Abstract
We recently demonstrated highly efficient antitumor immunity against dermal tumors of B16F10 murine melanoma with the use of dendritic cells (DCs) activated by replication-competent, as well as nontransmissible-type, recombinant Sendai viruses (rSeV), and proposed a new concept, “immunostimulatory virotherapy,” for cancer immunotherapy. However, there has been little information on the efficacies of thismethod: 1) inmore clinically relevant situations including metastatic diseases, 2) on other tumor types and other animal species, and 3) on the related molecular/cellular mechanisms. In this study, therefore, we investigated the efficacy of vaccinating DCs activated by fusion gene-deleted nontransmissible rSeV on a rat model of lung metastasis using a highly malignant subline of Dunning R-3327 prostate cancer, AT6.3. rSeV/dF-green fluorescent protein (GFP)-activated bone marrow-derived DCs (rSeV/dF-GFP-DC), consistent with results previously observed in murine DCs. Vaccination of rSeV/dF-GFP-DC was highly effective at preventing lung metastasis after intravenous loading of R-3327 tumor cells, compared with the effects observed with immature DCs or lipopolysaccharide-activated DCs. Interestingly, neither CTL activity nor DC trafficking showed any apparent difference among groups. Notably, rSeV/dF-DCs expressing a dominant-negative mutant of retinoic acid-inducible gene I (RIG-I) (rSeV/dF-RIGIC-DC), an RNA helicase that recognizes the rSeV genome for inducing type I interferons, largely lost the expression of proinflammatory cytokines without any impairment of antitumor activity. These results indicate the essential role of RIG-I-independent signaling on antimetastatic effect induced by rSeV-activated DCs and may provide important insights to DC-based immunotherapy for advanced malignancies.
Introduction
Prostate cancer is one of the most common malignancies among Western populations, and its incidence in Japan is increasing. Since the first report by Huggins and Hodges in 1941 [1], androgen ablation has been the initial treatment of choice for men with metastatic prostate cancer. However, these tumors eventually become androgen-independent and progressed despite androgen ablation [2]. After all, only 10% to 20% of patients with stage D2 prostate cancer are alive 5 years after diagnosis, by which time the options for treatment are limited and most are palliative [2]. Conventional chemotherapy for prostate cancer that is no longer responsive to androgen deprivation was initially found to produce only marginal responses [3] but is now being reevaluated with interest as new agents and better supportive care become available [4,5]. Nevertheless, palliation remains the primary goal of current chemotherapy, and overall response rates are low and associated with general toxicity [4]. Therefore, there has been a serious demand for novel and well-tolerated strategies to treat patients with advanced and hormone-refractory prostate cancer.
Dendritic cells (DCs) have received much attention as a new therapeutic tool for cancer immunotherapy against advanced and hormone-insensitive prostate cancers [6–8]. Although early clinical studies successfully demonstrated cancer cell-specific immune responses in tumor-bearing patients, the related clinical outcome of DC-based immunotherapy has been unsatisfactory [6–8], similar to findings observed in other tumors [9]. These results, together with those of clinical studies all over the world, indicate that a number of concerns need to be examined, including practical issues (optimal numbers of DCs and route, interval, and frequency of administration) as well as DC qualities (subtype, phenotype, and activation status) [9].
To overcome these hurdles, we recently proposed a new concept, “immunostimulatory virotherapy,” that provides efficient, DC-based antitumor immunity against highly malignant B16F10 melanoma; this immunity is induced by a new DC-activating modality, the replication-competent [10] as well as nontransmissible [11] recombinant Sendai viruses (rSeVs). SeV, a member of the family Paramyxoviridae, has a nonsegmented negative-strand RNA genome and shows a broad spectrum of gene transfer [12–14], including DCs. rSeV can mediate gene transfer and expression to a cytoplasmic location using cellular tubulin [15], thereby avoiding possible malignant transformation due to the genetic alteration of host cells; this is a safety advantage of rSeV. Importantly, cytoplasmic replication and transcription of rSeV would also be recognized by a DExD/H-box RNA helicase, retinoic acid-inducible gene-I (RIG-I), leading cells to a variety of cellular responses, including induction of type I interferons (IFNs) [16,17].
Such unique features of rSeV modulation of DC functions may explain the efficient antitumor immunity of rSeV-DCs. However, questions remain 1) whether rSeV-DC is also effective for clinically more relevant situations, including lung metastasis, and 2) whether the induction of antitumor immunity through rSeV-DC can be expanded to other malignancies and animal species.
To answer these questions, we here investigated the antimetastatic activity of DCs modified by nontransmissible rSeV lacking the F-gene (rSeV/dF) [18]. Here, this rSeV/dF that has been used in the first clinical gene therapy study in Japan was used to treat a rat model of lung metastasis of a highly malignant subline of Dunning R-3327 (AT6.3) [19,20]. To examine the biologic functions of DCs modified by rSeV/dF, we here used rSeV expressing a typical reporter gene, GFP. This study successfully demonstrated that rSeV/dF-GFP-DC was representatively effective for preventing pulmonary tumor metastasis in a rat model of prostate cancer. Furthermore, we here show evidence that the antitumor effect of rSeV/dF-DC was independent of the RIG-I-related signal transduction pathway.
Materials and Methods
Animals and Tumor Cells
Male 6- to 8-week-old Copenhagen rats were obtained from the Institute for Animal Reproduction (Tsukuba, Ibaraki, Japan). In some experiments, male 8-week-old Wistar rats were also used as controls. Rats were kept under specific pathogen-free and humane conditions in the animal care facility of Chiba University's Inohana campus. The animal experiment was reviewed and approved by the Institutional Animal Care and Use Committee and by the Biosafety Committee for Recombinant DNA experiments of Chiba University. These experiments were also done in accordance with recommendations for the proper care and use of laboratory animals and according to The Law (No. 105) and Notification (No. 6) of the Japanese Government.
The AT6.3 clone, a highly metastatic, androgen-independent subline from Dunning R-3327 rat prostate adenocarcinoma, served as a model for experimental metastasis subsequent to intravascular tumor inoculation. The development and characteristics of the AT6.3 subline were described previously [20].
Monoclonal Antibodies
The following mouse antirat monoclonal antibodies (mAbs) were used in this study. Biotin-conjugated OX35 (CD4), OX8 (CD8), and OX17 (MHC class II) were purchased from BD Pharmingen (San Diego, CA). Phycoerythrin (PE)-conjugated OX42 (CD11b/c), which recognizes most DCs, monocytes/macrophages, and granulocytes in part, was purchased fromGeneTex (San Antonio, TX). Biotin-conjugated B7-1 (CD80) and B7-2 (CD86) were from Cedarlane Laboratories (Hornby, Ontario, Canada), and antirat pan-granulocyte (RK-4) were from BMA (Augst, Switzerland). PE-conjugated OX62 (an integrin on DCs) was from Serotec (Oxford, UK), and PE-conjugated antirat ICAM-1 (intracellular adhesion molecule 1/CD54) was from R&D Systems (Minneapolis, MN). Anti-CD45R (on B cells) was obtained from e-Bioscience (San Diego, CA). Hamster antimouse CD40 (crossreacting with rat CD40) mAbs were purchased from Pharmingen.
