Abstract
Background
Cerebrospinal fluid (CSF) protein values decline over the first few months of life as the infant's blood-CSF barrier matures. However, published studies differ in the reported rate, timing, and magnitude of this decline.
Objective
To quantify the age-related changes in CSF protein concentration and to determine accurate, age-specific reference values for neonates and young infants.
Design, Setting and Patients
This cross-sectional study included infants age 56 days or younger who had a lumbar puncture performed in the emergency department of an urban tertiary care children's hospital between January 1, 2005 and June 30, 2007. Infants with conditions associated with elevated CSF protein concentrations, including traumatic lumbar puncture and bacterial or viral meningitis, were excluded.
Results
Of 1,064 infants undergoing lumbar puncture, 375 (35%) met inclusion criteria. The median CSF protein value was 58 mg/dL (interquartile range: 48–72 mg/dL). In linear regression, the CSF protein concentration decreased 6.8% (95% confidence interval: 5.4%–8.1%; p<0.001) with each 1 week increase in age. The 95th percentile values were 115 mg/dL for infants ≤28 days and 89 mg/dL for infants 29–56 days. The 95th percentile values by age category were as follows: ages 0–14 days, 132 mg/dL; ages 15–28 days, 100 mg/dL; ages 29–42 days, 89 mg/dL; and ages 43–56 days, 83 mg/dL.
Conclusions
We quantify the age-related decline in CSF protein concentration among infants 56 days of age and younger and provide age-specific reference values. The values reported here represent the largest series to-date for this age group.
Keywords: cerebrospinal fluid; infant, newborn; reference values; lumbar puncture
Introduction
Emergency department evaluation of a febrile neonate or young infant routinely includes lumbar puncture and cerebrospinal fluid (CSF) analysis to diagnose meningitis or encephalitis. In addition to CSF Gram stain and culture, clinicians generally request a laboratory report for the CSF cell count, glucose content and protein concentration. Interpretation of these ancillary tests requires knowledge of normal reference values. In adult medicine, the accepted reference value for CSF protein concentration at the level of the lumbar spine is 15–45 mg/dL.1 There is general consensus among reference texts and published original studies dating back to Widell2 in 1958 that adult CSF protein reference values are not valid in the pediatric population. A healthy neonate's CSF protein concentration is normally twice to three times that of an adult, and declines with age from birth to early childhood. The most rapid rate of decline is thought to occur in the first six months of life as the infant's blood-CSF barrier matures.3 However, published studies4–7 differ in the reported rate, timing, and magnitude of this decline; on close review these studies have significant limitations which call into question the appropriateness of using these values in clinical practice. Perhaps in recognition of the limited evidence, textbooks of general pediatrics,8–10 hospital medicine,11–13 emergency medicine,14, 15 infectious diseases,16, 17 neonatology,18 and neurology19, 20 frequently publish norms for pediatric CSF protein concentration without reference to any original research studies.
Because ethical considerations prohibit subjecting young infants to a potentially painfully procedure (i.e., lumbar puncture) before they are able to assent, we sought to define a study population that approximates a group of healthy infants. Our objectives were to quantify age-related declines in CSF protein concentration and to determine accurate, age-specific reference values for CSF protein concentration in a population of neonates and young infants who presented for medical care with an indication for lumbar puncture and were subsequently found to have no condition associated with elevated or depressed CSF protein concentration.
Methods
Study Design and Setting
This cross-sectional study was performed at The Children's Hospital of Philadelphia (Philadelphia, PA), an urban, tertiary-care children's hospital. The Committees for the Protection of Human Subjects approved this study with a waiver of informed consent.
Study Participants
Infants 56 days of age or younger were eligible for inclusion if they had a lumbar puncture performed as part of their emergency department evaluation between January 1, 2005 and June 30, 2007. Children in this age range were selected as they routinely undergo lumbar puncture when presenting with fever at our institution.21, 22 Patients undergoing lumbar puncture in the emergency department were identified using two different data sources to ensure accurate identification of all eligible infants: 1) Emergency department computerized order entry records identified all infants with CSF testing (including CSF Gram stain, culture, cell count, glucose, or protein) performed during the study period, and 2) Clinical Virology Laboratory records identified all infants in whom CSF herpes simplex virus or enterovirus testing was performed. Medical records of infants identified by these two sources were reviewed to determine study eligibility.
