Abstract
Emerging data is suggesting that estrogens, in addition to androgens, may also be contributing to the development of prostate cancer (PCa). In view of this notion agents that target estrogens, in addition to androgens, may be a novel approach for PCa chemoprevention and treatment. Thus, the identification and development of non-toxic dietary agents capable of disrupting androgen receptor (AR) in addition to estrogen receptor (ER) could be extremely useful in the management of PCa. Through molecular modeling we found carnosol, a dietary diterpene fits within the ligand binding domain of both AR and ER-α. Using a TR-FRET assay we found that carnosol interacts with both AR and ER-α and additional experiments confirmed that it functions as a receptor antagonist with no agonist effects. LNCaP, 22Rv1, and MCF7 cells treated with carnosol (20–40 µM) showed decreased protein expression of AR and ER-α. Oral administration of carnosol at 30 mg/kg five days weekly for 28 days to 22Rv1 PCa xenografted mice suppressed tumor growth by 36% (p = 0.028) and was associated with a decrease in serum PSA by 26% (p=0.0042). These properties make carnosol unique to any known anti-androgen or anti-estrogen investigated so far for the simultaneous disruption of AR and ER-α. We suggest that carnosol may be developed or chemically modified through more rigorous structure activity relationship studies for a new class of investigational agents - a dual AR/ER modulator.
INTRODUCTION
Evidence is emerging that androgens may not be the only hormone responsible for the pathogenesis of prostate cancer (PCa); recent data has suggested that estrogens may be another important consideration in the PCa puzzle (1–3). Using Noble rats the combined treatment of testosterone and estradiol, but not the separate administration of each has been shown to significantly induce dysplasia and increase the mitotic index in the dorsolateral prostate (4). Another animal model has evaluated the de novo synthesis of estradiol from testosterone via the aromatase enzyme by generating ARKO (aromatase knockout) mice. Three characteristics were associated with these mice that included 1) increased serum levels of androgens,2) complete absence of serum estradiol, and 3) they were incapable of developing PCa (5). More recently ER-α has been receiving increased attention in PCa as evidenced by clinical trials using estrogen antagonists as a monotherapy or in combination with androgen antagonists with encouraging results (2). Further support to the concept of estrogen as a target in PCa comes from a clinical trial in participants (n = 447) randomized to the anti-estrogen toremfiene (20 mg/day) as a monotherapy for one year which showed a decreased cumulative risk of progressing from high-grade prostatic intraepithelial neoplasia (HG-PIN) to PCa by 21.8% (p <0.05) (6).
Simultaneous disruption of both androgen and estrogen receptors has been proposed with FDA approved drugs such as toremifene and fulvestrant as well as other agents, however, there are significant limitations with these chemical entities. Toremifene has a bimodal effect where it functioned as an antagonist at lower concentrations, however, as the dose was increased, it functioned as an agonist in LNCaP cells (7). Limitations of fulvestrant as a dual AR and ER modulator include 1) the AR antagonist effect is saturable regardless of increasing the dose, 2) it can not bind to the mutated AR (T877) that is found in LNCaP cells, and 3) it did not exhibit any effect in a Phase II clinical trial in castration resistant PCa (8, 9). Aromatase inhibitors such as exemestane have also been proposed as a way to target both estrogen and androgen signaling, however, they also function as AR agonists (10, 11).
Carnosol (Figure 1A) is a dietary diterpene isolated from culinary herbs that include rosemary, basil, sage, and oregano and has been noted for its potent antioxidant activity and anti-cancer properties. Approximately 5% of the dry weight of rosemary leaves are the diterpenes carnosol and carnosic acid (12) being responsible for 90% of the antioxidant activity found in rosemary (13, 14). In traditional Chinese medicine rosemary extracts containing high amounts of diterpenes and triterpenes are used to treat inflammatory conditions such as arthritis. Additionally, dietary supplements of rosemary extracts standardized to carnosol and/or carnosic acid are available in health food markets. An in vivo study that evaluated the anti-mutagenic activity of rosemary and carnosol was associated with a significant decrease, 74% and 65%, respectively, in the number of DMBA-induced mammary adenocarcinomas when compared to controls (15). Another in vivo study showed that dietary carnosol (0.1%) decreased APC associated adenoma formation by 46% in the C57BL/6J/Min/+ (Min/+) mouse compared to controls (16). Recently, we have shown that carnosol induces cell cycle arrest by targeting AMPK leading to an inhibition of the mTOR pathway (17).
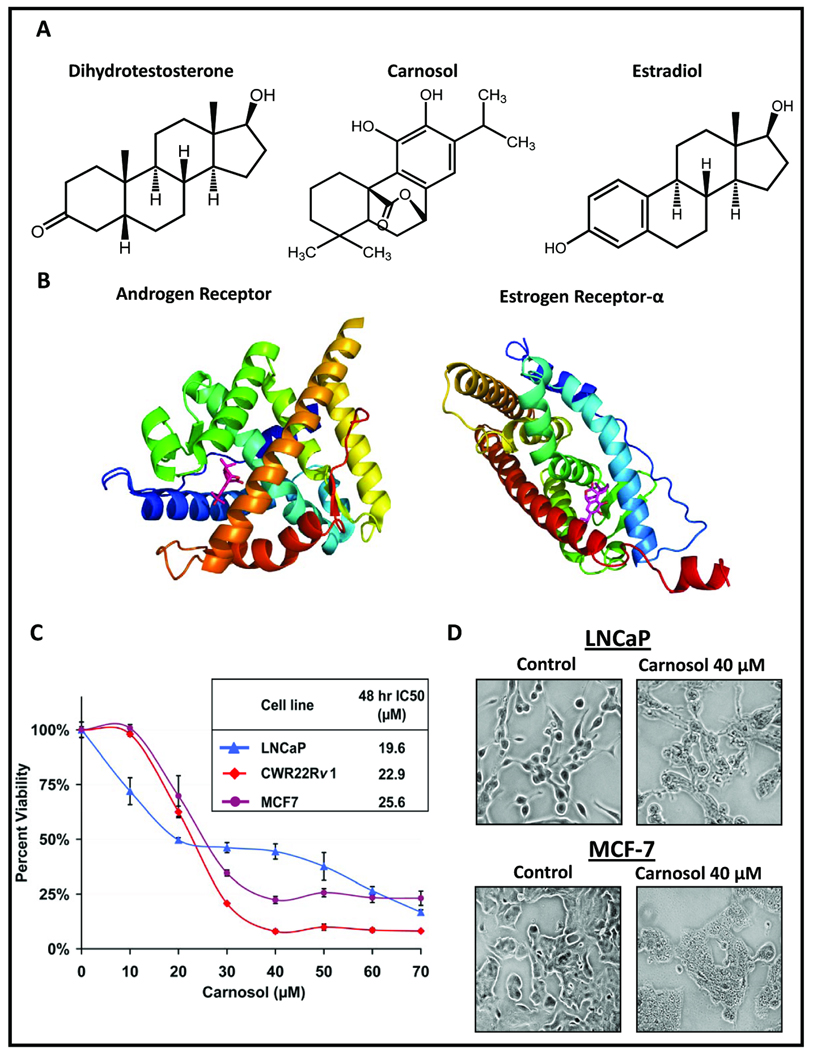
Figure 1.
A, Chemical structures of dihydrotestosterone (DHT), estradiol (E2) and carnosol. B, Crystal structures of androgen and estrogen receptors bound to DHT and E2 respectively were acquired from the protein database bank (www.rcsb.org) and the native ligand was removed and replaced with carnosol. C, The PCa cell lines LNCaP and 22Rv1 and breast cancer cell line MCF7 that express both AR and ER-α were treated with carnosol for 48 h and evaluated for cell viability using an MTT assay. D, Cells were grown to 60–70% confluence and treated with or without carnosol (40µM) for 48 h. Effect of carnosol on cellular morphology in LNCaP and MCF7 cells can be visualized.
Here we provide evidence that carnosol, a dietary diterpene, functions as a dual AR and ER-α modulator by binding and disrupting AR and ER-α receptor leading to a decrease in protein stability of AR and ER-α. Carnosol along with other diterpenes (18) may perhaps prove useful by themselves or as a lead compound for the development of a dual AR/ER-α modulator for use in the chemoprevention and/or chemotherapy of hormone responsive cancers.
