Abstract
Background
Clinicopathologic studies of Parkinson disease dementia (PDD) and dementia with Lewy bodies (DLB) commonly reveal abnormal β-amyloid deposition in addition to diffuse Lewy bodies (α-synuclein aggregates), but the relationship among these neuropathologic features and the development of dementia in these disorders remains uncertain.
Objective
To determine whether amyloid-βdeposition detected by PET imaging with Pittsburgh Compound B (PIB) distinguishes clinical subtypes of Lewy body-associated disorders.
Methods
Nine healthy controls (HC), eight PD with no cognitive impairment (PD-noCI), nine PD with mild cognitive impairment (PD-MCI), six dementia with Lewy bodies (DLB) and fifteen PD with dementia (PDD) patients underwent [11C]-PIB PET imaging, clinical examination, and cognitive testing. The binding potential (BP) of PIB for predefined regions and the mean cortical BP (MCBP) were calculated for each participant. Annual longitudinal follow-up and postmortem examinations were performed on a subset of participants.
Results
Regional PIB BPs and the proportion of individuals with abnormally elevated MCBP were not significantly different across participant groups. Elevated PIB binding was associated with worse global cognitive impairment in participants with Lewy body disorders but was not associated with any other clinical or neuropsychological features, including earlier onset or faster rate of progression of cognitive impairment.
Conclusions
These results suggest that the presence of fibrillar amyloid-βdoes not distinguish between clinical subtypes of Lewy body-associated disorders, although larger numbers are needed to more definitively rule out this association. Amyloid-βmay modify the severity of global cognitive impairment in individuals with Lewy body-associated dementia.
Keywords: Parkinson’s disease, Parkinson’s disease with dementia, Dementia with Lewy bodies, PET
Introduction
Cognitive impairment is common in Parkinson disease (PD) and can range in severity from mild executive dysfunction to dementia1, 2. Individuals with PD are nearly six times more likely to develop dementia than age-matched individuals2, and greater than 80% of individuals who survive over 20 years with PD will become demented3. Dementia also co-occurs with parkinsonism in dementia with Lewy bodies (DLB), a disorder in which cognitive impairment begins before or within one year of motor parkinsonism4.
Neuropathologic studies of PD dementia (PDD) and DLB reveal advanced synucleinopathy with cortical Lewy bodies in the majority of cases5–7. Alzheimer disease (AD) pathology including amyloid-βplaques and neurofibrillary tangles are observed in some cases of PDD and DLB5–7. The role of AD pathology in the pathogenesis of these Lewy body-associated dementias is thus unclear. Some have hypothesized that the presence of fibrillar amyloid-βmay exacerbate cortical Lewy body deposition and thereby accelerate cognitive decline7–9. Postmortem studies do not permit determination of the relative timing of these events.
In vivo PET imaging of the amyloid-binding tracer, Pittsburgh Compound B (PIB), can potentially elucidate the consequences of amyloid-βdeposition in the context of Lewy body-associated neurodegeneration. Since PIB does not bind to cortical Lewy bodies10–12, PIB PET is a reliable method for identifying amyloid-βdeposition in patients with Lewy body disorders12. Previous PIB PET studies found high rates of elevated PIB binding in DLB but not PDD patients13, 14 and concluded that amyloid deposition contributes selectively to the pathogenesis of DLB. However, not all data are consistent with this pattern15 and PDD patients demonstrate some degree of PIB binding, suggesting these clinical phenotypes may both be influenced by coexisting amyloid-βpathology.
The current study evaluates whether amyloid-βdeposition, as determined by PIB PET, distinguishes among clinical phenotypes in patients with Lewy body disorders. We compare PIB binding in patients with a range of cognitive impairment and time course of symptom progression. Longitudinal follow up is ongoing, and all participants have consented to postmortem neuropathologic examination.
Methods
This study was approved by the Human Research Protection Office at Washington University in St. Louis, and written informed consent was obtained for all participants.
Design and Participants
Participants with Lewy body disorders (n = 40) were recruited from the Movement Disorders Center at Washington University School of Medicine and the community, and healthy control participants (HC; n = 10) were recruited through patient participants and the community. Eligibility criteria included age at least 55 years, no major neurological or psychiatric diseases other than PD or DLB, ability to lie still for 90 minutes, no contraindications to MRI, and consent to brain donation. Patients were diagnosed with idiopathic PD based on modified United Kingdom Parkinson’s Disease Society Brain Bank clinical diagnostic criteria16 or DLB according to McKeith criteria4. All DLB patients in this study had parkinsonian motor manifestations and onset of dementia either before or within one year of the onset of these motor manifestations. HC participants were required to have no family history of PD, normal neurologic examination and a Clinical Dementia Rating scale (CDR)17 score of 0. Participants underwent structural MRI, PIB PET imaging, clinical interview, neurological examination and neuropsychological testing.
