Abstract
Mutations in the leucine-rich repeat kinase 2 gene (LRRK2, PARK8) are the most commonly identified monogenic etiology of Parkinson disease (PD). Over-represented in the Ashkenazi Jewish (AJ) population, these mutations are transmitted in an autosomal dominant manner with age-dependent reduced penetrance. The natural history and penetrance of these mutations in the elderly is controversial and inadequately studied. We conducted a nested cohort study in a community-based aging study (the Einstein Aging Study, EAS). Six elderly, initially non-manifesting carriers (NMC) of the LRKK2 G2019S mutation were identified (average age 82.1±7.0, range 72.7-90.8), and five had available longitudinal data. We matched 5 non-carrier controls to each NMC and followed them for an average of 4.7 years with annual cognitive and motor examinations. PD was identified in one NMC at age 95 and in no control subjects. The remaining carriers did not differ from controls on motor scores at baseline or follow-up. The baseline Unified Parkinson's Disease Rating Scale motor subscore (UPDRS-III) in cases was 6.2±6.9 (range 1-19) and in controls was 4.5±6.6 (1-30), p=0.6; the mean difference in UPDRS-III slopes over time between cases and controls was 0.1±1.3 and was not statistically significant. Our data, while limited by a small sample size, show that in LRKK2 G2019S mutation carriers, phenoconversion to PD can occur late in life. However, most NMC have motor decline which indistinguishable from their age mates, suggesting that the larger subset of elderly non-manifesting carriers is not on the motor trajectory to disease.
Keywords: LRRK2, Parkinson's disease, parkinsonism, non-manifesting carriers, penetrance, cognition, clinical
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disease with an overall prevalence of approximately 1.8% over the age of 65 (1,2). While the cause of PD is usually unknown, mutations in the leucine-rich repeat kinase 2 gene (3,4) (LRRK2, PARK8) are the most frequently identified monogenic cause of PD. A glycine to serine substitution located in the highly conserved kinase region of exon 41 (G2019S) is responsible for approximately 4% of familial and with 1% of apparently sporadic PD (5,6) worldwide. In Ashkenazi Jews (AJ) and North African Arabs, the mutation is overrepresented (7-13) due to a common founder mutation (14-16).
While an autosomal dominant mode of transmission is accepted, the lifetime penetrance of LRRK2 mutations is debated, with estimates ranging from 17% to 100% (7-9,17). The broad estimates may be due to methodological differences including subject ascertainment and diagnostic criteria, especially in the very elderly, where parkinsonism needs to be distinguished from normal motor aging.
The natural history of clinical expression in elderly gene carriers without diagnosed PD (non-manifesting carriers, NMC) has not been well studied. We present cross-sectional and longitudinal data on motor and cognitive features in LRRK2 G2019S NMC and compare their motoric progression to age and gender matched subjects.
Methods
Subjects were ascertained from the cohort individuals followed annually as part of the longitudinal Einstein Aging Study (EAS) from 1993 to present. Recruitment and examination methods have been previously described (18). We first sought to identify EAS subjects who had phlebotomy for genetic studies, were screened for the LRRK2 G2019S mutation, and had complete neurological and neuropsychological examination. We then compared each mutation positive subjects without PD (non-manifesting carriers, NMC) to five mutation negative subjects without PD matched for age (± 5 years), gender and year of study recruitment (±1 year). The matches were randomly selected from the EAS and at baseline did not have dementia or history of stroke or neuroleptic use.
Yearly neurological examinations conducted by physicians included, but were not limited to, the motor section of the Unified Parkinson's Disease Rating Scale (19) (UPDRS-III) and the Clinical Dementia Rating Scale (20,21) (CDR). History of prior diagnosis of PD and treatment with parkinsonian medications and dopamine blockers or depleters was queried, and parkinsonian gait and features were rated by the examiner based on history and examination. Diagnosis of parkinsonism was confirmed using research criteria (22,23). Dementia diagnosis was assigned at consensus case conferences using information from the neuropsychological test battery, the neurological exam and medical and social history data using the Diagnostic and Statistical Manual of Mental Disorders fourth edition criteria (24).
