Abstract
Ketamine exerts powerful anesthetic, psychotic and anti-depressant effects in both healthy volunteers and clinically-depressed patients. Although ketamine targets particular glutamate receptors, there is a dearth of evidence for additional, alternative molecular substrates for the behavioral actions of this NMDA receptor antagonist drug. Here, we provide behavioral and molecular evidence for the actions of ketamine using a new vertebrate model for psychiatric disorders: the zebrafish. Sub-anesthetic doses of ketamine produced a variety of abnormal behaviors in zebrafish that were qualitatively analogous to those previously measured in humans and rodents treated with drugs that produce transient psychosis. In addition, we revealed that the transcription factor Phox2b is a molecular substrate for the actions of ketamine, particularly during periods of hypoxic stress. Finally, we also show that SIRT1, a histone deacetylase widely recognized for its link to cell survival is also affected by hypoxia crises. These results establish a relevant assay system in which the effects of psychotomimetic drugs can rapidly be assessed, and provide a plausible and novel neuronal mechanism through which ketamine affects critical sensory circuits that monitor breathing behavior.
Keywords: Circling Behavior, Gill Movement, Hypoxia, Phox2b, Sirtuins
INTRODUCTION
Ketamine is a dissociative anesthetic capable of inducing post-operative hallucination, psychosis, and cognitive deficits in healthy individuals that bear an uncanny resemblance to those observed in schizophrenic patients (Moghaddam, 2003). Yet, ketamine also appears to exert rapid and relatively sustained antidepressant-like effects at sub-anesthetic doses in patients with major depression (Berman et al., 2000; Amiel and Mathew, 2007; Pilc et al., 2007; Pittenger et al., 2007). Because ketamine is an antagonist of the N-Methyl-D-Aspartate (NMDA) ion channel receptor, one of several subtypes of the glutamate receptor system (Leheste et al., 2008), it is thought that derangements in glutamate neurotransmission may contribute to the pathophysiology of both schizophrenia and major depressive disorder (Ross et al., 2006; Sanacora et al., 2007).
Evidence for glutamate's role in psychiatric disorders also comes from animal models with varying resemblance to the clinical features of a disease. For example, pharmacological or transgenic manipulation of NMDA receptor signaling pathways in the rodent brain indicate that ionotropic glutamate receptors have a profound influence on a wide-range of behaviors that are relevant to schizophrenia (Moghaddam, 2003; Engin et al., 2009). In this regard, there is a significant need to develop novel and better animal models for testing drugs to treat psychiatric disorders, including agents that target glutamate-based communication in specific brain circuits. In the case of ketamine, we are using the freshwater zebrafish (Danio rerio) to study the neural networks that mediate the actions of psychotropic drugs and to identify specific candidate genes that underlie the basis of ketamine-induced behaviors. The zebrafish offers unrivaled opportunities for this line of investigation, primarily because of its amenability to high throughput in vivo drug screening (Guo, 2004; Flinn et al., 2008). This feature, together with a well-characterized stereotypical neuromuscular system and genetic tractability offers a quick, easy and cost-effective way for modeling psychiatric disorders in a vertebrate organism. Thus, the present study describes a series of experiments in which acute and chronic sub-anesthetic doses of ketamine were used to analyze species-specific behaviors, and to identify behaviorally relevant molecular substrates for ketamine in the adult zebrafish.
MATERIALS AND METHODS
Animals
Adult wild-type zebrafish (4-8 months) of mixed genders were obtained locally and handled in compliance with the NIH Guide for the Care and Use of Laboratory Animals and with approval from the NYIT/NYCOM IACUC. Fish were kept at 28 °C in a recirculation aquaculture system equipped with carbon filtration, ultraviolet light sterilizers, and bio-filtration (Aquatic Habitats) under a 12 hr light:dark cycle (lights on 0700) and fed twice daily with a commercial fish diet. All experiments were performed during the lights on period. All efforts were made to minimize animal stress and to reduce the number of fish used for the experiments detailed below.
Ketamine Administration
On test day, zebrafish were removed from their aquatic habitat and placed individually in 250 ml glass-beakers (10 cm length × 8 cm width × 7 cm depth) containing temperate, recirculation aquaculture normoxic water. After a 10-min acclimation phase, ketamine (Vetalar-HCl, Amtech Phoenix Scientific, St Joseph, MO) was dissolved in the aquaculture water and then after a 5-min waiting period species-specific behaviors (e.g., swimming behavior, gill movement) were recorded and videotaped for further behavioral analyses. We conducted several pilot studies to determine optimal, sub-anesthetic doses of ketamine for producing desired degrees of behavioral effects. The studies described herein have utilized this experience and knowledge base. First, we determined that a ketamine dose of 200 μl dissolved in 100 ml of aquaculture normoxic water (0.2% solution) was the optimal, sub-anesthetic dose for our experimental purposes. Second, we determined that a solution concentration of 0.8% ketamine was a physiological anesthetic dose for this particular freshwater animal as it produced a deep level of unconsciousness. Thus, the ketamine doses were selected because they are sub-threshold (0.2%) or above threshold for an anesthetic effect in wild-type zebrafish. The above ketamine dose paradigm was instituted acutely and chronically for 5 consecutive days. To our knowledge, ketamine has not yet been applied to zebrafish for pharmacological studies.
