Abstract
Introduction
Evidence has been inconsistent regarding the impact of social networks on survival after breast cancer diagnosis. We prospectively examined the relation between components of social integration and survival in a large cohort of breast cancer survivors.
Methods
Women (N=4,589) diagnosed with invasive breast cancer were recruited from a population-based, multi-center, case-control study. A median of 5.6 years (Interquartile Range 2.7–8.7) after breast cancer diagnosis, women completed a questionnaire on recent post-diagnosis social networks and other lifestyle factors. Social networks were measured using components of the Berkman-Syme Social Networks Index to create a measure of social connectedness. Based on a search of the National Death Index, 552 deaths (146 related to breast cancer) were identified. Adjusted hazard ratios (HR) and 95% confidence intervals (CI) were estimated using Cox proportional hazards regression.
Results
Higher scores on a composite measure of social connectedness as determined by the frequency of contacts with family and friends, attendance of religious services, and participation in community activities was associated with a 15–28% reduced risk of death from any cause (p-trend=0.02). Inverse trends were observed between all-cause mortality and frequency of attendance at religious services (p-trend =0.0001) and hours per week engaged in community activities (p-trend =0.0005). No material associations were identified between social networks and breast cancer-specific mortality.
Conclusions
Engagement in activities outside the home was associated with lower overall mortality after breast cancer diagnosis.
Keywords: cancer, oncology, breast cancer, survival, mortality, social networks
Introduction
Evidence suggests that social networks may be associated with reduced overall mortality[1]. Improvements in breast cancer detection and treatment have led to a growing number of cancer survivors, resulting in a need to elucidate strategies for improving outcomes, including lifestyle factors. A recent meta-analysis of 87 studies summarizing the literature on the association between social networks and cancer survival reported having high levels of perceived social support, larger social networks, and being married were associated with decreases in risk ratios for mortality of 25%, 20%, and 12%, respectively [2]. Relationships varied by cancer site, with stronger associations of number of social contacts observed in studies of breast cancer [2].
The impact of social networks on breast cancer specific survival has been insufficiently studied: a systematic review suggested characteristics associated with better breast cancer prognosis include social support, marriage, minimizing, and denial; however, the role of these factors has not been supported in all studies [3]. Previous observational studies were based on selected populations, did not have adequate information on potential confounders, or had limited power [4–12], leading to inconclusive results [3]. A few randomized controlled trials have been conducted to investigate the role of social networks, including social supports, in breast cancer survival, but the results have been mixed. These studies, however, may not have simulated natural social networks or have intervened at the appropriate time point [13–16].
The biological and social mechanisms for the role of social networks require further study [17, 18]. There are several potential pathways by which social networks may reduce mortality. Social networks may alleviate depressive symptoms [19, 20], promote adoption of health behaviors through peer support [21–23], and/or improve resistance to infection through reduction in stress [24–26]. Social networks could impact cancer outcomes by influencing stage at detection or progression by affecting treatment decisions. Recent reports suggest social networks are a key factor in seeking cancer screening [27, 28], which could lead to detection at earlier stages and improved prognosis. Five of seven studies from a recent review supported the hypothesis that social networks influences cancer progression [29].
In order to better understand how social networks relate to survival after a breast cancer diagnosis, we examined the relation of social networks and survival in a large, population-based cohort of breast cancer survivors. Data collected enabled us to evaluate whether the type, number, and frequency of social contacts have any discernable impact on breast cancer outcomes.
Methods
As previously described [30–32], study participants were recruited from three consecutive population-based case-control studies of invasive breast cancer conducted between 1988 and 2001 in New Hampshire, Massachusetts, and Wisconsin. Cases were enrolled in the survivorship cohort after participating in the case-control study. Following an initial vital status search to identify decedents, the survivorship cohort was constructed of all women living with a history of breast cancer who completed a mailed questionnaire that captured information on factors of interest including social networks. A total of 4,589 women were included in the analysis following exclusions for metastatic breast cancer at initial diagnosis (n=34); unknown disease stage at diagnosis (n=615); or recurrence of breast cancer before completion of the questionnaire (n=553). The vital status of women was documented up to December 31, 2005 by a search of the National Death Index [33]. Women were followed a median of 5.6 (inter-quartile range 2.7–8.7) years after the breast cancer diagnosis. This study was approved by the institutional review boards at both the Harvard School of Public Health and the University of Wisconsin.
Social networks were assessed using a modified version of the Berkman-Syme Social Networks Index, a composite measure of four types of social connection: marital status (married versus not); social integration (number and frequency of contacts with children, close relatives, confidant and close friends); frequency of attending religious meetings or services; and membership in other community organizations [34, 35]. Summary scores were constructed using previously published methodology [35] assessing 1) network size: number of members in the network; 2) frequency of contacts: estimation of members seen at least once per month; and 3) overall social connectedness: number of frequent (at least once per month) social contacts with confidants or close friends, and/or attendance of religious or community services.
Hazard rate ratios and 95% confidence intervals were estimated using Cox proportional hazards regression. Adjusted models accounted for potential confounding factors at diagnosis, interval between diagnosis and enrollment in the cohort study, and characteristics at the time of social networks assessment, and weight change from pre-diagnosis to after treatment).
Results
Of the 4,589 women included in the analysis, approximately two-thirds of the women (n=2,995) had localized disease at diagnosis. During follow-up, 552 died after completing the questionnaire, 146 from breast cancer.
Table 1 shows participant characteristics by category of overall social connectedness. Those at higher categories of social connectedness were more likely to be married, have children, be non-smokers, and have a history of chemotherapy and tamoxifen treatment (Table 1).
Table 1.