An extensive neuropsychological test battery validated in our and other aging populations (25) was also administered. This battery, detailed elsewhere (25) included the Trail Making Test (26,27) (TMT). The TMT Part B (Trails B) was used as a test of motor speed and visual attention (26,27).
Informed consent was obtained from all subjects for participation in the EAS; the study was approved by the Committee on Clinical Investigations at The Albert Einstein College of Medicine and was conducted in accordance with the Declaration of Helsinki. DNA was extracted from white blood cells using QIAamp DNA Blood Maxi Kit according to manufacturer's instructions (Qiagen, Valencia, CA). The G2019S mutation in LRRK2 corresponds to a G6055A SNP in exon 41 and was genotyped by pyrosequencing using methods and primers described previously (8).
For statistical data analysis, SAS 9.1 (SAS Institute Inc., Cary, N.C) and STATA10 (STATA Corp., College Station, TX) were used. Non-parametric tests were employed when variables were not normally distributed. Inverse transformation was used for Trails A and B time to eliminate severe skewness and was interpreted as Trails A and B speed. To compare the longitudinal UPDRS-III scores and Trails speed between the matched LRRK2 G2019S mutation carriers and controls, the slope over time in years was calculated for each subject and the average slope among controls in each matched set was obtained. The difference in slopes between each case and the mean in the control group was then calculated for each matched set and evaluated using a matched-pair t-test.
Results
A total of 791 subjects were screened for mutations, including 355 AJ individuals. 192 of the AJ subjects were previously reported (28). Twelve AJ subjects had definite parkinsonism (3.3%) (Table 1). Heterozygous LRRK2 G2019S mutations were identified in 7 AJ individuals (2.1% carrier frequency among AJs) and at baseline none of them had definite parkinsonism. One of the mutation carriers was deceased shortly after intake, therefore only six of the seven mutation carriers had complete follow-up information.
TABLE 1.
Characteristics of the screening population
| N | Female (%, n) | Age (Years±SD) | Parkinsonism | Type of parkinsonism | |
|---|---|---|---|---|---|
| AJ* | 355 | 58.9% (209) | 83.2±5.7 | 12/355 | 7 IPD** 1 drug-induced parkinsonism 2 AD-related parkinsonism*** 1 diffuse Lewy body disease 1 mild axial parkinsonism at age 100 |
| Carriers | 7 | 85.7% (6) | 87.7±6.9 | 0/7 | |
| Non carriers | 348 | 58.3% (203) | 83.1±5.7 | 12/348 | |
| Non AJ | 436 | 63.8% (278) | 81.4±5.5 | 15/436 | 9 IPD** 1 drug-induced parkinsonism 2 AD-related parkinsonism*** 2 vascular parkinsonism 1 NPH**** |
| Carriers | 0 | ||||
| Non carriers | 436 | 63.8% (278) | 81.4±5.5 | 15/436 |
AJ: Ashkenazi Jews
IPD: Idiopathic Parkinson's disease
AD-related parkinsonism: Alzheimer's disease related parkinsonism
NPH: Normal pressure hydrocephalus
Baseline characteristics are summarized in Table 2. The G2019S mutation carriers were not different from the overall matched control group except for Trails B speed, where cases had slightly shorter times (faster speed) than the control group.
TABLE 2.
Baseline characteristics of LRRK2 mutation carriers and controls
| LRRK2 carriers (n=6) | Control subjects (n=30) | All subjects (n=36) | |
|---|---|---|---|
| UPDRS-III* | 6.2 ± 6.9 (1-19) | 4.5 ± 6.6 (1-30) | 4.8 ± 6.6 (1-30) |
| Trails B speed** | 42.0 ± 8.9 | 31.0 ± 3.1 | 36.0 ± 8.4 |
| CDR* | 0.1 ± 0.2 | 0.1 ± 0.2 | 0.1 ± 0.2 |
| BIMC* | 0.8 ± 0.7 | 2.3 ± 2.4 | 2.1 ± 2.3 |
| FCSRT* (free recall) | 31.8 ± 3.2 | 31.6 ± 5.4 | 31.6 ± 5.1 |
LRRK2: Leucine Rich Repeat Kinase 2; UPDRS-III: Unified Parkinson's Disease Rating Scale, motor subscale, mean±SD (range); CDR: Clinical Dementia Rating Scale, mean±SD; BIMC: Blessed Information Memory Concentration test, mean±SD; FCSRT: Free and Cued Selective Reminding test, mean±SD.