MATERIALS AND METHODS
The AR and PSA antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) and ER-α antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA). Carnosol (>99% pure) was purchased from Caymen Chemical (Ann Arbor, MI). Tamoxifen (≥99%) and flutamide (≥99%) were purchased from Sigma (St. Louis, MO). Anti-mouse and anti-rabbit secondary antibody horseradish peroxidase conjugate was obtained from Cell Signaling Technology. Protein assay kit was obtained from Pierce (Rockford, IL). PSA ELISA kit was purchased from Anogen (Mississauga, Ontario, Canada).
Cell culture and treatment
The LNCaP, R22Rv1, MCF7 cells were obtained from American Type Culture Collection. These cells were cultured in RPMI supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Human normal prostate epithelial cells (PrEC) were obtained from Lonza (Basel, Switzerland) and grown according to the manufacturer's instructions. All cells were maintained under standard cell culture conditions at 37°C and 5% CO2 environment. Carnosol dissolved in DMSO was used for the treatment of cells. The final concentration of DMSO used was 0.1% (v/v) for each treatment. The cells (60–70% confluent) were treated with carnosol (10–60 µM) for 24–48 h in complete growth medium.
Cell Viability
Cell viability of human cells treated with carnosol was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay as described previously (17).
Molecular Modeling
Molecular modeling of carnosol was performed with the appropriate nuclear receptor from a protein database bank and was performed using AutoDock 4.2 by the Small Molecule Screening Facility at the University of Wisconsin-Madison.
Biochemical Nuclear Receptor Activity Assay
To identify AR and ER-α ligands a FRET based assay was used to test the anti-androgenic and anti-estrogenic potential of carnosol using purified protein of AR and ER-α that contain the ligand binding domains (LBD). R1881 was included as the control competitor. Briefly, in this assay the ligands for AR or ER-α are identified by their ability to compete with and displace a strong-affinity AR or ER-α ligand (called fluormone) from the receptor. A purified, glutathione S-transferase (GST)–tagged, AR-LBD or ER-α-LBD (1 nmol/L) is indirectly labeled with a terbium-labeled anti-GST tag antibody (5 nmol/L). Binding of fluormone (1 µmol/L solution) to AR or ER-α is then measured by monitoring fluorescence resonance energy transfer (FRET) from the terbium-labeled anti-GST tag antibody to the red fluorescent ligand, resulting in a high time-resolved FRET ratio (570 nm fluorescent emission/546 nm fluorescent emission). Competitors will displace Fluormone AL Red and disrupt FRET, resulting in a lower time-resolved FRET ratio. Serial dilutions of R1881 and carnosol were done in 100% DMSO at 100X the final screening concentration. Each dilution series was then diluted to 4X the final screening concentration in assay buffer (Invitrogen). To perform the assay, 5 µL of 4X carnosol and R1881 were dispensed in the 384-well assay plate. Following this, 5 µL of 4 nmol/L AR or ER-α LBD were dispensed in the assay wells followed by the addition of 10 µL of 10 nmol/L terbium-anti-GST antibody/20 nmol/L fluormone mixture. The plates were incubated for 2 h at room temperature and read using 546- and 570-nm emission intensities on Tecan Infinite F500 plate reader, following excitation at 340 nm. A 100 µs delay followed by a 200 µs integration time was used to collect the time-resolved signal. Three-fold recurrently increasing concentrations of reference competitor R1881 (starting from 0.001 to 10,000 nmol/L, 10 different concentrations) and carnosol (starting from 0.001 to 100,000 nmol/L) were used to test for competition with a constant 10 nmol/L concentration of the labeled competitor fluormone. The concentration of tracer corresponds to its KD value for binding to our AR-LBD. Compound interference is defined in these cases as >50% quenching of the terbium reference emission intensity measured at 546 nm. No interference with carnosol was observed during the assay.
Cell-Based Nuclear Receptor Reporter Assays
AR-UAS-bla GripTite™ 293 cells contain the LBD of the human AR fused to the DNA-binding domain (DBD) of GAL4 stably integrated in the GeneBLAzer® UAS-bla GripTite™ 293 cell line. ER-α-UAS-bla GripTite™ 293 cells contain the LBD of ER-α fused to the DBD GAL4 stably integrated in the GeneBLAzer® UAS-bla GripTite™ 293 cell line. GeneBLAzer® UAS-bla GripTite™ cells stably express a β-lactamase (bla) reporter gene under the transcriptional control of an upstream activator sequence (UAS). When an agonist binds to the LBD of the GAL4 (DBD)-AR (LBD) fusion protein, the protein binds to the UAS, resulting in expression of bla. With expression of bla the substrate is cleaved leading to a separation of the fluorophores, disrupting the energy transfer. The manufacturer’s protocol was followed for screening of carnosol in agonist and antagonist modes.
Agonist Screen - 4 µL of a 10X serial dilution of R1881 or estradiol and carnosol were added to appropriate wells of a 384-well TC-Treated assay plate. 32 µL of cell suspension (10,000 cells) was added to each well. 4 µL of Assay Media was added to all wells to bring the final assay volume to 40 µL. The plate was incubated for 16 h at 37°C/5% CO2 in a humidified incubator. 8 µL of 1 µM Substrate Loading Solution was added to each well and the plate was incubated for 2 h at room temperature. The plate was read on a fluorescence plate reader with two scans performed at 409/460 and 409/530 nm.
Antagonist Screen - 4 µL of a 10X serial dilution of 4-hydroxytamoxifen or Cyproterone Acetate and carnosol were added to appropriate wells of a 384-well TC-Treated assay plate. 32 µL of cells were added to the wells and pre-incubated at 37°C/5% CO2 in a humidified incubator with compounds and control antagonist for 30–60 minutes. 4 µL of 10X control agonist R1881 at the pre-determined EC80 concentration was added to wells containing the control antagonist or compounds. The plate was incubated for 16 h at 37°C/5% CO2 in a humidified incubator. 8 µL of 1 µM Substrate Loading Solution was added to each well and the plate is incubated for 2 h at room temperature and was read on a fluorescence plate reader with two scans performed 409/460 and 409/530 nm.
Western Blot
Following treatment of cells by carnosol whole cell lysates were collected and used to perform Western blot analysis as described previously (17). Cells were lysed and centrifuged at 14,000 × g for 25 min, and the supernatant fraction was used for immunoblotting. Proteins were resolved on a 12% Tris glycine gel and transferred onto a nitrocellulose membrane. After blocking with 5% nonfat dry milk in blocking solution the membrane was incubated with the desired primary antibody for 2 h at room temperature. The membrane was then incubated with the appropriate peroxidase-conjugated secondary antibody and the immunoreactive bands were visualized using the SuperSignal® West Pico chemiluminescent substrate (Pierce, Rockford, Illinois) kit. To ensure equal protein loading, each membrane was stripped and reprobed with β-actin antibody to normalize for differences in protein loading.
AR and ER-α protein stability assay
LNCaP cells were treated with 40 µM carnosol and 50 µg/mL cycloheximide for 0, 6, 12, and 24 h, followed by the preparation of whole cell lysates. Cycloheximide (CHX) was added to the media 30 min before the addition of carnosol. AR and ER-α protein levels were determined by Western blot analysis with antibody specifically against AR and ER-α and were normalized to β-actin control.
Immunoflourescense Microscopy
Cells were seeded on a two-chamber tissue culture-treated glass slides as described previously (19). The following day, media was replaced with or without carnosol and cultured for 24 h. After removing the chamber, slides were rinsed with phosphate-buffered saline (PBS) and cells were fixed with 2% paraformaldehyde and permeabilized in methanol. After washing with PBS, slides were blocked with 2% donkey serum. Primary and secondary antibodies were incubated in 5% donkey serum. SlowFadeGold-DAPI (Invitrogen; Madison, WI) was used as mounting and counterstaining media. For analysis, Bio-Rad Radiance 2100 MP Rainbow system in the W.M. Keck Laboratory for Biological Imaging at the University of Wisconsin–Madison was used.