Clinical evaluation
Movement disorders specialists performed all clinical and neurological evaluations. Neuropsychological and initial motor evaluations were completed off antiparkinsonian medications overnight (mean = 13.2 hours), with repeat motor evaluation following medication (mean time on meds = 1.0 hour). Motor evaluation involved the Unified Parkinson’s Disease Rating Scale Part III (UPDRS)18 and the Hoehn and Yahr scale19. Levodopa equivalent daily dose (LEDD) was calculated using the following corrections: levodopa with a COMT-inhibitor*1.3; sustained release levodopa*0.75; pramipexole or pergolide*100; ropinirole*20; selegiline*10. Control participants had a full neurological examination and UPDRS.
PDD was diagnosed according to published criteria2. This study was initiated before diagnostic criteria for mild cognitive impairment in PD (PD-MCI) were published. The CDR17 was used to assess the presence and severity of cognitive impairment. The “sum of boxes” score (CDR-SB) is the sum of the category ratings. The global CDR score is the weighted average of the category ratings, where CDR 0 indicates no dementia and CDR 1, 2, and 3 indicate mild, moderate, and severe dementia, respectively. A global CDR score of 0.5 indicated PD-MCI if cognitive impairment did not interfere with activities of daily living (ADL) or very mild dementia if cognitive impairment interfered with ADL. Additional standardized assessments included the Mayo fluctuations screen20, Geriatric Depression Scale (GDS)21, Neuropsychiatric Inventory (NPIQ)22 and Mini-Mental State Examination (MMSE).
Neuropsychological evaluation
Trained raters blinded to the clinical evaluation, CDR and PET results administered a neuropsychological battery, mostly from the National Alzheimer’s Coordination Center’s Unified Data Set, which included standardized tests of memory (Logical Memory23; California Verbal Learning Test II (CVLT)24), language (Boston Naming Test25), attention (Digit Span26), visuospatial processing (Spatial Relations Test 27), and executive function (Category Fluency). The Spatial Relations Test was added because visuospatial impairments are highly associated with DLB28. Six participants (one PD-MCI, four PDD, one DLB) did not complete neuropsychological testing due to difficulties remaining off antiparkinsonian medication or severity of dementia.
Imaging
[11C]-PIB was synthesized according to published methods29. PET imaging was performed using a Siemens 961 HR ECAT PET scanner (CTI, Knoxville, TN). Approximately 12 mCi of radiotracer (range, 10.4–14.5; specific activity ≥ 1,200Ci/mmol) was injected via an antecubital vein, and a 60-minute, three-dimensional (septa retracted) dynamic PET scan was collected in 5 minute frames. Emission data were corrected for scatter and randoms. Image reconstruction produced images with a final resolution of 7.5mm full width half-maximum in all directions at the center of the field of view. Frame alignment was corrected for head motion using in-house software and co-registered to each person’s T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) MR scan.
For quantitative analyses, three-dimensional regions of interest (prefrontal cortex, gyrus rectus, lateral temporal cortex, precuneus, occipital lobe, caudate nucleus, brainstem, and cerebellum) were created by a blinded observer for each subject based on the individual’s MRI scans, with boundaries defined as previously described30. Time-activity curves were analyzed using Logan graphical analysis, with the cerebellum as the reference tissue input function30, 31. Binding potentials (BP) were calculated from the tracer distribution volume (DV, reflected in the slope of the Logan graphical analysis) as BP = DV – 1. Mean cortical binding potentials (MCBP) were calculated for each subject as the average of all cortical regions except the occipital lobe. MCBP values greater than 0.2 are associated with low cerebrospinal fluid Aβ42 levels32 and are considered abnormally elevated.
Statistical Analyses
Non-parametric tests (Chi-square, Kruskal-Wallis, Wilcoxon rank sum, Mann-Whitney U) were used for group comparisons of relevant variables. Bivariate correlations (Pearson r) were calculated to examine the relationships between PIB binding and other factors. All tests were 2-tailed, and p < 0.05 was considered significant.
Results
Participant characteristics
Two PDD participants did not complete PET imaging and one HC participant was CDR 0.5; these participants were excluded from analysis. The final sample (N = 47) consisted of nine healthy controls (HC), eight PD with no cognitive impairment (PD-noCI), nine PD-MCI, fifteen PDD and six DLB patients (Table 1). Groups were equivalent in age and education (ps > 0.36). The PDD and DLB groups had worse scores on most cognitive and neuropsychiatric scales. The PDD and DLB groups did not differ from each other in terms of demographics, motor severity, neuropsychiatric features (NPIQ, GDS) and cognitive functioning (CDR, MMSE, fluctuations) (ps > 0.23); however, as expected due to the shorter duration of parkinsonian motor manifestations (p < 0.001) the DLB group took lower doses of antiparkinsonian medication (p = 0.01).
Table 1.
