Abstract
The development of metastases has been shown to be associated with the microvascular density of the primary tumor in some clinical studies and with the extent of hypoxia in others. The aim of this study was to investigate the validity of these apparently inconsistent observations and to reveal possible links between them. Xenografted tumors of nine melanoma cell lines established from patients with diseases differing in aggressiveness were studied. The aggressiveness of the cell lines was assessed by measuring their lung colonization potential, invasiveness, angiogenic potential, and tumorigenicity. Spontaneous metastasis was assessed in untreated mice and mice treated with neutralizing antibody against vascular endothelial growth factor A (VEGF-A) or interleukin 8 (IL-8). Microvascular density was scored in histologic preparations. Hypoxic fractions were measured by using a radiobiologic assay and a pimonidazole-based immunohistochemical assay. The aggressiveness of the melanoma lines reflected the aggressiveness of the donor patients' tumors. The metastatic propensity was associated with the microvascular density but not with the hypoxic fraction. Anti-VEGF-A and anti-IL-8 treatments resulted in decreased microvascular density and reduced incidence of metastases in all lines. Large hypoxic fractions were not a secondary effect of high cellular aggressiveness, whereas the microvascular density was associated with the cellular aggressiveness. The metastatic propensity was governed by the angiogenic potential of the tumor cells. The differences in microvascular density among the lines were most likely a consequence of differences in the constitutive angiogenic potential rather than differences in hypoxia-induced angiogenesis. VEGF-A and IL-8 may be important therapeutic targets for melanoma.
Introduction
Tumors gradually accumulate stable and unstable genomic alterations during growth, resulting in the development of an increasing number of aggressive phenotypic traits with time, a process termed malignant progression [1,2]. The final stage of the malignant progression is the development of cell variants showing invasive growth in surrounding normal tissues and metastatic spread to distant organ sites. The development of metastatic cell phenotypes has been suggested to be a result of genomic instability caused by defects in regulatory genes, including oncogenes, tumor suppressor genes, and other genes involved in signal transduction, DNA replication, cell cycle regulation, DNA repair, and apoptosis [2,3].
Side by side with the generation of aggressive cell phenotypes, tumors develop an abnormal microvasculature showing loss of hierarchy, increased intervessel distances, elevated geometric resistance, and inadequate blood flow. These abnormalities result in a pathophysiological tumor microenvironment characterized by low glucose concentrations, high lactate concentrations, acidic extracellular pH, elevated interstitial fluid pressure, and low oxygen tensions [4,5]. Regions with hypoxic tissue are a characteristic feature of many primary tumors [6,7].
Clinical investigations involving several histologic types of cancer have shown that patients with highly hypoxic primary tumors may have an increased frequency of locoregional treatment failure after radiation therapy, elevated incidence of regional and distant metastases, and poor disease-free and overall survival rates [7]. Multiple mechanisms may link tumor hypoxia to metastasis [8–10]. Hypoxia followed by reoxygenation may induce DNA double-strand breaks leading to genomic instability and provide a physiological pressure selecting for aggressive cell phenotypes [11,12]. Furthermore, tumor hypoxia activates several transcription factors, including hypoxia-inducible factor 1, leading to upregulated expression of a large number of gene products known to promote invasive growth and metastatic dissemination [13].
There is also significant evidence that cancer metastasis may be determined by the angiogenic potential of the primary tumor rather than the extent of hypoxia. Thus, clinical studies involving primary tumors in many organs have shown associations between incidence of metastases and the microvascular density in vascular hot spots in the tumor periphery [14,15]. The metastatic propensity of tumors may be influenced by the angiogenic potential of the tumor cells by several mechanisms. High angiogenic activity in the tumor periphery may promote invasive growth, and the microvascular networks of highly angiogenic tumors may show high vessel density and are composed of immature vessels that are leaky and have fragmented basement membranes and, therefore, may facilitate tumor cell intravasation [16]. Furthermore, elevated capacity to induce neovascularization may increase the probability of tumor cells trapped in secondary organ capillary beds to give rise to macroscopic tumor growth [16].
Although tumor hypoxia is partly a consequence of poor oxygen supply owing to inadequate vascularization [4], the associations between tumor hypoxia and metastasis and the associations between microvascular density and metastasis observed in clinical studies are not necessarily mutually exclusive. However, the mechanisms connecting these two sets of observation are not understood. It has been hypothesized that the most aggressive cell phenotypes may give rise to tumors with particularly large fractions of hypoxic tissue as well as particularly high microvascular density as a consequence of hypoxiainduced angiogenesis [17]. To investigate the validity of this hypothesis, we established cell lines from metastases of melanoma patients with diseases differing significantly in aggressiveness and measured the metastatic propensity, the fraction of hypoxic cells, and the microvascular density of xenografted tumors initiated from the cell lines. Because melanoma cells show large secretion of the proangiogenic factors vascular endothelial growth factor A (VEGF-A) and interleukin 8 (IL-8) [18,19], effects of anti-VEGF-A treatment and anti-IL-8 treatment on microvascular density and metastasis were also investigated. In this article, we show that the aggressiveness of the cell lines and the metastatic propensity of the xenografted tumors reflected the aggressiveness of the donor patients' tumors. Moreover, we present significant evidence that the metastatic propensity of the xenografted tumors was determined primarily by the microvascular density in the tumor periphery rather than by the fraction of hypoxic tumor cells and that anti-VEGF-A treatment and anti-IL-8 treatment resulted in reduced microvascular density in the tumor periphery and decreased incidence of metastases.
Materials and Methods
Donor Patients' Tumors
Nine patients admitted to the Norwegian Radium Hospital for the treatment of malignant melanoma were donors of tumor tissue (Table 1). The patients had developed regional subcutaneous and lymph node metastases at the time of initial diagnosis. Surgical specimens were obtained from large subcutaneous lesions before initiation of chemotherapy.
Table 1.