Transient Transfection and Luciferase Assays
Transient transfections were performed using Lipofectamine (Invitrogen) per manufacturer’s protocol using an AR luc (~6 kb promoter) in the pGL3-Basic vector and ER-α Luc (−5.8-kb) in the pGL2-Basic vector reporter plasmids provided by Donald J. Tindall and Ronald J. Weigel respectively. Co-transfections were performed with 0.5 µg of reporter plasmid and 15 ng of Renilla luciferase plasmid (Promega) and assays performed after 24 h using dual luciferase assay kit (Promega). Cells were co-transfected and normalized with renilla Luciferase plasmid (Promega). Following transfection cells were treated with carnosol for 24 h and samples were prepared per manufacturer’s protocol.
PSA ELISA
The human PSA ELISA kit (Anogen) was used for the quantitative determination of PSA levels in the mouse serum per manufacturer’s protocol. Following collection of blood the samples were allowed to sit at room temperature for one hour and the sera were separated by centrifuging at 2000 rpm for 15 min at 4°C and then stored at −20°C until assayed for secreted PSA. A standard curve was plotted using available PSA standards (2, 10, 20, 40, and 80 ng/mL) in order to quantify individual samples.
Cleaved (active) caspase-3 ELISA
Lysates were prepared with the same lysis buffer for Western blotting. Two hundred micrograms of lysates were analyzed using PathScan cleaved caspase-3 sandwich ELISA (Cell Signaling) following manufacturer's instructions. Lysates were mixed with 100 µL of sample diluent and incubated in antibody-coated microwell strips. One hundred microliters of cleaved caspase-3 detection antibodies were added to each well. Binding was detected with 100 µL of horseradish peroxidase–linked streptavidin antibody and 100 µL of TMB substrate solution. The colored reaction product was measured in a standard ELISA plate reader at 450 nm.
In vivo 22Rv1 tumor xenograft model
Athymic (nu/nu) male nude mice (Harlan Laboratory, Madison, WI) seven weeks old were housed under pathogen-free conditions with a 12 h light/12 h dark schedule and fed with an autoclaved diet ad libitum. AR and ER-α positive 22Rv1 cells were used for determining the in vivo effects of carnosol based on the fact that these cells form rapid and reproducible tumors in nude mice and secrete significant amounts of PSA in the bloodstream of the host. To establish tumor xenografts, mice were injected s.c. with 1×106 22Rv1 cells mixed with 50 µL RPMI + 50 µL Matrigel (BD, Franklin Lakes, NJ) in the right and left flanks of each mouse. Fourteen animals were then randomly divided into two groups, with seven animals in group 1 and seven animals in group 2. The animals in group 1 received cottonseed oil (100 µL) by oral gavage and served as control. The animals in group 2 received carnosol (30 mg/kg) in 100 µL of cottonseed oil by oral gavage 5 days weekly. Body weights were recorded five days weekly throughout the study. Once xenografts started growing, their sizes were measured thrice weekly. The tumor volume was calculated by the formula 0.5238L1L2H, where L1 is the long diameter, L2 is the short diameter, and H is the height of the tumor. This formula is derived from an equation for calculating the volume of a hemi-ellipsoid, the geometric figure most nearly approximating the shape of tumors (20). All animals in group 1 and group 2 were sacrificed when tumors reached an average tumor volume of ~1,200 mm3 in the control group. All procedures conducted were in accordance with the guidelines for the use and care of laboratory animals. Blood samples were collected by the “mandibular bleed.” The blood was allowed to sit at room temperature for 1 h and the sera were separated by centrifuging blood for at 2000 rpm for 15 min at 4°C and then stored at −20°C for future assays.
Statistical Analysis
All statistical analysis was performed by using GraphPad QuickCals software. Statistical significance of differences in all measurements between control and treated groups was determined. Student's paired t test was used for pair wise group comparisons for analyzing Luciferase assays, tumor measurements, and serum PSA levels. All statistical tests were two-sided, and P < 0.05 was considered statistically significant.
RESULTS
Carnosol decreases cell viability of LNCaP, 22Rv1, and MCF7 cells in a dose dependent manner
Given the structural similarity of carnosol to dihydrotestosterone (DHT) and estradiol (E2) (Figure 1A) we hypothesized that carnosol may have the ability to interact with AR and ER-α. Molecular modeling showed that carnosol is able to fit within the LBD of both AR and ER-α (Figure 1B). Based on this premise three different cancer cell lines were selected that express both AR and ER-α that included PCa cell lines LNCaP and 22Rv1 and the breast cancer cell line MCF7 (Figure 1C). Carnosol decreased cell viability in a dose dependent manner with an IC50 of 19.6 µM in LNCaP cells, 22.9 µM in 22Rv1, and 25.6 µM in MCF7 cells (Figure 1C). This decrease in cell viability was accompanied by a change in cellular morphology in carnosol treated cells (Figure 1D).
Carnosol binds to the AR and ER-α in a cell-free based biochemical assay
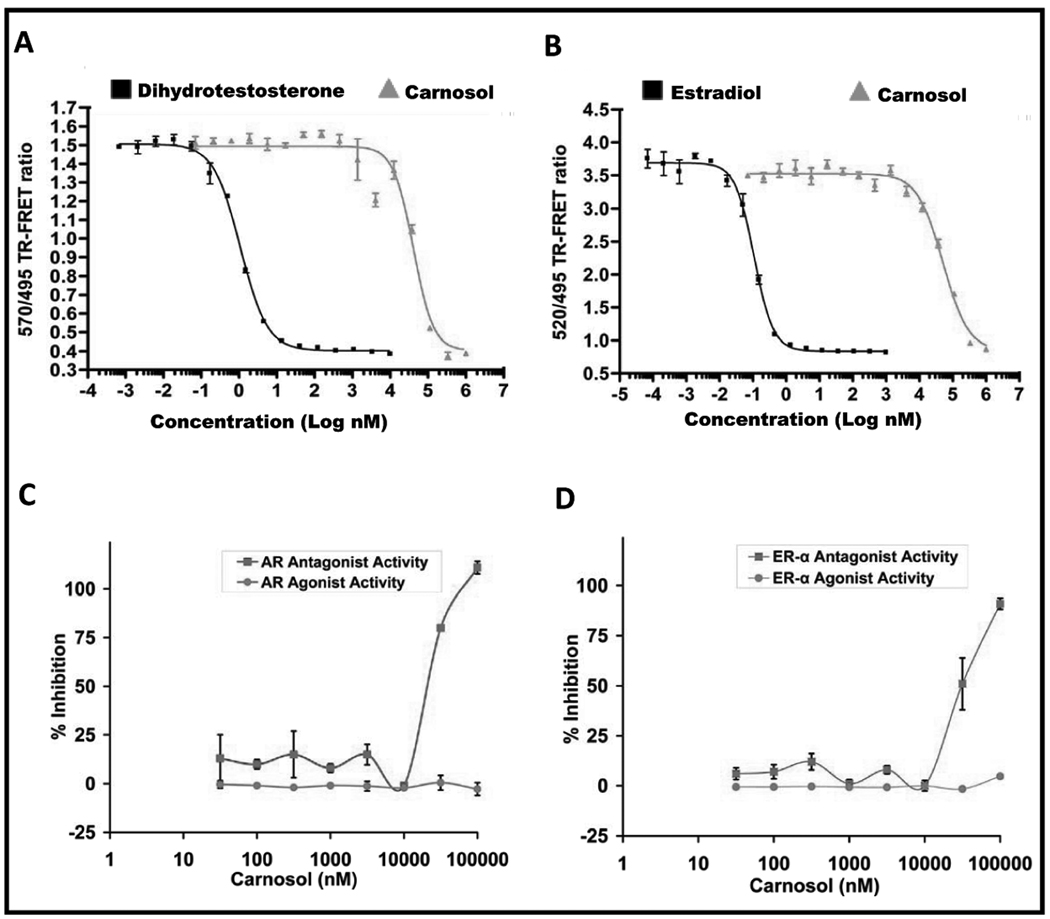
The role of AR and ER-α in PCa is well established and subsequently androgen depletion pharmacotherapy as well as estrogen depletion pharmacotherapy is emerging as a target of interest in both prostate and breast cancer. We evaluated carnosol for its interaction with the AR and ER-α using a LanthaScreen™ TR-FRET (i.e. cell-free based biochemical assay) competitive binding assay. Using this assay, we found that carnosol bound to the AR with an EC50 of 40.8 µM and to the ER-α with an EC50 of 51 µM as shown in Figure 2A and 2B, respectively. These data provided evidence that carnosol can bind to the AR and ER-α receptor, however, further evaluation was needed to determine if carnosol displays agonist/antagonist properties.