Behavioral Testing Procedures
Behavioral activity (i.e., circling behavior) was monitored for 5-min and the number of complete, full (right or left) 360° circles were scored and videotaped following ketamine (experimental group; n = 20 fish) or no ketamine exposure (control group; n = 20 fish). A stress response test (i.e., hypoxic stress) was also conducted either acutely or chronically for 5 consecutive days. In brief, after ketamine or no ketamine exposure, individual zebrafish were removed from the aquaculture water for a 20-sec testing period during which time the number of gill movements (breaths) were recorded as well as the number of body pulses (“flops”). This particular stress response test was chosen because it provokes a ventilatory chemoreflex response in zebrafish. Thus, we established first a functional ventilatory chemoreflex response frequency in drug-naïve animals and then compared this baseline response frequency to that of ketamine-treated fish. After the hypoxic stress response test, animals were transferred to aquaculture fish chambers for 90-min and then sacrificed by decapitation. Subsequently, their brains were excised from the skulls and processed for quantitative polymerase chain reaction (QPCR) procedures.
Gene Expression Analysis by QPCR Procedures
Zebrafish brain tissue was homogenized and RNA extracted using RNeasy® Plus Mini Kit (Qiagen, Carlsbad, CA), and QIAshredder™ (Qiagen Carlsbad, CA). RT-PCR was performed using the cDNA made with Superscript® III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA). Expression of Phox2b, SIRT1 and Actin genes was determined by QPCR with Power SYBR® Green PCR Master Mix (Applied Biosystems, Warrington, UK). Gene-specific DNA primers were manufactured using Integrated DNA Technologies (Coralville, IA). The phox2b primer sequence was forward 5′- ACA ATC CCA TCA GGA CGA CGT TTG -3′ and reverse 5′- TTC AAG CCT CCG TGA TCG GTG AAA -3′. The SIRT1 primer sequence was: forward 5′- ACA GTT CCA GCC ATC TCC ATG TCA -3′ and reverse 5′- AAG ACC CGT GGC ACT GAA TGA TCT -3′. The β-actin primer sequence was forward 5′- CAG CCA TGT ACG TTG CTA TCC AGG -3′ and reverse 5′- AGG TCC AGA CGC AGG ATG GCA TG -3′.
Data Analyses
Behavioral data are reported as means ± SEM. Analyses of Variance (ANOVA) followed by Mann-Whitney Rank Sum Tests were performed with the assumption of unequal variance to test for differences between group means. Statistically significant differences were defined as P ≤ 0.05. Relative gene expression was analyzed using 2ΔΔCt methods (Livak and Schmittgen, 2001).
RESULTS
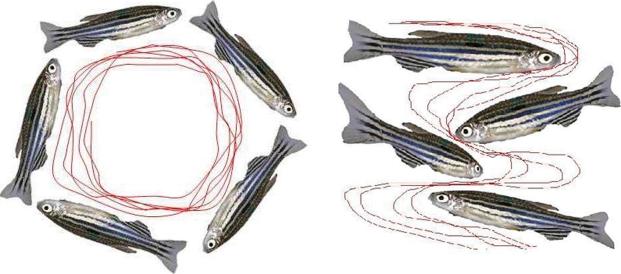
Antagonist drugs of the NMDA-type glutamate receptor, such as ketamine and phencyclidine (PCP), can induce psychosis, as well as some of the behavioral signs catalogued in non-medicated schizophrenia patients, including rotational (circle) behavior. In this study, a sub-anesthetic dose of ketamine (0.2%) also produced a consistent circling behavioral phenotype in zebrafish, with individual turning rates ranging from 35 to 50 full body turns per 5-min testing period (Figs. 1, 2). This aberrant behavioral phenotype, in contrast, was completely absent in control animals (P ≤ 0.01). Instead, spontaneous behavioral activity in control zebrafish showed chamber exploration with oblique turns and half-turn rotations accompanied by high percentage frequencies of apparent exploratory tasks. The high degree of circling behavior and hyperactivity in ketamine-treated zebrafish persisted for longer than the 5-min testing period and were still observed even after the animals had been transferred to aquaculture fish chambers (i.e., less than 2 min). Further, the aforementioned locomotor activity syndrome was characterized by lateralized circling behavior (e.g., a right-preference population bias), postural asymmetry, and hyperactivity to sensory stimuli. These initial findings indicate that application of ketamine to zebrafish produces an abnormal behavioral phenotype that resembles that of healthy volunteers using ketamine (Krystal et al., 1994; Newcomer et al., 1999; Lahti et al., 2001), and mouse models with varying resemblance to the clinical features of schizophrenia (Mohn et al., 1999; Torres et al, 2004; Torres et al., 2005). Further, the fact that zebrafish were especially sensitive to the psychotomimetic effects of ketamine, suggest that ionotropic NMDA receptor sub-units in this vertebrate animal might be homologous in function to those occurring in mammalian brains.