p>0.05;
p=0.02
The performance in UPDRS among the overall control group declined (score increased) over time with an average slope of 0.7 ± 0.5 points per year (Table 3). Among the LRRK2 mutation carriers, UPDRS scores increased slightly faster (difference in slope of 0.1 ± 1.3 points per year, 95%CI: -1.2, 1.5) but the difference was not statistically significant. The motor slope for Subject 2, who at the end of the follow-up period met criteria for PD, was 2.8 UPDRS points per year, compared to a mean of 0.5 points per year among the other mutation positive subjects.
TABLE 3.
Clinical characteristics and UPDRS-III total and subscores of LRRK2 G2019S carriers and matched controls*
| Subject | Sex (n) |
Family history** |
Follow up time (yrs) |
UPDRS-III at First Exam | UPDRS-III at Last Exam | Meets PD criteria |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (yrs) |
Total score |
RT | R | B | P/G | Age (yrs) |
Total score |
RT | R | B | P/G | |||||
| 1 | M | + | 5.67 | 78 | 1 | 0 | 0 | 0 | 1 | 84 | 5 | 0 | 0 | 0 | 5 | No |
| Controls-1 | M(5) | 6.1±0.9 | 76.9±2.9 | 3.2 (2-6) | 0 | 0 | 0.4 (0,2) | 2.5 (2,4) | 82.9±3.3 | 8 (2,16) | 0 | 0.2 (0,1) | 3.4 (0,8) | 4 (2,8) | No | |
| 2 | F | + | 8.28 | 86 | 8 | 0 | 0 | 0 | 7 | 95 | 29 | 0 | 5 | 16 | 7 | Yes |
| Controls-2 | F(5) | 5.4±1.8 | 84.2±1.1 | 5 | 0 | 0 | 2 | 2 (1,3) | 89.6±1.0 | 7.5 (1,11) | 0 | 0 | 5.2 (0,10) | 3.25 (1,8) | No | |
| 3 | F | + | 7.33 | 81 | 6 | 0 | 0 | 2 | 4 | 88 | 14 | 0 | 0 | 6 | 6 | No |
| Controls-3 | F(5) | 7.0±0.2 | 81.1±3.0 | 2.4 (1,6) | 0 | 0 | 0 | 2.4 (1,6) | 88.1±3.0 | 7.2 (3,18) | 0.2 (0,1) | 0 | 4.4 (0,9) | 2.4 (0,9) | No | |
| 4 | F | - | 4.31 | 72 | 1 | 0 | 0 | 0 | 1 | 77 | 1 | 0 | 0 | 1 | 0 | No |
| Controls-4 | F(5) | 4.0±0.1 | 72.3±2.7 | 1.6 (1,3) | 0 | 0 | 0 | 1.2 (1,2) | 76.3±2.6 | 2.4 (0,7) | 0 | 0 | 0.6 (0,2) | 1 (0,5) | No | |
| 5 | F | - | 3.9 | 90 | 19 | 0 | 2 | 11 | 6 | 94 | 25 | 0 | 0 | 15 | 10 | No |
| Controls-5 | F(5) | 2.6±0.5 | 86.9±1.4 | 8.2 (2,30) | 0 | 0.6 (0,2) | 3.6 (0,16) | 4 (1,12) | 89.6±1.7 | 12 (1,27) | 0 | 0 | 5.8 (0,12) | 5.8 (1,15) | No | |
| 6 | F | - | 0 | 85 | 2 | 0 | 0 | 1 | 1 | - | - | - | - | - | - | No |
| Controls-6 | F(5) | 2.7±0.5 | 83.3±2.7 | 6.6 (1,20) | 0 | 0 | 3.6 (0,14) | 1.8 (1,4) | 86.1±2.3 | 9.6 (5,15) | 0 | 1.2 (0,4) | 4.4 (0,8) | 3.6 (1,7) | No | |
UPDRS-III: Unified Parkinson's Disease Rating Scale, Motor Subscale; RT: Rest tremor component of UPDRS-III; R: Rigidity component of UPDRS-III; B: bradykinesia component of UPDRS-III, not including speech and facial expression; P/G: postural changes and gait component of UPDRS-III. Years of follow up and age are expressed in mean years ± S.D. UPDRS and subscores are expressed in mean points and range.