Subjects with conditions known or suspected to cause abnormal CSF protein concentration were systematically excluded from the final analysis. Exclusion criteria included traumatic lumbar puncture (defined as CSF sample with greater than 500 red blood cells per mm3), serious bacterial infection (including meningitis, urinary tract infection, bacteremia, pneumonia, osteomyelitis or septic arthritis), congenital infection, CSF positive for enterovirus by polymerase chain reaction (PCR) testing, seizure prior to presentation, presence of a ventricular shunt device, elevated serum bilirubin, and absent CSF protein measurements or CSF red blood cell counts. The presence of lysed red blood cells in the CSF secondary to a traumatic lumbar puncture or subarachnoid hemorrhage alters the CSF protein.23 We also excluded subjects who had CSF assays done on samples drawn by accessing a ventricular shunt device, as there may be up to a 300% regional difference in CSF protein concentration between the cranial and caudal ends of the neuroaxis.1 Bilirubin in the CSF sample at a concentration of 5 mg/dL biases the CSF protein concentration measurement by an average of 13.7 mg/dL.24 Quantitative protein assay was performed on the institution's standard Vitros chemistry system; the protein assay is a modified biuret reaction.
Study Definitions
CSF pleocytosis was defined as a CSF white blood cell count >22/mm3 (for infants age ≤28 days) or >15/mm3 (for infants 29–56 days of age).25 Bacterial meningitis was defined as isolation of a bacterial pathogen from the CSF. Bacteremia was defined as isolation of a bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Bacterial pneumonia was defined as a new discrete infiltrate on chest radiograph as documented by an attending pediatric radiologist in conjunction with growth of a respiratory bacterial pathogen from blood culture. Urinary tract infection was defined as growth of a single known pathogen in culture as follows: (1) ≥ 1,000 colony-forming units/ml for cultures obtained by suprapubic aspiration, (2) ≥ 50,000 cfu/mL from a catheterized specimen, or (3) ≥ 10,000 cfu/mL from catheterized specimen in conjunction with a positive urinalysis.26 Positive urinalysis was defined as trace or greater leukocyte esterase by dip stick, or >9 WBC per high-power filed on standard microscopic exam of centrifuged urine, or >10 WBC/mm3 by hemocytometer count of uncentrifuged urine.27, 28 We defined osteomyelitis as growth of pathogenic bacteria from blood, bone, or subperiosteal aspirate culture in a subject with fever and localized tenderness, edema or erythema at the site of bony infection, and compatible imaging; and septic arthritis as growth of pathogenic bacteria from synovial fluid or blood culture from a subject with purulent synovial fluid or positive Gram stain of synovial fluid.
A temperature ≥38.0°C by any method qualified as fever. Prematurity was defined as a gestational age less than 37 weeks. Seizure included any clinical description of the event within 48 hours of presentation to the Emergency Department, or documented seizure activity on electroencephalogram. Enterovirus season was defined as June 1st to October 31st of each year.29
Data Collection and Statistical Analysis
Information collected included the following: demographics, vital signs, history of present illness, birth history, clinical findings, results of laboratory testing and imaging within 48 hours of presentation, antibiotics administered, and duration of visit to the Emergency Department or admission to the hospital.
Categorical data were described using frequencies and percents, and continuous variables were described using mean, median, interquartile range, and 90th and 95th percentile values. Linear regression was used to determine the association between age and CSF protein concentration. Because the CSF protein concentrations had a skewed distribution (p<0.001, Shapiro-Δ analyses were performed using logarithmically transformed CSF protein values as the dependent variable. The resulting beta-coefficients were transformed to reflect the percent change in CSF protein with increasing age. Two-sample Wilcoxon rank-sum tests were subsequently used to compare the distribution of CSF protein concentrations amongst four predefined age categories to facilitate implementation of our results into clinical practice: 0–14 days, 15–28 days, 29–42 days, and 43–56 days. The analyses were repeated while excluding preterm infants, patients receiving antibiotics before lumbar puncture, and patients with CSF pleocytosis to determine the impact of these factors on CSF protein concentrations. Data were analyzed using STATA v10 (Stata Corporation, College Station, Texas). Two-tailed p-values <.05 were considered statistically significant.