Clinical Characteristics of the Donor Patients.
| Patient | Age (Year) | Sex | Primary Tumor | Breslow Index (mm) | Stage | Cause of Death |
| A-07 | 50 | Male | Upper limb | 3.8 | III-N3 | Lung Mets |
| C-10 | 27 | Female | Trunk | 4.5 | III-N2 | Liver Mets |
| D-12 | 38 | Female | Trunk | 8.3 | III-N3 | Lung Mets |
| R-18 | 45 | Female | Neck | 7.4 | IV-M1a | Lymph node Mets |
| S-20 | 64 | Male | Face | 4.2 | III-N3 | Lymph node Mets |
| T-22 | 62 | Male | Neck | 5.2 | III-N3 | Lung Mets |
| U-25 | 49 | Male | Trunk | 5.0 | III-N3 | Brain Mets |
| V-27 | 35 | Female | Lower limb | 6.5 | III-N2 | Lung Mets |
| Z-98 | 22 | Male | Trunk | 4.0 | IV-M1a | Lung Mets |
Mets indicates metastases.
Cell Lines
Permanent cell lines, one from each of the nine patients, were established in monolayer culture. The experimental procedure and four of the cell lines have been described in detail previously [20]. Beyond passage 50, the cell lines were found to be stable with respect to several parameters, including growth rate, plating efficiency in vitro, DNA content, radiation sensitivity in vitro, and lung colonization potential in vivo. Large stocks of cells in passages 50 to 100 were frozen and stored in liquid nitrogen. These stocks were verified to be free from Mycoplasma contamination. The cells used in the present experiments were taken from our frozen stock and were maintained in monolayer culture in RPMI 1640 (25 mM HEPES and l-glutamine) supplemented with 13% bovine calf serum, 250 mg/L penicillin, and 50 mg/L streptomycin.
Mice
Adult (8–10 weeks) female BALB/c nu/nu mice, bred and maintained as described elsewhere [21], were used as host animals for xenografted tumors. The animal experiments were approved by the institutional committee on research animal care and were done according to the USPHS Policy on Humane Care and Use of Laboratory Animals.
Tumors
Xenografted tumors for assessment of volumetric growth rate, fraction of hypoxic cells, microvascular density, and metastatic propensity were initiated by injecting aliquots of 5.0 x 104 to 2.5 x 106 cells intradermally (ID) into the left mouse flank. Tumor volume (V) and tumor volume doubling time (Td) were calculated as V = π/6 x a x b2 and Td = ln2 x t/(lnVt - lnV0), where a is the longer and b is the shorter of two perpendicular diameters, and Vt and V0 represent the tumor volume at time t and time zero, respectively.
Invasiveness
Cell invasiveness was determined by using 24-well Matrigel invasion chambers with 8-µm pore polycarbonate membranes precoated with a thin layer of Matrigel Basement Membrane Matrix (BD Biosciences, Cowley, UK). The chambers were rehydrated in serum-free medium as described by the manufacturer. Complete medium (750 µl) was used as chemoattractant. Suspensions of 2.5 x 104 to 7.5 x 104 cells in 500 µl of complete medium were added to the wells and incubated for 24 hours at 37°C in 5% CO2 in air. Cells remaining on the upper membrane surface were removed, whereas the cells on the lower surface were fixed in methanol, stained with hematoxylin, and counted by examining 10 randomly selected fields at x40 magnification. Further details have been reported elsewhere [22].
Lung Colonization Potential
Aliquots of 1.0 x 104 to 2.0 x 106 cells in 0.2 ml of HBSS were inoculated into the lateral tail vein [22]. The mice were killed and autopsied 2 to 8 weeks after the cell inoculation. The lungs were removed and fixed in the Bouin solution for 24 hours, and the number of surface colonies was determined by stereomicroscopy.
Tumorigenicity
Serial dilutions of cell suspensions were inoculated ID or intracranially (IC) for tumor formation [23]. The inoculation point in the intradermal assay lay above the subcutaneous muscle tissue in the deeper part of the dermis. The mice were examined twice weekly for up to 120 days after the inoculations, and an inoculation was scored as positive if a tumor with a longest diameter of at least 8 mm was observed. The inoculation point in the intracranial assay lay ∼2 mm anterior to the coronal and ∼2 mm lateral to the sagittal suture lines. The mice were examined daily for up to 90 days after the inoculations. Positive transplantation was detected by the development of progressive neurologic signs, which were evident 1 to 2 days before death. Moribund mice were killed, and the presence of tumor was confirmed by autopsy and histologic examination. In both assays, the percentage of positive inoculations was plotted versus cell number, and the cell number resulting in 50% positive inoculations (ID TD50 or IC TD50) was determined by probability regression analysis.
Angiogenesis
Angiogenic potential was measured by using an intradermal angiogenesis assay [20]. Aliquots of 1.0 x 106 cells in 10 µl of HBSS were inoculated, and the recipient mice were killed 7 days later when small tumors had developed in the inoculation sites. The tumors with surrounding skin were removed, and the vessels in the dermis oriented toward the tumors were counted by using a stereomicroscope.
Fraction of Radiobiologically Hypoxic Cells
Hypoxic fractions were determined by using the paired survival curve method [24]. Tumors were irradiated at a dose rate of 5.1 Gy/min by using an x-ray unit operated at 220 kV, 19 to 20 mA, and with 0.5-mm Cu filtration. Hypoxic tumors were obtained by occluding the blood supply with a clamp 5 minutes before irradiation. Tumor cell survival was measured in vitro [25]. Briefly, the tumors were resected immediately after irradiation, minced in cold HBSS, and treated with an enzyme solution (0.2% collagenase, 0.05% Pronase, and 0.02% DNase) at 37°C for 2 hours. Trypan blue-negative cells were plated in 25-cm2 tissue culture flasks and incubated at 37°C for 14 days for colony formation. The cell-surviving fraction of an irradiated tumor was calculated from the plating efficiency of the cells of the tumor and the mean plating efficiency of the cells of six untreated control tumors. Cell survival curves were established for clamped and unclamped tumors, and the fraction of radiobiologically hypoxic cells was calculated from the vertical displacement of the curves [24].