Figure 2.
A, TR-FRET AR competitive binding assay with carnosol and B, TR-FRET ER-α competitive binding assay with carnosol. A 100X stock concentration of carnosol in 100% DMSO, was diluted to 4X concentration (4% DMSO) in assay buffer and challenged with a control competitor. C, To verify if carnosol is an inhibitor of native ligand binding properties(i.e. antagonist) or binding properties (i.e. agonist) we stimulated AR-UAS-bla GripTite™ HEK 293 cells with R1881, a known agonist of AR, using cells that express the LBD of AR. A standard curve was performed using an agonist (R1881) and an antagonist (cyproterone acetate). The EC50 of R1881 was 0.127 nM and the EC50 of cyproterone acetate was 11.5 nM. The standard curve for R1881 was performed in triplicate spanning 10 doses ranging from 0.001 nM to 30 nM. The standard curve for cyproterone acetate was performed in triplicate spanning 10 doses ranging from 0.1 nM to 3 µM. D, To verify if carnosol is an inhibitor of native ligand binding properties(i.e. antagonist) or binding properties (i.e. agonist) we stimulated ER-α -UAS-bla GripTite™ HEK 293 cells with estradiol, a known agonist of ER-α, using cells that express the LBD of ER-α. A standard curve was performed using an agonist (estradiol) and an antagonist (4-hydroxytamoxifen). The EC50 of estradiol was 0.027 nM and the EC50 of 4-hydroxytamoxifen was 1.77 nM. The standard curve for estradiol was performed in triplicate spanning 10 doses ranging from 0.0002 nM to 10 nM. The standard curve for 4-hydroxytamoxifen was performed in triplicate spanning 10 doses ranging from 0.002 nM to 100 nM.
Carnosol does not display agonist activity at the AR or ER-α in a cell based assay
In order to understand if carnosol possesses agonist activity we used the AR-UAS-bla Grip Tite™ 293 cells and the ER-α-UAS-bla Grip Tite™ 293 cells that have a LBD for the appropriate nuclear receptor. Carnosol was not found to display any agonist activity in either the AR assay (Figure 2C) or the ER-α assay (Figure 2D) at doses up to 100 µM. These results suggest that carnosol altogether physically interacts with the AR and the ER-α as shown by the cell free biochemical based assay and it possesses no agonist activity.
Carnosol displays antagonist activity at the AR and ER-α in a cell based assay
In order to determine if carnosol has antagonist activity we used the AR-UAS-bla Grip Tite™ 293 as described above with a slight modification where cells were stimulated with the known agonist R1881 (AR) or estradiol (ER-α) and treated with carnosol. A dose response effect in regard to antagonist activity was observed with carnosol with an estimated EC50 of 29.6 µM for the AR (Figure 2C) and an EC50 of 32.9 µM for ER-α (Figure 2D). The results of this further support that carnosol does not display agonist activity at doses up to 100 µM and functions as an antagonist for both AR and the ER-α.
Carnosol decreases the expression of AR, ER-α and PSA in LNCaP, 22Rv1, and MCF7 cells
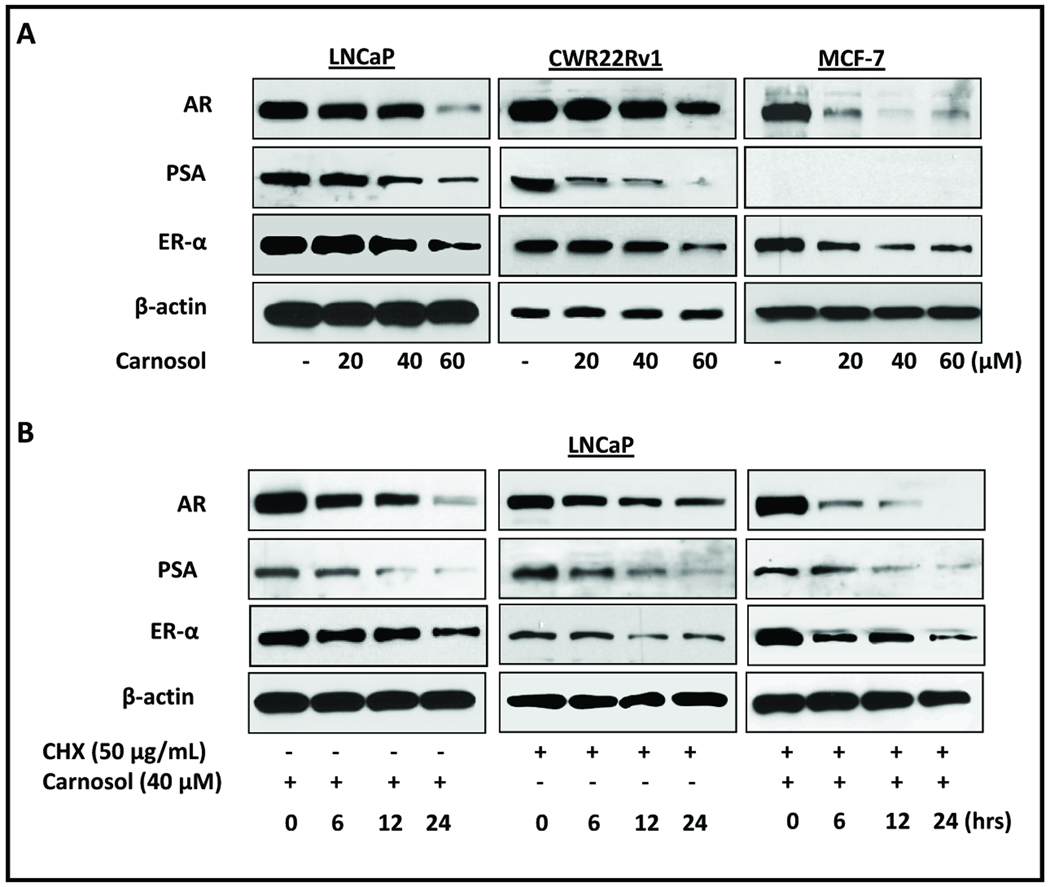
As described above, we used cells stimulated with a known agonist (R1881 or estradiol) and found that carnosol has antagonist properties and no agonist properties. Next, we evaluated three different cell lines to determine if carnosol can decrease the functionality (i.e. protein expression) of AR and ER-α. First, whole cell lysates were evaluated for the intracellular expression of AR, ER-α, and PSA after a 48 h carnosol treatment (20, 40 and 60 µM) using PCa cell lines (LNCaP and 22Rv1) and a breast cancer cell line (MCF7). Our results show that after 48 h carnosol decreased the protein expression of AR and ER-α in a dose dependent manner in LNCaP and 22Rv1 prostate cell lines and MCF7 breast cancer cell line (Figure 3A). In addition, we evaluated the time dependent (0, 6, 12, and 24 h) decrease in AR and ER-α in LNCaP cells and observed a decrease in protein expression as early as 6 h after treatment (Figure 3B).
Figure 3.
Cells were grown to 60–70% confluence and treated with carnosol up to 24 h and whole cell lysates were prepared. 40 µg of protein were subjected to SDS-PAGE followed by Western blot analysis and chemiluminescence detection as described in materials and methods. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. A, LNCaP, 22Rv1, and MCF7 cells were treated with carnosol for 24 h and whole cell lysates were collected and prepared as described in materials and methods. Intracellular protein expression of AR, ER-α, and PSA was evaluated by Western Blot after carnosol treatment. B, LNCaP cells were treated with cycloheximide, carnosol, or carnosol/cycloheximide and whole cell lysates were prepared as described above with lysates collected at 0, 6, 12, and 24 h. AR, ER-α, and PSA intracellular protein expression was evaluated by Western Blot Analysis.
Carnosol alone or in combination with cycloheximide decrease the expression of AR and PSA in a time-dependent manner
To further understand the effect of carnosol on AR stability we performed a pulse chase experiment using the translation inhibitor, cycloheximide (CHX). LNCaP cells treated with either carnosol or CHX led to a time dependent decrease in AR and ER-α protein expression as expected (Figure 3B). Interestingly, co-treatment of LNCaP cells with CHX and carnosol to LNCaP cells further increased the rate of AR and ER-α degradation suggesting that carnosol destabilizes AR and ER-α protein levels.