Results
During the study period, 1064 infants age 56 days of age or younger underwent lumbar puncture in the emergency department. Of these, 689 (65%) met sequential exclusion criteria as follows: traumatic lumbar puncture (n=330); transported from an outside medical facility (n=90); bacterial meningitis (n=6); non-central nervous system serious bacterial infections (n=135); CSF positive for herpes simplex virus by PCR (n=2); CSF positive for enterovirus by PCR (n=45); congenital syphilis (n=1); seizures (n=28); abnormal central nervous system imaging (n=2); and ventricular shunt device (n=1). An additional 44 patients had lumbar puncture and CSF testing but the protein assay was never done or never reported and 5 patients did not have a CSF red blood cell count available. No cases were excluded for elevated serum bilirubin. Infants may have met multiple exclusion criteria. The remaining 375 (35%) subjects were included in the final analysis. The median patient age was 36 days (interquartile range: 22–47 days); 139 (37%) were 28 days of age or younger. Overall, 205 (55%) were male, 211 (56%) were black, and 145 (39%) presented during enterovirus season. Most (43 of 57) preterm infants were born between 34–37 weeks gestation. Antibiotics were administered before lumbar puncture to 42 (11%) infants and 312 (83%) infants had fever.
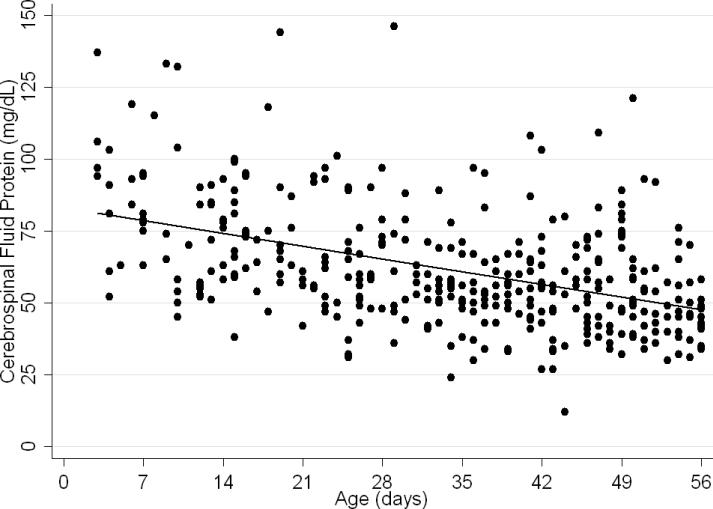
The median CSF protein value was 58 mg/dL (interquartile range: 48–72 mg/dL). There was an age-related declined in CSF protein concentration (Figure 1). In linear regression, the CSF protein concentration decreased 6.8% (95% CI: 5.4%–8.1%; p<0.001) for each 1 week increase in age.
Figure 1.
Relationship of cerebrospinal fluid protein concentration and age. Each circle represents data from one infant. A linear regression line shows the rate of decline in protein concentration with age.
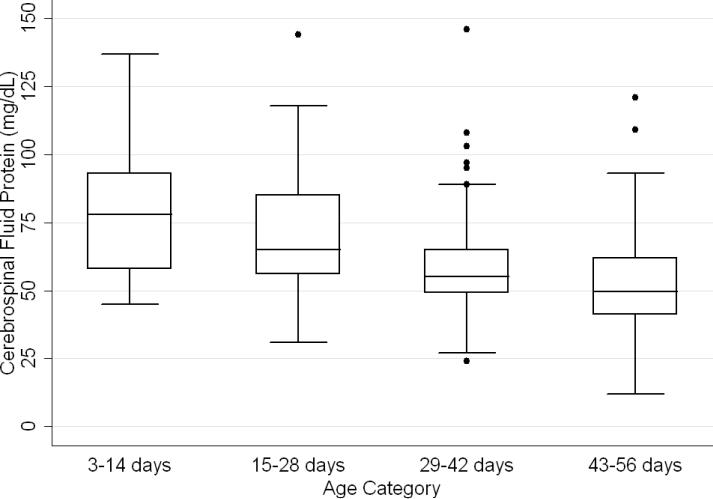
CSF protein concentrations were higher for infants ≤28 days of age than for infants 29–56 days of age (p<0.001, Wilcoxon rank-sum test). The median CSF protein concentrations were 68 mg/dL (95th percentile value, 115 mg/dL) for infants ≤28 days of age and 54 mg/dL (95th percentile value, 89 mg/dL) for infants 29–56 days. CSF protein concentrations by 2-week age intervals are shown in Table 1. The 95th percentile CSF protein concentrations were as follows: ages 0–14 days, 132 mg/dL; ages 15–28 days, 100 mg/dL; ages 29–42 days, 89 mg/dL; and ages 43–56 days, 83 mg/dL (Table 1). CSF protein concentration decreased significantly across each age interval when compared with infants in the next highest age category (P<0.02 for all pairwise comparisons, Wilcoxon rank-sum test).