Immunohistochemical Assessment of Tumor Hypoxia and Microvascular Density
Pimonidazole [1-[(2-hydroxy-3-piperidinyl)-propyl]-2-nitroimidazole], administered as described previously [24], was used as a marker of tumor hypoxia, and CD31 was used as a marker of tumor endothelial cells. Tumors were fixed in phosphate-buffered 4% paraformaldehyde for detection of hypoxia and in liquid nitrogen for detection of endothelial cells. Immunohistochemistry was carried out by using an avidin-biotin peroxidase-based staining method [17]. An anti-pimonidazole rabbit polyclonal antibody (gift from Professor J. Raleigh, Department of Radiation Oncology, University of North Carolina School of Medicine, Chapel Hill, NC) or an antimouse CD31 rat monoclonal antibody (Research Diagnostics, Flanders, NJ) was used as primary antibody. Diaminobenzidine was used as chromogen, and hematoxylin was used for counterstaining. Controls included omission of the primary antibody and incubation with blocking peptide before staining. Quantitative studies of hypoxia were based on four cross sections of each tumor. The fraction of hypoxic cells, defined as the area fraction of the nonnecrotic tissue showing positive pimonidazole staining, was assessed by image analysis [24]. The microvascular density was scored in the invasive front by counting the vessels located within a 1-mm-thick band in the tumor periphery. Three cross sections were analyzed for each tumor. Microvessels were defined and scoredmanually as described earlier by Weidner [14].
Metastasis
Spontaneous metastasis was studied as described elsewhere [25]. Briefly, the primary tumors were resected when they had grown to a volume of ∼400 mm3. The host mice were then examined daily for clinical signs of metastases, and when they became moribund, they were killed and autopsied. All organs were removed and inspected for macroscopic tumor growth by stereomicroscopy, and the body was examined for external and internal lymph node metastases. All metastasis-positive mice became moribund within 3 months after the primary tumor was resected. The remaining mice were autopsied 6 months after the primary tumor resection, and they were all metastasis-negative. The presence of metastases was confirmed by histologic examination.
Treatment with Neutralizing Antibody
The specific roles of VEGF-A and IL-8 in tumor angiogenesis and metastasis were investigated by treating tumor-bearing mice with neutralizing antibody against these angiogenic factors, using an anti-human VEGF-A mouse monoclonal antibody (R&D Systems, Abingdon, UK) and an anti-human IL-8 mouse monoclonal antibody (R&D Systems). The treatments were given in daily doses of 25 µg (VEGF-A) or 100 µg (IL-8) for predetermined time intervals. The antibody solutions were diluted in PBS and administered to the mice in volumes of 0.25 ml by intraperitoneal injection. Control mice were treated with an irrelevant antihuman monoclonal antibody of the same isotype as the neutralizing antibody.
Statistical Analysis
Statistical analyses were carried out by one-way analysis of variance followed by the Student-Newman-Keuls test (comparison of tumor groups) or by the paired Student's t test (effect of treatment) when the data complied with the conditions of normality and equal variance. Under other conditions, comparisons were done by nonparametric analysis using the Kruskal-Wallis one-way analysis of variance on ranks or the Wilcoxon signed rank test. Probability values of P < .05 were considered significant. The statistical analysis was carried out by using the SigmaStat statistical software (Jandel Scientific, Erkrath, Germany).
Results
Clinical Aggressiveness
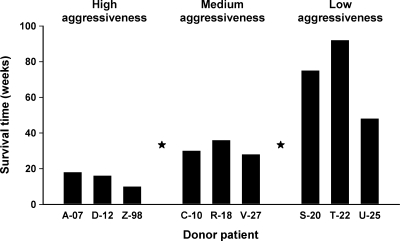
The donor patients showed different metastatic patterns independent of the location and thickness of the primary tumor (Table 1). Two of the patients (R-18 and Z-98) presented with metastases in distant lymph nodes. The other patients developed distant metastases subsequent to the initial diagnosis, first in lymph nodes and then in lung, liver, and/or brain. The rate of disease progression differed significantly among the patients. Thus, the time from the initial diagnosis until the patients died of distant metastases ranged from 10 to 92 weeks. Based on this time, the tumors were divided into three groups of three tumors each: A-07, D-12, and Z-98 (high aggressiveness); C-10, R-18, and V-27 (medium aggressiveness); and S-20, T-22, and U-25 (low aggressiveness). The survival time was significantly shorter for the patients in the high aggressiveness group than for those in the medium aggressiveness group (P < .05) and significantly shorter for the patients in the medium aggressiveness group than for those in the low aggressiveness group (P < .05; Figure 1).
Figure 1.
Donor patients' survival time determined as the time from the initial diagnosis until the patients died of metastatic disease. On the basis of this time, the tumors were divided into a high aggressiveness group, a medium aggressiveness group, and a low aggressiveness group. The stars between the groups indicate the significance level of the difference between the groups: one star indicates P < .05.