Nontransmissible rSeVs (rSeV/dF)
Preparation and recovery of F-defective nontransmissible recombinant SeVs (rSeV/dF-GFP, rSeV/dF-null, and rSeV/dF-RIGIC) were performed as previously described [18]. Briefly, LLC-MK2 cells stably transfected with pCALNdLw/F (the Cre/loxP-inducible F-gene expression plasmid) were maintained under G418 (400 µg/ml). F protein expression was induced by infecting an adenovirus-expressing Cre recombinase (AxCANCre) at a multiplicity of infection (MOI) of 3. Approximately 107 LLC-MK2 cells were infected with psoralen- and long-wave UV-inactivated vaccinia virus expressing T7 RNA polymerase (vTF7-3) at MOI = 2. The cells were then transfected with a plasmid mixture containing pSeV18+b(+)/dF, pGEM-NP, pGEM-P, and pGEM-L by lipid-mediated gene transfer. The transfected cells were maintained for 3 hours and incubated for 60 hours in minimum essential medium containing ara-C. The cells were collected by centrifugation, resuspended, and lysed by three cycles of freezing and thawing. Subsequent genomic RNA nuclear protein complex transfection was performed by cationic lipid into F-expressing LLC-MK2/F7 cells. Human RIG-IC complementary DNA, a dominant-negative form of RIG-I that was subcloned to the vector template pSeV18+b(+)/dF, was cloned by reverse transcription-polymerase chain reaction (RT-PCR) as previously described [21].
Generation of Rat Bone Marrow-Derived DCs
DCs were obtained as previously described with additional negative selection [22]. Briefly, bone marrow cells were harvested from femurs and tibias. Red blood cells were lysed with VersaLyse lysing solution (Beckman Coulter, Fullerton, CA). After washing, lineage antigen-positive (CD45R, CD4, and CD8) cells and granulocytes were depleted using the biotinylated primary antibodies and streptavidin-coated magnetic beads (DynabeadsMyOne Streptavidin; Invitrogen, Carlsbad, CA). These cells were cultured under 20 ng/ml rat granulocyte/macrophage colony-stimulating factor (GM-CSF; Peprotech, London, UK) and 10 ng/ml rat interleukin 4 (IL-4; Peprotech) in RPMI 1640 medium with 10% fetal calf serum in six-well plates (5–10 x 104/5 ml per well). On day 4, the culture medium was refreshed using medium supplemented with GM-CSF and IL-4 at the same concentrations. On day 7, obtained immature DCs (iDCs) were collected and used for subsequent experiments. For rSeV/dF-mediated transfection, DCs were incubated with rSeV vectors at MOI 100 or as indicated. For lipopolysaccharide (LPS)-mediated DC activation, DCs were cultured in the same medium supplemented with 1 µg/ml LPS (Sigma-Aldrich Japan, Tokyo, Japan). For tumor lysate preparation, AT6.3 cells were processed by three rapid cycles of freezing and thawing. DCs were pulsed with tumor lysate (DC number vs number of tumor cells for lysate = 1:3) for 18 hours and then incubated with rSeV/dF or LPS for 48 hours, as previously described [10]. For all injections, materials were suspended in a 200-µl volume of phosphate-buffered saline (PBS). When preparing rat bone marrow-derived DC (rBM-DCs), we paid serious attention to maintaining an endotoxin-free condition using endotoxin-free reagents throughout this study.
Flow Cytometric Analysis
rBM-DCs were analyzed by flow cytometry. The cells were incubated with primary antibodies for 30 minutes at 4°C, and biotinylated Abs were detected by subsequent staining with streptavidin-PE or fluorescein isothiocyanate (FITC) (Pharmingen). Cells were analyzed using a FACS Calibur with the CellQuest software (D Biosciences Japan, Tokyo, Japan). Data analysis was performed using FlowJo 4.5 software (TREE STAR, Inc, San Carlos, CA). For surface markers, DCs were replated in fresh medium (1 x 106/ml) and incubated with SeV/dF without a foreign gene (MOI = 100) or 1 µg/ml LPS for 48 hours. Biotin-conjugated antirat CD40, CD80, CD86, MHC class II, and PE-conjugated OX62 (Pharmingen) mAbs were used for each primary antibody, and biotinylated Abs were detected by subsequent staining with streptavidin-FITC (Pharmingen). OX62 expression of rSeV/dF-GFP-DCs was observed in comparison with that of LPS at different times after stimulation.
Real-time Quantitative RT-PCR
The cultured DCs were plated in fresh medium and were incubated with rSeV/dF-GFP, rSeV/dF-RIGIC, or LPS for 48 hours. Total RNA was extracted from each DC using a Micro-to-Midi Total RNA purification kit (Invitrogen) according to the manufacturer's instructions. After spectrophotometric quantification, reverse transcription was carried out using random primers and SuperScript III reverse transcriptase (Invitrogen). The expression of cytokines was examined by real-time quantitative PCR using LightCycler (Roche Diagnostics, Mannheim, Germany). The glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene served as an internal control. The PCR mixture consisted of DNA Master SYBR Green I Mix (SYBR Green Two-Step qRT-PCR Kit; Invitrogen), 1 µM of each primer, and complementary DNA. The PCR processes were as follows: initial denaturation at 95°C for 1 minute, followed by 45 cycles of denaturation at 95°C for 10 seconds, annealing at 57°C for 10 seconds, and elongation at 72°C for 10 seconds. To check the specificity of the amplified products, melting curve analysis was performed immediately after the completion of the PCR. The melting protocol consisted of heating from 65 to 95°C at a rate of 0.2°C per step and holding for 1 second at each step for data acquisition. The primer sequences are listed on the Table W1.
ELISA
The DCs were plated in fresh medium and incubated with rSeV/dF-GFP or LPS (1 ng/ml) for 48 hours. The culture media were harvested, and the concentration of rat tumor necrosis factor a (TNF-α) was measured by a rat-specific ELISA kit (BioSource, Camarillo, CA) according to the manufacturer's instructions.