Carnosol decreases the mRNA expression of AR and ER-α
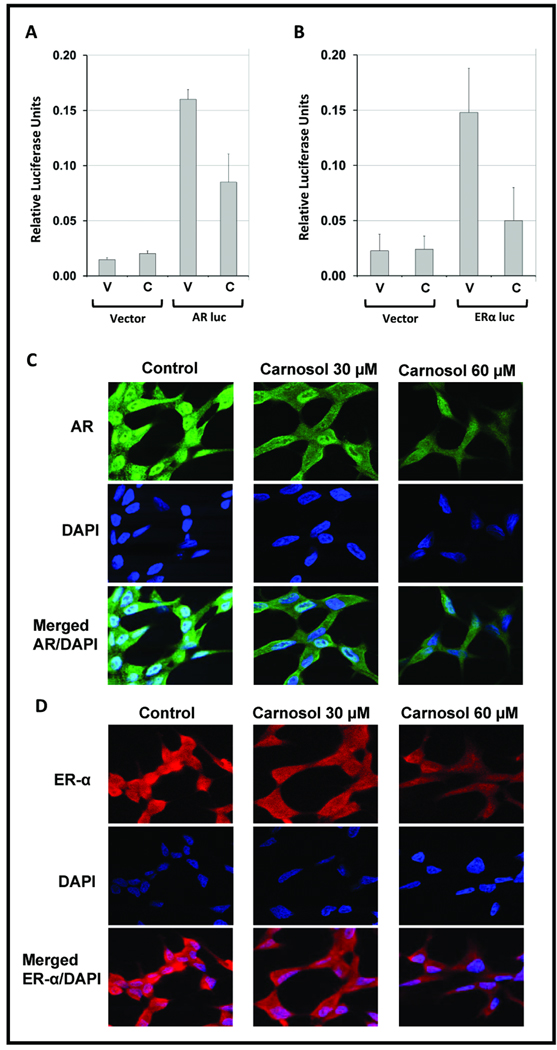
To determine if carnosol acts directly on the AR and ER-α promoter to inhibit gene transcription we examined the effect of carnosol on the activity of an AR and ER-α promoter-reporter construct transfected into LNCaP cells. LNCaP cells were transiently transfected with promoter-reporter constructs for AR (~6-kb) and ER-α (~5.8-kb). A renilla Luciferase plasmid (pRL) was used as a control for transfection efficiency. As shown in Figure 4A and B carnosol treatment significantly (p<0.05) decreased both AR and ER-α promoter Luciferase activity compared to vehicle treatment.
Figure 4.
LNCaP cells were transiently transfected with an ~6-kb AR promoter-luciferase reporter plasmid in a pGL3 Basic Vector or ~5.8-kb ER-α promoter-luciferase reporter plasmid in a pGL2 Basic Vector. 18 h after transfection, cells were treated with DMSO (vehicle) or 30 µM carnosol and analyzed for Luciferase activity after 24 h treatment with carnosol. The mean represents three individual samples with bars representing standard deviation (SD); * = p <0.01. C–D, LNCaP cells were grown in chamber slides at 100,000 cells per chamber overnight and treated with carnosol (30 µM) for 24 h. For labeling, anti-AR antibody (Santa Cruz Biotechnology, Santa Cruz, CA.) and Alexa Fluor 488 goat anti-rabbit IgG (Invitrogen) were used as primary and secondary antibodies after 24 h carnosol treatment, respectively. For labeling anti-ER-α (Cell Signaling Technology, Danvers, MA) and Alexa Fluor 594 goat anti-mouse IgG were utilized after 24 h treatment. 4,6-Diamidino-2-phenylindole (DAPI) was used to counter stain the nucleus.
Carnosol decreases the protein expression of AR and ER-α in LNCaP cells
To further confirm whether carnosol can decrease the expression of AR and ER-α, LNCaP cells were grown in chamber slides for 24 h and treated with carnosol for 24 h and prepared as described in the materials and methods. We observed a decrease in overall protein expression of both receptors when LNCaP cells were treated with carnosol (figure 4C and D). Also, the translocation from the cytoplasm to the nucleus of AR and ER-α was inhibited by carnosol as shown by a diminished fluorescence in the AR and ER-α panels along with the merged panel containing the cells counterstained with DAPI.
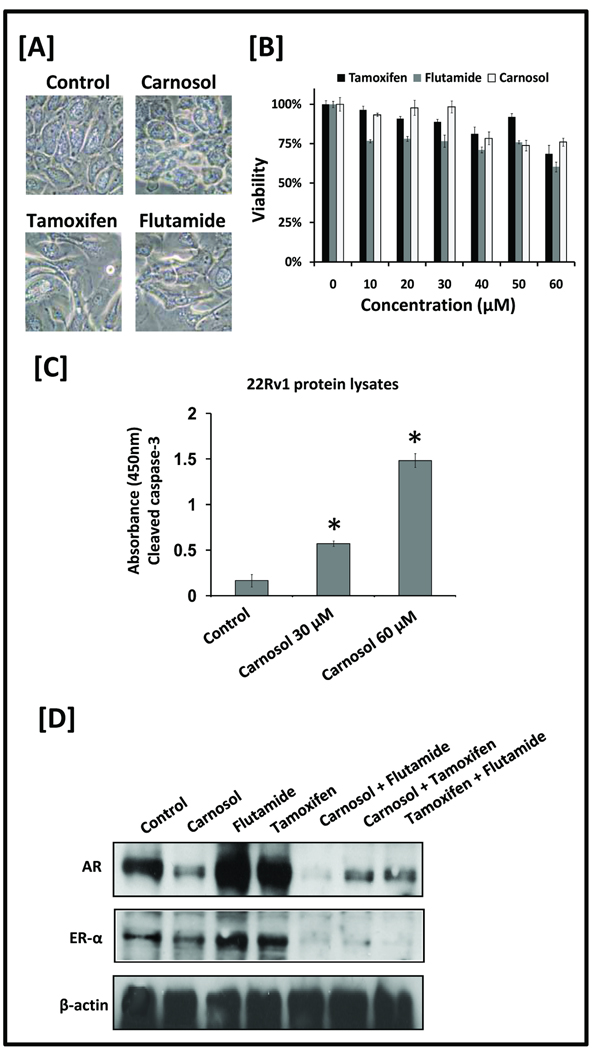
Carnosol displays limited effects on prostate epithelial cells
To determine if carnosol decreases cellular viability of non-tumorigenic cells, prostate epitheial cells (PrEC) were treated with increasing concentrations of carnosol, flutamide, or tamoxifen for 48 h and remained intact as visualized in Figure 5A. At the highest dose of 60 µM the decrease in cell viability was 76.0% ± 2.6 (carnosol), 60.3% ± 3.2 (flutamide), and 68.4% ± 5.6 (tamoxifen) as shown in Figure 5B. These results were unique compared to tumorigenic cell lines as shown in Figure 1. Whole cell lysates were prepared and used to detected cleaved (activated) caspase-3 of carnosol treated 22Rv1 cells. Carnosol treatment for 24 hours led to a significant increase (p<0.01) in cleaved caspase-3 versus control as shown in Figure 5C. These results suggest that carnosol is as tolerable to non-tumorigenic cell lines (e.g. PrEC) as flutamide and tamoxifen that are used regularly in clinical settings.
Figure 5.
A, Prostate epithelial cells (PrEC) were treated with DMSO (i.e. vehicle control), carnosol (30 µM), flutamide (30 µM), or tamoxifen (30 µM). Phase contrast images at 40× magnification were taken 48 h after treatment. B, PrEC cells were treated with increasing concentrations of carnosol, tamoxifen, or flutamide and MTT assay was performed to determine the effect on cell viability. Mean represents three individual samples; bars represent standard deviation. C, Cleaved (activated) caspase-3 was detected by ELISA. 22Rv1 cells were treated with vehicle, carnosol 30 uM, Carnosol 60 uM for 24 hours and protocol was followed per manufacturer’s directions. Mean represents average of three individual samples; bars represent standard deviation. *, P <0.01 of carnosol treated samples versus control. D, LNCaP cells were grown to 60–70% confluence and medium was replaced containing carnosol, flutamide, and tamoxifen, or combinations of carnosol/flutamide, carnosol/tamoxifen, or flutamide/tamoxifen. Total cell lysates were prepared and 40 µg of protein were subjected to SDS-PAGE followed by Western blot analysis. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin.