Participant characteristics by category of social connectedness
| Category of Overall Social Connectedness1 | |||||
|---|---|---|---|---|---|
| N | Low (5–12) 620 |
Low- Medium (13–15) 1171 |
Medium (16–18) 1377 |
Medium- High (19–20) 709 |
High (21–26) 467 |
| Characteristic2 | -Mean (SD) or %-3 | ||||
| Age, y | 59 (9) | 58 (10) | 59 (10) | 59 (10) | 59 (9) |
| Time since diagnosis, y | 6 (3) | 6 (3) | 6 (3) | 6 (1) | 6 (3) |
| BMI at social support assessment, kg/m2 | 25.4 (4.8) | 25.7 (5.1) | 25.5 (4.7) | 25.95 (4.7) | 25.7 (4.5) |
| Education | |||||
| Less than high school | 8 | 9 | 8 | 8 | 7 |
| High School | 43 | 42 | 44 | 45 | 40 |
| Some college | 23 | 23 | 23 | 24 | 27 |
| College graduate | 25 | 27 | 24 | 23 | 25 |
| Smoker | 26 | 20 | 16 | 12 | 8 |
| Marital Status | |||||
| Yes | 62 | 70 | 70 | 71 | 70 |
| No | 38 | 30 | 29 | 28 | 28 |
| Parity | |||||
| Nulliparous | 19 | 15 | 10 | 9 | 6 |
| 1 to 2 | 42 | 42 | 37 | 32 | 29 |
| 3 to 5 | 36 | 38 | 48 | 49 | 52 |
| ≥6 | 3 | 4 | 5 | 9 | 12 |
| Post-menopausal | 75 | 69 | 73 | 73 | 76 |
| Localized disease | 63 | 65 | 66 | 66 | 65 |
| Treatment | |||||
| Radiation | 49 | 50 | 52 | 49 | 48 |
| Chemotherapy | 31 | 34 | 32 | 31 | 37 |
| Tamoxifen | 55 | 57 | 61 | 59 | 60 |
Summary score estimating relative frequency of contact with confidants, frequency of attending religious services, frequency of attending community group(s), and number of children, relatives, and friends seen at least once per month[35]
Characteristics assessed at breast cancer diagnosis unless otherwise noted.
Percents don’t sum to 100% due to missing values.
Social network size and the number of regular (at least once per month) social contacts were not associated with overall or breast-cancer specific survival (Table 2). However, the composite measure of social connectedness that incorporated both number of regular contacts and frequency of social activities was inversely associated with overall (p=0.02), but not breast cancer specific (p=0.93), survival.
Table 2.
Hazard rate ratios (and 95% confidence intervals) for all-cause and breast cancer mortality according to composite measures of social connectedness.
| All-cause | Breast Cancer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Deaths | HR1 | 95% CI | HR2 | 95% CI | Deaths | HR1 | 95% CI | HR2 | 95% CI | |
| Social Network Size3 | |||||||||||
| Small (5–9) | 935 | 133 | 1.00 | 1.00 | 33 | 1.00 | 1.00 | ||||
| Small-medium (9) | 854 | 115 | 1.01 | 0.78 to 1.29 | 1.04 | 0.81 to 1.34 | 30 | 0.97 | 0.59 to 1.60 | 1.01 | 0.61 to 1.66 |
| Medium (10) | 860 | 84 | 0.76 | 0.58 to 1.01 | 0.88 | 0.67 to 1.17 | 21 | 0.67 | 0.39 to 1.17 | 0.70 | 0.40 to 1.22 |
| Medium-large (11–12) | 1,188 | 117 | 0.76 | 0.60 to 0.98 | 0.92 | 0.71 to 1.20 | 39 | 0.93 | 0.59 to 1.49 | 0.97 | 0.59 to 1.58 |
| Large (13–16) | 564 | 68 | 0.94 | 0.70 to 1.27 | 1.33 | 0.97 to 1.82 | 19 | 0.98 | 0.56 to 1.73 | 1.07 | 0.59 to 1.96 |
| P-trend | 0.08 | 0.55 | 0.80 | 0.98 | |||||||
| Number of social ties seen at least once per month4 | |||||||||||
| Low (3–5) | 802 | 92 | 1.00 | 1.00 | 22 | 1.00 | 1.00 | ||||
| Low-medium (6) | 919 | 130 | 1.30 | 0.99 to 1.70 | 1.24 | 0.95 to 1.62 | 34 | 1.35 | 0.79 to 2.32 | 1.20 | 0.70 to 2.07 |
| Medium (7) | 1,022 | 124 | 1.14 | 0.87 to 1.49 | 1.19 | 0.90 to 1.56 | 34 | 1.22 | 0.71 to 2.08 | 1.14 | 0.66 to 1.96 |
| Medium-high (8–9) | 1,244 | 130 | 1.01 | 0.77 to 1.32 | 1.10 | 0.83 to 1.44 | 34 | 1.00 | 0.59 to 1.72 | 0.96 | 0.55 to 1.67 |
| High (10–13) | 494 | 54 | 0.94 | 0.67 to 1.32 | 1.08 | 0.77 to 1.53 | 18 | 1.37 | 0.73 to 2.56 | 1.33 | 0.70 to 2.55 |
| P-trend | 0.29 | 0.89 | 0.87 | 0.86 | |||||||
| Overall social connectedness5 | |||||||||||
| Low (5–12) | 620 | 93 | 1.00 | 1.00 | 27 | 1.00 | 1.00 | ||||
| Low-medium (13–15) | 1,171 | 151 | 0.92 | 0.71 to 1.20 | 1.01 | 0.78 to 1.32 | 35 | 0.68 | 0.41 to 1.12 | 0.69 | 0.41 to 1.14 |
| Medium (16–18) | 1,377 | 146 | 0.70 | 0.54 to 0.91 | 0.78 | 0.60 to 1.02 | 33 | 0.55 | 0.33 to 0.92 | 0.58 | 0.34 to 0.97 |
| Medium-high (19–20) | 709 | 78 | 0.72 | 0.53 to 0.97 | 0.85 | 0.62 to 1.15 | 25 | 0.83 | 0.48 to 1.43 | 0.94 | 0.54 to 1.65 |
| High (21–26) | 467 | 43 | 0.56 | 0.39 to 0.81 | 0.72 | 0.49 to 1.04 | 16 | 0.81 | 0.43 to 1.50 | 0.85 | 0.45 to 1.62 |
| P-trend | 0.002 | 0.02 | 0.65 | 0.93 | |||||||
Hazard Ratios and 95% Confidence Intervals estimated from models adjusted for age at diagnosis and state of residence.
