]
- 12.Nieman LK. Cushing’s syndrome. Curr Ther Endocrinol Metab. 1997;6:161–164. [PubMed] [Google Scholar]
- 13.Orth DN. Cushing’s syndrome. N Engl J Med. 1995;332:791–803. doi: 10.1056/NEJM199503233321207. [DOI] [PubMed] [Google Scholar]
- 14.Ross RJ, Trainer PJ. Endocrine investigation: Cushing’s syndrome. Clin Endocrinol. 1998;49:153–155. doi: 10.1046/j.1365-2265.1998.00516.x. [DOI] [PubMed] [Google Scholar]
- 15.Friedman TC. Pseudo-Cushing syndrome. In: Margioris AN, Chrousos GP, editors. Contemporary Endocrinology: Adrenal Disorders. Totowa, NJ: Humana Press; 2001. pp. 203–218. [Google Scholar]
- 16.Pall ME, Lao MC, Patel SS, Lee ML, Ghods DE, Chandler DW, Friedman TC. Testosterone and Bioavailable Testosterone Help to Distinguish between Mild Cushing’s Syndrome and Polycystic Ovarian Syndrome. Horm Metab Res. 2008;40:813–818. doi: 10.1055/s-0028-1087186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burke CW. Biologically active cortisol in plasma of oestrogen-treated and normal subjects. Br Med J. 1969;2:798–800. doi: 10.1136/bmj.2.5660.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doe RP, Zinneman HH, Flink HB, Ulstrom RA. Significance of the concentration of non protein bound plasma cortisol in normal subjects, Cushing’s syndrome, pregnancy and during estrogen therapy. J Clin Endocrinol Metab. 1960;20:1484–1492. doi: 10.1210/jcem-20-11-1484. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield EH, Doppman JL, Nieman LK, Chrousos GP, Miller DL, Katz DA, Cutler GB, Jr, Loriaux DL. Petrosal sinus sampling with and without corticotropin-releasing hormone for the differential diagnosis Cushing’s syndrome. N Engl J Med. 1991;325:897–905. doi: 10.1056/NEJM199109263251301. [DOI] [PubMed] [Google Scholar]
- 20.Esoterix Directory of Services. Vol. 136 2004. [Google Scholar]
- 21.Foster LB, Dunn RT. Single-antibody technique for radioimmunoassay of cortisol in unextracted serum or plasma. Clin Chem. 1974;20:365–368. [PubMed] [Google Scholar]
- 22.Papanicolaou DA, Yanovski JA, Cutler G, Jr, Chrousos GP, Nieman LK. A single midnight serum cortisol measurement distinguishes Cushing’s syndrome from pseudo-Cushing states. J Clin Endocrinol Metab. 1998;83:1163–1167. doi: 10.1210/jcem.83.4.4733. [DOI] [PubMed] [Google Scholar]
- 23.Raff H, Homar PJ, Skoner DP. New enzyme immunoassay for salivary cortisol. Clin Chem. 2003;49:203–204. doi: 10.1373/49.1.203. [DOI] [PubMed] [Google Scholar]
- 24.Raff H, Raff JL, Findling JW. Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab. 1998;83:2681–2686. doi: 10.1210/jcem.83.8.4936. [DOI] [PubMed] [Google Scholar]
- 25.Esoterix Directory of Services. Vol. 135. 2004. p. 135. [Google Scholar]
- 26.Van Herle AJ, Birnbaum JA, Slomowitz LA, Mayes D, Chandler DW, Rosenblit PD, Nissenson A. Paper chromatography prior to cortisol RIA allows for accurate use of the dexamethasone suppression test in chronic renal failure. Nephron. 1998;80:79–84. doi: 10.1159/000045132. [DOI] [PubMed] [Google Scholar]
- 27.Boscaro M, Arnaldi G. Approach to the patient with possible Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94:3121–3131. doi: 10.1210/jc.2009-0612. [DOI] [PubMed] [Google Scholar]
- 28.Esoterix Directory of Services. Vol. 239 2004. [Google Scholar]
- 29.Silber RH, Porter CC. The determination of 17, 21-dihydroxy-20-keto-steroids in urine and plasma. J Biol Chem. 1954;210:923–932. [PubMed] [Google Scholar]
- 30.Clopper CJ, Pearson E. The use of confidence or fiducial limits illustrated in the case of binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 31.McNemar Q. Note on the sampling error of the differences between correlated proportions or percentages. Psycometrika. 1947;12:153–157. doi: 10.1007/BF02295996. [DOI] [PubMed] [Google Scholar]
- 32.McCance DR, Gordon DS, Fannin TF, Hadden DR, Kennedy L, Sheridan B, Atkinson AB. Assessment of endocrine function after transsphenoidal surgery for Cushing’s disease. Clin Endocrinol (Oxf) 1993;38:79–86. doi: 10.1111/j.1365-2265.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 33.Streeten DH, Anderson GH, Jr, Dalakos T, Joachimpillai AD. Intermittent hypercortisolism: a disorder strikingly prevalent after hypophysial surgical procedures. Endocr Pract. 1997;3:123–129. doi: 10.4158/EP.3.3.123. [DOI] [PubMed] [Google Scholar]
- 34.Atkinson AB, Kennedy AL, Carson DJ, Hadden DR, Weaver JA, Sheridan B. Five cases of cyclical Cushing’s syndrome. Br Med J (Clin Res Ed) 1985;291:1453–1457. doi: 10.1136/bmj.291.6507.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nunes ML, Vattaut S, Corcuff JB, Rault A, Loiseau H, Gatta B, Valli N, Letenneur L, Tabarin A. Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab. 2009;94:456–462. doi: 10.1210/jc.2008-1542. [DOI] [PubMed] [Google Scholar]
- 36.Mengden T, Hubman P, Muller J, Greminger P, Vetter W. Urinary free cortisol versus 17-hydroxycorticosteroids: a comparative study of their diagnostic value in Cushing’s syndrome. Clin Investig. 1992;70:545–548. doi: 10.1007/BF00184788. [DOI] [PubMed] [Google Scholar]
- 37.Friedman TC. An update on the overnight dexamethasone suppression test for the diagnosis of Cushing’s syndrome: limitations in patients with mild and/or episodic hypercortisolism. Exp Clin Endocrinol Diabetes. 2006;114:356–360. doi: 10.1055/s-2006-924281. [DOI] [PubMed] [Google Scholar]
- 38.Kruse A, Klinken L, Holck S, Lindholm J. Pituitary histology in Cushing’s disease. Clin Endocrinol (Oxf) 1992;37:254–259. doi: 10.1111/j.1365-2265.1992.tb02319.x. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield EH, Vortmeyer AO. Development of a histological pseudocapsule and its use as a surgical capsule in the excision of pituitary tumors. J Neurosurg. 2006;104:7–19. doi: 10.3171/jns.2006.104.1.7. [DOI] [PubMed] [Google Scholar]
- 40.Meeran K, Hattersley A, Mould G, Bloom SR. Venepuncture causes rapid rise in plasma ACTH. Br J Clin Pract. 1993;47:246–247. [PubMed] [Google Scholar]
- 41.Patel PM, Shahinian H, Friedman TC. Cushing’s disease is not necessarily a progressive and fatal disease: A case of documented Cushing’s disease untreated for 26 years. Endocrinologist. 2005;15:343–344. [Google Scholar]
- 42.Gabrilove JL. The continuum of adrenocortical disease: a thesis and its lesson to medicine. J Mt Sinai Hosp NY. 1965;32:634–636. [PubMed] [Google Scholar]