Vaccination
Rats were intravenously or intradermally vaccinated three times, every other week, with appropriate numbers of immature, LPS-, rSeV/dF-GFP-, or rSeV/dF-RIGIC-DCs pulsed with or without tumor lysate. A day after the final vaccination, 5 x 105 AT6.3 cells suspended in a 200-µl volume of PBS were inoculated intravenously. Three weeks after injection, rats were killed, and their lung metastases were counted by measuring lung weight and the number of metastatic nodules on the lung surface. For immunologic assessment, a Cr51 release assay for cytolytic activity of CTL was performed.
Cr51 Release Assay for CTL
Seven days after the third immunization, splenocytes were obtained, and contaminated erythrocytes were depleted by VersaLyse lysing solution. The effector splenocytes (4 x 106 cells/ml) were cocultured with irradiated (100 Gy) AT6.3 cells (stimulator; 3 x 105 cells/ml) for 5 days. Consequently, cells containing CTLs were harvested and used as effector cells in a Cr51 release assay. Briefly, target tumor cells (1 x 106) were labeled with 100 µCi of Na2 51CrO4 (Amersham Biosciences, Piscataway, NJ) in 200 µl of medium for 90 minutes at 37°C. The labeled target cells (1 x 104 cells per well) were incubated with the effector cells for 4 hours at 37°C in 96-well plates in 200 µl of T-cell medium at various E/T ratios. The plates were then centrifuged, and the radioactivity of the supernatants was counted using a gamma counter. The maximum or spontaneous release was defined as counts from samples incubated with 2% Triton X-100 or medium alone, respectively. Cytolytic activity was calculated using the following formula: percentage of specific Cr51 release = (experimental release - spontaneous release) x 100 / (maximum release - spontaneous release). Assays were performed in triplicate wells.
γ-Camera Imaging and Biodistribution Analysis
DCs were labeled with 111In oxinate (Nihon Medi-Physics, Hyogo, Japan) in PBS for 20 minutes at room temperature. Cells were washed, and the labeling efficiency was calculated as the percentage of activity that remained associated with the cell pellet. For the in vivo studies, five rats per experimental group were given intravenous injection of 3 x 106 DCs (30 µCi) in 200 µl of PBS per rat. At different time points, images were recorded with a CdTe semiconductor γ-camera (MGC1500; Acrorad, Tokyo, Japan). For tissue biodistribution, groups of five rats were analyzed 48 hours after injection of the 111In-labeled DCs. Lungs, liver, kidneys, spleen, thymus, and lymph nodes (axillar, hilar/mediastinal, abdominal, and pelvic/inguinal) were collected and counted in the γ-counter. The measured activity in tissues and samples was expressed as a percentage of total detected dose.
Statistical Analysis
All data were expressed as means ± SD and were evaluated statistically by repeated-measures one-way analysis of variance. The statistical difference among groups was determined using Scheffé test, and P<.05 was considered statistically significant.
Results
Characterization of rSeV/dF-Treated DCs Derived from Rat Bone Marrow
At the initial stage of this study, we characterized rBM-DCs treated with rSeV/dF, because this was the first time for us to examine the rat DCs (Figure W1).
Two days after rSeV/dF-GFP exposure to a possible iDC-rich population (>95% of OX42+ in triplicate experiments) at an MOI of 30, microscopic examination occasionally demonstrated large GFP-positive cells showing typical dendrites. FACS analysis exhibited an apparent dose-dependent increase in the GFP-positive cell ratio, up to approximately 80%, without an apparent cytopathic effect as assessed by 7-amino-actinommycin D (7AAD) assay.
Next, we assessed whether rSeV/dF-GFP treatment led rBM-DCs to an activated and mature state, as was demonstrated with murine BM-DCs (mBM-DCs) in our previous study [10,11]. Similar to the case of the mBM-DCs, nontransmissible rSeV/dF-GFP upregulated CD80/B7-1 and CD86/B7-2 of rBM-DCs, findings that were not apparent for CD40 and OX17 (MHC class II). Interestingly, the expression level of the αE2 subunit of integrin on rat DCs, which was recognized by mAb OX62, was more pronounced on rSeV/dFGFP- DCs than on LPS-activated DCs (LPS-DCs; data not shown; representative data are demonstrated in Figure 4D). Proinflammatory cytokines, including IL-1β, IL-6, and TNF-α, were also upregulated by rSeV/dF-GFP treatment. However, the level did not reach those observed by LPS treatment (data not shown; representative data are demonstrated in Figure 4B). FITC-dextran uptake assay demonstrated time-dependent impairment of endo-/phagocytotic activity of DCs treated by LPS as well as rSeV/dF-null (for exclusion of GFP background), whereas no significant reduction was observed in the case of iDCs, a finding that conflicts with our previous data in use of mBM-DCs [11], probably because of the species' specificity. Furthermore, an allogeneic mixed lymphocyte reaction assay revealed that both DC-activated LPS and rSeV/dF-GFP could stimulate allogeneic T-cell proliferation.
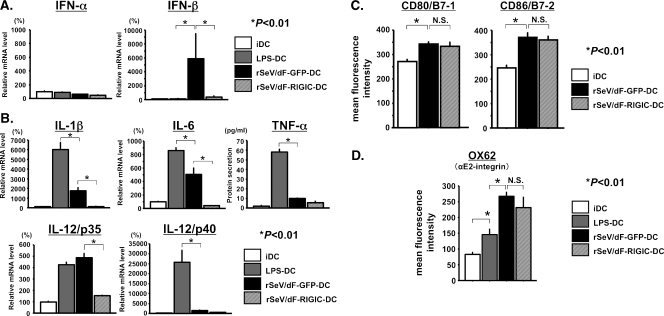
Figure 4.
Type I IFN (A), typical proinflammatory cytokine (B), and surface marker (C and D) expression profiles in iDC, LPS-DC, rSeV/dF-GFP-DC, and rSeV/dF-RIGIC-DC. Two days after treatment, gene or protein expression was determined by real-time quantitative RT-PCR or ELISA (A or B) or by FACS analyses (C and D). *P < .01. (A) Gene expression of type I IFNs. rSeV/dF stimulated IFN-β but not IFN-α expression, a finding diminished by rSeV/dF expressing RIG-IC, which is a dominant-negative inhibitor of a RNA helicase RIG-I. Each group contained n = 4. (B) Protein secretion (ELISA: TNF-α) and gene expression (real-time quantitative RT-PCR: others). Note that rSeV/dF-related up-regulation of IL-1β, IL-6, and IL-12/p35 was almost completely abolished by RIG-IC expression. Each group contained n = 3. (C and D) RIG-IC had no effect on surface markers of DCs, CD80/B7-1 and CD86/B7-2 (C), and αE2-integrin, a target antigen of OX62 (D). Each group contained n = 4 (C) and n = 12 (D, total of three independent experiments).
These results indicate that, largely similar to the findings obtained by the use of mBM-DCs [10,11], transfection of rSeV/dF could lead to an activated and mature state of rBM-DCs.