Hazard Ratios and 95% Confidence Intervals estimated from models adjusted for factors at diagnosis (age, breast cancer stage, state of residence, menopausal status, smoking, hormone replacement therapy use, parity, education), interval between diagnosis and social support assessment, and factors at the time of social support assessment (treatment (radiation, chemotherapy, tamoxifen), physical activity(quintiles), BMI (quintiles), weight change from pre-diagnosis to follow-up).
Ranking of relative network size, including spouse/partner, confidants, children, and close relatives and friends.
Ranking of relative number of children, relatives, and friends seen at least once per month.
Summary score estimating relative frequency of contact with confidants, frequency of attending religious services, frequency of attending community group(s), and number of children, relatives, and friends seen at least once per month.[35]
Among specific types of social contacts, increased participation in religious or community activities was significantly associated with improved overall survival (Table 3). Attending religious gatherings more than once a week (n=670, 14%) was associated with a 34% (95% CI = 10%–51%, p-trend=0.0001) reduction in death from any cause when compared to no religious participation (n=1125, 25%). Greater community participation, as measured in hours per week, was also associated with improved overall survival (p-trend=0.0005).
Table 3.
Hazard rate ratios (and 95% confidence intervals) for all-cause and breast cancer mortality according to components of social connectedness.
| All-cause | Breast Cancer | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | Deaths | HR2 | 95% CI | HR3 | 95% CI | Deaths | HR2 | 95% CI | HR3 | 95% CI | |
| Religious participation | |||||||||||
| Never | 1,125 | 160 | 1.00 | 1.00 | 46 | 1.00 | 1.00 | ||||
| < Once per month | 461 | 52 | 0.78 | 0.57 to 1.06 | 0.89 | 0.65 to 1.22 | 16 | 0.85 | 0.48 to 1.51 | 0.87 | 0.49 to 1.54 |
| 1–3 times per month | 455 | 49 | 0.66 | 0.48 to 0.92 | 0.74 | 0.53 to 1.03 | 9 | 0.51 | 0.25 to 1.04 | 0.57 | 0.28 to 1.19 |
| Once a week | 1,822 | 207 | 0.62 | 0.50 to 0.76 | 0.67 | 0.54 to 0.83 | 52 | 0.74 | 0.49 to 1.11 | 0.84 | 0.55 to 1.27 |
| > Once a week | 670 | 68 | 0.54 | 0.40 to 0.72 | 0.66 | 0.49 to 0.90 | 21 | 0.84 | 0.49 to 1.42 | 1.00 | 0.58 to 1.73 |
| P-trend | <0.0001 | 0.0001 | 0.23 | 0.67 | |||||||
| Community group participation | |||||||||||
| None | 1,980 | 269 | 1.00 | 1.00 | 65 | 1.00 | 1.00 | ||||
| 1–2 hours per week | 1,233 | 137 | 0.82 | 0.66 to 1.01 | 0.93 | 0.76 to 1.15 | 48 | 1.20 | 0.82 to 1.74 | 1.31 | 0.89 to 1.93 |
| 3–5 hours per week | 827 | 93 | 0.75 | 0.59 to 0.95 | 0.84 | 0.66 to 1.07 | 22 | 0.82 | 0.50 to 1.32 | 0.86 | 0.53 to 1.42 |
| 6–10 hours per week | 286 | 24 | 0.49 | 0.33 to 0.75 | 0.58 | 0.38 to 0.88 | 4 | 0.43 | 0.16 to 1.19 | 0.44 | 0.16 to 1.23 |
| 11–15 hours per week | 82 | 4 | 0.30 | 0.11 to 0.81 | 0.40 | 0.15 to 1.08 | 1 | 0.36 | 0.05 to 2.58 | 0.42 | 0.06 to 3.02 |
| ≥16 hours per week | 91 | 4 | 0.36 | 0.13 to 0.96 | 0.39 | 0.15 to 1.06 | 2 | 0.62 | 0.15 to 2.55 | 0.58 | 0.14 to 2.38 |
| P-trend | <0.0001 | 0.0005 | 0.07 | 0.11 | |||||||
| Cancer support group participation | |||||||||||
| Never | 3,828 | 476 | 1.00 | 1.00 | 114 | 1.00 | 1.00 | ||||
| In the past | 423 | 36 | 0.94 | 0.67 to 1.33 | 1.06 | 0.75 to 1.50 | 18 | 1.32 | 0.80 to 2.19 | 1.22 | 0.73 to 2.05 |
| Currently | 257 | 21 | 0.82 | 0.53 to 1.28 | 1.00 | 0.64 to 1.56 | 10 | 1.37 | 0.71 to 2.62 | 1.15 | 0.59 to 2.24 |
| Number of close relatives | |||||||||||
| None | 363 | 58 | 1.00 | 1.00 | 14 | 1.00 | 1.00 | ||||
| 1–2 | 1,484 | 198 | 0.92 | 0.68 to 1.23 | 0.95 | 0.71 to 1.28 | 51 | 0.84 | 0.47 to 1.53 | 0.82 | 0.45 to 1.49 |
| 3–5 | 1,559 | 157 | 0.74 | 0.55 to 1.00 | 0.81 | 0.60 to 1.10 | 45 | 0.71 | 0.39 to 1.29 | 0.70 | 0.38 to 1.29 |
| 6–9 | 633 | 76 | 0.91 | 0.65 to 1.29 | 1.09 | 0.77 to 1.54 | 20 | 0.77 | 0.38 to 1.52 | 0.73 | 0.36 to 1.48 |