Demographic and clinical characteristics of sample, N=47.
| HC | PD-noCI | PD-MCI | PDD | DLB | |
|---|---|---|---|---|---|
| n | 9 | 8 | 9 | 15 | 6 |
| Sex, M/F | 2/7a | 4/4a | 4/5a | 14/1 | 6/0 |
| Age (years) | 72.0 (6.7) | 69.4 (7.3) | 73.2 (8.5) | 74.8 (6.0) | 71.0 (9.1) |
| Education (years) | 13.9 (2.7) | 14.8 (2.3) | 14.8 (2.7) | 14.5 (2.6) | 14.5 (2.9) |
| WTARd | 102.9 (9.9) | 106.9 (9.9) | 113.3 (7.1)a | 97.1 (13.7) | 94.2 (18.9) |
| CDR global 0/0.5/1/2/3 | 9/0/0/0/0 | 8/0/0/0/0 | 0/9/0/0/0 | 0/5/8/2/0 | 0/1/4/0/1 |
| CDR-SB | 0 (0)a | 0.1 (0.2)a | 1.2 (1.0)a | 5.5 (3.6) | 6.3 (4.8) |
| MMSE | 28.2 (1.4)a | 28.6 (1.1)a | 27.6 (1.3)a | 23.2 (4.8) | 21.0 (6.3) |
| GDS | 1.2 (1.1)a | 2.1 (2.0)a | 4.0 (2.4) | 5.9 (3.7) | 4.5 (2.6) |
| NPIQ | 1.0 (1.7)a | 2.4 (1.8)a | 5.9 (4.6)a | 12.9 (8.3) | 13.2 (6.9) |
| Mayo Fluctuation score | 0 (0)a | 0 (0)a | 0.9 (0.9) | 1.8 (1.4) | 2.0 (1.5) |
| Duration of motor impairment (years) | --- | 7.3 (5.0)b | 8.7 (3.6) | 13.1 (4.8) | 5.4 (2.4)b |
| Duration of cognitive impairment (years) | --- | --- | 3.1 (1.3)a | 5.7 (2.9) | 5.8 (2.3) |
| Levodopa equivalent daily dose (mg) | --- | 669 (375)b | 676 (326)b | 1217 (517) | 608 (240)b |
| UPDRS III off medicatione | 0.78 (1.7)c | 23.0 (9.9)a | 34.2 (12.8) | 36.1 (9.5) | 36.7 (7.1) |
| UPDRS III on medicationf | --- | 21.1 (7.2) | 25.1 (14.2) | 31.1 (11.9) | 35.9 (11.3) |
| Hoehn & Yahr 1/2/2.5/3/4 | --- | 1/7/0/0/0 | 0/5/3/1/0 | 0/9/1/2/3 | 0/3/1/2/0 |
Values are shown as mean (SD) with the exception of sex, CDR global, and Hoehn & Yahr, which are shown as numbers of participants in each category. Significant group differences (p < 0.05) and variables with missing data are indicated with superscript letters as follows:
= different from PDD and DLB;
= different from PD-D;
= different from PD-noCI, PD-MCI, PDD and DLB;
= PD-MCI n=8, PDD n=11, DLB n=5;
= PDD n=12, DLB n=5;
= PD-noCI n=7.
WTAR = Weschler Test of Adult Reading scaled score; CDR = Clinical Dementia Rating Scale; CDR-SB = CDR Sum of Boxes; MMSE = Mini-Mental State Examination; GDS = Geriatric Depression Scale (15-item); NPIQ = Neuropsychiatric Inventory Questionnaire; UPDRS III = Unified Parkinson Disease Rating Scale, Motor subscale; HC = healthy control; PD-noCI = Parkinson disease without cognitive impairment; PD-MCI = Parkinson disease with mild cognitive impairment; PDD = Parkinson disease with dementia, DLB = Dementia with Lewy bodies.
Neuropsychological test performance
Age-scaled scores for the neuropsychological tests were compared across groups (Table 2). The PDD and DLB groups performed worse on most tests compared to the HC, PD-noCI and PD-MCI groups, but there were no differences between the PDD and DLB groups for any of the tests (ps > 0.21). There were no group differences on the Digit Span or Boston Naming Test (ps > 0.58). There were also no differences among the HC, PD-noCI, and PD-MCI groups for any of the tests (ps > 0.35).
Table 2.