Subjects 1-2 had a family history of Parkinson's disease in first-degree relatives. Subject 6's mother carried a diagnosis of Pick's disease.
Trails B speed did not decline yearly among the control group. While the rate of decline in Trails B speed was faster for the LRRK2 mutation carriers, the difference was not statistically significant (difference in slopes= -0.8 ± 0.8, 95%CI: -1.8, 0.2). The decline slope in Trails B speed for Subject 2 was -1.7 compared to an average slope of 0.1 in the other cases. Similar results were obtained for Trails A (Table 4). Graphic representation of the progression of UPDRS-III scores for each subject compared to their controls as well as to the overall control group is depicted in Figure 1.
TABLE 4.
UPDRS-III and Trails A and B difference in baseline and slopes between LRRK2 G2019S mutation carriers and controls*
| Mean difference ±SD | 95% CI | Minimum | Maximum | |
|---|---|---|---|---|
| UPDRS-III Baseline | 0.7 ± 5.4 | -4.1, 7.4 | -4.6 | 10.8 |
| UPDRS-III Slopes | 0.1 ± 1.3 | -1.2, 1.5 | -1.6 | 2.2 |
| Trails A speed Baseline | -2.8 ± 7.8 | -11, 5.4 | -14.8 | 7.3 |
| Trails A speed Slopes | -0.3 ± 5.2 | -5.8, 5.2 | -9.8 | 5.6 |
| Trails B speed Baseline | 10.4 ± 8.3 | 0.04, 20.7 | -1.8 | 16.8 |
| Trails B speed Slopes | -0.8 ± 0.8 | -1.8, 0.2 | -1.9 | 0.2 |
Free recall (31.8±3.2 for cases and 31.6±5.4 for controls) and Blessed scores (0.8±0.7 for cases and 2.3±2.4 for controls) at baseline and at the end of the follow up period (31.3±4.1 for cases and 32.6±6.8 for controls; 2.17±1.5 for cases and 2.1±2.7 for controls) as well as the calculated progression slopes for both were not significantly different between cases and controls.
Figure 1.