Table 1.
Cerebrospinal fluid protein concentrations in infants age 56 days of age and younger.a
| Value | 0 – 14 days | 15 – 28 days | 29 – 42 days | 43 – 56 days | All Infants |
|---|---|---|---|---|---|
| (N=52) | (N=87) | (N=110) | (N=126) | (N=375) | |
| Mean (± SD) | 79 (±23) | 69 (±20) | 58 (±17) | 53 (±17) | 62 (±21) |
| Median (IQR) | 78 (58–93) | 65 (56–85) | 55 (49–65) | 50 (41–62) | 58 (48–72) |
| 90th percentile | 106 | 95 | 79 | 75 | 91 |
| 95th percentile | 132 | 100 | 89 | 83 | 99 |
| 95th percentileb | 132 | 101 | 89 | 82 | 97 |
| 95th percentilec | 132 | 100 | 87 | 74 | 97 |
Cerebrospinal fluid protein values presented as mg/dL
Excluding patients with antibiotics before lumbar puncture
Excluding preterm infants
Abbreviations: IQR, interquartile range; SD, standard deviation
Age-specific 95th percentile CSF protein values changed by <1% when infants receiving antibiotics before lumbar puncture were excluded (Table 1). Age-specific CSF protein values changed minimally when preterm infants were excluded with the exception of infants 43–56 days of age where the 95th percentile value was 9.7% lower than when all infants were included (Table 1); the 90th percentile values in this age group were more comparable at 75 mg/dL and 71 mg/dL, respectively, in the subgroups with and without preterm infants. Age-specific 95th percentile CSF protein values changes by <1% when patients with CSF pleocytosis were excluded.
Discussion
We examined CSF protein values in neonates and young infants to establish reference values and to bring the literature up to date at a time when molecular tools are commonly used in clinical practice. We also quantified the age-related decline in CSF protein concentrations over the first two months of life. Our findings provide age-specific reference ranges for CSF protein concentrations in neonates and young infants. These findings are particularly important because a variety of infectious (e.g., herpes simplex virus infection) and non-infectious (e.g., subarachnoid or intraventricular hemorrhage) conditions may occur in the absence of appreciable elevations in the CSF white blood count.
Cerebrospinal fluid protein concentrations depend on serum protein concentrations and on the permeability of the blood-CSF barrier. Immaturity of the blood-CSF barrier is thought to result in higher CSF protein concentrations for neonates and young infants compared with older children and adults. Though previous studies agree that CSF protein concentrations depend on age, the reported age-specific values and rates of decline vary considerably.4–7, 30–32 Additionally, these prior studies are limited by 1) small sample size, 2) variable inclusion and exclusion criteria, 3) variable laboratory techniques to quantify protein concentration in a CSF sample, and 4) presentation of mean, standard deviation and range values rather than the 75th, 90th, or 95th percentile values necessary to define a clinically meaningful reference range.
The median and mean values found in this study were generally comparable to previously published values (Table 2). In addition, we have quantified the age-related decline in CSF protein concentrations identified in previous studies. While our large sample size allowed us to define narrower reference intervals than most previous studies, direct comparison of values used to define reference ranges was hampered by lack of consistent reporting of data across studies. Ahmed et al5 and Bonadio et al4 reported only mean and standard deviation values. When data are skewed, as is the case for CSF protein values, the standard deviation will be grossly inflated, making extrapolation to percentile values unreliable. The 90th percentile value of 87 mg/dL reported by Wong et al7 for infants 0–60 days of age was similar to the value of 91 mg/dL for infants 56 days of age and younger found in this study. Biou et al6 reported the following 95th percentile values: ages 1–8 days, 108 mg/dL; ages 8–30 days, 90 mg/dL; and ages 1–2 months, 77 mg/dL. These values are lower than those reported in our study. The reason for such differences is not clear. The exclusion criteria were similar between the two studies though Biou et al6 did not include preterm infants. When we excluded preterm infants from our analysis, no age-specific result decreased by more than 5%, making the inclusion of this population an unlikely explanation for the differences between the two studies.