Optimization of rSeV/dF-GFP-DC Vaccination in Experimental Lung Metastasis of AT6.3
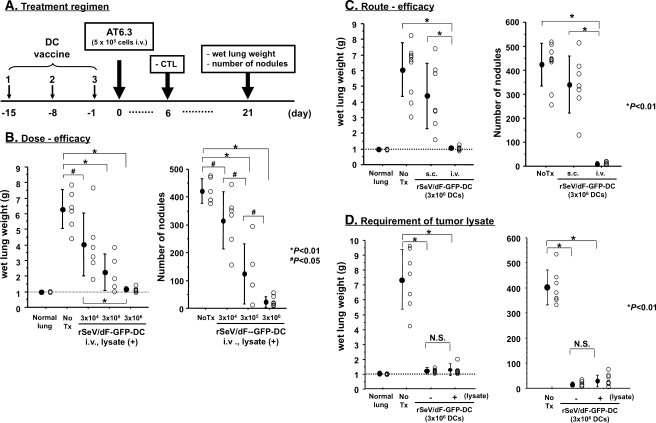
Next, we optimized rSeV/dF-based DC vaccination with tumor lysate pulsation according to the number of DCs and route of administration. The treatment regimen was demonstrated in Figure 1A. Apparent and statistically significant reductions in wet lung weight and the number of metastatic nodules were observed in a dose-dependent manner; the optimized effect was observed at 3 x 106 cells of rSeV/dF-GFP-DCs by intravenous vaccination through the tail vein (Figure 1B). The effect was markedly diminished when such DCs were administered subcutaneously (Figure 1C); therefore, all subsequent experiments were done by intravenous administration of 3 x 106 cells of DCs. We additionally examined whether ex vivo pulsation of tumor lysate was required. As shown in Figure 1D, rSeV/dF-GFP-DCs constantly showed an antimetastatic effect on AT6.3 irrespective of tumor lysate pulsation.
Figure 1.
Optimization of tumor lysate-pulsed rSeV/dF-GFP-DC vaccine on rat model of lung metastasis of AT6.3 prostate cancer. *P < .01. (A) Treatment regimen. (B) Optimization of dose-efficacy relationship. DCs were administered through the tail vein. The following numbers of animals were used: normal lung, n = 6; No Tx, n = 6; and rSeV/dF, n = 17 (3 x 104 cells, n = 6; 3 x 105 cells, n = 5; and 3 x 106 cells, n = 6). (C) Optimization of administration route. A total of 3 x 106 DCs were used per vaccine. The following numbers of animals were used: normal lung, n = 6; No Tx, n = 9; and rSeV/dF, n = 16 (subcutaneous [s.c.] n = 7 and intravenous [i.v.] n = 9). (D) Requirement of ex vivo pulsation of tumor lysate. A total of 3 x 106 DCs were used per vaccine. The following numbers of animals were used: normal lung, n = 6; No Tx, n = 7; and rSeV/dF, n = 16 (-lysate n = 8 and +lysate n = 8). N.S. indicates not significant.
Direct Comparison of Antimetastasis Efficacies
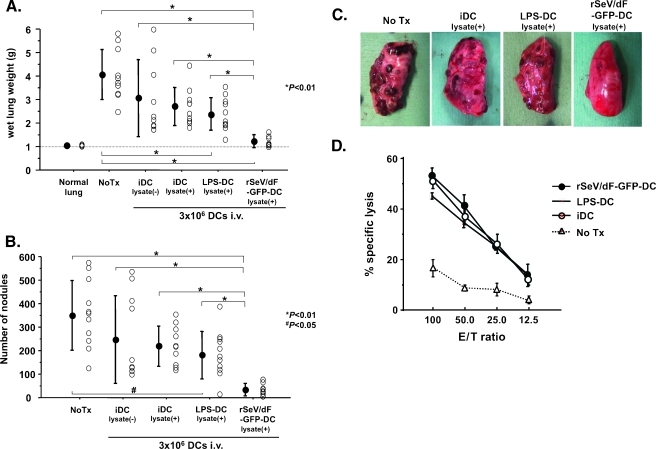
Next, we directly compared the antimetastatic effect of DCs (iDC, LPS-DC, and rSeV/dF-GFP-DC) by the same treatment regimen demonstrated in Figure 1A. We here included another iDC group, iDC without tumor lysate, to exclude spontaneous activation of DCs because of endo-/phagocytosis of the tumor antigen.
As shown in Figure 2, A to C, experimental lung metastasis was significantly prevented in groups of LPS-DCs as well as in rSeV/dF-GFP-DCs but not in any iDCs, and the finding was highly pronounced in the use of rSeV/dF-GFP-DCs. A similar effect was observed in the use of rSeV/dF-DCs without exogenous gene (data not shown). It was of interest that CTL activity in splenocytes assessed at 3 weeks after tumor inoculation showed no difference among the groups, indicating that systemic tumor-specific CTL responses, at least, could be effectively induced by intravenous administration of any types of DCs.
Figure 2.
Direct comparison of antimetastatic ability of iDC, LPS-DC, and rSeV/dF-GFP-DC on rat model of lung metastasis of AT6.3 prostate cancer. (A–C) Direct comparison of efficacy of iDC with or without tumor lysate pulsation (n = 10, respectively), LPS-DC with lysate (n = 11), and rSeV/dF-GFP-DC with lysate (n = 11): total wet lung weight (g) (A), number of metastatic nodules (B), and gross observation of left upper lobe (C). Normal lung, n = 6; and No Tx, n = 11. The treatment regimen was the same as that in panel A. All DCs were administered intravenously through the tail vein at 3 x 106 cells per vaccine. Note that the iDC group was included so as to exclude the effect of spontaneous activation through tumor antigen capture. *P < .01 and #P <.05. (D) CTL activity assessed in use of splenocytes. Each group contained n = 3.
Therefore, these results suggest that other factors might contribute to the strong antimetastatic ability of rSeV/dF-GFP-DCs.
In Vivo Trafficking of DCs
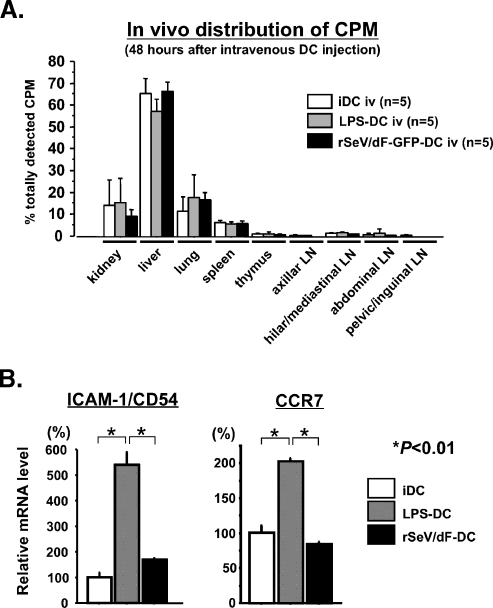
To explain the highly antimetastatic activity of rSeV/dF-GFP-DCs, we first hypothesized that these DCs might be retained in the lung, resulting in more pronounced antitumor responses at local sites. To examine this possibility, we assessed the time course of in vivo trafficking of DCs that were labeled by 111In oxinate.