Neuropsychological test performance (age-scaled scores).
| HC | PD-noCI | PD-MCI | PDD | DLB | |
|---|---|---|---|---|---|
| n | 9 | 8 | 8 | 11 | 5 |
| Logical Memory I (percentile equivalent) | 50.9 (20.0)a | 58.1 (28.0)a | 48.0 (26.1)a | 11.6 (7.8) | 9.6 (10.5) |
| Logical Memory II (percentile equivalent) | 47.2 (14.3)a | 54.0 (25.0)a | 52.3 (25.9)a | 21.5 (16.5) | 14.8 (7.3) |
| CVLT Short delay free recall (Z) | 0.78 (1.4)b | 1.2 (1.1)a | 0.31 (1.1)b | −1.4 (0.9) | −0.8 (0.6) |
| CVLT Long delay free recall (Z) | 0.83 (0.75)b | 0.81 (1.0)b | 0.19 (1.0) | −0.9 (0.9) | −0.4 (0.8) |
| Spatial Relations standardized score (100±15) | 111.4 (7.2)c | 109.9 (8.3) | 112.9 (9.9)c | 103.2 (9.3) | 96.8 (8.6) |
| Category Fluency (T) | 54.0 (11.0)b | 52.9 (10.3)b | 49.1 (7.3) | 37.2 (11.2) | 43.4 (17.4) |
| Digit Span Total standardized score (10±3) | 10.2 (2.2) | 10.3 (2.4) | 10.0 (1.4) | 9.7 (1.3) | 9.0 (1.4) |
| Boston Naming Test standardized score (10±3) | 12.9 (2.4) | 13.3 (2.9) | 12.1 (4.1) | 12.3 (3.0) | 12.2 (4.0) |
Values are shown as mean (SD). Significant group differences (p < 0.05) are indicated with superscript letters as follows:
= different from PDD and DLB;
= different from PDD;
= different from DLB.
CVLT = California Verbal Learning Test, II; HC = healthy control; PD-noCI = Parkinson disease without cognitive impairment; PD-MCI = Parkinson disease with mild cognitive impairment; PDD = Parkinson disease with dementia; DLB = Dementia with Lewy bodies.
PIB binding and its association with clinical and cognitive factors
No differences were identified in regional PIB binding or MCBP among the HC, PD-noCI, PD-MCI, PDD and DLB groups (ps > 0.64; see Table 3). There were also no differences among the Lewy body disorders groups after removing the HC group from the analysis (ps > 0.54) or between the PDD and DLB groups (ps > 0.38).
Table 3.
Regional PIB binding potentials and MCBP.
| HC | PD-noCI | PD-MCI | PDD | DLB | |
|---|---|---|---|---|---|
| n | 9 | 8 | 9 | 15 | 6 |
| Occipital | 0.14 (0.08) | 0.10 (0.09) | 0.14 (0.19) | 0.08 (0.09) | 0.08 (0.06) |
| Prefrontal* | 0.08 (0.18) | 0.05 (0.18) | 0.05 (0.24) | 0.11 (0.27) | 0.18 (0.32) |
| Gyrus Rectus* | 0.00 (0.10) | 0.00 (0.11) | 0.00 (0.20) | 0.08 (0.25) | 0.16 (0.27) |
| Temporal* | 0.08 (0.11) | 0.03 (0.12) | 0.05 (0.18) | 0.10 (0.19) | 0.17 (0.21) |
| Precuneus* | 0.15 (0.13) | 0.09 (0.17) | 0.22 (0.25) | 0.17 (0.28) | 0.21 (0.28) |
| Caudate | −0.08 (0.11) | −0.05 (0.19) | −0.08 (0.13) | −0.00 (0.18) | 0.02 (0.22) |
| MCBP | 0.08 (0.13) | 0.04 (0.14) | 0.08 (0.21) | 0.11 (0.24) | 0.18 (0.26) |
Binding potentials are shown as mean (SD). Cortical regions included in MCBP calculation are indicated with an asterisk (*).
PIB = Pittsburgh Compound B; MCBP = mean cortical binding potential; HC = healthy control; PD-noCI = Parkinson disease without cognitive impairment; PD-MCI = Parkinson disease with mild cognitive impairment; PDD = Parkinson disease with dementia; DLB = Dementia with Lewy bodies.
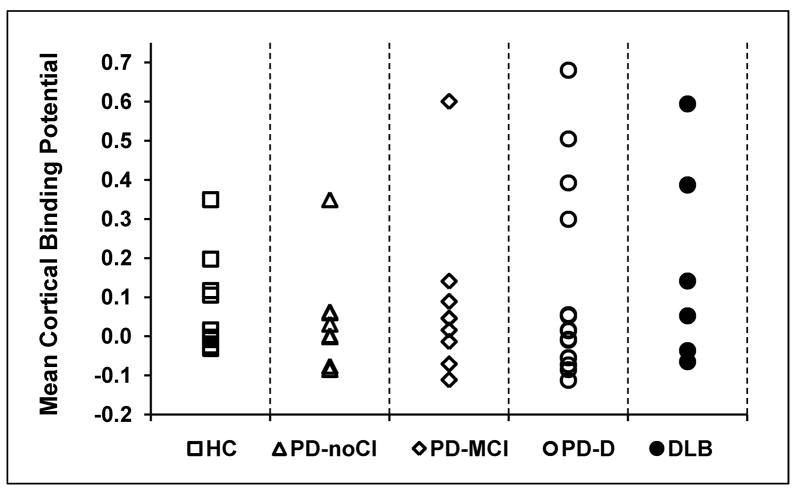
Using the cutoff of MCBP ≥ 0.2 32, 1/9 HC, 1/8 PD-noCI, 1/9 PD-MCI, 4/15 PDD and 2/6 DLB participants had abnormally elevated cortical PIB binding (PIB+; see Figure 1). The proportion of PIB+ participants was similar for groups without (PD-noCI and PD-MCI) and with dementia (PDD and DLB) (Chi-square = 1.60, p = 0.26) and for groups with early (DLB) and later (PDD) onset of dementia (Chi-square = 0.09, p = 0.58). If we assumed that the two PDD patients that did not complete PET imaging either both had positive or negative PIB scans, the statistical findings would still be similar.