Carnosol reverses the upregulation of AR and ER-α induced by flutamide or tamoxifen
LNCaP cells were treated with carnosol, flutamide, tamoxifen, carnosol/flutamide, carnosol/tamoxifen, or tamoxifen/flutamide for 24 h. In agreement with other investigators (7, 21–23), we observed an increase in AR and ER-α activity with treatment of flutamide or tamoxifen individually as shown by Western blot analysis in Figure 5C. However, upon co-treatment of flutamide or tamoxifen with carnosol, a decrease in protein expression of AR and ER- α was observed. We also observed a similar decrease in protein expression of AR and ER- α when co-treatment of tamoxifen and flutamide was performed.
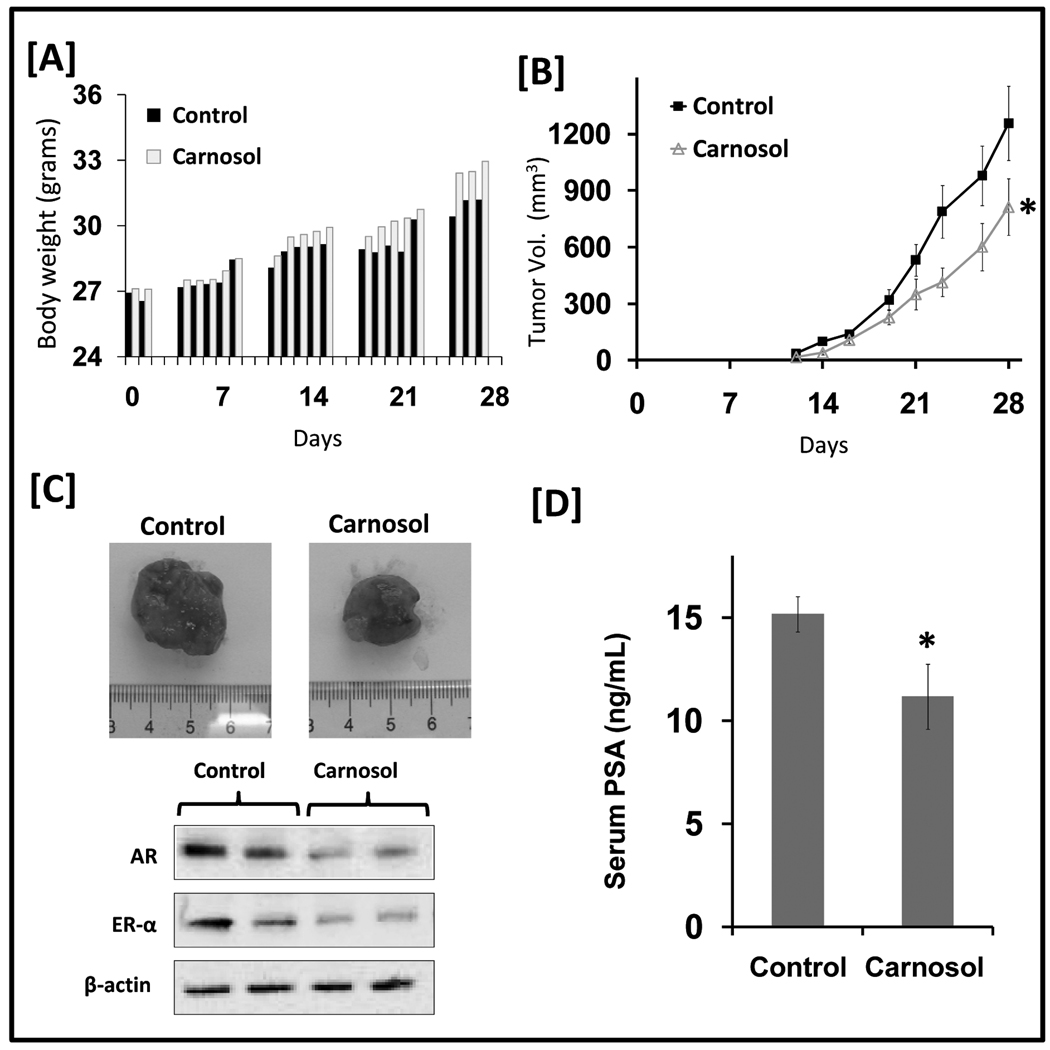
Carnosol inhibits the growth of human prostate carcinoma 22Rv1 cells and PSA secretion in athymic nude mice
Athymic nude mice were implanted with AR and ER-α positive 22Rv1 cells and were divided into two cohorts receiving either cotton seed oil (i.e. vehicle) or carnosol (30 mg/kg). Carnosol administered orally five days weekly appeared to be tolerable as depicted by daily body weight measurements (Figure 6A). On day 12, the appearance of small tumors was observed in both cohorts with consistently a smaller average tumor volume being observed in the mice treated with carnosol as depicted in Figure 6B. The average computed tumor volume was significantly inhibited in mice receiving carnosol. In the control group the average tumor volume of 1257 mm3 was reached at day 28 after tumor cell inoculation while mice receiving carnosol had an average tumor volume of 813 mm3 representing a significant suppression in tumor growth by 36% (p=0.028). In addition, protein lysates were evaluated from carnosol treated mice and analyzed by western blot analysis. Overall we observed a decrease in AR and ER-alpha protein expression in mice treated with carnosol as shown in Figure 6C.
Figure 6.
Effect of carnosol on 22Rv1 tumor growth and PSA secretion in athymic nude mice. Fourteen animals were s.c. injected in each flank of the mouse with approximately 1×106 22Rv1 cells to initiate tumor growth. The animals were divided into two cohorts with seven animals in each cohort received either cottonseed oil (control) or carnosol. 24 h after cell implantation, the animals in cohort 1 received cotton seed (100 uL) by oral gavage five times weekly and served as control. The animals in cohort 2 received carnosol 30 mg/kg by oral gavage in cottonseed oil five times weekly. Once tumors started to grow, tumors were measured three times weekly and the tumor volumes were calculated. A, Body weights were recorded five days weekly and were plotted over days after tumor cell inoculation. B, the average tumor volume of control and carnosol-treated mice were plotted over days after tumor cell inoculation. Data points represent the mean of 14 tumors in seven mice; bars represent standard deviation (SD), * = p < 0.05. C, Representative tumors of control and carnosol treated mice. Total cell lysates were prepared and 10 µg of protein were subjected to SDS-PAGE followed by Western blot analysis. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for β-actin. D, serum PSA levels were analyzed by ELISA at the conclusion of the study. The mean of seven animals per cohort is shown; bars represent SD, * = p < 0.01.
At the conclusion of the study on day 28 blood was collected through mandibular bleed. A quantitative sandwich ELISA was used to determine total circulating PSA in mouse serum secreted by the implanted 22Rv1 tumor cells. Significant inhibition of PSA secretion was observed in carnosol treated mice as shown in Figure 5D. At day 28 PSA levels were 15.2 ng/mL in the control cohort and 11.2 ng/mL in the carnosol treated cohort. Taken together, we show that the treatment of mice with carnosol when given orally inhibited tumor formation by 36% (p=0.028) and suppressed serum levels of PSA by 26% (p=0.0042).