| ≥10 | 515 | 54 | 0.80 | 0.55 to 1.15 | 0.98 | 0.67 to 1.43 | 16 | 0.77 | 0.37 to 1.57 | 0.77 | 0.37 to 1.61 |
| P-trend | 0.20 | 0.91 | 0.42 | 0.46 | |||||||
| Number of close relatives seen at least once per month | |||||||||||
| None | 1,109 | 139 | 1.00 | 1.00 | 35 | 1.00 | 1.00 | ||||
| 1–2 | 1,745 | 231 | 1.11 | 0.90 to 1.37 | 1.13 | 0.91 to 1.39 | 58 | 1.06 | 0.70 to 1.62 | 0.99 | 0.65 to 1.51 |
| 3–5 | 1,149 | 111 | 0.89 | 0.69 to 1.15 | 0.98 | 0.76 to 1.27 | 34 | 0.92 | 0.57 to 1.48 | 0.90 | 0.56 to 1.46 |
| 6–9 | 376 | 44 | 1.00 | 0.71 to 1.41 | 1.12 | 0.79 to 1.58 | 9 | 0.74 | 0.35 to 1.54 | 0.74 | 0.35 to 1.56 |
| ≥10 | 173 | 18 | 0.97 | 0.59 to 1.58 | 1.13 | 0.69 to 1.86 | 9 | 1.62 | 0.78 to 3.38 | 1.41 | 0.67 to 3.00 |
| P-trend | 0.48 | 0.74 | 0.95 | 0.97 | |||||||
| Number of close friends | |||||||||||
| None | 133 | 19 | 1.00 | 1.00 | 4 | 1.00 | 1.00 | ||||
| 1–2 | 1,007 | 124 | 0.96 | 0.59 to 1.56 | 1.11 | 0.68 to 1.82 | 35 | 1.12 | 0.40 to 3.15 | 1.25 | 0.44 to 3.56 |
| 3–5 | 1,971 | 228 | 0.87 | 0.55 to 1.40 | 1.06 | 0.65 to 1.71 | 64 | 1.06 | 0.39 to 2.92 | 1.24 | 0.45 to 3.46 |
| 6–9 | 828 | 95 | 0.81 | 0.49 to 1.32 | 1.04 | 0.63 to 1.72 | 18 | 0.74 | 0.25 to 2.18 | 0.86 | 0.29 to 2.58 |
| ≥10 | 621 | 78 | 0.84 | 0.51 to 1.39 | 1.19 | 0.71 to 1.99 | 25 | 1.40 | 0.49 to 4.01 | 1.71 | 0.58 to 5.01 |
| P-trend | 0.21 | 0.69 | 0.82 | 0.53 | |||||||
| Number of close friends seen at least once per month | |||||||||||
| None | 311 | 37 | 1.00 | 1.00 | 9 | 1.00 | 1.00 | ||||
| 1–2 | 1,616 | 207 | 1.13 | 0.80 to 1.60 | 1.21 | 0.85 to 1.72 | 52 | 1.10 | 0.54 to 2.24 | 1.21 | 0.60 to 2.48 |
| 3–5 | 1,766 | 204 | 0.97 | 0.69 to 1.38 | 1.13 | 0.79 to 1.61 | 56 | 1.09 | 0.54 to 2.20 | 1.27 | 0.62 to 2.59 |
| 6–9 | 555 | 64 | 0.89 | 0.59 to 1.33 | 1.04 | 0.69 to 1.57 | 17 | 1.10 | 0.49 to 2.46 | 1.29 | 0.57 to 2.93 |
| ≥10 | 294 | 30 | 0.81 | 0.50 to 1.32 | 1.05 | 0.64 to 1.71 | 11 | 1.35 | 0.56 to 3.26 | 1.50 | 0.61 to 3.67 |
| P-trend | 0.05 | 0.54 | 0.62 | 0.40 | |||||||
| Number of children | |||||||||||
| None | 495 | 62 | 1.00 | 1.00 | 16 | 1.00 | 1.00 | ||||
| 1–2 | 1,869 | 214 | 0.95 | 0.72 to 1.26 | 0.87 | 0.61 to 1.23 | 62 | 1.01 | 0.59 to 1.76 | 0.93 | 0.48 to 1.80 |
| 3–5 | 1,960 | 225 | 0.90 | 0.68 to 1.19 | 0.92 | 0.58 to 1.46 | 60 | 1.00 | 0.58 to 1.74 | 0.99 | 0.40 to 2.44 |
| ≥6 | 232 | 40 | 1.19 | 0.80 to 1.78 | 1.22 | 0.71 to 2.11 | 6 | 0.91 | 0.35 to 2.35 | 0.90 | 0.27 to 2.98 |
| P-trend | 0.98 | 0.49 | 0.89 | 0.89 | |||||||
| Number of living children seen at least once per month | |||||||||||
| None | 876 | 101 | 1.00 | 1.00 | 26 | 1.00 | 1.00 | ||||
| 1–2 | 2,391 | 271 | 1.09 | 0.87 to 1.37 | 1.12 | 0.86 to 1.45 | 72 | 1.00 | 0.64 to 1.57 | 0.93 | 0.56 to 1.55 |
| 3–5 | 1,146 | 144 | 1.19 | 0.92 to 1.53 | 1.29 | 0.93 to 1.78 | 41 | 1.25 | 0.76 to 2.04 | 1.33 | 0.70 to 2.53 |
| ≥6 | 134 | 26 | 1.56 | 1.02 to 2.41 | 1.48 | 0.93 to 2.37 | 5 | 1.40 | 0.53 to 3.64 | 1.41 | 0.50 to 3.95 |
| P-trend | 0.05 | 0.05 | 0.26 | 0.27 | |||||||
| Have confidant | |||||||||||
| Yes | 4128 | 483 | 1.00 | 1.00 | 130 | 1.00 | 1.00 | ||||
| No | 366 | 52 | 1.06 | 0.80 to 1.41 | 0.93 | 0.70 to 1.25 | 13 | 1.19 | 0.67 to 2.11 | 1.23 | 0.69 to 2.19 |
| Frequency of seeing confidant | |||||||||||
| Daily | 2,042 | 245 | 1.00 | 1.00 | 73 | 1.00 | 1.00 | ||||
| Weekly | 1,551 | 181 | 0.92 | 0.76 to 1.11 | 0.91 | 0.75 to 1.11 | 46 | 0.85 | 0.59 to 1.23 | 0.89 | 0.61 to 1.29 |
| Monthly | 299 | 33 | 0.92 | 0.64 to 1.33 | 0.94 | 0.65 to 1.35 | 7 | 0.65 | 0.30 to 1.42 | 0.66 | 0.30 to 1.45 |
| Several times/year | 188 | 18 | 0.72 | 0.45 to 1.17 | 0.76 | 0.47 to 1.22 | 0 | na | na | ||
| Once a year or less | 25 | 3 | 0.90 | 0.29 to 2.82 | 1.11 | 0.35 to 3.51 | 0 | na | na | ||
| P-trend | 0.18 | 0.26 | 0.01 | 0.01 | |||||||
| Currently Married | |||||||||||
| Yes | 3130 | 306 | 1.00 | 1.00 | 99 | 1.00 | 1.00 | ||||
| No | 1393 | 230 | 1.21 | 1.02 to 1.45 | 0.96 | 0.79 to 1.15 | 44 | 1.08 | 0.74 to 1.55 | 1.02 | 0.70 to 1.49 |
Numbers do not sum to 4589 due to missing values.