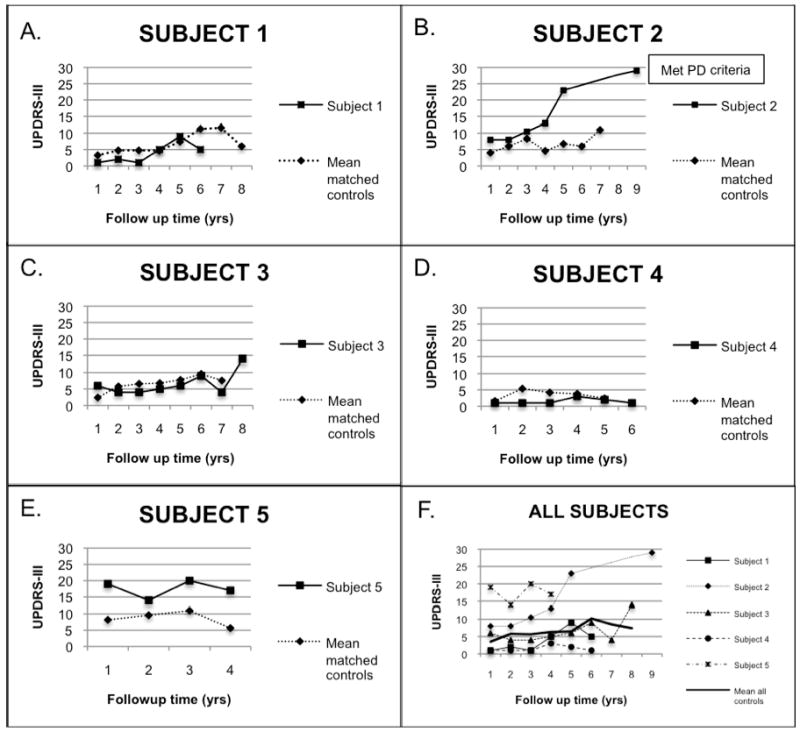
Longitudinal UPDRS-III ratings in LRRK2 G2019S carriers and controls
Figures A-E are graphic representations of UPDRS-III scores over time of the different subjects compared to the mean of their matched control set. Subject 2 had missing UPDRS-III points from years 6-8. In Figure F, mean UPDRS-III scores are presented for all subjects and controls using the same Y-axis scale.
None of the mutation carriers or controls developed dementia by the end of the follow up period.
Clinical description
Only subject 2 developed PD by research criteria during follow-up. She is a 95 year-old woman, previously described at age 91 (28), when she was living independently, had mild slowness of gait, kyphosis and limited mobility of her leg which was attributed to a mild peripheral neuropathy, knee pain and spine osteoarthritis. At her last exam at age 95, she remained independent in her activities of daily living and lived alone. She had definite bradykinesia in her arms and legs with decrementing movement amplitude and occasional movement arrests on finger taps. She had mild rigidity in neck and both arms, and needed to push herself out of a chair to stand. Her gait was markedly slow with decreased right arm swing and shortened stride length. She recovered unaided from the pull test in two steps. Despite the lack of rest tremor, she met UK Brain Bank clinical diagnostic criteria for PD (22).
Discussion
We identified and followed a cohort of very old carriers of the common G2019S LRRK2 mutation by screening a systematically recruited community based sample. Of our 5 LRKK2 mutation carriers free of PD at baseline, only one developed PD. She showed gradual worsening of UPDRS-III scores suggesting progressive motor worsening during the preclinical onset of PD. For the other participants, mild motor features and change in mild motor features were similar for those without the LRKK2 mutation.
All subjects had a mild increase in their UPDRS-III ratings over time, whether or not they carried the LRKK2 mutation: this supports the known progression of mild parkinsonian features with aging (29); it also is consistent with reduced penetrance of PD in elderly carriers of the LRKK2 mutation. The slope of progression of motor features was similar between mutation carriers and controls for all except Subject 2, whose steeper progression led to PD at last follow-up. That is, most of the LRRK2 non-manifesting mutation carriers did not show evidence of accelerated motor decline. Our mutation carriers' ages at last follow-up ranged from 77 to 95 years. Several individuals with age of onset of LRRK2 PD in their early to mid-eighties have been reported (5,7,30-32) including several with onset after age 90 (5,33). As Subject 2 did not meet criteria for PD until age 95, it is possible then that our carriers may still develop PD in the upcoming years. As a pre-clinical period of PD has been posited (34), and Subject 2, unlike the other carriers, had a steep increase in her motor scores starting five years before she met PD criteria that differentiated her curve from her matched controls, the change in UPDRS is in excess of normal motor aging.
Consistent with the known prevalence of approximately ∼14% of LRRK2 mutations among all Ashkenazi Jewish individuals with PD (2), in the 355 AJ subjects screened for the G2019S mutation, seven had idiopathic PD but none of them were carriers. Therefore even though mutations in the LRRK2 gene are the most common genetic determinant of PD identified to date among the Ashkenazim (6), the etiology of PD in most cases remains largely unexplained.