Figure 1. PIB uptake for each participant subgroup.
Mean cortical binding potentials (MCBPs) for the participant groups. Each point represents an individual participant.
Because the PDD and DLB groups did not differ in terms of cognitive and neuropsychiatric functioning or PIB binding, we pooled them into a single Lewy body-type dementia (LB-Dem) group for the remaining analyses. A comparison of the PIB+ and PIB- LB-Dem participants is shown in Table 4. The PIB+ participants had worse global CDR (Chi-square= 8.6, p = 0.03) and tended to have worse CDR-SB (U = 21.5, p = 0.07) scores compared to the PIB- group, but there were no differences for any of the other demographic, clinical or neuropsychiatric variables. Neuropsychological test performance could not be compared across PIB binding groups because three of the six PIB+ participants were too impaired to complete testing.
Table 4.
Comparison of the PIB- and PIB+ LB-Dem participants (N=21).
| PIB- | PIB+ | p | |
|---|---|---|---|
| n | 15 | 6 | |
| Sex, M/F | 15/0 | 5/1 | 0.29 |
| Age (years) | 73.6 (7.7) | 74.0 (5.4) | 0.91 |
| CDR global score 0/0.5/1/2/3 | −/5/10/0/0 | −/1/2/2/1 | 0.03 |
| CDR-SB | 4.4 (1.9) | 8.9 (5.6) | 0.07 |
| MMSE | 23.7 (3.6) | 18.0 (7.7) | 0.17 |
| GDS | 5.2 (3.3) | 6.2 (3.7) | 0.55 |
| NPIQ | 12.4 (7.8) | 14.5 (7.9) | 0.52 |
| Mayo Fluctuation scale | 1.6 (1.3) | 2.3 (1.6) | 0.35 |
| Duration of motor impairment (years) | 9.8 (4.9) | 13.5 (6.4) | 0.24 |
| Duration of cognitive impairment (years) | 5.1 (2.2) | 7.3 (3.2) | 0.21 |
| Duration between onset of motor and cognitive impairment (years) | 4.5 (4.6) | 6.1 (5.6) | 0.73 |
| Levodopa equivalent daily dose (mg) | 1071 (548) | 974 (524) | 0.68 |
| UPDRS III off antiparkinsonian medications* | 37.5 (7.4) | 30.2 (13.5) | 0.36 |
| UPDRS III on antiparkinsonian medications | 31.7 (10.9) | 34.5 (14.3) | 0.62 |
| Hoehn & Yahr 1/2/2.5/3/4 | −/8/2/3/2 | −/4/0/1/1 | 0.79 |
Values are shown as mean (SD) with the exception of sex, CDR global, and Hoehn & Yahr, which are shown as numbers of participants in each category.
One PIB- participant and 3 PIB+ participants were unable to perform the UPDRS III off antiparkinsonian medications.
PIB- = absence of elevated Pittsburgh Compound B binding; PIB+ = elevated cortical Pittsburgh Compound B binding; LB-Dem = Lewy body dementia (PDD and DLB); CDR = Clinical Dementia Rating Scale; CDR-SB = CDR Sum of Boxes; MMSE = Mini-Mental State Examination; GDS = Geriatric Depression Scale (15-item); NPIQ = Neuropsychiatric Inventory Questionnaire; UPDRS III = Unified Parkinson Disease Rating Scale, Motor subscale.