Fig. 1.
Spontaneous behavioral activity of zebrafish is significantly affected by ketamine exposure (digitized video-images). Individual zebrafish were treated with either aquaculture water (right panel) or 0.2% ketamine (left panel) for 5 min as described in methods. Note the circling behavioral phenotype in adult animals treated with a sub-anesthetic dose of ketamine.
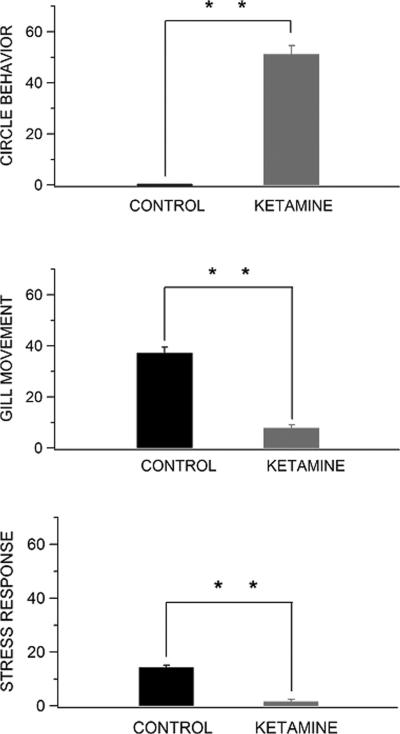
Fig. 2.
Behavioral abnormalities produced by acute ketamine exposure in zebrafish. Treatment with 0.2 % ketamine led to statistically significant differences in all primary outcome measures: circling behavior (t18 = -14.9, P ≤ 0.01), gill movement (ventilatory response to hypoxia, t18 = 11.3, P ≤ 0.01), and stress response to hypoxia (body pulses or “flops”, t18 = 11.0, P ≤ 0.01) when compared to control, drug-naïve zebrafish. ** P ≤ 0.01 Mann-Whitney Rank Sum Tests. Data are means ± SEM.
The actions of ketamine and the ventilatory response to hypoxia are critically dependent on ionotropic NMDA glutamate receptors (Ohtake et al., 2000; Turesson et al., 2006; Maeng and Zarate, 2007; Leheste et al., 2008). This commonality in molecular substrates suggests that ketamine exposure might affect the general ventilatory response to hypoxia in zebrafish. To test this possibility, we assessed gill movement and the stress response (i.e., body “flops”) to a 20-sec hypoxic challenge. In this context, most of the sensory receptors responsible for triggering an oxygen ventilatory chemoreflex response in fish are located to gill filaments (Sundin and Nilsson, 2002). Under this experimental condition, we found that individual animals treated acutely with 0.2% ketamine exhibited a profound (P ≤ 0.01) reduction in both gill movement and stress response to hypoxia relative to control, drug-naive zebrafish (Fig. 2). In particular, we observed an almost complete lack of gill movement in ketamine-treated zebrafish, indicating a significantly decreased respiratory drive in these behaving animals. It should be noted that the 20-sec hypoxic challenge instituted in this experiment did not precipitate any deaths as all ketamine-treated zebrafish survived the procedure (data not shown). Within this brief period of hypoxia, we also noted a significant decrease in behaving activities (e.g., rapid turns and body “flops”) in animals exposed to 5-min of ketamine. This suggests that ketamine either reduces the acute hypoxic stress in zebrafish or suppresses the contribution of NMDA receptors to respiratory drive transmission at respiratory motor neurons in intact animals. Regardless, the parallel decrease in gill movement and the stress response we observed during hypoxia crisis suggests that these events are profoundly affected by previous ketamine exposure.