Hazard Ratios and 95% Confidence Intervals estimated from models adjusted for age at diagnosis and state of residence.
Hazard Ratios and 95% Confidence Intervals estimated from models adjusted for factors at diagnosis (age, breast cancer stage, state of residence, menopausal status, smoking, hormone replacement therapy use, parity, education), interval between diagnosis and social support assessment, and factors at the time of social support assessment (treatment (radiation, chemotherapy, tamoxifen), physical activity (quintiles), BMI (quintiles), weight change from pre-diagnosis to follow-up).
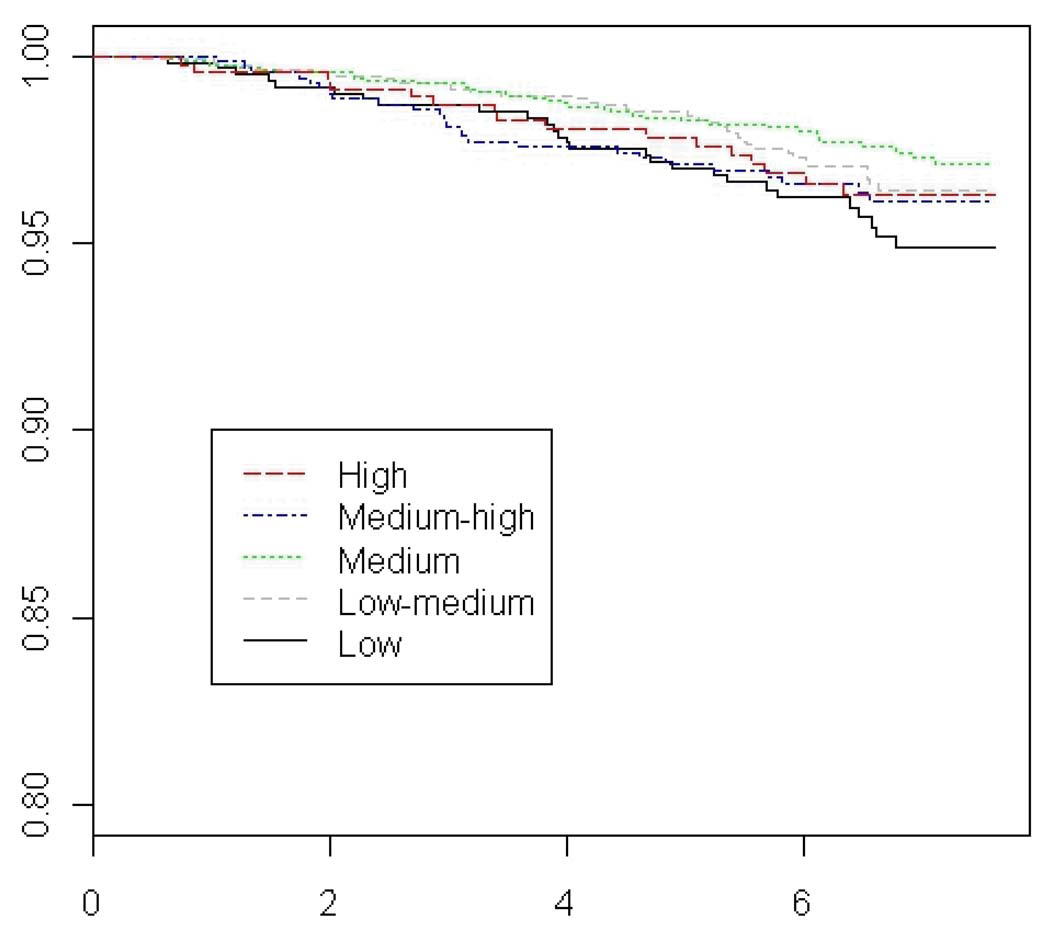
Weaker and no significant trends were observed for breast cancer-specific survival. Number of close friends, relatives, or confidants, and number of contacts with such persons had no impact on overall survival rates, nor did marital status. Cancer support group participation, either in the past or currently, had no association with mortality after breast cancer.
Discussion
In this large cohort of breast cancer survivors, we found some evidence for an association between social connectedness and overall mortality. Whereas participation in community and religious activities was significantly associated with lower overall mortality, such interactions had no material influence on breast cancer specific mortality. The number of close friends, relatives and living children, the frequency of contacts with significant others, and marital status had no significant impact on subsequent breast cancer-specific or overall survival rates.