MCBP and caudate BP from the pooled sample of all participants with Lewy body disorders modestly correlated with MMSE (MCBP: r = −0.37, p = 0.02; caudate BP: r = −0.37, p = 0.03), CDR global (MCBP: r = 0.44, p = 0.006; caudate BP: r = 0.40, p = 0.01) and CDR-SB (MCBP: r = 0.38, p = 0.02; caudate BP: r = 0.37, p = 0.02) such that higher PIB binding was associated with worse global cognitive functioning. There were no significant correlations with occipital BP. We did not explore cognitive measure correlations with the individual cortical regions because these regional BPs were highly correlated with each other and with MCBP (r = 0.88 to 0.98, p < 0.001). Within PD-MCI, caudate BP (r = −0.82, p = 0.007), but not MCBP (r = −0.24, p = 0.52), correlated with MMSE. Within LB-Dem, MCBP correlated with MMSE (r = −0.47, p = 0.04), CDR global (r = 0.55, p = 0.01), and CDR-SB (r = 0.45, p = 0.04) and caudate BP correlated with MMSE (r = −0.44, p = 0.05) and CDR global (r = 0.51, p = 0.02). There were no significant correlations between PIB binding and global cognitive functioning within the HC and PD-noCI groups (ps > 0.15). PIB binding did not correlate with severity or duration of motor impairment, duration of cognitive impairment, neuropsychiatric functioning, LEDD or neuropsychological test performance (ps > 0.10) and did not correlate with duration between onset of motor and cognitive impairment within the PD-MCI and LB-Dem groups (ps > 0.43).
Follow-up data
One year follow-up data are available for 31 participants; 11 of these also have two year follow-up data. The proportion of participants whose CDR scores increased over time (indicating appearance or progression of dementia) was not different for the PIB- and PIB+ groups (6/26 PIB- vs. 1/5 PIB+; Chi-square = 0.45, p = 0.8).
Autopsy Data
We have autopsy data on five participants (three PIB+ and two PIB-; see 12 for a detailed report on the first three cases). All three PIB+ cases (two PDD, one DLB) had diffuse Lewy body and amyloid plaque pathology, but only the DLB case had sufficient tau pathology to warrant a pathologic diagnosis of AD according to NIA-Reagan criteria. One PIB- case (PDD) had diffuse Lewy body pathology and minimal cortical amyloid plaque pathology. The other PIB- case (PD-MCI) had neither Lewy body nor AD pathology but rather had hypoxic-ischemic pathologic changes in striatum. Review of this patient’s clinical history revealed chronic obstructive pulmonary disease and neurologic presentation with typical PD clinical features including asymmetric rest tremor, rigidity, bradykinesia and levodopa responsiveness (greater than 29% reduction of UPDRS motor score with levodopa).
Discussion
We found elevated PIB PET in almost a third of patients with Lewy body-associated dementia, but PIB binding did not distinguish between DLB and PDD patients. Increased PIB uptake was associated with increased severity of global cognitive impairment but not with any other specific clinical or neuropsychological features. Furthermore, there were no differences in progression of cognitive impairment at follow-up based on PIB binding.
Our rate of elevated PIB binding in PDD patients (27%) is consistent with previous studies (range 17–33%)13, 15, 33. Our rate in DLB patients (33%) is strikingly lower than previous studies in which DLB samples were recruited primarily from dementia centers (85–88%)13, 14 but more comparable to that of a recent study conducted in a movement disorders center (44%)15. Others have found higher PIB binding in DLB compared to PDD13, 14, but we did not. Our results suggest considerable overlap between the neuropathological features of DLB and PDD and support using a single disorder model when studying the underlying pathobiology of Lewy body-associated dementia34. However, the differences among PIB PET studies in this population also illustrate the potential for bias due to recruitment source. Individuals presenting to dementia centers have a higher risk for AD than those presenting to movement disorders centers. In addition, all of the DLB participants in our cohort had parkinsonism; in dementia clinics, 10–15% of individuals meeting criteria for DLB lack significant parkinsonian motor findings35. Differences in the pretest probability of AD and the diagnostic accuracy of DLB across settings may produce varying PIB PET results.
Similar to other PIB studies, we did not correct our PET data for partial volume effects, which could cause an underestimation of amyloid burden in patients with brain atrophy that is worse with more severe dementia. The association between PIB binding and dementia severity suggests this did not play a major role in our analysis. Consistent with previous studies14, 15, 36, we found that higher PIB uptake was associated with greater cognitive impairment in patients with MCI or dementia. These results suggest that amyloid burden may modify dementia severity in Lewy body disorders, potentially by altering the pathophysiology or effects of α-synucleinopathy8, 9. We also found an association between greater cognitive impairment and increased caudate, but not cortical, PIB uptake within MCI, suggesting that the striatum may be an early site for amyloid deposition in Lewy body dementias13.
The large proportion of Lewy body-associated dementia patients without elevated PIB binding (71%) and the lack of association between PIB binding and neuropsychological test performance or progression of cognitive impairment in our study suggest that amyloid deposition is not central to the pathogenesis of dementia in Lewy body disorders. This interpretation is in concordance with clinicopathological examinations, which conclude that diffuse Lewy body pathology is the main substrate of dementia, as it is present in nearly all cases and correlates better with cognitive decline than amyloid plaque burden1, 5.
Amyloid plaques may nonetheless influence the evolution of dementia in individuals with Lewy body disease. They are commonly found in patients with DLB and PDD and are positively correlated with cortical Lewy body counts, suggesting the two pathologies share common origins or interact6, 7. Increased cortical PIB uptake has been associated with faster development of the full DLB clinical phenotype36. Understanding the role of amyloid pathology in Lewy body dementias will increase in importance as anti-amyloid agents become available for therapeutic investigations and clinical application.