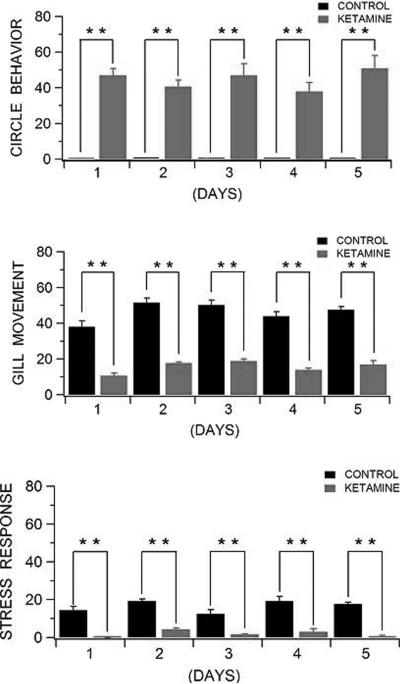
Next, we determined the chronic effects of ketamine on spontaneous behavioral activity using the same experimental design used for the acute drug treatment. Thus, the primary difference between this experiment and the previous one is that instead of acute ketamine exposure, individual zebrafish were now exposed to 5 consecutive days of 0.2% ketamine. Under this chronic drug paradigm, we observed that repeated administration of ketamine did not cause tolerance or sensitization to specific drug effects (Fig. 3). In general, the pharmacological effects of chronic ketamine administration were almost identical to those previously observed in the acute drug preparation. That is, ketamine produced a consistent circling behavioral phenotype in zebrafish that was completely absent in control, drug-naive animals (P ≤ 0.01). As described in the previous acute experiment, the recorded locomotor activity syndrome was also characterized by lateralized circling behavior (e.g., a right-preference population bias), postural asymmetry, and hyperactivity to sensory stimuli. Further, we observed that animals treated chronically with 0.2% ketamine exhibited a profound (P ≤ 0.01) reduction in both gill movement and stress response to hypoxia relative to control, drug-naive zebra fish (Fig. 3). In particular under this chronic drug administration, we observed an almost complete lack of gill movement in ketamine-treated zebrafish, indicating a significantly decreased respiratory drive in these behaving animals. We also noted a significant decrease in behavioral activities (e.g., rapid turns and body “flops”) in animals exposed to 5 consecutive days of ketamine (Fig. 3). However, none of these deficits (i.e., diminished breathing capacity and reduced ventilatory chemoreflex response) were recorded in control animals kept in aquaculture fish chambers. Thus regardless of temporal drug treatment, ketamine not only seems to affect spontaneous behavioral and breathing activity but also acts very swiftly throughout single or multiple drug exposures. This is consistent with the clinical view that single intravenous ketamine infusions rapidly produce optimal therapeutic effects in treatment-resistant major depression (Liebrenz et al., 2007).
Fig. 3.
Behavioral abnormalities produced by chronic ketamine exposure in zebrafish. Treatment with 0.2 % ketamine led to statistically significant differences in all primary outcome measures: circling behavior (F9,40 = 36.4, P ≤ 0.01) , gill movement (ventilatory response to hypoxia, F9,40 = 60.8, P ≤ 0.01), and stress response to hypoxia (body pulses or “flops”, F9,40 = 31.8, P ≤ 0.01) when compared to control, drug-naïve zebrafish. Under this chronic drug paradigm, we observed that repeated administration of ketamine did not cause tolerance or sensitization to specific drug effects (F4,40 = 0.89, P ≥ 0.05; F4,40 = 0.60, P ≥ 0.05; F4,40 = 1.4, P ≥ 0.05, respectively) . ** P ≤ 0.01 by repeated ANOVA followed by Mann-Whitney Rank Sum Tests. Data are means ± SEM.
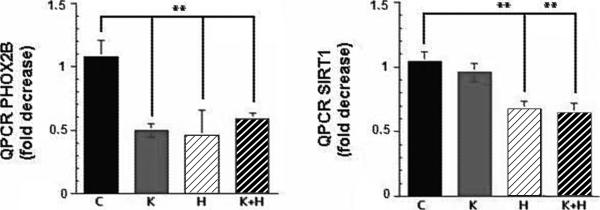
To identify novel molecular substrates for the actions of ketamine during hypoxia crisis, we performed QPCR in brains of drug-naïve and ketamine-exposed zebrafish. We selected gene-specific primers for Phox2b, a hindbrain transcription factor predominantly expressed in neurons controlling breathing behavior (Guyenet, 2008) and sirtuin 1 (SIRT1), an oxidation-reduction (REDOX)-sensitive deacetylase that stimulates the activity of hypoxia-inducible factor 2α (HIF-2α) during oxygen scarcity (Dioum et al., 2009). Against this background, acute administration of ketamine to zebrafish caused a significantly greater reduction (P ≤ 0.01) in the expression of both Phox2b and SIRT1 during hypoxia crisis than that seen in brains from control, drug-naïve animals (Fig. 4). More specifically, QPCR analysis of zebrafish treated with ketamine and then exposed to hypoxia showed a significant reduction in Phox2b expression relative to normalized levels of β-actin in zebrafish collected immediately from aquaculture fish chambers. This transcriptional repression was similar in magnitude to levels observed in brains collected from animals exposed only to ketamine or hypoxia (i.e., ~41% vs. 44% and 54%, respectively; P ≥ 0.05). Thus, no synergistic effects were measured as the combined actions of ketamine and hypoxia failed to produce a greater diminution of Phox2b transcription levels than the sum of ketamine or hypoxia alone. These data support a mechanism in which NMDA receptor inhibition accounts for the reduction of Phox2b transcripts in the zebrafish brain. These data also advance the idea that Phox2b is a novel molecular substrate target for ketamine.