The current findings are consistent with some, but not all, previous studies that addressed the impact of social networks on breast cancer outcome, as reviewed by Falagas et al [3]. Of nine published studies, four reported a survival advantage associated with one or more index of social networks [4–7] whereas five reported no association [8–12]. All of the null studies had limited power to detect associations (<250 participants). A recent meta-analysis combining data from 87 studies of social support, social networks, and marital status and cancer outcomes reported stronger inverse associations with cancer mortality among breast cancer survivors compared with other cancer sites [2]. Possible mechanisms suggested included that those with social networks would be more inclined to seek and/or follow through with treatment. Social networks may also favorably change hormone concentrations, thereby preferentially acting on hormonal cancers [2]. An alternative explanation is that more studies have been conducted in breast cancer relative to other sites, thereby providing sufficient power to detect weak associations between social networks and survival relative to other sites.
An analysis of Nurses’ Health Study (NHS) data by Kroenke et al [7] also based on the Berkman-Syme Index found that the number of close friends and relatives as reported prior to breast cancer diagnosis was associated with improved survival subsequent to breast cancer diagnosis even after adjusting for breast cancer stage at diagnosis. In contrast to present findings, participation in religious and community activities had no important influence on breast cancer or overall survival. Differences in results in the NHS and current study could be related to differences in the age (NHS women were about 10 years older) or economic levels (the NHS was comprised of nurses who were presumably more educated) between the study populations, and the timing of collection of social network data with respect to the breast cancer diagnosis (data collected two years post diagnosis in the NHS versus six years for the current study). For example, having larger numbers of friends and close relatives could be associated with better treatment compliance, accounting for the inverse association with number of friends and relatives noted in the NHS. In contrast, numbers of friends and relatives may be less important after treatment is completed. As with our results, Kroenke et al reported religious and community participation was significantly associated with improved all-cause survival after diagnosis.
Randomized controlled trials assessing the impact of improved social support on breast cancer survival have produced mixed results. An initial randomized controlled trial that enrolled 86 women with metastatic breast cancer reported a mean doubling in survival time after weekly group therapy for one year [13]. However, subsequent trials in similar populations have suggested that while group therapy may improve quality of life, it does not prolong survival [14–16].
One possible explanation for the mixed findings from previous studies is measurement error in the assessment of social networks. Previous research, including the current study, ranked women on the number of close relationships and marital status. A recent study found that the quality, rather than quantity, of close relationships was associated with improved survival among ninety breast cancer survivors; a composite measure of marital confiding and close relationships had a strong inverse effect (RR= 0.41 (95% CI 0.21–0.80)) [6]. In measuring the quality of social networks, it may be important to account for specific informational, instrumental, and emotional support being provided by members of the network [29].
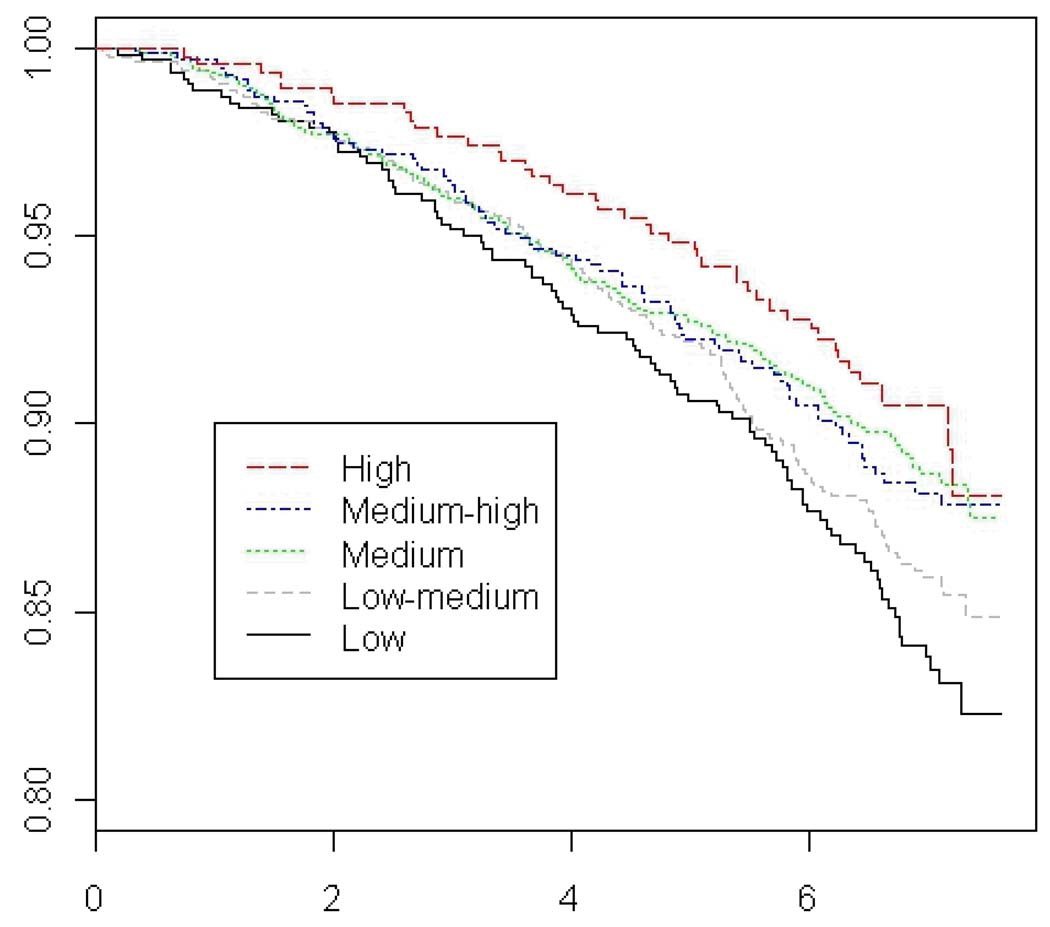
Taken together with previous studies, the available evidence suggests that social networks, as measured by the number of close relationships, may have an influence on survival after a breast cancer diagnosis. After accounting for confounders, women in the highest category of social connectedness had a 28% (95% CI, -4%-51%, p-trend 0.02) lower risk of overall mortality compared to those in the lowest category of social connectedness. There was an observed benefit for increased community and religious participation, a finding that may reflect better health and extended longevity of women able to engage in activities outside the home (e.g. reverse-causation). Moreover, the suggestions of higher death rates observed in women that had regular visits from larger numbers of their children (p=0.05), which may be surrogate for declining health or physical incapacity, is also consistent with a confounding influence of general health on the results, despite adjustment for smoking, BMI, and other predictors of mortality.