Aggressiveness of the Melanoma Lines Reflected the Clinical Aggressiveness
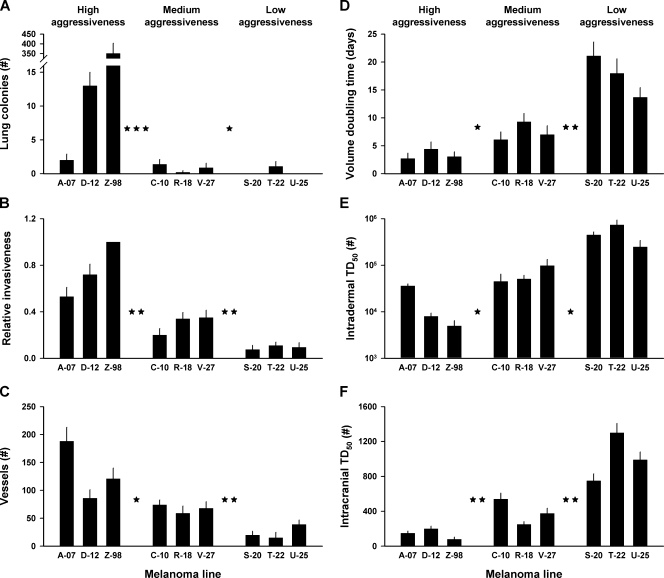
To investigate whether the cellular aggressiveness of the melanoma lines reflected the clinical aggressiveness of the donor patients' tumors, the following biologic parameters were measured: lung colonization potential, cell invasiveness, angiogenic potential, tumor growth rate, intradermal tumorigenicity, and intracranial tumorigenicity. The lung colonization potential, measured as the number of lung colonies per 1.0 x 105 cells, was significantly higher for the high aggressiveness group than for the medium aggressiveness group (P < .001) and significantly higher for the medium aggressiveness group than for the low aggressiveness group (P < .05; Figure 2A). Cell invasiveness was measured in vitro by using Z-98 cells as a reference in all experiments. The percentage of cells penetrating a polycarbonate membrane relative to that of Z-98 cells was used as a parameter for cell invasiveness. This parameter was significantly higher for the high aggressiveness group than for the medium aggressiveness group (P < .01) and significantly higher for the medium aggressiveness group than for the low aggressiveness group (P < .01; Figure 2B). The angiogenic potential was determined by inoculating 1.0 x 106 cells ID and counting the tumor-oriented vessels 7 days later. The number of vessels was significantly higher for the high aggressiveness group than for the medium aggressiveness group (P < .05) and significantly higher for the medium aggressiveness group than for the low aggressiveness group (P < .01; Figure 2C). Tumor growth rate was measured as the volume doubling time from a volume of 200 to a volume of 400 mm3. The volume doubling time was significantly shorter for the high aggressiveness group than for the medium aggressiveness group (P < .05) and significantly shorter for the medium aggressiveness group than for the low aggressiveness group (P < .01; Figure 2D). The intradermal and intracranial tumorigenicities were measured by using the ID TD50 and IC TD50 values as parameters. These parameters were significantly lower for the high aggressiveness group than for the medium aggressiveness group (P < .05 [ID TD50], Figure 2E; P < .01 [IC TD50], Figure 2F) and significantly lower for the medium aggressiveness group than for the low aggressiveness group (P < .05 [ID TD50], Figure 2E; P < .01 [IC TD50], Figure 2F).
Figure 2.
Lung colonization potential (A), relative cell invasiveness (B), angiogenic potential (C), tumor volume doubling time (D), ID TD50 (E), and IC TD50 (F) for high aggressiveness, medium aggressiveness, and low aggressiveness melanomas. Columns indicate means of five experiments involving 10 mice each (A), six experiments (B), 20 inoculations (C), 20 tumors (D), or five to six experiments (E and F). Bars, SEM. The stars between the groups indicate the significance level of the difference between the groups: one star indicates P < .05; two stars, P < .01; three stars, P < .001.
High Fraction of Radiobiologically Hypoxic Cells Was Not Associated with High Cellular Aggressiveness
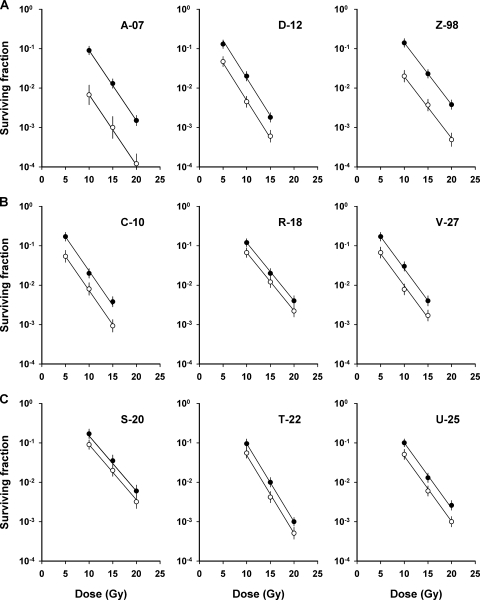
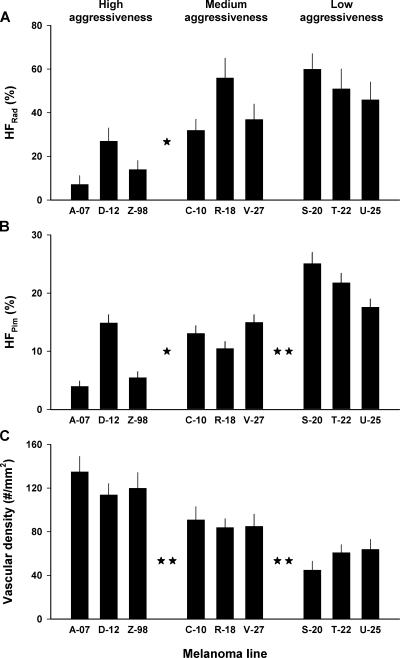
To investigate whether the extent of hypoxia in xenografted tumors of the melanoma lines was associated with the cellular aggressiveness, the fraction of radiobiologically hypoxic cells was measured in tumors with volumes of ∼400 mm3 by using the paired survival curve method. The results are presented in Figure 3, which shows the paired survival curves for the high aggressiveness melanomas (Figure 3A), the medium aggressiveness melanomas (Figure 3B), and the low aggressiveness melanomas (Figure 3C). The cellular radiation sensitivity, given by the slopes of the curves (a steep curve corresponds to a high radiation sensitivity), and the fraction of hypoxic cells, given by the vertical distance between the curves (a short distance corresponds to a high hypoxic fraction), differed substantially among the melanoma lines. The three melanoma groups did not differ significantly in cellular radiation sensitivity (P > .05; data not shown). The fraction of radiobiologically hypoxic cells was significantly lower for the high aggressiveness group than for the medium (P < .05) and the low (P < .05) aggressiveness groups, but it did not differ significantly between the medium and the low aggressiveness groups (P > .05; Figure 4A).
Figure 3.
Cell survival curves for xenografted tumors of high aggressiveness melanomas (A), medium aggressiveness melanomas (B), and low aggressiveness melanomas (C) irradiated under air breathing (○) or hypoxic (●) conditions. Points indicate geometric means of five to seven tumors. Bars, SEM. Solid curves indicate curves fitted to the data by regression analysis.
Figure 4.
Fraction of radiobiologically hypoxic cells, HFRad (A), fraction of pimonidazole-positive hypoxic cells, HFPim (B), and microvascular density (C) in xenografted tumors of high aggressiveness, medium aggressiveness, and low aggressiveness melanomas. The HFRad values were calculated from the survival curves in Figure 3. The HFPim values refer to 10 tumors of each line. The microvascular densities refer to the periphery of eight tumors of each line. Columns indicate means. Bars, SEM. The stars between the groups indicate the significance level of the difference between the groups: no star indicates P > .05; one star, P < .05; two stars, P < .01.