Fig. 4.
Quantitative PCR analysis of zebrafish brain Phox2b and SIRT1 transcripts following acute ketamine exposure (n = 5/group). Bathing zebrafish with 0.2% ketamine alone or in conjunction with hypoxic stress led to a profound and significant reduction in Phox2b transcription levels (F3,36 = 8.5 , P ≤ 0.01 as detected by one-way ANOVA). Phox2b is a transcription factor specifically expressed and required in neurons controlling breathing behavior. Ketamine administration, however, failed to affect levels of SIRT1 transcripts in zebrafish brain. SIRT1 transcription levels were only affected by ketamine exposure in conjunction with hypoxia (n = 12-27/group). Thus, an increase rather than a decrease in SIRT1 transcription levels is revealed during episodes of ketamine exposure and hypoxia crises. Hypoxia alone, in contrast, led to a profound and significant reduction in SIRT1 transcription levels (F3,74 = 7.3, P ≤ 0.01 as detected by one-way ANOVA). SIRT1 is a histone deacetylase widely recognized for its link to cell survival and more recently to hypoxic stress. * P ≤ 0.05 Mann-Whitney Rank Sum Tests. Data are means ± SEM of three consecutive experiments. C = control group; K = ketamine group; H = hypoxia group; K + H = ketamine + hypoxia group.
Our QPCR measurements also showed SIRT1 transcripts to be significantly (P ≤ 0.01) reduced in brains of animals exposed to both ketamine and hypoxic stress (Fig. 4). It is noteworthy that zebrafish exposed only to ketamine showed a modest (i.e., 5%), but not statistically significant reduction (P ≥ 0.05) in SIRT1 transcription levels relative to normalized levels of β-actin measured in drug-naïve animals. Hypoxia alone, in contrast, produced a profound and statistically significant reduction (P ≤ 0.01) in brain SIRT1 transcription levels, suggesting that during oxygen scarcity, substrates for SIRT1 are either degraded or destabilized in order to maintain transcription of the deacetylase. Further, these data suggest that SIRT1 itself is negatively influenced by hypoxic stress. Thus, levels of SIRT1 transcripts in the zebrafish brain are proportional to conditions of hypoxia but not ketamine. A possible reason for the augmentation of SIRT1 transcription levels following 0.2 % ketamine and hypoxic stress could be explained by the persistent increase of neuronal superoxide and other reactive oxygen species (ROS) generated by NMDA receptor antagonists (Behrens et al., 2007; de Oliveira et al., 2009).
DISCUSSION
Schizophrenia and major depression have, for a long time, been regarded as having very different origins because of their distinct clinical symptoms and modes of drug treatment. However, these disorders are closely related to each other because their etiologies are clearly related to functional alterations in catecholamine and glutamate systems that can spread from one system to another and are precipitated by environmental insults in genetically-prone individuals. Thus, common characteristics of schizophrenia and major depression suggest parallel modes of disease pathogenesis and parallel approaches to treatment. If this is the case, then sensible models of certain aspects of the neurobiological underpinning of these psychiatric disorders should be useful in establishing high-throughput drug screening for novel molecule probes that modulate a specific psychiatric disease mechanism. Results from this study strongly suggest that despite very different physiology and habitat, the relatively simpler and short-lived zebrafish appears to be an adequate model for studying some psychiatric disorders, especially with respect to biochemistry (i.e., glutamate signaling pathways) and response to treatment (i.e., ketamine). In this regard, ketamine elicited an aberrant circling behavioral phenotype in zebrafish that was reminiscent of rodents treated with NMDA receptor antagonists (e.g., PCP also known as angel dust), experimental drugs whose effects recapitulate certain aspects of psychosis (Moghaddam, 2003). Ketamine and PCP also trigger psychosis in healthy volunteers that often resemble acute paranoid schizophrenia, and low doses of these two psychotomimetic agents exacerbate preexisting symptoms of individuals with schizophrenia (Ross et al., 2006). Thus, basic and clinical evidence with NMDA receptor antagonists suggest that the behavioral effects following ketamine treatment seem to be conserved over large evolutionary distances as transient changes in NMDA receptor function may be sufficient to elicit an abnormal motor state in zebrafish as well.