The reduced risk of overall mortality with increasing levels of social connectedness was likely driven by cardiovascular disease-specific mortality. In analyses restricted to mortality from cardiovascular disease in this cohort (n=123), there was a suggestion of reduced risk of cardiovascular disease-specific mortality among those in the highest category of overall social connectedness (scores=21–26) compared to those with low scores (5–12) (Adjusted HR=0.41, 95% CI 0.16 to 1.04, p-trend 0.01). This is consistent with recent reports that increasing levels of social engagement confer protection against stroke in women [37] and cardiovascular mortality in men [38].
The present analysis had some limitations. Not all eligible women responded to the study invitation, and respondents were generally more highly educated and demonstrated a healthier profile; i.e. fewer current smokers, greater proportion of BMI values in the normal range than those who did not participate [39]. Thus, although this is the only study to target a population-wide cohort of breast cancer survivors, results may not be applicable to all breast cancer survivors. In addition, women were enrolled several years after breast cancer diagnosis and those with more rapidly fatal, aggressive breast cancers would have been underrepresented in the study cohort. Finally, few women reported having no friends or close relatives, and the majority were married. This may have reduced power to detect associations at extreme levels of social isolation.
Identification of factors that improve the health and longevity of breast cancer survivors is an important public health challenge. Our study supports the hypothesis that social networks reduce the likelihood of death from any cause among breast cancer survivors…
Figure 1.
Adjusted Overall Survival Curves by Level of Overall Social Connectedness
Figure 2.
Adjusted Breast-Cancer Specific Survival Curves by Level of Overall Social Connectedness
Acknowledgements
This work was supported by The Susan B. Komen For the Cure Foundation (POP 0504234) and NIH grants CA47147 and CA47305.
Literature Cited
- 1.House JS, Landis KR, Umberson D. Social relationships and health. Science (New York, NY. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 2.Pinquart M, Duberstein PR. Associations of social networks with cancer mortality: A meta-analysis. Critical reviews in oncology/hematology. 2009 doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falagas ME, Zarkadoulia EA, Ioannidou EN, Peppas G, Christodoulou C, Rafailidis PI. The effect of psychosocial factors on breast cancer outcome: a systematic review. Breast cancer research: BCR. 2007;9:R44. doi: 10.1186/bcr1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marshall JR, Funch DP. Social environment and breast cancer. A cohort analysis of patient survival. Cancer. 1983;52:1546–1550. doi: 10.1002/1097-0142(19831015)52:8<1546::aid-cncr2820520835>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds P, Boyd PT, Blacklow RS, Jackson JS, Greenberg RS, Austin DF, et al. The relationship between social ties and survival among black and white breast cancer patients. National Cancer Institute Black/White Cancer Survival Study Group. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 1994;3:253–259. [PubMed] [Google Scholar]
- 6.Weihs KL, Enright TM, Simmens SJ. Close relationships and emotional processing predict decreased mortality in women with breast cancer: preliminary evidence. Psychosomatic medicine. 2008;70:117–124. doi: 10.1097/PSY.0b013e31815c25cf. [DOI] [PubMed] [Google Scholar]
- 7.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 8.Greer S, Morris T, Pettingale KW. Psychological response to breast cancer: effect on outcome. Lancet. 1979;2:785–787. doi: 10.1016/s0140-6736(79)92127-5. [DOI] [PubMed] [Google Scholar]
- 9.Cassileth BR, Walsh WP, Lusk EJ. Psychosocial correlates of cancer survival: a subsequent report 3 to 8 years after cancer diagnosis. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1988;6:1753–1759. doi: 10.1200/JCO.1988.6.11.1753. [DOI] [PubMed] [Google Scholar]
- 10.Barraclough J, Osmond C, Taylor I, Perry M, Collins P. Life events and breast cancer prognosis. BMJ (Clinical research ed) 1993;307:325. doi: 10.1136/bmj.307.6899.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butow PN, Coates AS, Dunn SM. Psychosocial predictors of survival: metastatic breast cancer. Annals of Oncology: Official Journal of the European Society for Medical Oncology / ESMO. 2000;11:469–474. doi: 10.1023/a:1008396330433. [DOI] [PubMed] [Google Scholar]
- 12.Osborne RH, Sali A, Aaronson NK, Elsworth GR, Mdzewski B, Sinclair AJ. Immune function and adjustment style: do they predict survival in breast cancer? Psycho-oncology. 2004;13:199–210. doi: 10.1002/pon.723. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- 14.Kissane DW, Grabsch B, Clarke DM, Smith GC, Love AW, Bloch S, et al. Supportive-expressive group therapy for women with metastatic breast cancer: survival and psychosocial outcome from a randomized controlled trial. Psycho-oncology. 2007;16:277–286. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- 15.Coyne JC, Hanisch LJ, Palmer SC, et al. Psychotherapy does not promote survival (Kissane et al., 2007): now what? Psycho-oncology. 2007;16:1050–1052. doi: 10.1002/pon.1285. [DOI] [PubMed] [Google Scholar]
- 16.Spiegel D, Butler LD, Giese-Davis J, Koopman C, Miller E, DiMiceli S, et al. Effects of supportive-expressive group therapy on survival of patients with metastatic breast cancer: a randomized prospective trial. Cancer. 2007;110:1130–1138. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- 17.Seeman TE, Kaplan GA, Knudsen L, Cohen R, Guralnik J. Social network ties and mortality among the elderly in the Alameda County Study. American Journal of Epidemiology. 1987;126:714–723. doi: 10.1093/oxfordjournals.aje.a114711. [DOI] [PubMed] [Google Scholar]