High Fraction of Pimonidazole-Positive Hypoxic Cells Was Not Associated with High Cellular Aggressiveness
To investigate further whether the extent of hypoxia in xenografted tumors of the melanoma lines was associated with the cellular aggressiveness, the fraction of pimonidazole-positive cells was assessed in ∼400-mm3 tumors by using an immunohistochemical assay. The tumors of all lines showed highly heterogeneous staining for pimonidazole. Necrotic regions were encompassed by a rim of pimonidazole-positive cells, two to four cell layers thick. The periphery of tumor chords also stained positive for pimonidazole. Moreover, foci of pimonidazole-positive cells scattered throughout the tissue were observed in tumor regions without necrosis. The fraction of pimonidazole-positive hypoxic cells was significantly lower for the high aggressiveness group than for the medium aggressiveness group (P < .05) and was significantly lower for the medium aggressiveness group than for the low aggressiveness group (P < .01; Figure 4B).
High Microvascular Density Was Associated with High Cellular Aggressiveness
To investigate whether the microvascular density in xenografted tumors of the melanoma lines was associated with the cellular aggressiveness, the microvascular density was measured in the periphery of tumors with volumes of ∼400 mm3. It differed substantially among the melanoma lines and was significantly higher for the high aggressiveness group than for the medium aggressiveness group (P < .01) and significantly higher for the medium aggressiveness group than for the low aggressiveness group (P < .01; Figure 4C).
High Metastatic Propensity Was Associated with High Microvascular Density and Not with High Hypoxic Fraction
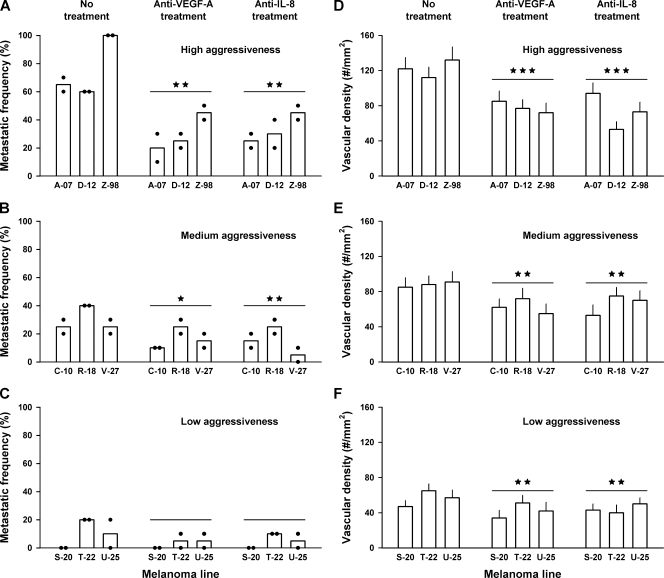
To investigate whether the metastatic propensity of xenografted tumors of the melanoma lines was associated with the microvascular density or the extent of hypoxia, the development of spontaneous metastases was studied. The fraction of metastasis-positive mice was significantly higher for the high aggressiveness group than for the medium aggressiveness group (P < .01; Figure 5, A and B, no treatment) and significantly higher for the medium aggressiveness group than for the low aggressiveness group (P < .05; Figure 5, B and C, no treatment). The propensity of the xenografted tumors to form spontaneous metastases thus reflected the clinical aggressiveness of the donor patients' tumors (Figure 1), and it was associated with a high microvascular density in the tumor periphery (Figure 4C) but not with a high hypoxic fraction (Figure 4, A and B).
Figure 5.
Metastatic frequency (A–C) and tumor microvascular density (D–F) in untreated mice, mice given anti-VEGF-A treatment, and mice given anti-IL-8 treatment for high aggressiveness (A, D), medium aggressiveness (B, E), and low aggressiveness (C, F) melanomas. Columns and points in panels A to C indicate means and individual values of two experiments involving 10 mice each. Columns and bars in panels D to F indicate means and SEM of 20 tumors. The stars above the treatment groups indicate the significance level of the difference between the treatment group and the untreated control group: no star indicates P > .05; one star, P < .05; two stars, P < .01; three stars, P < .001.
Metastatic Propensity Was Reduced by Anti-VEGF-A and Anti-IL-8 Treatments
To investigate whether VEGF-A and/or IL-8 played a significant role in the metastatic process, the development of spontaneous metastases was studied in mice treated with neutralizing antibody against these angiogenic factors. The antibody treatments were given in daily fractions from the primary tumors reached a volume of 50 mm3 (high aggressiveness group), 100 mm3 (medium aggressiveness group), or 200 mm3 (low aggressiveness group) until they reached a volume of 400 mm3 and were resected. Compared with untreated control mice, mice given anti-VEGF-A treatment and mice given anti-IL-8 treatment showed decreased incidence of metastatic disease. The decreases were significant for the high aggressiveness group (P = .0011, VEGF-A; P = .0013, IL-8; Figure 5A) and the medium aggressiveness group (P = .031, VEGF-A; P = .0011, IL-8; Figure 5B) and on the borderline of being significant for the low aggressiveness group (P = .10, VEGF-A; P = .08, IL-8; Figure 5C).
Microvascular Density Was Reduced by Anti-VEGF-A and Anti-IL-8 Treatments
To investigate whether the decreased metastasis after the anti-VEGF-A and anti-IL-8 treatments was associated with decreased microvascular density, the microvascular density in the periphery of the primary tumors was assessed. Compared with the tumors in untreated control mice, the tumors in mice given anti-VEGF-A treatment and the tumors in mice given anti-IL-8 treatment showed decreased microvascular density. The decreases were significant for both treatments and all three tumor groups (P < .01; Figure 5, D–F).
Discussion
Tumors develop aggressive cell phenotypes during growth as a consequence of stable and unstable genomic alterations in regulatory genes [1–3]. Furthermore, they develop abnormal microvascular networks and regions with hypoxic tissue [4–7]. The present study showed that highly aggressive cell variants may give rise to tumors with high microvascular density but may not necessarily give rise to tumors with large fractions of hypoxic cells. Consequently, high microvascular density in tumors may reflect the presence of particularly aggressive cell phenotypes, whereas large hypoxic fractions are not a secondary effect of high cellular aggressiveness.