Given the small sample, subgroup differences (or lack thereof) should be interpreted with caution and remain to be confirmed in a larger study. Increased statistical power is needed to definitively exclude amyloid deposition as a feature that distinguishes Lewy body-associated disorders. Likewise, our limited follow-up does not permit us to determine the utility of PIB PET as an antecedent marker for dementia in PD, as has been indicated for AD37.
Amyloid burden may contribute to an increased severity of cognitive impairment in Lewy body disease; however, neuropathological features other than amyloid, such as α-synucleinopathy, likely underlie most of the associated dementia. Further investigations in larger cohorts are needed to determine the role of amyloid deposition in the pathogenesis and clinical course of Lewy body-associated dementia and the clinical utility of PIB PET in Lewy body diseases. Improved understanding of synucleinopathy and its association with cognitive decline is also required.
Acknowledgments
This study was supported by NIH NCRR UL1RR024992, NINDS P30NS05710 (Neuroscience Blueprint Grant),the American Parkinson Disease Association (APDA) Advanced Research Center for Parkinson Disease at Washington University in St. Louis, the Greater St. Louis Chapter of the APDA, and the Barnes-Jewish Hospital Foundation (the Elliot H. Stein Family Fund, the Handelman Fund and the Jack Buck Fund for PD Research). We thank the Alzheimer’s Disease Research Center at Washington University School of Medicine for their time in training and certifying members of our team in CDR administration.
Footnotes
All authors report no financial conflicts of interest related to this research.
Author Roles: 1. Research project: A. Conception, B. Organization, C. Execution
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique
3. Manuscript: A. Writing of the first draft, B. Review and Critique
Foster: 1A, B, C; 2A, B, C; 3A, B
Campbell: 1A, B, C; 2A, B, C; 3B
Burack: 1A, B, C; 2A, C; 3B
Hartlein: 1B, C; 2C; 3C
Flores: 1C, 2B, C; 3B
Cairns: 1C; 2B, C; 3B
Hershey: 1A, B; 2A, C; 3B
Perlmutter: 1A, B; 2A, C; 3B
Financial Disclosures: Dr. Foster receives salary and research support from an NIH KL2 career development award (through the Washington University Institute of Clinical and Translational Sciences), American Parkinson Disease Association (APDA) and the Greater St. Louis Chapter of the APDA.
Dr. Campbell receives salary and research support from NIH, NARSAD, Tourette Syndrome Association, McDonnell Foundation, American Parkinson Disease Association (APDA), and the Greater St. Louis Chapter of the APDA.
Dr. Burack receives salary and research support from an American Academy of Neurology Clinical Research Training Fellowship. During the study enrollment period she received salary support from a Medtronic training fellowship and her spouse held stock in Amgen, Medtronic, Novartis, Pfizer, Wyeth, and Astra-Zeneca. She has received honoraria from Healthcare Research Consulting Group.
J. Hartlein receives salary support from NIH, American Parkinson Disease Association (APDA), and the Greater St. Louis Chapter of the APDA.
H. Flores receives salary support from NIH, American Parkinson Disease Association (APDA), Greater St. Louis Chapter of the APDA, and the Michael J. Fox Foundation.
Dr. Cairns receives salary and research support from NIH and the Charles and Joanne Knight Alzheimer Research Fund.
Dr. Hershey receives salary and research support from NIH, the American Parkinson Disease Association (APDA), and the Greater St. Louis Chapter of the APDA.
Dr. Perlmutter receives salary and research support from NIH, HiQ Foundation, APDA, Greater St. Louis Chapter of the APDA, McDonnell Foundation, Barnes-Jewish Hospital Foundation, Washington University, Huntington’s disease Society of America, Michael J. Fox Foundation, and the Bander Foundation for Medical Business Ethics. He has NIH subcontracts via Emory and the University of Rochester. He has received honoraria from the University of Maryland, University of Saskatoon, Parkinson Study Group (University of Rochester), Society of Nuclear Medicine, Movement Disorders Society, and the American Academy of Neurology and is on the DMRF and APDA advisory boards.