Table 2.
Summary of prior studies reporting age-specific cerebrospinal fluid protein concentrations.a
| Author | Year | Number of Infants | Age (days) | Median (mg/dL) | Mean ± SD (mg/dL) |
|---|---|---|---|---|---|
| Bonadio et al | 1992 | 35 | 0–30 | … | 84 ± 45 |
| 40 | 30–60 | … | 59 ± 25 | ||
| Ahmed et al | 1996 | 17 | 0–7 | … | 81 ± 31 |
| 33 | 8–14 | … | 69 ± 23 | ||
| 25 | 15–21 | … | 60 ± 23 | ||
| 33 | 22–30 | … | 54 ± 16 | ||
| Biou et al | 2000 | 26 | 1–8 | 71 | … |
| 76 | 8–30 | 59 | … | ||
| 155 | 30–60 | 47 | … | ||
| Wong et al | 2000 | 99 | 0–60 | 60 | 59 ± 21 |
Ellipses indicate that the value was not reported by the authors
CSF protein concentration is a method-dependent value; the results depend a great deal on what technique the laboratory uses. Two common methods used in the past few decades are Biuret Colorimetry and Turbidimetric; reported values are approximately 25% higher with the Biuret method compared with the Turbidimetric method.33 A CSF protein reference value is only clinically useful if the method used to define the norm is specified and equivalent to currently used methods. Similar to our study, Biou et al6 and Wong et al7 used the Biuret (Vitros) method. The method of protein measurement was not specified by other studies.4, 5
This study had several limitations that could cause us to overestimate the upper bound of the reference range. First, spectrum bias is possible in this observational study. Individual physicians determined whether lumbar puncture was warranted, a limitation that could potentially lead to the disproportionate inclusion of infants with conditions associated with higher CSF protein concentrations. We do not believe that this limitation would meaningfully affect our results because febrile infants 56 days of age or younger routinely undergo lumbar puncture at our institution, regardless of illness severity, and patients diagnosed with conditions known or suspected to increase CSF protein concentrations were excluded. Second, infants with aseptic meningitis- a condition that can be associated with elevated CSF protein concentrations-may have been misclassified as uninfected. Though we excluded patients with positive CSF enteroviral PCR tests, some infants were not tested and other viruses (e.g., parechoviruses)34 not detected by the enterovirus PCR may also cause aseptic meningitis. Third, certain antibiotics including ampicillin and vancomycin are known to interfere with the CSF protein assay used in our laboratory.24 Forty-two of the 375 subjects included in our final analysis received antibiotics prior to lumbar puncture. When receiving antibiotics prior to lumbar puncture were excluded from analysis, the CSF protein concentrations were within 1% of the overall study population, suggesting that antibiotic administration before lumbar puncture did not influence our results in any meaningful way. We would not expect any of these limitations to disproportionately affect patients in one particular age category.
In conclusion, the CSF protein concentration values reported here represent the largest series to-date for this young age group. Our study quantifies the age-related decline in CSF protein concentration from birth to 56 days of life. Our work designing this study, specifically the exclusion criteria, refines the approach to defining normal CSF protein values in children. As CSF protein values decline steadily with increasing age, the selection of reference values is a balance of accuracy and convenience. Age-specific reference values by two-week increments would be most accurate. However, considering reference values by month of age, as is the convention for CSF white blood cell counts, is far more practical. The 95th percentile values by age category in our study were as follows: ages 0–14 days, 132 mg/dL; ages 15–28 days, 100 mg/dL; ages 29–42 days, 89 mg/dL; and ages 43–56 days, 83 mg/dL. The 95th percentile values were 115 mg/dL for infants ≤28 days and 89 mg/dL for infants 29–56 days. We feel that either approach is reasonable. These values can be used to accurately interpret the results of CSF studies in neonates and young infants.
Figure 2.