Clinically relevant preclinical studies of associations between the metastatic propensity of primary tumors and their physiological properties require the use of multiple tumor lines because each tumor line represents a single patient, whereas individual tumors of the same line represent copies of the same tumor. By including many copies of the same tumor line in preclinical studies, the uncertainty in the measurements can be reduced significantly compared with that in the clinic. Cell lines established from subcutaneous metastases of nine melanoma patients were used as model systems in the present work. On the basis of the aggressiveness of the donor patients' diseases, the cell lines were divided into three groups of high, medium, or low aggressiveness. By measuring the lung colonization potential, invasive potential, angiogenic potential, ID TD50, and IC TD50 of the cell lines and the growth rate and metastatic propensity of xenografted tumors initiated from the cell lines, it was shown that the aggressiveness of the melanoma lines mirrored the aggressiveness of the donor patients' tumors. Consequently, the melanoma lines should be adequate models for answering the questions addressed in the work reported here.
Microvascular density was scored by counting the vessels located within a 1-mm-thick band in the periphery of the melanoma xenografts. In most clinical studies showing associations between microvascular density and incidence of metastases, the microvascular density was defined as the vessel density in vascular hot spots in the invasive front of the primary tumor [14,15]. Thus, the parameter used to describe microvascular density in the present work deviates from that used in the clinical studies. The clinical parameter for microvascular density was not applicable to the melanoma xenografts because the tumors of most of the nine lines did not show distinct vascular hot spots.
The melanoma xenograft studies showed that the incidence of spontaneous metastases was strongly associated with the angiogenic potential of the tumor cells and the microvascular density of the primary tumor. Furthermore, treatment with neutralizing antibody against VEGF-A or IL-8 resulted in reduced primary tumor microvascular density and decreased incidence of metastatic disease. These observations suggest that the metastatic propensity of the melanoma xenografts was determined primarily by the angiogenic potential of the melanoma cells and, moreover, that melanoma cells with a high angiogenic potential gave rise to tumors with high microvascular density. The latter statement is not an obvious statement because the volumetric growth rate of the melanoma xenografts also increased with increasing angiogenic potential. The present study is thus consistent with the clinical studies having shown associations between tumor microvascular density, incidence of metastases, and prognosis, studies that involve several histologic types of cancer [14,15] including malignant melanoma [26–28].
Tumor hypoxia was assessed by measuring the fraction of radiobiologically hypoxic cells using the paired survival curve method and by measuring the fraction of pimonidazole-positive cells using an immunohistochemical assay. The radiobiologic assay detects acutely as well as chronically hypoxic cells and measures the fraction of the clonogenic cells in tumors that are hypoxic during the radiation exposure. However, because 50% radiosensitization occurs at an oxygen tension of ∼3 mm Hg, this assay detects only the most hypoxic tumor cells [29]. Nevertheless, the fraction of radiobiologically hypoxic cells is considered to be of greater clinical significance than hypoxic fractions derived from nonradiobiologic assays because only clonogenic cells are of relevance for tumor growth, metastasis, and response to treatment [30]. The immunohistochemical assay detects primarily chronically hypoxic cells and does not distinguish between nonclonogenic and clonogenic cells and may thus give an erroneous estimate of the extent of hypoxia in tumors [30]. However, because pimonidazole staining occurs at an oxygen tension of 7.5 to 10 mm Hg, this assay detects a broader level of tumor hypoxia [24]. Furthermore, in many of the clinical studies having shown associations between tumor hypoxia and incidence of metastases or metastasis-free survival, the extent of hypoxia was measured immunohistochemically by using pimonidazole as an exogenous hypoxia marker or hypoxia-inducible factor 1 or carbonic anhydrase IX as endogenous hypoxia markers [31].
Our study did not provide any evidence that the metastatic propensity of the melanoma xenografts increased with increasing fraction of hypoxic cells. In fact, the fraction of radiobiologically hypoxic cells did not differ between the medium and the low aggressiveness groups and was significantly higher for these two groups than for the high aggressiveness group. Moreover, the fraction of pimonidazole-positive hypoxic cells was significantly higher for the low aggressiveness group than for the medium aggressiveness group and was significantly higher for the medium aggressiveness group than for the high aggressiveness group. This strong tendency toward an inverse relationship between metastatic propensity and tumor hypoxia may be a consequence of the elevated angiogenic potential of the melanoma lines in the high and medium aggressiveness groups. These observations thus suggest that the fraction of hypoxic cells was not a primary determinant of the metastatic propensity of the melanoma xenografts.
However, this suggestion does not imply that the extent of tumor hypoxia is an unimportant factor in the metastasis of melanoma xenografts. In previous studies of the D-12 and R-18 lines, we have shown that tumors with large fractions of hypoxic cells have a higher metastatic potential than tumors with low hypoxic fractions [17,32]. In the D-12 line, this effect was primarily due to hypoxia-induced upregulation of IL-8 [17], whereas the hypoxia-induced metastasis in the R-18 line was mainly a consequence of upregulated expression of urokinase-type plasminogen activator receptor [32]. Moreover, we have observed that normoxic A-07 tumors exposed to cyclic hypoxic stress in vivo show enhanced angiogenesis and metastasis, primarily as a result of hypoxia-induced up-regulation of VEGF-A [33]. These observations suggest that tumor hypoxia may promote metastasis in melanoma xenografts by upregulating the expression of gene products stimulating angiogenesis. Consequently, the angiogenic activity may be a major determinant of the metastatic propensity of melanoma xenografts, both in normoxic and hypoxic tissue regions.
The malignant progression of melanoma is characterized by distinct sequential steps from commonly acquired melanocytic nevus through dysplastic nevus, radial growth phase primary melanoma, and vertical growth phase primary melanoma to melanoma metastasis [34]. There is substantial evidence that melanoma progression requires induction of new blood vessels. Thus, the transition from the radial to the vertical growth phase, which represents a significant worsening of the prognosis, has been shown to be dependent on neovascularization [35,36]. The preclinical study reported here suggests that VEGF-A and IL-8 are important proangiogenic factors in malignant melanoma and, consequently, VEGF-A, IL-8, and their receptors may be important targets for the treatment of this disease.