DISCUSSION
For a long time the role of androgens in PCa has been associated with androgens stimulating the AR. Recently, it has become increasingly clear that there are two significant shortcomings to the strategy of inhibiting a single isolated target for PCa. First, the role of anti-androgens is limited because androgen antagonists (e.g. flutamide, bicalutamide) are used clinically for only 6–12 months to control the initial “flare” of PCa. This is often followed by an eventual conversion of PCa to androgen independent status with studies suggesting an upregulation of AKT (24) and AR (25). Second, a host of adverse events associated with androgen antagonists can be observed that include hyperlipidemia (26), osteoporosis (26, 27), gynecomastia (28) as well as others. More recently a role for estrogens stimulating the ER-α in the prostate gland is being more closely scrutinized in the etiology of PCa. Specifically, pre-clinical models have begun to shed light on the role of androgens and estrogens in etiology of PCa. This has been observed in Noble rats that require the co-administration of both testosterone and estradiol for the initiation of PCa (4). Furthermore, animal studies have shown that the aromatase enzyme responsible for the conversion of testosterone to estradiol is essential for the development of PCa (5). In human clinical settings considerable evidence has emerged that supports an association with estrogen and PCa. It is interesting that the relative levels of estradiol increase with age as does the risk of prostate cancer and may contribute to PCa risk (29). Epidemiology studies have suggested that African Americans, who are at the highest risk of PCa, display the highest levels of estrogens (30). Further support of the role for estrogens in PCa initiation and progession come from a study where participants were diagnosed with HG-PIN, a risk factor for PCa, when treated with an anti-estrogen toremifene showed a decreased cumulative risk of PCa diagnosis by 21.8% (6).
As a strategy to decrease unwanted effects of hormone deprivation therapy, clinical trials have combined anti-androgens and anti-estrogens to improve lipid profile (26), increase bone mineral density (27, 31), and reduce gynecomastia (28, 32) in human subjects. These side effects along with other considerations such as androgen resistance represent a significant limitation and are often the explanation for their limited clinical use of 6–12 months. By incorporating both an anti-estrogen and an anti-androgen this strategy may allow for the decrease of known side effects of anti-androgens, a reduction in resistance to anti-androgens due to the multi-target strategy, and possibly the potential to introduce regimen earlier in the disease development as a chemopreventive agent. In addition, by limiting the adverse events it may not be unreasonable to hypothesize that targeting AR and ER-α will allow for the therapy to be used earlier and for a longer period of time.
Here, for the first time we report that carnosol, a novel dietary diterpene, inhibits the growth of androgen-dependent PCa cells. More importantly, the possible role of carnosol to be developed as a chemopreventive agent is strengthened by our observation that carnosol displays minimal effect on the growth of normal prostate epithelial cells (Figure 5A and B). In this context, when HEK293 cells were treated with carnosol up to 1 mM there was not a noticeable decrease in cell viability or change in cellular morphology (data not shown). Carnosol was found to physically interact with the LBD of both the AR and ER-α. In cells stimulated with R1881 or estradiol, carnosol was shown to display purely antagonist properties with no evidence of agonist properties at the AR and ER-α. This is a unique property compared to tamoxifen, toremifene, and fulvestrant that have been evaluated for the dual disruption of AR and ER-α. When administered orally to athymic nude mice with 22Rv1 xenografts carnosol inhibited tumor formation by 36%.
It is possible that carnosol upon binding to the LBD of AR and ER-α could result in the destabilization of AR and ER-α thereby decreasing its expression. Support this idea was obtained from the results of a pulse chase experiment that suggested carnosol accelerated the decay of AR and ER-α in the absence of protein synthesis. Hence, carnosol mediated a decrease in AR and ER-α protein expression seems to be primarily due to a decrease in promoter activity, interference at the LBD, and accelerating the decay of AR and ER-α leading to a decrease in transactivation and protein stability. This indicates that carnosol could serve as a more effective disruptor of hormone signaling by disrupting AR and ER-α at multiple levels compared to anti-androgens (e.g. flutamide and bicalutamide) or anti-estrogens (e.g. tamoxifen or toremifene) that function as antagonists. In our cell free biochemical based assays (Figures 2A and B) the concentration of carnosol was higher than traditional antagonists (e.g. tamoxifen, flutamide, etc.) however, this could be explained by the physico-chemical properties of carnosol. Carnosol is a very hydrophobic molecule as evidenced by a high distribution coefficient (logP) suggesting that it is going to favor hydrophobic environments. In a complex biological environment that is primarily made of water, it may not be unreasonable to hypothesize that carnosol will partition into the hydrophobic binding pockets of AR and ER-α. We would like to add that the selected doses of all our in vitro cell culture work performed with carnosol are within the ranges for in vitro work performed with other agents that have been evaluated as dual AR and ER-α modulators that include tamoxifen, toremifene, and fuvlestrant.
In this study, treatment of LNCaP cells treated with an anti-androgen and anti-estrogen led to an increase in AR and ER-α activity that has been observed by others (7, 21–23) and ourselves. This stimulatory effect was reversed when LNCaP cells were co-treated with carnosol (30 µM) and flutamide (30 µM) or tamoxifen (30 µM) with a decrease in AR and ER- α protein expression (Figure 5C) suggesting that carnosol could reverse the stimulatory action of flutamide and tamoxifen. Interestingly, when LNCaP cells were co-treated with flutamide and tamoxifen a similar inhibition of AR and ER-α protein expression was observed suggesting combination treatment could reverse the stimulatory action of individual treatments. This further indicates that carnosol could serve as a more effective disruptor of AR and ER-α activity compared to flutamide or tamoxifen by inhibiting multiple aspects of hormone signaling.
Although further studies are needed to determine the optimal dose of carnosol in future animal studies, it should be noted that several studies of anti-estrogens and anti-androgens have used oral doses up to 100 mg/kg daily. In fact, oral administration of anti-androgens and anti-estrogens in animal settings often use doses that are 166% to 333% higher than the doses of carnosol used in this study (33–36). Given this line of reasoning, future studies comparing carnosol with anti-androgens and anti-estrogens should be performed using equivalent doses to determine if there is any benefit to increasing the dose of carnosol. Oral delivery for this study was favored over intraperitoneal administration because this is the most likely means of administration in human clinical settings. Oral administration does have several shortcomings compared to intraperitineal administration in pre-clinical studies. Most significantly the maximum concentration (Cmax) of an investigational agent achieved after oral administration is likely to be significantly lower than that achieved after intraperitoneal administration.
Treatment with carnosol (30 mg/kg) significantly slowed the progression of 22Rv1 tumor growth in nude mice as shown in Figure 6B. Based on this study as well as others (15, 16) when carnosol is administered orally our observations indicate carnosol is tolerable and able to decrease tumorigenicity. The inhibition of tumor growth was coupled with a decrease in serum levels of PSA, a clinical diagnostic marker for monitoring PCa in human patients (Figure 6D). Using dose scaling as advised in FDA guidance the human equivalent dose of carnosol used in our study would be 97 mg of carnosol in a 60 kg adult and represents a reasonable starting point for future clinical investigations (37).
The major finding of the study is the demonstration that carnosol can modulate both AR and ER-α activity. Using both prostate and breast cancer cell lines that each express AR and ER-α we found carnosol decreases the expression of AR and ER-α in a dose dependent manner. In addition, we have provided evidence that carnosol induces apoptosis in prostate cancer cells, however, further studies are needed to determine if there is any role for the ER-β which has been shown to have pro-apoptotic properties. We have also observed carnosol to be unique to other proposed dual AR and ER-α antagonists (e.g. tamoxifen, toremifene, fulvestrant) in that there is no evidence of carnosol acting as an agonist at the AR or ER-α. When carnosol was administered orally tumor formation was inhibited in athymic nude mice implanted with PCa cells. The attributes of carnosol to simultaneously disrupt AR and ER-α are unique compared to other FDA approved agents and may be further developed or chemically modified to develop a dual AR/ER-α disruptor.
ACKNOWLEDGEMENTS
This work was supported by a Clinical and Translational Science Award (CTSA) KL2 program (J.Johnson) at the University of Wisconsin (NIH 1KL2RR025012-01). We also thank Donald J. Tindall and Ronald J. Weigel for generously providing the reporter plasmids.