Boxplot showing variation in cerebrospinal fluid protein concentrations by age category. The line in the middle of the box denotes the median value. The ends of the boxes represent the interquartile range (i.e., 25th and 75th percentile) values. The whiskers extend 1.5 times the interquartile range values and the circles denote extreme outlying values.
Acknowledgments
Sources of Funding: Dr. Kestenbaum received support from the Clinical Neurosciences Scholars Track at the University of Pennsylvania School of Medicine. Dr. Shah received support from the National Institute of Allergy and Infectious Diseases (K01 AI73729) and the Robert Wood Johnson Foundation under its Physician Faculty Scholar Program. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Robert Wood Johnson Foundation.
References
- 1.McPherson RA, Pincus MR. Henry's Clinical Diagnosis and Management by Laboratory Methods. 21st W.B. Saunders, Inc.; Philadelphia, PA: 2006. [Google Scholar]
- 2.Widell S. On the cerebrospinal fluid in normal children and in patients with acute abacterial meningo-encephalitis. Acta Paediatr Suppl. 1958 May;47(Suppl 115):1–102. [PubMed] [Google Scholar]
- 3.Statz A, Felgenhauer K. Development of the blood-CSF barrier. Dev Med Child Neurol. 1983 Apr;25(2):152–161. doi: 10.1111/j.1469-8749.1983.tb13738.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonadio WA, Stanco L, Bruce R, Barry D, Smith D. Reference values of normal cerebrospinal fluid composition in infants ages 0 to 8 weeks. Pediatr Infect Dis J. 1992 Jul;11(7):589–591. doi: 10.1097/00006454-199207000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed A, Hickey SM, Ehrett S, et al. Cerebrospinal fluid values in the term neonate. Pediatr Infect Dis J. 1996 Apr;15(4):298–303. doi: 10.1097/00006454-199604000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Biou D, Benoist JF, Nguyen-Thi C, Huong X, Morel P, Marchand M. Cerebrospinal fluid protein concentrations in children: age-related values in patients without disorders of the central nervous system. Clin Chem. 2000 Mar;46(3):399–403. [PubMed] [Google Scholar]
- 7.Wong M, Schlaggar BL, Buller RS, Storch GA, Landt M. Cerebrospinal fluid protein concentration in pediatric patients: defining clinically relevant reference values. Arch Pediatr Adolesc Med. 2000 Aug;154(8):827–831. doi: 10.1001/archpedi.154.8.827. [DOI] [PubMed] [Google Scholar]
- 8.Behrman RE, Kliegman R, Jenson HB. Nelson Textbook of Pediatrics. 17th Saunders; Philadelphia: 2004. [Google Scholar]
- 9.McMillan JA, Feigin RD, DeAngelis C, Jones MD. Oski's pediatrics: principles & practice. 4th Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 10.Robertson J, Shilkofski N, editors. Johns Hopkins: The Harriet Lane Handbook: A Manual for Pediatric House Officers. 17 Elsevier Mosby; Philadelphia: 2005. [Google Scholar]
- 11.Frank G, Shah SS, Catallozzi MC, Zaoutis LB. The Philadelphia Guide: Inpatient Pediatrics. Lippincott Williams & Wilkins; Philadelphia: 2005. [Google Scholar]
- 12.Perkin RM, Swift JD, Newton DA, Anas NG. Pediatric Hospital Medicine: Textbook of Inpatient Management. Lippincott Williams & Wilkins; Philadelphia: London: 2008. [Google Scholar]
- 13.Zaoutis LB, Chiang VW. Comprehensive pediatric hospital medicine. Mosby Elsevier; Philadelphia: 2007. [Google Scholar]
- 14.Fleisher GR, Ludwig S, Henretig F. Textbook of Pediatric Emergency Medicine. 5th Lippincott Williams & Wilkins; Philadelphia: 2006. [Google Scholar]
- 15.Baren JM, Brennan JA, L. B, Rothrock SG. Pediatric Emergency Medicine. Saunders Elsevier; Philadelphia: 2008. [Google Scholar]