Unexpectedly, the results were negative. At 3 hours after DC administration, γ-camera imaging analysis revealed that most radioactivities were distributed at the chest and, 21 hours later, at the upper abdomen, irrespective of DC type (data not shown). To assess this more precisely, these animals were killed, and the lysate of each organ was subjected to the γ-counter 48 hours after DC administration (Figure 3A). Most radioactivities were accumulated in the liver, and 15% to 20% was observed in the lung with the use of any DCs. No significant differences were found in other lymphatic organs.
Figure 3.
Trafficking of DCs in vivo. At 3 and 24 hours after intravenous administration of 3 x 106 111In oxinate-labeled DCs, the whole body was screened by a γ-camera (data not shown). Twenty-four hours later, the tissue samples were harvested and subjected to a γ-counter. Each group contained n = 5. (A) Tissue distribution of radioactivity 48 hours after DC administration. No difference in accumulation was observed in any of the tissues sampled in this study. (B) Gene expression of ICAM-1/CD54 and CCR7 in DCs after treatment with LPS or rSeV/dF. Forty-eight hours after treatment, total RNA was extracted and subjected to quantitative real-time PCR. The GAPDH expression level was used for internal control of relative expression, and the data were expressed as percentages against relative expression of iDC.
Subsequently, we also examined the expression of theDC-trafficking-related molecules, including ICAM-1/CD54 and chemokine receptor CCR7. Real-time PCR analyses revealed that messenger RNA expression of ICAM-1/CD54 and CCR7 was stimulated by LPS but not by rSeV (Figure 3B); these findings differed from those observed in DC surface expression of murine DCs [10].
These results suggested that the expression of some DC trafficking-related molecules, ICAM-1/CD54 and CCR7, as well as the actual trafficking of DCs in vivo were not likely to be important in the differences in the antimetastatic activities among types of DCs in the rat model of AT6.3 lung metastasis.
Effect of Dominant-Negative Mutant of RIG-I, RIG-IC, on the Expression of IFN-β and Proinflammatory Cytokines in rBM-DCs
Next, we hypothesized that the unique feature of rSeV, a negative-strand RNA vector that replicates in the cellular cytoplasm [12–14], might explain the superior antitumor effect of rSeV/dF-GFP-DCs compared with other DCs. Since important studies recently revealed that an RNA helicase, RIG-I, was critical for recognition of the RNA genome of SeV for inducing various cellular responses, including expression of type I IFNs [16,17], we constructed a new rSeV/dF expressing a dominant-negative mutant of RIG-I, namely RIG-IC (rSeV/dF-RIGIC) [21], to knock down the RIG-I-related signaling in rSeV/dF-activated DCs.
As expected, rSeV/dF-GFP but not LPS selectively induced IFN-β messenger RNA expression of rBM-DCs, an effect that was almost completely diminished in DCs transfected by rSeV/dF-RIGIC (Figure 4A, right graph). In contrast, neither rSeV/dF-GFP nor LPS affected the gene expression of IFN-α in DCs (Figure 4A, left graph). Interestingly, the expression of RIG-IC almost completely diminished rSeV/dF-related up-regulation of IL-1β, IL-6, and IL-12/p35 gene expression, too (Figure 4B). Because the enhancement of TNF-α protein secretion and IL-12/p40 gene expression through rSeV/dF was modest, the effect of RIG-IC expression on these was minimal. These results indicated that RIG-I-related signaling induced by rSeV/dF was critical not only for the expression of IFN-β but also for some typical proinflammatory cytokines on rBM-DCs. We confirmed these findings also in human peripheral blood-derived DCs and murine BM-DCs (unpublished data).
DC surface markers, however, showed opposite results.
As shown in Figure 4C, upregulated expression of CD80/B7-1 and CD80/B7-2 on DCs through rSeV/dF was not affected by the expression of RIG-IC. The expression of αE2-integrin on rBM-DCs, which mAb OX62 recognized as an important marker of rat DCs, was much accelerated by rSeV/dF-GFP compared with LPS. Importantly, rSeV/dF-mediated up-regulation of αE2-integrin was not significantly affected by the expression of RIG-IC at all (Figure 4D).
These results strongly suggested that RIG-I-related signaling was critical for inducing IFN-β and some proinflammatory cytokines on DCs. However, the RNA genome of rSeV/dF might possibly be recognized by an RIG-I-independent mechanism that might contribute to the expression of some surface markers, including CD80/B7-1 and CD86/B7-2, as well as αE2-integrin.
RIG-IC Does Not Impair Antimetastatic Activity of rSeV/dF-DCs
As a final assessment, we tested the antimetastatic activity of the vaccination of rSeV/dF-RIGIC-DCs on the AT6.3 experimental lung metastasis model in the same regimen as that demonstrated in Figure 1A.
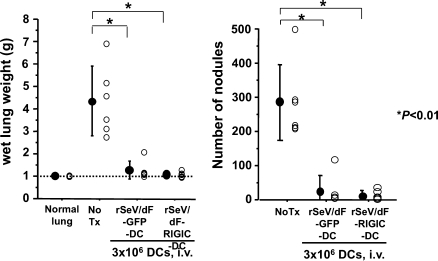
As shown in Figure 5, the expression of RIG-IC did not affect the reduction of wet lung weight or the reduction in the number of metastatic nodules at all. This indicated the essential contribution of RIG-I-independent signaling on the antimetastatic ability of DCs activated by rSeV/dF.
Figure 5.
Antimetastatic activity of rSeV/dF-modified DC does not depend on RIG-I-related signaling. Comparison of the efficacy of rSeV/dF-modified DC expressing a reporter gene (GFP: n = 6) or RIG-IC (n = 6); total wet lung weight (g) (left graph) and the number of metastatic nodules (right graph) were demonstrated. Normal lung, n = 6; and No Tx, n = 11. Treatment regimen was followed as in Figure 1A. All DCs were administered intravenously through the tail vein at 3 x 106 cells per vaccine. *P < .01.