References
- 1.Emre M. What causes mental dysfunction in Parkinson's disease? Movement Disorders. 2003;18(Suppl. 6):S63–S71. doi: 10.1002/mds.10565. [DOI] [PubMed] [Google Scholar]
- 2.Emre M, Aarsland D, Brown R, et al. Clinical diagnostic criteria for dementia associated with Parkinson's disease. Mov Disord. 2007;22(12):1689–1707. doi: 10.1002/mds.21507. quiz 1837. [DOI] [PubMed] [Google Scholar]
- 3.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 4.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 5.Aarsland D, Perry R, Brown A, Larsen JP, Ballard C. Neuropathology of dementia in Parkinson's disease: a prospective, community-based study. Ann Neurol. 2005;58(5):773–776. doi: 10.1002/ana.20635. [DOI] [PubMed] [Google Scholar]
- 6.Apaydin H, Ahlskog JE, Parisi JE, Boeve BF, Dickson DW. Parkinson disease neuropathology: later-developing dementia and loss of the levodopa response. Arch Neurol. 2002;59(1):102–112. doi: 10.1001/archneur.59.1.102. [DOI] [PubMed] [Google Scholar]
- 7.Ballard C, Ziabreva I, Perry R, et al. Differences in neuropathologic characteristics across the Lewy body dementia spectrum. Neurology. 2006;67(11):1931–1934. doi: 10.1212/01.wnl.0000249130.63615.cc. [DOI] [PubMed] [Google Scholar]
- 8.Masliah E, Rockenstein E, Veinbergs I, et al. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98(21):12245–12250. doi: 10.1073/pnas.211412398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pletnikova O, West N, Lee MK, et al. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in Lewy body diseases. Neurobiol Aging. 2005;26(8):1183–1192. doi: 10.1016/j.neurobiolaging.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Fodero-Tavoletti MT, Smith DP, McLean CA, et al. In vitro characterization of Pittsburgh compound-B binding to Lewy bodies. J Neurosci. 2007;27(39):10365–10371. doi: 10.1523/JNEUROSCI.0630-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ye L, Velasco A, Fraser G, et al. In vitro high affinity alpha-synuclein binding sites for the amyloid imaging agent PIB are not matched by binding to Lewy bodies in postmortem human brain. J Neurochem. 2008;105(4):1428–1437. doi: 10.1111/j.1471-4159.2008.05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 74(1):77–84. doi: 10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edison P, Rowe CC, Rinne JO, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J Neurol Neurosurg Psychiatry. 2008;79(12):1331–1338. doi: 10.1136/jnnp.2007.127878. [DOI] [PubMed] [Google Scholar]
- 14.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71(12):903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maetzler W, Liepelt I, Reimold M, et al. Cortical PIB binding in Lewy body disease is associated with Alzheimer-like characteristics. Neurobiol Dis. 2009;34(1):107–112. doi: 10.1016/j.nbd.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson's disease. Am J Med Genet. 1999;88(5):539–543. [PubMed] [Google Scholar]
- 17.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 18.Fahn S, Elton RL Members of the UDC, Marsden CD, Goldstein M, Calne DB. Recent developments in Parkinson's disease. New York: Macmillan; 1987. Unified Parkinson's disease rating scale; pp. 153–163. [Google Scholar]
- 19.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 20.Ferman TJ, Smith GE, Boeve BF, et al. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181–187. doi: 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- 21.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 22.Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci. 2000;12(2):233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Manual for the Wechsler Memory Scale - Revised. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- 24.Delis D, Kaplan E, Kramer J, Ober B. California Verbal Learning Test-II. San Antonio: Psychological Corporation; 2000. [Google Scholar]
- 25.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. 2. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 26.Wechsler D. Manual for the Wechsler Adult Intelligence Scale - Revised. New York: Psychological Corporation; 1981. [Google Scholar]
- 27.Woodcock RW, Johnson MB. The Woodcock-Johnson Psycho-Educational Battery -Revised. Texas: DLM Teaching Resources; 1990. [Google Scholar]
- 28.Collerton D, Burn D, McKeith I, O'Brien J. Systematic review and meta-analysis show that dementia with Lewy bodies is a visual-perceptual and attentional-executive dementia. Dement Geriatr Cogn Disord. 2003;16(4):229–237. doi: 10.1159/000072807. [DOI] [PubMed] [Google Scholar]
- 29.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C-labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J Med Chem. 2003;46(13):2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 30.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 31.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16(5):834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 33.Maetzler W, Reimold M, Liepelt I, et al. [11C]PIB binding in Parkinson's disease dementia. Neuroimage. 2008;39(3):1027–1033. doi: 10.1016/j.neuroimage.2007.09.072. [DOI] [PubMed] [Google Scholar]
- 34.Lippa CF, Duda JE, Grossman M, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68(11):812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- 35.Noe E, Marder K, Bell KL, Jacobs DM, Manly JJ, Stern Y. Comparison of dementia with Lewy bodies to Alzheimer's disease and Parkinson's disease with dementia. Mov Disord. 2004;19(1):60–67. doi: 10.1002/mds.10633. [DOI] [PubMed] [Google Scholar]
- 36.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68(20):1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 37.Okello A, Koivunen J, Edison P, et al. Conversion of amyloid positive and negative MCI to AD over 3 years: an 11C-PIB PET study. Neurology. 2009;73(10):754–760. doi: 10.1212/WNL.0b013e3181b23564. [DOI] [PMC free article] [PubMed] [Google Scholar]