A strength of our study is that our sample was derived from a community based cohort, and thus we may have selected a more benign group, as our carriers were not ascertained based on the presence of PD or family history of PD. While this would not detract from our finding of incomplete penetrance, our sample cannot be used to estimate overall penetrance in the elderly, as individuals with PD might be more likely to be living in care facilities than at home. Finally, because this elderly group has survived for the longest period without PD, they constitute a unique cohort possibly enriched for increased protective or a lower burden of deleterious factors.
The motoric progression of the carrier who manifested PD suggests that there is a period of motoric worsening which precedes clinical LRRK2 PD and can be distinguished from normal aging. Further prospective evaluation will allow us to determine whether similar motor slopes are observed prior to the development of PD in other carriers.
Acknowledgments
We are indebted to our subjects for participating in the study and to Charlotte Magnotta for her assistance with the research subjects. Dr. San Luciano is supported by an American Academy of Neurology Foundation Clinical Research Training Fellowship. This study was supported by Grant Number K23NS047256 from the National Institute of Neurological Disorders and Stroke (RSP), the Michael J. Fox Foundation (RSP, SBB), the Thomas Hartman Foundation (RSP) and The Einstein Aging Study is funded by the National Institute on Aging (AG03949, Principal Investigator: R.B. Lipton).
Footnotes
Financial disclosures: None to report pertaining to this manuscript
Drs. San Luciano, Wang, Katz, Zimmerman, Sanders, Ozelius, Bressman and Saunders-Pullman have nothing to disclose. Dr. Lipton has received grants and honorarium for AstraZeneca, Merck, OrthoMcNeill, GSK, Allergan, MAP and Minster among other companies, but not in connection with this study.
Documentation of Author Roles
1. Research project: A. Conception, B. Organization, C. Execution
2. Statistical analysis: A. Design, B. Execution, C. Review and Critique
3. Manuscript: A. Writing of first and subsequent drafts, B. Review and Critique M. San Luciano: 1A, 1B, 1C, 2A, 2B, 3A; R. Lipton: 1A, 1C, 2A, 2C, 3B; C. Wang: 2A, 2B, 2C; M. Katz: 1B, 1C, 2C, 3B; M. Zimmerman: 1C, 3B; A. Sanders: 1C, 3B; L. Ozelius: 1C, 3B; S. Bressman: 1A, 2C, 3B; R. Saunders-Pullman: 1A, 1B, 1C, 2A, 2C, 3B.
References
- 1.de Rijk MC, Breteler MMB, Graveland GA, Ott A, Grobbee DE, van der Meche FGA, Hofman A. Prevalence of Parkinson's disease in the elderly: The Rotterdam Study. Neurology. 1995;45:2143–2146. doi: 10.1212/wnl.45.12.2143. [DOI] [PubMed] [Google Scholar]
- 2.de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M. Prevalence of Parkinson's disease in Europe: A collaborative study of population-based cohorts. Neurology. 2000;54:S21–S23. [PubMed] [Google Scholar]
- 3.Funayama M, Hasegawa K, Kowa H, Saito M, Tsuji S, Obata F. A new locus for Parkinson's disease (PARK8) maps to chromosome 12p11.2-q13.1. Ann Neurol. 2002;51:296–301. doi: 10.1002/ana.10113. [DOI] [PubMed] [Google Scholar]
- 4.Paisan-Ruiz C, Jain S, Evans EW, et al. Cloning of the gene containing mutations that cause PARK8-linked Parkinson's disease. Neuron. 2004;44:595–600. doi: 10.1016/j.neuron.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Healy DG, Falchi M, O'Sullivan SS, et al. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson's disease: a case-control study Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Correia Guedes L, Ferreira JJ, Rosa MM, Coelho M, Bonifati V, Sampaio C. Worldwide frequency of G2019S LRRK2 mutation in Parkinson's disease: A systematic review. Parkinsonism Relat Disord. 2009 doi: 10.1016/j.parkreldis.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Lesage S, Ibanez P, Lohmann E, et al. G2019S LRRK2 mutation in French and North African families with Parkinson's disease Ann Neurol. 2005;58:784–787. doi: 10.1002/ana.20636. [DOI] [PubMed] [Google Scholar]