- 16.Feigin RD, Cherry JD, Demmler GJ, Kaplan SL. Textbook of Pediatric Infectious Diseases. 5th Saunders; Philadelphia: 2004. [Google Scholar]
- 17.Remington JS, Klein JO. Infectious Diseases of the Fetus and Newborn Infant. 6th Elsevier Saunders; Philadelphia: 2006. [Google Scholar]
- 18.Taeusch HW, Ballard RA. Avery's diseases of the newborn. 7th Saunders; Philadelphia: 1998. [Google Scholar]
- 19.Menkes JH, Sarnat HB. Child Neurology. 6th Lippincott Williams & Wilkins; Philadelphia: 2000. [Google Scholar]
- 20.Swaiman KF, Ashwal S. Pediatric Neurology: Principles and Practice. 3rd Mosby; St. Louis: 1999. [Google Scholar]
- 21.Baker MD, Bell LM. Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age. Arch Pediatr Adolesc Med. 1999 May;153(5):508–511. doi: 10.1001/archpedi.153.5.508. [DOI] [PubMed] [Google Scholar]
- 22.Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics. 1999 Mar;103(3):627–631. doi: 10.1542/peds.103.3.627. [DOI] [PubMed] [Google Scholar]
- 23.Palazzi DL, Klein JO, Baker CJ. Bacterial sepsis and meningitis. In: Remington JS, Klein JO, Wilson CB, Baker CJ, editors. Infectious Diseases of the Fetus and Newborn Infant. 6th Elsevier, Inc.; Philadelphia, PA: 2006. pp. 247–295. [Google Scholar]
- 24.NCCLS . Interference testing in Clinical Chemistry, NCCLS Document EP7. NCCLS; Wayne, PA: 1986. [Google Scholar]
- 25.Seiden JA, Zorc JJ, Hodinka RL, Shah SS. Lack of cerebrospinal fluid pleocytosis in young infants with enterovirus infections of the central nervous system. Pediatr Emerg Care. 2010 Feb;26(2):77–81. doi: 10.1097/PEC.0b013e3181ce2fad. [DOI] [PubMed] [Google Scholar]
- 26.Zorc JJ, Levine DA, Platt SL, et al. Clinical and demographic factors associated with urinary tract infection in young febrile infants. Pediatrics. 2005 Sep;116(3):644–648. doi: 10.1542/peds.2004-1825. [DOI] [PubMed] [Google Scholar]
- 27.Hoberman A, Wald ER, Penchansky L, Reynolds EA, Young S. Enhanced urinalysis as a screening test for urinary tract infection. Pediatrics. 1993 Jun;91(6):1196–1199. [PubMed] [Google Scholar]
- 28.Shaw KN, McGowan KL, Gorelick MH, Schwartz JS. Screening for urinary tract infection in infants in the emergency department: which test is best? Pediatrics. 1998 Jun;101(6):E1. doi: 10.1542/peds.101.6.e1. [DOI] [PubMed] [Google Scholar]
- 29.King RL, Lorch SA, Cohen DM, Hodinka RL, Cohn KA, Shah SS. Routine cerebrospinal fluid enterovirus polymerase chain reaction testing reduces hospitalization and antibiotic use for infants 90 days of age or younger. Pediatrics. 2007 Sep;120(3):489–496. doi: 10.1542/peds.2007-0252. [DOI] [PubMed] [Google Scholar]
- 30.Stewart D. The normal cerebro-spinal fluid in children. Archives of Disease in Childhood. 1928:96–108. doi: 10.1136/adc.3.14.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo BT. The cerebrospinal fluid in the healthy newborn infant. S Afr Med J. 1968 Sep 14;42(35):933–935. [PubMed] [Google Scholar]
- 32.Sarff LD, Platt LH, McCracken GH., Jr. Cerebrospinal fluid evaluation in neonates: comparison of high-risk infants with and without meningitis. J Pediatr. 1976 Mar;88(3):473–477. doi: 10.1016/s0022-3476(76)80271-5. [DOI] [PubMed] [Google Scholar]
- 33.Lott JA, Warren P. Estimation of reference intervals for total protein in cerebrospinal fluid. Clin Chem. 1989 Aug;35(8):1766–1770. [PubMed] [Google Scholar]
- 34.Verboon-Maciolek MA, Krediet TG, Gerards LJ, de Vries LS, Groenendaal F, van Loon AM. Severe neonatal parechovirus infection and similarity with enterovirus infection. Pediatr Infect Dis J. 2008 Mar;27(3):241–245. doi: 10.1097/INF.0b013e31815c1b07. [DOI] [PubMed] [Google Scholar]