Ketamine not only induces psychosis but more recently in a randomized, double-blind trial study produced a robust and rapid antidepressant effect in patients with major depression. This is of significant interest as the therapeutic effects of ketamine were manifested after only a single intravenous dose of 0.5 mg/kg (Zarate et al., 2006). In our zebrafish assay preparation, the pharmacological effects of ketamine were also immediate and sustained as chronic administration of the drug did not reduce or amplify the behavioral responsiveness to the drug. Thus, ketamine leads to rapid, robust, and relatively sustained species-specific effects in both humans and zebrafish. A major advantage of this finding is that there is no further need to implement chronic regimens of ketamine administration in zebrafish either. In addition, the fact that ketamine can, at least in a subgroup of depressed patients, have an immediate effect on clinical symptoms suggests that new, small-molecule probes with acute effects can be developed and tested in zebrafish. In this regard, a sub-anesthetic dose of ketamine also affected gill movement and the stress response to a 20-sec hypoxic challenge in zebrafish. In particular, we found that a parallel fixed dose experiment of ketamine greatly reduced the ventilatory chemoreflex response to hypoxia in these animals. Treatment with ketamine led to statistically significant reduction in the ventilatory response to hypoxia in all primary outcome measures (e.g., gill movement and body pulses) compared to drug-naïve zebrafish either immediately after ketamine exposure or at the 5-day endpoint. Again, chronic administration of the drug did not reduce or amplify the ventilatory responsiveness to reduced oxygen availability. In this context, the ventilatory system has received relatively little attention in psychiatry despite the fact that panic attacks and abnormal respiratory sensations constitute a discrete cluster of panic symptoms and play a major role in the pathophysiology of depression, as defined in the DMS IV criteria. While admitting that our zebrafish assay preparation only addresses the actions of a psychotomimetic drug, the finding that ketamine reduces the ventilatory chemoreflex response to hypoxia suggests that one mechanism underlying the intriguing effects of ketamine in treatment-resistant depression might be the ability of this drug to reduce exaggerated panic and respiratory symptoms in these patients. The benefits of ketamine therapy for panic attacks and deranged respiratory sensations will need to be further examined in the clinic.
The ventilatory response to hypoxia in both fish and mammals appears to involve the glutamate system, in particular NMDA-type receptor signaling pathways (Ohtake et al., 2000; Panigraphy et al., 2000; Turesson et al., 2006). In humans, the parabrachial/Kolliker fuse nucleus of the pons modulates breathing and ventilatory responses to hypoxia and there is evidence that direct administration of NMDA receptor antagonists to this anatomical nucleus reduces breathing frequency and amplitude in rats (Boon and Milsom, 2008). These findings, together with our current results, suggest that selected glutamate-sensitive neuronal systems in zebrafish participate in breathing activity and that global NMDA receptor antagonism diminishes the ventilatory response to reduced oxygen availability. It is worth noting that our molecular data also implicate the glutamate system as being relevant for the actions of ketamine during oxygen scarcity. For instance, ketamine administration led to a significant reduction in the expression of Phox2b transcripts in the fish brain. This transcription factor is housed in glutamate-containing neurons of the mammalian retrotrapezoid nucleus (RTN), a small nucleus located ventral to the medulla in the vicinity of the facial motor nucleus (Guyenet, 2008; Thoby-Brisson et al., 2009). RTN neurons and Phox2b in particular play a critical role in respiratory automaticity, and heterozygous mutations in Phox2b cause the congenital hypoventilation syndrome (CCHS), a human disease characterized by respiratory distress during sleep (Weese-Mayer et al., 2008). In zebrafish, Phox2b appears to coordinate the development of norepinephrine (NE) cells in the locus coeruleus (LC) and of sympathetic neurons in ganglia (Guo et al., 1999; Lucas et al., 2006). It should be noted that NE in the LC and NE in the nucleus of the solitary tract of the mammalian brain also modulates central respiratory chemo-receptors during chronic, sustained hypoxia (Zhang et al., 2009). Thus, it is conceivable that Phox2b-expressing catecholamine neurons of the LC might be playing a similar respiratory role in the zebrafish brain. The actions of ketamine during acute hypoxia surely support this possibility as transcription levels of Phox2b were significantly reduced relative to drug-naïve zebrafish. Thus, pharmacological inhibition of NMDA receptor function induces a respiratory deficit in behaving zebrafish that resembles that of CCHS or other disorders of respiratory control. This in turn provides the first biological evidence for Phox2b being a molecular substrate for the hypnotic actions of ketamine. Further study of Phox2b in relationship to psychiatric disorders appears warranted, as does detailed investigation of the role of Phox2b in glutamate neurotransmission.