- 8.Ozelius LJ, Senthil G, Saunders-Pullman R, et al. LRRK2 G2019S as a cause of Parkinson's disease in Ashkenazi Jews. N Engl J Med. 2006;354:424–425. doi: 10.1056/NEJMc055509. [DOI] [PubMed] [Google Scholar]
- 9.Lesage S, Durr A, Tazir M, et al. LRRK2 G2019S as a cause of Parkinson's disease in North African Arabs. N Engl J Med. 2006;354:422–423. doi: 10.1056/NEJMc055540. [DOI] [PubMed] [Google Scholar]
- 10.Orr-Urtreger A, Shifrin C, Rozovski U, et al. The LRRK2 G2019S mutation in Ashkenazi Jews with Parkinson disease: is there a gender effect? Neurology. 2007;69:1595–1602. doi: 10.1212/01.wnl.0000277637.33328.d8. [DOI] [PubMed] [Google Scholar]
- 11.Hulihan MM, Ishihara-Paul L, Kachergus J, et al. LRRK2 Gly2019Ser penetrance in Arab-Berber patients from Tunisia: a case-control genetic study. Lancet Neurol. 2008;7:591–594. doi: 10.1016/S1474-4422(08)70116-9. [DOI] [PubMed] [Google Scholar]
- 12.Lesage S, Belarbi S, Troiano A, et al. Is the common LRRK2 G2019S mutation related to dyskinesias in North African Parkinson disease? Neurology. 2008;71:1550–1552. doi: 10.1212/01.wnl.0000338460.89796.06. [DOI] [PubMed] [Google Scholar]
- 13.Thaler A, Ash E, Gan-Or Z, Orr-Urtreger A, Giladi N. The LRRK2 G2019S mutation as the cause of Parkinson's disease in Ashkenazi Jews. J Neural Transm. 2009;116:1473–1482. doi: 10.1007/s00702-009-0303-0. [DOI] [PubMed] [Google Scholar]
- 14.Zabetian CP, Hutter CM, Yearout D, et al. LRRK2 G2019S in families with Parkinson disease who originated from Europe and the Middle East: evidence of two distinct founding events beginning two millennia ago. Am J Hum Genet. 2006;79:752–758. doi: 10.1086/508025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren L, Gibson R, Ishihara L, et al. A founding LRRK2 haplotype shared by Tunisian, US, European and Middle Eastern families with Parkinson's disease. Parkinsonism Relat Disord. 2008;14:77–80. doi: 10.1016/j.parkreldis.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Bar-Shira A, Hutter CM, Giladi N, Zabetian CP, Orr-Urtreger A. Ashkenazi Parkinson's disease patients with the LRRK2 G2019S mutation share a common founder dating from the second to fifth centuries. Neurogenetics. 2009;10:355–358. doi: 10.1007/s10048-009-0186-0. [DOI] [PubMed] [Google Scholar]
- 17.Clark LN, Wang Y, Karlins E, et al. Frequency of LRRK2 mutations in early- and late-onset Parkinson disease. Neurology. 2006;67:1786–1791. doi: 10.1212/01.wnl.0000244345.49809.36. [DOI] [PubMed] [Google Scholar]
- 18.Lipton RB, Katz MJ, Kuslansky G, et al. Screening for dementia by telephone using the memory impairment screen. J Am Geriatr Soc. 2003;51:1382–1390. doi: 10.1046/j.1532-5415.2003.51455.x. [DOI] [PubMed] [Google Scholar]
- 19.Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent development in Parkinson's disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–164. [Google Scholar]