Clinical investigations have shown that invasive growth, development of metastatic disease, and poor disease-free and overall survival rates are associated with extensive hypoxia in the primary tumor in several cancer types, including cervical carcinoma, head and neck carcinoma, and soft tissue sarcoma [37–39]. In contrast, no association was found betweenmetastatic propensity and hypoxic fraction in the present study, most likely because the metastatic propensity of melanoma xenografts is determined by the tumor microvascular density, and the differences in microvascular density among lines are governed by the constitutive angiogenic potential of the tumor cells rather than by hypoxia-induced angiogenesis. There are several possible explanations of this disparity. First, the metastatic propensity of cervical carcinoma, head and neck carcinoma, and soft tissue sarcoma may be governed by tumor properties other than the angiogenic potential and the microvascular density. Thus, preclinical studies have suggested that tumor hypoxia also may promote metastasis by increasing the resistance to apoptosis by upregulating Mdm2 (murine double minute 2, an inhibitor of p53 transcriptional activation) [40] and by increasing cell invasiveness and motility by upregulating lysyl oxidase [41]. Furthermore, patients with cervical carcinoma, head and neck carcinoma, and soft tissue sarcoma are generally treated with radiation therapy, and the poor outcome of the patients with the most hypoxic primary tumors may be a result of hypoxia-induced radiation resistance rather than hypoxia-induced metastasis, a suggestion that is consistent with a study of advanced squamous cell carcinoma of the uterine cervix in our hospital [42].
In summary, the present preclinical study involving nine human melanoma xenograft lines showed that the metastatic propensity of the lines was associated with the microvascular density in the tumor periphery and not with the fraction of hypoxic tumor cells, most likely because the differences in microvascular density among the lines were governed by the constitutive angiogenic potential rather than by hypoxia-induced angiogenesis. Moreover, VEGF-A and IL-8 were identified as important angiogenic factors in malignant melanoma as the vascularization and metastasis of the xenografts were inhibited significantly by anti-VEGF-A and anti-IL-8 treatments.
Acknowledgments
The authors thank Kristin Henriksen, Kristil Kindem, Liv Nordli, and Mai Nguyen for excellent technical assistance.
Abbreviations
- IL-8
interleukin 8
- TD50
tumor dose 50 (cell number resulting in tumor growth in 50% of the inoculation sites)
- VEGF-A
vascular endothelial growth factor A
Footnotes
This work was supported by research funding from the Norwegian Cancer Society. The authors declare no conflicts of interest.
References
- 1.Nowell PC. Tumor progression: a brief historical perspective. Semin Cancer Biol. 2002;12:261–266. doi: 10.1016/s1044-579x(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 3.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 4.Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res. 1998;58:1408–1416. [PubMed] [Google Scholar]
- 5.Rofstad EK, Gaustad JV, Brurberg KG, Mathiesen B, Galappathi K, Simonsen TG. Radiocurability is associated with interstitial fluid pressure in human tumor xenografts. Neoplasia. 2009;11:1243–1251. doi: 10.1593/neo.91152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 7.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 8.Rofstad EK. Microenvironment-induced cancer metastasis. Int J Radiat Biol. 2000;76:589–605. doi: 10.1080/095530000138259. [DOI] [PubMed] [Google Scholar]
- 9.Subarsky P, Hill RP. The hypoxic tumour microenvironment and metastatic progression. Clin Exp Metastasis. 2003;20:237–250. doi: 10.1023/a:1022939318102. [DOI] [PubMed] [Google Scholar]
- 10.Huang CH, Yang WH, Chang SY, Tai SK, Tzeng CH, Kao JY, Wu KJ, Yang MH. Regulation of membrane-type 4 matrix metalloproteinase by SLUG contributes to hypoxia-mediated metastasis. Neoplasia. 2009;11:1371–1382. doi: 10.1593/neo.91326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bindra RS, Glazer PM. Genetic instability and the tumor microenvironment: towards the concept of microenvironment-induced mutagenesis. Mutat Res. 2005;569:75–85. doi: 10.1016/j.mrfmmm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 13.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–634. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weidner N. Intratumor microvessel density as a prognostic factor in cancer. Am J Pathol. 1995;147:9–19. [PMC free article] [PubMed] [Google Scholar]
- 15.Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, van Mark E, Magnani E, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564–1579. doi: 10.1016/s0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 16.Geiger TR, Peeper DS. Metastasis mechanisms. Biochim Biophys Acta. 2009;1796:293–308. doi: 10.1016/j.bbcan.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Rofstad EK, Halsør EF. Hypoxia-associated spontaneous pulmonary metastasis in human melanoma xenografts: involvement of microvascular hot spots induced in hypoxic foci by interleukin 8. Br J Cancer. 2002;86:301–308. doi: 10.1038/sj.bjc.6600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merighi S, Simioni C, Gessi S, Varani K, Mirandola P, Tabrizi MA, Baraldi PG, Borea PA. A2B and A3 adenosine receptors modulate vascular endothelial growth factor and interleukin-8 expression in human melanoma cells treated with etoposide and doxorubicin. Neoplasia. 2009;11:1064–1073. doi: 10.1593/neo.09768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaustad JV, Simonsen TG, Brurberg KG, Huuse EM, Rofstad EK. Blood supply in melanoma xenografts is governed by the morphology of the supplying arteries. Neoplasia. 2009;11:277–285. doi: 10.1593/neo.81400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rofstad EK. Orthotopic human melanoma xenograft model systems for studies of tumour angiogenesis, pathophysiology, treatment sensitivity and metastatic pattern. Br J Cancer. 1994;70:804–812. doi: 10.1038/bjc.1994.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rofstad EK, Ruud EBM, Mathiesen B, Galappathi K. Associations between radiocurability and interstitial fluid pressure in human tumor xenografts without hypoxic tissue. Clin Cancer Res. 2010;16:936–945. doi: 10.1158/1078-0432.CCR-09-2718. [DOI] [PubMed] [Google Scholar]