Interestingly, hypoxic stress precipitated a significant and profound reduction of SIRT1 transcription levels in the vertebrate brain. This raises the prospect that REDOX pathways, under acute conditions of oxygen scarcity, may actually reduce the transcriptional activity of SIRT1 in glutamate containing-neurons. SIRT1 is widely recognized for its link to eukaryotic life span and more recently for its role in deacetylating HIF-2α during hypoxic stress (Guarente and Picard, 2005; Michan and Sinclair, 2007; Dioum et al., 2009). In addition, SIRT1 is expressed in the mammalian brain in a relatively wide pattern, including branchio-motor neurons controlling breathing behavior (Zakhary et al., 2010). Thus, there is evidence to support the idea that sirtuins and their metabolic pathways might be involved in hypoxic signaling pathways that regulate the transcription of HIF members. In our zebrafish assay preparation, we also found that bathing these animals in ketamine followed by a brief hypoxia crisis led to an increase in brain SIRT1 transcription levels. At first glance, this finding is less clear-cut, but suggests that ketamine restores SIRT1 transcriptional activity in REDOX sites of NMDA receptor-based proteins whose biochemical compositions had been silenced by hypoxia. In this context, there is in vivo and in vitro evidence that ketamine and other NMDA receptor analogs (e.g., MK-801) can substantially increase the synthesis of toxic free radicals (e.g., superoxide) that can disrupt normal cell function, including transcriptional events (Behrens et al., 2007; de Oliveira et al., 2009; Girouard et al., 2009). It is conceivable therefore that the increased levels of SIRT1 transcripts seen after combined ketamine and hypoxic stress could be due to NMDA ion channel receptor-derived ROS production. Substantial increases of superoxide in the brain would trigger SIRT1 transcription, thus making this deacetylase available to provide neuroprotection from oxidative insults. Disentangling the effects of hypoxia from the effects of ketamine is an important task for future studies in sirtuin deacetylase biology.
All together, our results provide to the best of our knowledge, the first direct evidence that pharmacological inhibition of NMDA receptor function in the zebrafish has a profound effect on spontaneous behavioral activity and cellular REDOX mechanisms related to oxygen availability and subsequent breathing behavior. This raises the prospect that a zebrafish assay model provides an adequate experimental tool in which neurochemical dysfunction of either catecholamine or glutamate systems can properly be studied. This vertebrate model-system has already told us much about other physiological processes, such as development and immunity. Now, the zebrafish can be used as a model of psychiatric disorders, especially for testing and developing drugs that modulate new types of cellular targets.
ACKNOWLEDGMENTS
This work was supported in part by NIH grant #R15MH064513-02A1 (JMH).
REFERENCES
- Amiel JM, Mathew SJ. Glutamate and anxiety disorders. Curr. Psychiatry Rep. 2007;9:278–283. doi: 10.1007/s11920-007-0033-7. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, Dugan LL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1646. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- Boon JA, Milsom WK. NMDA receptor-mediated processes in the Parabrachial/Kölliker fuse complex influence respiratory responses directly and indirectly via changes in cortical activation state. Respir. Physiol. Neurobiol. 2008;162:63–72. doi: 10.1016/j.resp.2008.04.002. [DOI] [PubMed] [Google Scholar]
- de Oliveira L, Spiazzi CM, Bortolin T, Canever L, Petronilho F, Mina FG, Dal-Pizzol F, Quevedo J, Zugno AI. Different sub-anesthetic doses of ketamine increase oxidative stress in the brain of rats. Prog. Neuro-Psychoph. 2009;33:1003–1008. doi: 10.1016/j.pnpbp.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Dioum EM, Chen R, Alexander MS, Zhang Q, Hogg RT, Gerard RD, Garcia JA. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase Sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D, Dickson CT. Anxiolytic- and antidepressant-like properties of ketamine in behavioral and neurophysiological animal models. Behav. Neurosci. 2009;161:359–369. doi: 10.1016/j.neuroscience.2009.03.038. [DOI] [PubMed] [Google Scholar]
- Flinn L, Bretaud S, Lo C, Ingham PW, Bandmann O. Zebrafish as a new animal model for movement disorders. J. Neurochem. 2008;106:1991–1997. doi: 10.1111/j.1471-4159.2008.05463.x. [DOI] [PubMed] [Google Scholar]
- Girouard H, Wang G, Gallo EF, Anrather J, Zhou P, Pickel VM, Iadecola C. NMDA receptor activation increases free radical production through nitric oxide and NOX2. J. Neurosci. 2009;29:2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L, Picard F. Calorie restriction-the SIR2 connection. Cell. 2005;120:473–482. doi: 10.1016/j.cell.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Guo S. Linking genes to brain, behavior and neurological diseases: what can we learn from zebrafish? Genes Brain Behav. 2004;3:63–74. doi: 10.1046/j.1601-183x.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- Guo S, Brush J, Teraoka H, Goddard A, Wilson SW, Mullins MC, Rosenthal A. Development of noradrenergic neurons in the zebrafish hindbrain requires BMP, FGF8, and the homeodomain protein soulless/Phox2a. Neuron. 1999;24:555–566. doi: 10.1016/s0896-6273(00)81112-5. [DOI] [PubMed] [Google Scholar]