Discussion
We here investigated the potential of our recently developed DC-based immunostimulatory virotherapy using nontransmissible rSeV/dF in an immune-competent rat model of lung metastasis of highly malignant prostate cancer AT6.3. The key observations obtained in this study were as follows: 1) nontransmissible rSeV/dF transfected efficiently to rBM-DCs without significant toxicity, and led them to the activation/maturation state, findings that were representative of those observed in murine DCs demonstrated in our previous study; 2) rSeV/dF-modified DCs effectively prevented lung metastasis in a rat model of prostate cancer; 3) the efficient antimetastatic activity of rSeV/dF-DCs over iDCs and LPS-DCs was likely to depend on neither CTL activity nor DC trafficking; and 4) RIG-I-related signaling in rSeV/dF-modified DCs was critical for cytokine production but not for their antimetastatic activity.
Although there remain concerns regarding the molecular and immunologic mechanisms related to the antitumor activity of rSeV/dF-DCs, this is the first report to indicate the high efficacy of prevention for lung metastasis through vaccination of rSeV/dF-modified DCs. Therefore, we believe that rSeV/dF-mediated immunostimulatory virotherapy warrants further investigation as a new concept and a promising tool for enhancing DC-mediated cancer immunotherapy.
The major concerns arising from the current study should focus on the possible molecular mechanisms of rSeV/dF-related activation/maturation of DCs. Of interest, we here demonstrated the divergent expression patterns between cytokines and DC surface markers; the former was RIG-I-dependent, and the latter, including the target of OX62 αE2-integrin, was independent. According to recent extensive studies done by Kawai et al. [16], Kato et al. [17], and Yoneyama et al. [21], the RIG-I-dependent expressional regulation of proinflammatory cytokines, as well as that of IFN-β, has been expected in our cases of rSeV/dF-DCs. Unexpectedly, however, the up-regulation of DC surface proteins induced by rSeV/dF was independent of RIG-I-related signaling in rat DCs. There are two possible explanations: 1) the fusion of the envelope, but not of the genomic ribonucleotide protein complex, of rSeV/dF stimulated the expression of surface proteins; or 2) an alternative pathway that recognizes the replication/transcription of the genome of rSeV/dF stimulated surface marker expression but not proinflammatory cytokines. The former seems unlikely because some studies from different laboratories demonstrated that the use of the envelope of SeV did not evoke the up-regulation of surface markers, thus suggesting DC activation/maturation [23–25]. We also confirmed that UV-inactivated rSeVs stimulated neither cytokines nor activation markers on DCs derived from human peripheral blood and murine bone marrow (data not shown). Furthermore, rSeV/dF-mediated up-regulation of CD80/B7-1, CD86/B7-2, and αE2-integrin was not a result of the autocrine effect of cytokines because the expression of these markers was not protected by RIG-IC expression. Therefore, it seems reasonable to hypothesize that the cytoplasmic alternative molecules, but not RIG-I, may recognize the RNA genome of rSeV/dF for inducing surface markers but not for inducing cytokine expression. Further studies should be conducted to clarify these questions.
The second question is which factor(s) and/or effector(s) determined the differences in antimetastatic activity among iDC, LPS-DC, and rSeV/dF-DC. As the present study demonstrated, splenic CTL activity as well as DC trafficking, at least, showed no apparent differences among these groups. It is hard for us, at present, to suggest a definitive answer because we have data indicating that direct cytolytic activity [26] could not be detected in these DCs (data not shown), and immunohistochemical studies have revealed the infiltration of similar numbers of CD4+ and CD8+ cells into the lung (data not shown). Although a large part of the possible mechanisms may be speculative, one possible explanation is the balance of expression between IL-12/p35 and IL-12/p40 in DCs. It has been well known that IL-12p75 (or IL-12p70), commonly IL-12 heterodimerically composed of a p35 subunit and a p40 subunit, is involved not only in the induction but also in the maintenance of TH1 responses, as well as in stimulating NK cell activity [27–29]. Both murine and human p40 homodimers, in contrast, are shown to act as antagonists of IL-12 [29]. In the present study, we demonstrated that LPS strongly stimulated both p35 and p40, whereas rSeV/dF-GFP selectively induced p35 dominantly, and lesser extent to p40 (Figure 4B), suggesting the more pronounced TH1 response and NK cell activity in DC vaccination using rSeV/dF than that observed by LPS. Particularly, NK cell activity seems possibly essential in the antimetastatic activity of rSeV/dF-DCs expressing lower levels of p40 because it was shown that IL-12p40 secretion suppressed NK cell activity, allowing the growth of cancer cells in vivo [30]. Some studies, however, have shown the divergent expression of p40 by SeV in human and murine macrophage-lineage cells [31,32], conflicting with our current findings obtained using rat BM-DCs. Therefore, because there are a number of limitations for us to assess regarding detailed immunologic aspects of the rat model, further studies are required using murine models of lung metastasis.
In this regard, we recently published an article demonstrating that rSeV-DC-based efficient prevention of lung metastasis of RM-9 prostate cancer was a NK/CD4+ cell-dependent mechanism [33]. Interestingly, the antimetastatic effect was sustained during 3 months, even when administered DCs were already cleared from the lung and organs related to the immune system. Furthermore, although NK cell activity had already declined to baseline at the time of tumor inoculation, antibody-mediated depletion studies revealed that CD4+ cells as well as the presence of, but not activation of, NK cells were crucial for prevention of lung metastasis. These results indicate that the efficient inhibition of lung metastasis through bolus administration of virally activated DCs that was sustained and NK/CD4+ cell-dependent, but not RIG-I-independent, suggesting a potentially new mechanism of DC-based immunotherapy for advanced malignancies.
Although some concerns remain to be clarified, we believe that a lack of information regarding some mechanistic aspects does not always diminish the value of rSeV-DC-based immunostimulatory virotherapy because this system representatively showed strong antitumor effects in some tumor models and species even when used for highly malignant tumor models [10,11]. Because rSeV/dF is now available for clinical study and because the preparation of rSeV/dF-modified DC does not require any specific techniques or materials, we should investigate whether this system may show a significant antitumor effect over the current cancer vaccines in clinical settings.
In conclusion, we here successfully demonstrated a highly efficient antimetastatic activity of nontransmissible rSeV-modified DC in a rat model of prostate cancer. These results prompt us to make further investigations toward clinical applications.
Supplementary Methods
Transfection Efficiency and Cytopathic Effect on rBM-DCs
The transfection efficiency of SeV/dF-GFP in rBM-DCs was evaluated. On day 7, immature rBM-DCs propagated in the presence of GM-CSF and IL-4 were collected. rBM-DCs were transfected with SeV/dF-GFP at MOI 1 to 300. Forty-eight hours after SeV/dF infection, rBM-DCs were analyzed by flow cytometry for GFP expression. Cell viability was also assessed with 7AAD to count living cells, as previously described [1].