- 22.Rofstad EK, Mathiesen B, Kindem K, Galappathi K. Acidic extracellular pH promotes experimental metastasis of human melanoma cells in athymic nude mice. Cancer Res. 2006;66:6699–6707. doi: 10.1158/0008-5472.CAN-06-0983. [DOI] [PubMed] [Google Scholar]
- 23.Rofstad EK. Metastatic behavior of human tumors in congenitally athymic nude mice: intrinsic properties of the tumor cells and host immune reactivity. Int J Cancer. 1995;63:744–749. doi: 10.1002/ijc.2910630523. [DOI] [PubMed] [Google Scholar]
- 24.Rofstad EK, Maseide K. Radiobiological and immunohistochemical assessment of hypoxia in human melanoma xenografts: acute and chronic hypoxia in individual tumours. Int J Radiat Biol. 1999;75:1377–1393. doi: 10.1080/095530099139250. [DOI] [PubMed] [Google Scholar]
- 25.Rofstad EK, Galappathi K, Mathiesen B, Ruud EBM. Fluctuating and diffusion-limited hypoxia in hypoxia-induced metastasis. Clin Cancer Res. 2007;13:1971–1978. doi: 10.1158/1078-0432.CCR-06-1967. [DOI] [PubMed] [Google Scholar]
- 26.Srivastava A, Laidler P, Davies RP, Horgan K, Hughes LE. The prognostic significance of tumor vascularity in intermediate-thickness (0.76–4.0 mm thick) skin melanoma: a quantitative histologic study. Am J Pathol. 1988;133:419–423. [PMC free article] [PubMed] [Google Scholar]
- 27.Denijn M, Ruiter DJ. The possible role of angiogenesis in the metastatic potential of human melanoma. Clinicopathological aspects. Melanoma Res. 1993;3:5–14. doi: 10.1097/00008390-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Graham CH, Rivers J, Kerbel RS, Stankiewicz KS, White WL. Extent of vascularization as a prognostic indicator in thin (<0.76 mm) malignant melanomas. Am J Pathol. 1994;145:510–514. [PMC free article] [PubMed] [Google Scholar]
- 29.Moulder JE, Rockwell S. Hypoxic fractions of solid tumors: experimental techniques, methods of analysis, and a survey of existing data. Int J Radiat Oncol Biol Phys. 1984;10:695–712. doi: 10.1016/0360-3016(84)90301-8. [DOI] [PubMed] [Google Scholar]
- 30.Fenton BM, Kiani MF, Siemann DW. Should direct measurements of tumor oxygenation relate to the radiobiological hypoxic fraction of a tumor? Int J Radiat Oncol Biol Phys. 1995;33:365–373. doi: 10.1016/0360-3016(95)00064-6. [DOI] [PubMed] [Google Scholar]
- 31.Bussink J, Kaanders JH, van der Kogel AJ. Tumor hypoxia at the micro-regional level: clinical relevance and predictive value of exogenous and endogenous hypoxic cell markers. Radiother Oncol. 2003;67:3–15. doi: 10.1016/s0167-8140(03)00011-2. [DOI] [PubMed] [Google Scholar]
- 32.Rofstad EK, Rasmussen H, Galappathi K, Mathiesen B, Nilsen K, Graff BA. Hypoxia promotes lymph node metastasis in human melanoma xenografts by up-regulating the urokinase-type plasminogen activator receptor. Cancer Res. 2002;62:1847–1853. [PubMed] [Google Scholar]
- 33.Rofstad EK, Gaustad JV, Egeland TAM, Mathiesen B, Galappathi K. Tumors exposed to acute cyclic hypoxic stress show enhanced angiogenesis, perfusion and metastatic dissemination. Int J Cancer. 2010;127:1535–1546. doi: 10.1002/ijc.25176. [DOI] [PubMed] [Google Scholar]
- 34.Herlyn M. Human melanoma: development and progression. Cancer Metastasis Rev. 1990;9:101–112. doi: 10.1007/BF00046337. [DOI] [PubMed] [Google Scholar]
- 35.Marcoval J, Moreno A, Graells J, Vidal A, Escribà JM, Garcia-Ramírez M, Fabra A. Angiogenesis and malignant melanoma. Angiogenesis is related to the development of vertical (tumorigenic) growth phase. J Cutan Pathol. 1997;24:212–218. doi: 10.1111/j.1600-0560.1997.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 36.Erhard H, Rietveld FJ, van Altena MC, Brocker EB, Ruiter DJ, de Waal RM. Transition of horizontal to vertical growth phase melanoma is accompanied by induction of vascular endothelial growth factor expression and angiogenesis. Melanoma Res. 1997;7(suppl 2):s19–s26. [PubMed] [Google Scholar]
- 37.Höckel M, Schlenger K, Aral B, Mitze M, Schäffer U, Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 38.Brizel DM, Dodge RK, Clough RW, Dewhirst MW. Oxygenation of head and neck cancer: changes during radiotherapy and impact on treatment outcome. Radiother Oncol. 1999;53:113–117. doi: 10.1016/s0167-8140(99)00102-4. [DOI] [PubMed] [Google Scholar]
- 39.Nordsmark M, Alsner J, Keller J, Nielsen OS, Jensen OM, Horsman MR, Overgaard J. Hypoxia in human soft tissue sarcomas: adverse impact on survival and no association with p53 mutations. Br J Cancer. 2001;84:1070–1075. doi: 10.1054/bjoc.2001.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang L, Hill RP. Hypoxia enhances metastatic efficiency by upregulating Mdm2 in KHT cells and increasing resistance to apoptosis. Cancer Res. 2004;64:4180–4189. doi: 10.1158/0008-5472.CAN-03-3038. [DOI] [PubMed] [Google Scholar]
- 41.Erler JT, Bennewith KL, Nicolau M, Dornhöfer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 42.Rofstad EK, Sundfør K, Lyng H, Tropé CG. Hypoxia-induced treatment failure in advanced squamous cell carcinoma of the uterine cervix is primarily due to hypoxia-induced radiation resistance rather than hypoxia-induced metastasis. Br J Cancer. 2000;83:354–359. doi: 10.1054/bjoc.2000.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]