- Guyenet PG. The 2008 Carl Ludwig Lecture: Retrotrapezoid nucleus, CO2 homeostasis and breathing automaticity. J. Appl. Physiol. 2008;105:404–416. doi: 10.1152/japplphysiol.90452.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers M, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Arch. Gen. Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara-Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacol. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Leheste JR, Curcio C, Baldinger L, Sarwar S, Zakhary SM, Hallas BH, Horowitz JM, Torres G. Glutamate-based drugs for the treatment of clinical depression. Curr. Med. Chem.: Cent. Nerv. Syst. Agents. 2008;8:170–176. [Google Scholar]
- Liebrenz M, Stohler R, Borgeat A. Repeated intravenous ketamine therapy in a patient with treatment-resistant major depression. J Biol. Psychiatry. 2007:1–4. doi: 10.1080/15622970701420481. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-Δ Δc (T) Method. Methods. 2001;4:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucas ME, Müller F, Rüdiger R, Henion PD, Rohrer H. The bHLH transcription factor hand2 is essential for noradrenergic differentiation of sympathetic neurons. Development. 2006;133:4015–4024. doi: 10.1242/dev.02574. [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA. The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr. Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- Michan S, Sinclair D. Sirtuins in mammals: insights into their biological function. Biochem. J. 2007;404:1–13. doi: 10.1042/BJ20070140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B. Bringing order to the glutamate chaos in schizophrenia. Neuron. 2003;40:881–884. doi: 10.1016/s0896-6273(03)00757-8. [DOI] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–436. doi: 10.1016/s0092-8674(00)81972-8. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacol. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- Ohtake PJ, Simakajornboon N, Fehniger MD, Xue YD, Gozal D. N-Methyl-D-Aspartate receptor expression in the nucleus tractus solitarii and maturation of hypoxic ventilatory response in the rat. Am. J. Respir. Crit. Care Med. 2000;162:1140–1147. doi: 10.1164/ajrccm.162.3.9903094. [DOI] [PubMed] [Google Scholar]
- Panigraphy A, Rosenberg PA, Assmann A, Foley EC, Kinney HC. Differential expression of glutamate receptor subtypes in human brainstem sites involved in perinatal hypoxia-ischemia. J. Comp. Neurol. 2000;427:196–208. doi: 10.1002/1096-9861(20001113)427:2<196::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Pilc A, Chaki S, Nowak G, Witkin JM. Mood disorders: regulation by metabotropic glutamate receptors. Biochem. Pharmacol. 2007;75:997–1006. doi: 10.1016/j.bcp.2007.09.021. [DOI] [PubMed] [Google Scholar]
- Pittenger C, Sanacora G, Krystal JH. The NMDA receptor as a therapeutic target in major depressive disorder. CNS Neurol. Disord. Drug Targets. 2007;6:101–115. doi: 10.2174/187152707780363267. [DOI] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of Schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Kendell SF, Levin Y, Simen AA, Fenton LR, Coric V, Krystal JH. Preliminary evidence of riluzole efficacy in antidepressant-treated patients with residual depressive symptoms. Biol. Psychiatry. 2007;61:822–825. doi: 10.1016/j.biopsych.2006.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin L, Nilsson S. Branchial innervations. J. Exp. Zool. 2002;293:232–248. doi: 10.1002/jez.10130. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Karlen M, Wu N, Charnay P, Champagnat J, Fortin G. Genetic identification of an embryonic parafacial oscillator coupling to the pre-Botzinger complex. Nat. Neurosci. 2009;12:1028–1035. doi: 10.1038/nn.2354. [DOI] [PubMed] [Google Scholar]
- Torres G, Hallas BH, Vernace VA, Jones C, Gross KW, Horowitz JM. A neurobehavioral screening of the ckr mouse mutant: implications for an animal model of schizophrenia. Brain Res. Bull. 2004;62:315–326. doi: 10.1016/j.brainresbull.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Torres G, Meeder BA, Hallas BH, Spernyak JA, Mazurchuk R, Jones C, Gross KW, Horowitz JM. Ventricular size mapping in a transgenic model of schizophrenia. Developmental Brain Research. 2005;154:35–44. doi: 10.1016/j.devbrainres.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Turesson J, Schwerte T, Sundin L. Late onset of NMDA receptor-mediated ventilatory control during early development in zebrafish (Danio rerio). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006;143:332–339. doi: 10.1016/j.cbpa.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Berry-Kravis EM, Ceccherini I, Rand CM. Congenital central hypoventilation syndrome (SIDS): kindred disorders of autonomic regulation. Respir. Physiol. Neurobiol. 2008;164:38–48. doi: 10.1016/j.resp.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Zakhary SM, Ayubcha D, Dileo JN, Jose R, Leheste JR, Horowitz JM, Torres G. Distribution analysis of deacetylase SIRT1 in rodent and human nervous systems. J of Anatomical Record. 2010;293:1024–1032. doi: 10.1002/ar.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-Methyl-D-Aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry. 2006;63:856–857. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zhang W, Carreno FR, Cunningham JT, Mifflin SW. Chronic sustained hypoxia enhances both evoked EPSCs and norepinephrine inhibition of glutamatergic afferent inputs in the nucleus of the solitary tract. J. Neurosci. 2009;29:3093–3102. doi: 10.1523/JNEUROSCI.2648-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]