FITC-Dextran Uptake
FITC-dextran uptake assay was performed to assess the endo-/phagocytotic activity of DCs. DCs were plated in fresh medium (1 x 106 cells/ml) and were incubated with or without rSeV/dF-GFP or LPS (1 ng/ml), and DCs were harvested at different times after stimulation. Acquired DCs were suspended in RPMI 1640 with 10% fetal calf serum and incubated with 1 mg/ml of FITC-dextran (MWt = 40,000; Sigma-Aldrich) for 30minutes at 4 or 37°C. Cells were washed three times with ice-cold PBS, and mean fluorescent intensity of FITC was analyzed by FACScalibur (BD Biosciences, Franklin Lakes, NJ). The uptake was calculated as the change in mean fluorescent intensity between cell samples incubated at 37°C and 4°C.
Allogeneic Mixed Lymphocyte Reactions
Allogeneic mixed lymphocyte reactions were performed to assess the antigen-presenting ability of DCs. Total splenocytes were obtained from allogeneic Wistar rats. Red blood cells were lysed using VersaLyse Lysing solution and served as responder cells. In vitro-generated immature rBM-DCs, as well as rSeV/dF-GFP-DCs and LPS-DCs stimulated on day 7, were collected at day 9. These DCs were irradiated with 3000 rad and then used as stimulator cells. Total splenocytes freshly extracted from Copenhagen rats were also irradiated and used as control stimulator APC. Allogeneic responder cells (1 x 105 cells/wells) were cultured in triplicate in a 96-well round-bottom microplate with different numbers of stimulator APCs (APC-to-T-cell ratios were 1:1, 1:10, and 1:100). Cultures were maintained in a humidified atmosphere at 37°C and 5% CO2. The thymidine analog bromodeoxyuridine (BrdU) was added on day 3 followed by quantitation of incorporated BrdU after a further 16 hours of culture using an ELISA-based cell proliferation kit (BrdU colorimetric, 1647229; Roche, Mannheim, Germany) according to the manufacturer's protocol.
Acknowledgments
The authors thank Drs. Akihiro Tagawa, Takumi Kanaya, Hiroshi Ban, and Takashi Hironaka, DNAVEC Corporation, for their excellent technical assistance in vector construction and large-scale production.
Abbreviations
- BM-DC
bone marrow-derived DC
- DC
dendritic cells
- GFP
green fluorescent protein
- i.v.
intravenous injection
- iDC
immature DC
- LPS
lipopolysaccharide
- LPS-DC
LPS-activated DC
- MOI
multiplicity of infection
- RIG-I
retinoic acid-inducible gene I
- rSeV
recombinant Sendai virus
- rSeV/dF
fusion gene-deleted rSeV
- rSeV/dF-RIGIC
rSeV/dF expressing dominant-negative mutant of RIG-I
- s.c.
subcutaneous injection
Footnotes
This work was supported in part by research grants from the 21st Century Center of Excellence Program, Chiba University Graduate School of Medicine; by grants-in-aid (to T.I. and Y.Y.) from the Japanese Ministry of Education, Culture, Sports, Science, and Technology; by research grants from Sankyo Foundation of Life Science (to Y.Y.), Mitsubishi Pharma Research Foundation (to Y.Y.), and Uehara Memorial Foundation (to Y.Y.).
Dr. Yonemitsu is a member of the Scientific Advisory Board of DNAVEC Corporation.
This article refers to supplementary materials, which are designated by Table W1 and Figure W1 and are available online at www.neoplasia.com.
References
- 1.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 2.Ichikawa T, Suzuki H, Ueda T, Komiya A, Imamoto T, Kojima S. Hormone treatment for prostate cancer: current issues and future directions. Cancer Chemother Pharmacol. 2005;56(suppl 1):58–63. doi: 10.1007/s00280-005-0100-x. [DOI] [PubMed] [Google Scholar]
- 3.Oh WK, Kantoff PW. Management of hormone refractory prostate cancer: current standards and future prospects. J Urol. 1998;160:1220–1229. [PubMed] [Google Scholar]
- 4.Borden LS, Jr, Clark PE, Lovato J, Hall MC, Stindt D, Harmon M, Mohler R, Torti FM. Vinorelbine, doxorubicin, and prednisone in androgenindependent prostate cancer. Cancer. 2006;107:1093–1100. doi: 10.1002/cncr.22078. [DOI] [PubMed] [Google Scholar]
- 5.Carducci MA, Padley RJ, Breul J, Vogelzang NJ, Zonnenberg BA, Daliani DD, Schulman CC, Nabulsi AA, Humerickhouse RA, Weinberg MA, et al. Effect of endothelin-A receptor blockade with atrasentan on tumor progression in men with hormone-refractory prostate cancer: a randomized, phase II, placebo-controlled trial. J Clin Oncol. 2003;21:679–689. doi: 10.1200/JCO.2003.04.176. [DOI] [PubMed] [Google Scholar]
- 6.Murphy GP, Tjoa BA, Simmons SJ, Ragde H, Rogers M, Elgamal A, Kenny GM, Troychak MJ, Salgaller ML, Boynton AL. Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate. 1999;39:54–59. doi: 10.1002/(sici)1097-0045(19990401)39:1<54::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Small EJ. The potential for prostate cancer immunotherapy. Crit Rev Oncol Hematol. 2003;46(suppl):S117–S125. doi: 10.1016/s1040-8428(03)00069-6. [DOI] [PubMed] [Google Scholar]
- 8.Pandha HS, John RJ, Hutchinson J, James N, Whelan M, Corbishley C, Dalgleish AG. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells: a phase I/II study. BJU Int. 2004;94:412–418. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata S, Okano S, Yonemitsu Y, Onimaru M, Sata S, Nagata-Takeshita H, Inoue M, Zhu T, Hasegawa M, Moroi Y, et al. Induction of efficient antitumor immunity using dendritic cells activated by Sendai virus and its modulation of exogenous interferon-β gene. J Immunol. 2006;177:3564–3576. doi: 10.4049/jimmunol.177.6.3564. [DOI] [PubMed] [Google Scholar]
- 11.Yoneyama Y, Ueda Y, Akutsu Y, Matsunaga A, Shimada H, Kato T, Kubota-Akizawa M, Okano S, Shibata S, Sueishi K, et al. Development of immunostimulatory virotherapy using non-transmissible Sendai virus-activated dendritic cells. Biochem Biophys Res Commun. 2007;355:129–135. doi: 10.1016/j.bbrc.2007.01.132. [DOI] [PubMed] [Google Scholar]
- 12.Yonemitsu Y, Kitson C, Ferrari S, Farley R, Griesenbach U, Judd D, Steel R, Scheid P, Zhu J, Jeffery PK, et al. Efficient gene transfer to airway epithelium using recombinant Sendai virus. Nat Biotechnol. 2000;18:970–973. doi: 10.1038/79463. [DOI] [PubMed] [Google Scholar]