- 20.Hughes C, Berg L, Danziger W, Coben L, Martin R. A new clinical scale for the staging of dementia. The British Journal of Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 21.Morris JC. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 22.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Rijk MC, Rocca WA, Anderson DW, Melcon MO, Breteler MM, Maraganore DM. A population perspective on diagnostic criteria for Parkinson's disease. Neurology. 1997;48:1277–1281. doi: 10.1212/wnl.48.5.1277. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Washington DC: 1994. [Google Scholar]
- 25.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, Buschke H. Abnormality of gait as a predictor of non-Alzheimer's dementia. N Engl J Med. 2002;347:1761–1768. doi: 10.1056/NEJMoa020441. [DOI] [PubMed] [Google Scholar]
- 26.Vickers D, Vincent N, Medvedev A. The geometric structure, construction, and interpretation of path-following (trail-making) tests. J Clin Psychol. 1996;52:651–661. doi: 10.1002/(SICI)1097-4679(199611)52:6<651::AID-JCLP7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 27.Misdraji EL, Gass CS. The Trail Making Test and its neurobehavioral components. J Clin Exp Neuropsychol. 2009:1–6. doi: 10.1080/13803390902881942. [DOI] [PubMed] [Google Scholar]
- 28.Saunders-Pullman R, Lipton RB, Senthil G, et al. Increased frequency of the LRRK2 G2019S mutation in an elderly Ashkenazi Jewish population is not associated with dementia. Neurosci Lett. 2006;402:92–96. doi: 10.1016/j.neulet.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 29.Bennett DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgrim DM, Evans DA. Prevalence of parkinsonian signs and associated mortality in a community population of older people. N Engl J Med. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- 30.Goldwurm S, Zini M, Di Fonzo A, et al. LRRK2 G2019S mutation and Parkinson's disease: a clinical, neuropsychological and neuropsychiatric study in a large Italian sample. Parkinsonism Relat Disord. 2006;12:410–419. doi: 10.1016/j.parkreldis.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Lesage S, Leclere L, Lohmann E, et al. Frequency of the LRRK2 G2019S mutation in siblings with Parkinson's disease. Neurodegener Dis. 2007;4:195–198. doi: 10.1159/000101844. [DOI] [PubMed] [Google Scholar]
- 32.De Rosa A, Criscuolo C, Mancini P, et al. Genetic screening for LRRK2 gene G2019S mutation in Parkinson's disease patients from Southern Italy. Parkinsonism Relat Disord. 2009;15:242–244. doi: 10.1016/j.parkreldis.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 33.Gan-Or Z, Bar-Shira A, Mirelman A, Gurevich T, Kedmi M, Giladi N, Orr-Urtreger A. LRRK2 and GBA mutations differentially affect the initial presentation of Parkinson disease. Neurogenetics. 2010;11:121–125. doi: 10.1007/s10048-009-0198-9. [DOI] [PubMed] [Google Scholar]
- 34.Tolosa E, Compta Y, Gaig C. The premotor phase of Parkinson's disease. Parkinsonism Relat Disord. 2007;13(Suppl):S2–7. doi: 10.1016/j.parkreldis.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Gilks WP, Abou-Sleiman PM, Gandhi S, et al. A common LRRK2 mutation in idiopathic Parkinson's disease. Lancet. 2005;365:415–416. doi: 10.1016/S0140-6736(05)17830-1. [DOI] [PubMed] [Google Scholar]
- 36.Ross OA, Toft M, Whittle AJ, et al. Lrrk2 and Lewy body disease. Ann Neurol. 2006;59:388–393. doi: 10.1002/ana.20731. [DOI] [PubMed] [Google Scholar]
- 37.Giasson BI, Covy JP, Bonini NM, Hurtig HI, Farrer MJ, Trojanowski JQ, Van Deerlin VM. Biochemical and pathological characterization of Lrrk2. Ann Neurol. 2006;59:315–322. doi: 10.1002/ana.20791. [DOI] [PubMed] [Google Scholar]
- 38.Papapetropoulos S, Singer C, Ross OA, Toft M, Johnson JL, Farrer MJ, Mash DC. Clinical Heterogeneity of the LRRK2 G2019S Mutation. Arch Neurol. 2006;63:1242–1246. doi: 10.1001/archneur.63.9.1242. [DOI] [PubMed] [Google Scholar]