REFERENCES
- 1.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102(4):899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 2.Ellem SJ, Risbridger GP. Treating prostate cancer: a rationale for targeting local oestrogens. Nat Rev Cancer. 2007;7(8):621–627. doi: 10.1038/nrc2174. [DOI] [PubMed] [Google Scholar]
- 3.Heldring N, Pike A, Andersson S, et al. Estrogen receptors: how do they signal and what are their targets. Physiol Rev. 2007;87(3):905–931. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 4.Leav I, Merk FB, Kwan PW, Ho SM. Androgen-supported estrogen-enhanced epithelial proliferation in the prostates of intact Noble rats. Prostate. 1989;15(1):23–40. doi: 10.1002/pros.2990150104. [DOI] [PubMed] [Google Scholar]
- 5.McPherson SJ, Wang H, Jones ME, et al. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142(6):2458–2467. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 6.Price D, Stein B, Sieber P, et al. Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol. 2006;176(3):965–970. doi: 10.1016/j.juro.2006.04.011. discussion 70–1. [DOI] [PubMed] [Google Scholar]
- 7.Kawashima H, Tanaka T, Cheng JS, et al. Effect of anti-estrogens on the androgen receptor activity and cell proliferation in prostate cancer cells. Urol Res. 2004;32(6):406–410. doi: 10.1007/s00240-004-0424-8. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharyya RS, Krishnan AV, Swami S, Feldman D. Fulvestrant (ICI 182,780) down-regulates androgen receptor expression and diminishes androgenic responses in LNCaP human prostate cancer cells. Mol Cancer Ther. 2006;5(6):1539–1549. doi: 10.1158/1535-7163.MCT-06-0065. [DOI] [PubMed] [Google Scholar]
- 9.Chadha MK, Ashraf U, Lawrence D, et al. Phase II study of fulvestrant (Faslodex) in castration resistant prostate cancer. Prostate. 2008;68(13):1461–1466. doi: 10.1002/pros.20813. [DOI] [PubMed] [Google Scholar]
- 10.Masri S, Lui K, Phung S, et al. Characterization of the weak estrogen receptor alpha agonistic activity of exemestane. Breast Cancer Res Treat. 2008 doi: 10.1007/s10549-008-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ariazi EA, Leitao A, Oprea TI, et al. Exemestane's 17-hydroxylated metabolite exerts biological effects as an androgen. Mol Cancer Ther. 2007;6(11):2817–2827. doi: 10.1158/1535-7163.MCT-07-0312. [DOI] [PubMed] [Google Scholar]
- 12.Huang MT, Ho CT, Wang ZY, et al. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994;54(3):701–708. [PubMed] [Google Scholar]
- 13.Aruoma OI, Halliwell B, Aeschbach R, Loligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: carnosol and carnosic acid. Xenobiotica. 1992;22(2):257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 14.Aruoma OI, Spencer JP, Rossi R, et al. An evaluation of the antioxidant and antiviral action of extracts of rosemary and Provencal herbs. Food Chem Toxicol. 1996;34(5):449–456. doi: 10.1016/0278-6915(96)00004-x. [DOI] [PubMed] [Google Scholar]
- 15.Singletary K, MacDonald C, Wallig M. Inhibition by rosemary and carnosol of 7,12-dimethylbenz[a]anthracene (DMBA)-induced rat mammary tumorigenesis and in vivo DMBA-DNA adduct formation. Cancer Lett. 1996;104(1):43–48. doi: 10.1016/0304-3835(96)04227-9. [DOI] [PubMed] [Google Scholar]
- 16.Moran AE, Carothers AM, Weyant MJ, Redston M, Bertagnolli MM. Carnosol inhibits beta-catenin tyrosine phosphorylation and prevents adenoma formation in the C57BL/6J/Min/+ (Min/+) mouse. Cancer Res. 2005;65(3):1097–1104. [PubMed] [Google Scholar]
- 17.Johnson JJ, Syed DN, Heren CR, Suh Y, Adhami VM, Mukhtar H. Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5'-AMP-activated protein kinase (AMPK) pathway. Pharm Res. 2008;25(9):2125–2134. doi: 10.1007/s11095-008-9552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin FM, Tsai CH, Yang YC, et al. A novel diterpene suppresses CWR22Rv1 tumor growth in vivo through antiproliferation and proapoptosis. Cancer Res. 2008;68(16):6634–6642. doi: 10.1158/0008-5472.CAN-08-0635. [DOI] [PubMed] [Google Scholar]
- 19.Suh Y, Afaq F, Johnson JJ, Mukhtar H. A plant flavonoid fisetin induces apoptosis in colon cancer cells by inhibition of COX2 and Wnt/EGFR/NF-kappaB-signaling pathways. Carcinogenesis. 2009;30(2):300–307. doi: 10.1093/carcin/bgn269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–154. doi: 10.1007/BF00300234. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen TV, Yao M, Pike CJ. Flutamide and cyproterone acetate exert agonist effects: induction of androgen receptor-dependent neuroprotection. Endocrinology. 2007;148(6):2936–2943. doi: 10.1210/en.2006-1469. [DOI] [PubMed] [Google Scholar]
- 22.Steinsapir J, Mora G, Muldoon TG. Effects of steroidal and non-steroidal antiandrogens on the androgen binding properties of the rat ventral prostate androgen receptor. Biochim Biophys Acta. 1991;1094(1):103–112. doi: 10.1016/0167-4889(91)90031-r. [DOI] [PubMed] [Google Scholar]
- 23.Wilkinson JM, Hayes S, Thompson D, Whitney P, Bi K. Compound profiling using a panel of steroid hormone receptor cell-based assays. J Biomol Screen. 2008;13(8):755–765. doi: 10.1177/1087057108322155. [DOI] [PubMed] [Google Scholar]
- 24.Festuccia C, Gravina GL, Muzi P, et al. Bicalutamide increases phospho-Akt levels through Her2 in patients with prostate cancer. Endocr Relat Cancer. 2007;14(3):601–611. doi: 10.1677/ERC-07-0118. [DOI] [PubMed] [Google Scholar]
- 25.Kawata H, Ishikura N, Watanabe M, Nishimoto A, Tsunenari T, Aoki Y. Prolonged treatment with bicalutamide induces androgen receptor overexpression and androgen hypersensitivity. Prostate. 70(7):745–754. doi: 10.1002/pros.21107. [DOI] [PubMed] [Google Scholar]
- 26.Smith MR, Malkowicz SB, Chu F, et al. Toremifene improves lipid profiles in men receiving androgen-deprivation therapy for prostate cancer: interim analysis of a multicenter phase III study. J Clin Oncol. 2008;26(11):1824–1829. doi: 10.1200/JCO.2007.13.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res. 2006;12(20 Pt 2):6315s–6319s. doi: 10.1158/1078-0432.CCR-06-0846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fradet Y, Egerdie B, Andersen M, et al. Tamoxifen as prophylaxis for prevention of gynaecomastia and breast pain associated with bicalutamide 150 mg monotherapy in patients with prostate cancer: a randomised, placebo-controlled, dose-response study. Eur Urol. 2007;52(1):106–114. doi: 10.1016/j.eururo.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Kaufman JM, Vermeulen A. The decline of androgen levels in elderly men and its clinical and therapeutic implications. Endocr Rev. 2005;26(6):833–876. doi: 10.1210/er.2004-0013. [DOI] [PubMed] [Google Scholar]
- 30.Rohrmann S, Nelson WG, Rifai N, et al. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab. 2007;92(7):2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 31.Smith MR, Malkowicz SB, Chu F, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179(1):152–155. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boccardo F, Rubagotti A, Battaglia M, et al. Evaluation of tamoxifen and anastrozole in the prevention of gynecomastia and breast pain induced by bicalutamide monotherapy of prostate cancer. J Clin Oncol. 2005;23(4):808–815. doi: 10.1200/JCO.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Kang IH, Kim HS, Shin JH, et al. Comparison of anti-androgenic activity of flutamide, vinclozolin, procymidone, linuron, and p, p'-DDE in rodent 10-day Hershberger assay. Toxicology. 2004;199(2–3):145–159. doi: 10.1016/j.tox.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 34.Terada N, Shimizu Y, Yoshida T, et al. Antiandrogen withdrawal syndrome and alternative antiandrogen therapy associated with the W741C mutant androgen receptor in a novel prostate cancer xenograft. Prostate. 70(3):252–261. doi: 10.1002/pros.21058. [DOI] [PubMed] [Google Scholar]
- 35.Yoneya T, Taniguchi K, Nakamura R, et al. Thiochroman derivative CH4986399, a new nonsteroidal estrogen receptor down-regulator, is effective in breast cancer models. Anticancer Res. 30(3):873–878. [PubMed] [Google Scholar]
- 36.Yoneya T, Tsunenari T, Taniguchi K, et al. Effects of CH4893237, a new orally active estrogen receptor downregulator, on breast cancer xenograft models with low serum estrogen levels. Oncol Rep. 2009;21(3):747–755. [PubMed] [Google Scholar]
- 37.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]