- 13.Nagai Y. Paramyxovirus replication and pathogenesis. Reverse genetics transforms understanding. Rev Med Virol. 1999;9:83–99. doi: 10.1002/(sici)1099-1654(199904/06)9:2<83::aid-rmv244>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Markwell MA, Svennerholm L, Paulson JC. Specific gangliosides function as host cell receptors for Sendai virus. Proc Natl Acad Sci USA. 1981;78:5406–5410. doi: 10.1073/pnas.78.9.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moyer SA, Baker SC, Lessard JL. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci USA. 1986;83:5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii KJ, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- 17.Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441(7089):101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 18.Li HO, Zhu YF, Asakawa M, Kuma H, Hirata T, Ueda Y, Lee YS, Fukumura M, Iida A, Kato A, et al. A cytoplasmic RNA vector derived from nontransmissible Sendai viruswith efficient gene transfer and expression. J Virol. 2000;74:6564–6569. doi: 10.1128/jvi.74.14.6564-6569.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doyle GM, Sharief Y, Mohler JL. Prediction of metastatic potential by cancer cell motility in the Dunning R-3327 prostatic adenocarcinoma in vivo model. J Urol. 1992;147:514–518. doi: 10.1016/s0022-5347(17)37291-9. [DOI] [PubMed] [Google Scholar]
- 20.Nihei N, Ichikawa T, Kawana Y, Kuramochi H, Kugo H, Oshimura M, Killary AM, Rinker-Schaeffer CW, Barrett JC, Isaacs JT, et al. Localization of metastasis suppressor gene(s) for rat prostatic cancer to the long arm of human chromosome 10. Genes Chromosome Cancer. 1995;14:112–119. doi: 10.1002/gcc.2870140205. [DOI] [PubMed] [Google Scholar]
- 21.Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 22.Grauer O, Wohlleben G, Seubert S, Weishaupt A, Kampgen E, Gold R. Analysis of maturation states of rat bone marrow-derived dendritic cells using an improved culture technique. Histochem Cell Biol. 2002;117:351–362. doi: 10.1007/s00418-002-0384-4. [DOI] [PubMed] [Google Scholar]
- 23.Lopez CB, Moltedo B, Alexopoulou L, Bonifaz L, Flavell RA, Moran TM. TLR-independent induction of dendritic cell maturation and adaptive immunity by negative-strand RNA viruses. J Immunol. 2004;173:6882–6889. doi: 10.4049/jimmunol.173.11.6882. [DOI] [PubMed] [Google Scholar]
- 24.Hiraoka K, Yamamoto S, Otsuru S, Nakai S, Tamai K, Morishita R, Ogihara T, Kaneda Y. Enhanced tumor-specific long-term immunity of hemagglutinating virus of Japan-mediated dendritic cell-tumor fused cell vaccination by coadministration with CpG oligodeoxynucleotides. J Immunol. 2004;173:4297–4307. doi: 10.4049/jimmunol.173.7.4297. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa T, Okada N, Tsujino M, Gao JQ, Hayashi A, Tsutsumi Y, Mayumi T, Yamamoto A, Nakagawa S. Vaccine efficacy of fusogenic liposomes containing tumor cell-lysate against murine B16BL6 melanoma. Biol Pharm Bull. 2006;29:100–104. doi: 10.1248/bpb.29.100. [DOI] [PubMed] [Google Scholar]
- 26.Trinite B, Voisine C, Yagita H, Josien R. A subset of cytolytic dendritic cells in rat. J Immunol. 2000;165:4202–4208. doi: 10.4049/jimmunol.165.8.4202. [DOI] [PubMed] [Google Scholar]
- 27.Park AY, Scott P. IL-12: keeping cell-mediated immunity alive. Scand J Immunol. 2001;53:529–532. doi: 10.1046/j.1365-3083.2001.00917.x. [DOI] [PubMed] [Google Scholar]
- 28.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-gamma production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 29.Brombacher F, Kastelein RA, Alber G. Novel IL-12 family members shed light on the orchestration of TH1 responses. Trends Immunol. 2003;24:207–212. doi: 10.1016/S1471-4906(03)00067-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt C, Brijs L, Cliquet P, De Baetselier P. Increased IL-12 P40 homodimer secretion by spleen cells during in vivo growth of the BW-19 T cell hybridoma accompanies suppression of natural immunity. Int J Cancer. 1998;77:460–466. doi: 10.1002/(sici)1097-0215(19980729)77:3<460::aid-ijc24>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 31.Pirhonen J, Matikainen S, Julkunen I. Regulation of virus-induced IL-12 and IL-23 expression in human macrophages. J Immunol. 2002;169:5673–5678. doi: 10.4049/jimmunol.169.10.5673. [DOI] [PubMed] [Google Scholar]
- 32.Walter MJ, Kajiwara N, Karanja P, Castro M, Holtzman MJ. Interleukin 12 p40 production by barrier epithelial cells during airway inflammation. J Exp Med. 2001;193:339–351. doi: 10.1084/jem.193.3.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komaru A, Ueda Y, Furuya A, Tanaka S, Yoshida K, Kato T, Kinoh H, Harada Y, Suzuki H, Inoue M, et al. Sustained and NK/CD4+ T-cell-dependent efficient prevention of lung metastasis induced by dendritic cells harboring recombinant Sendai virus. J Immunol. 2009;183:4211–4219. doi: 10.4049/jimmunol.0803845. [DOI] [PubMed] [Google Scholar]
Supplementary References
- 1.Herault O, Colombat P, Domenech J, Degenne M, Bremond JL, Sensebe L, Bernard MC, Binet C. A rapid single-laser flow cytometric method for discrimination of early apoptotic cells in a heterogenous cell population. Br J Haematol. 1999;104:530–537. doi: 10.1046/j.1365-2141.1999.01203.x. [DOI] [PubMed] [Google Scholar]
- 2.Shibata S, Okano S, Yonemitsu Y, Onimaru M, Sata S, Nagata-Takeshita H, Inoue M, Hasegawa M, Moroi Y, Furue M, et al. Induction of efficient antitumor immunity using dendritic cells activated by Sendai virus and its modulation of exogenous interferon-β gene. J Immunol. 2006;177:3564–3576. doi: 10.4049/jimmunol.177.6.3564. [DOI] [PubMed] [Google Scholar]
- 3.Yoneyama Y, Ueda Y, Akutsu Y, Matsunaga A, Shimada H, Kato T, Kubota-Akizawa M, Okano S, Shibata S, Sueishi K, et al. Development of immunostimulatory virotherapy using non-transmissible Sendai virus-activated dendritic cells. Biochem Biophys Res Commun. 2007;355:129–135. doi: 10.1016/j.bbrc.2007.01.132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.