- 18.Seeman TE, Berkman LF, Kohout F, Lacroix A, Glynn R, Blazer D. Intercommunity variations in the association between social ties and mortality in the elderly. A comparative analysis of three communities. Annals of Epidemiology. 1993;3:325–335. doi: 10.1016/1047-2797(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 19.Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, et al. No health without mental health. Lancet. 2007;370:859–877. doi: 10.1016/S0140-6736(07)61238-0. [DOI] [PubMed] [Google Scholar]
- 20.Spiegel D, Kato PM. Psychosocial influences on cancer incidence and progression. Harvard review of psychiatry. 1996;4:10–26. doi: 10.3109/10673229609030518. [DOI] [PubMed] [Google Scholar]
- 21.Christakis NA, Fowler JH. The collective dynamics of smoking in a large social network. The New England journal of medicine. 2008;358:2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. The New England journal of medicine. 2007;357:370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 23.Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM. Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:5814–5830. doi: 10.1200/JCO.2005.01.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature clinical practice Oncology. 2008;5:466–475. doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 25.Kopp MS, Skrabski A, Szekely A, Stauder A, Williams R. Chronic stress and social changes: socioeconomic determination of chronic stress. Annals of the New York Academy of Sciences. 2007;1113:325–338. doi: 10.1196/annals.1391.006. [DOI] [PubMed] [Google Scholar]
- 26.McGregor BA, Antoni MH, Boyers A, Alferi SM, Blomberg BB, Carver CS. Cognitive-behavioral stress management increases benefit finding and immune function among women with early-stage breast cancer. Journal of psychosomatic research. 2004;56:1–8. doi: 10.1016/S0022-3999(03)00036-9. [DOI] [PubMed] [Google Scholar]
- 27.Amorim VM, Barros MB, Cesar CL, Carandina L, Goldbaum M. [Factors associated with lack of mammograms and clinical breast examination by women: a population-based study in Campinas, Sao Paulo State, Brazil] Cadernos de saude publica / Ministerio da Saude, Fundacao Oswaldo Cruz, Escola Nacional de Saude Publica. 2008;24:2623–2632. doi: 10.1590/s0102-311x2008001100017. [DOI] [PubMed] [Google Scholar]
- 28.Fowler BA. The influence of social support relationships on mammography screening in African-American women. J Natl Black Nurses Assoc. 2007;18:21–29. [PubMed] [Google Scholar]
- 29.Nausheen B, Gidron Y, Peveler R, Moss-Morris R. Social support and cancer progression: a systematic review. Journal of psychosomatic research. 2009;67:403–15. doi: 10.1016/j.jpsychores.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Longnecker MP, Newcomb PA, Mittendorf R, Greenberg ER, Clapp RW, Bogdan GF, et al. Risk of breast cancer in relation to lifetime alcohol consumption. Journal of the National Cancer Institute. 1995;87:923–929. doi: 10.1093/jnci/87.12.923. [DOI] [PubMed] [Google Scholar]
- 31.Titus-Ernstoff L, Egan KM, Newcomb PA, Baron JA, Stampfer M, Greenberg ER, et al. Exposure to breast milk in infancy and adult breast cancer risk. Journal of the National Cancer Institute. 1998;90:921–924. doi: 10.1093/jnci/90.12.921. [DOI] [PubMed] [Google Scholar]
- 32.Sprague BL, Trentham-Dietz A, Newcomb PA, Titus-Ernstoff L, Hampton JM, Egan KM. Lifetime recreational and occupational physical activity and risk of in situ and invasive breast cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2007;16:236–243. doi: 10.1158/1055-9965.EPI-06-0713. [DOI] [PubMed] [Google Scholar]
- 33.Calle EE, Terrell DD. Utility of the National Death Index for ascertainment of mortality among cancer prevention study II participants. American Journal of Epidemiology. 1993;137:235–241. doi: 10.1093/oxfordjournals.aje.a116664. [DOI] [PubMed] [Google Scholar]
- 34.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda County residents. American Journal of Epidemiology. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 35.Sapp AL, Trentham-Dietz A, Newcomb PA, Hampton JM, Moinpour CM, Remington PL. Social networks and quality of life among female long-term colorectal cancer survivors. Cancer. 2003;98:1749–1758. doi: 10.1002/cncr.11717. [DOI] [PubMed] [Google Scholar]
- 36.Lehto US, Ojanen M, Dyba T, Aromaa A, Kellokumpu-Lehtinen P. Baseline psychosocial predictors of survival in localised breast cancer. British journal of cancer. 2006;94:1245–1252. doi: 10.1038/sj.bjc.6603091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutledge T, Linke SE, Olson MB, Francis J, Johnson BD, Bittner V, et al. Social networks and incident stroke among women with suspected myocardial ischemia. Psychosomatic medicine. 2008;70:282–287. doi: 10.1097/PSY.0b013e3181656e09. [DOI] [PubMed] [Google Scholar]
- 38.Ramsay S, Ebrahim S, Whincup P, Papacosta O, Morris R, Lennon L, et al. Social engagement and the risk of cardiovascular disease mortality: results of a prospective population-based study of older men. Annals of Epidemiology. 2008;18:476–483. doi: 10.1016/j.annepidem.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Holick CN, Newcomb PA, Trentham-Dietz A, Titus-Ernstoff L, Bersch AJ, Stampfer MJ, et al. Physical activity and survival after diagnosis of invasive breast cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2008;17:379–386. doi: 10.1158/1055-9965.EPI-07-0771. [DOI] [PubMed] [Google Scholar]