Abstract
Activation of extracellular signal-regulated kinase (ERK) and dopamine- and cAMP-regulated phosphoprotein (DARPP-32) pathways has been implicated in biochemical and behavioral effects induced by various drugs of abuse. In this study, we investigated the phosphorylation pathways of these two proteins in response to acute cocaine administration. A single cocaine administration (30 mg/kg) increased ERK-mediated signaling proteins, phosphoryation of cAMP response element-binding protein (CREB) kinase, pp90 ribosomal S6 kinase (RSK), and c-Fos protein levels in the caudate/putamen of Fischer rats. Acute cocaine administration also induced phosphorylation of the striatal-enriched protein tyrosine phosphatase (STEP) and decreased the phosphorylation of DARPP-32 protein at the Thr-75 site. The phosphorylation states of these inhibitors of ERK and DARPP-32 proteins may thus contribute to the effects of cocaine on ERK- and DARPP-32-mediated cascades, on gene expression and on behaviors.
Keywords: cocaine, ERK, Elk-1, DARPP-32, RSK, STEP
1. Introduction
Cocaine is primarily a drug of abuse in Western countries. The dopaminergic inputs from the ventral tegmental area to the nucleus accumbens and the nigrostriatal projections to the caudate-putamen (CPu) have been attributed to behavioral and biochemical effects of cocaine (reviewed in Hyman and Malenka 2001; Koob and Nestler 1997; Spanagel and Weiss 1999). For example, by binding to the dopamine transporter, cocaine leads to a buildup of extracellular dopamine levels in the mesocorticolimbic system (Maisonneuve and Kreek 1994; Reith et al., 1997). In addition, acute cocaine administration has been shown to alter synaptic glutamate concentration in the CPu (Kalivas and Duffy 1995; Smith et al., 1995; Zhang et al., 2001).
Accumulating studies have been demonstrated that extracellular signal-regulated kinase (ERK), a member of mitogen-activated protein kinase family, is critical in mediating cocaine-induced intracellular mechanisms. For instance, in rodents, both repeated and acute cocaine administrations increase ERK phosphorylation (p-ERK) in the mesocorticolimbic system and CPu (Berhow and Nestler 1996; Jenab et al., 2005; Valjent et al., 2000, 2004, 2005, Zhang et al., 2004). ERK1 knockout mice show enhanced behavioral and reward responses to morphine and cocaine (Ferguson et al., 2006; Mazzucchelli et al., 2002). Pharmacological inhibition of mitogen-activated protein kinase/ERK kinase (MEK), the upstream activator of ERK, blunts cocaine-induced p-ERK, ternary complex factor Elk-1 phosphorylation (p-Elk-1), and immediate early gene expression (such as c-Fos), suggesting that MEK activation is necessary for ERK activation and underlying transcriptional mechanisms (reviewed in Lu L et al., 2006; Mattson et al., 2005; Miller and Marshall 2005; Valjent et al., 2000; Zhang et al., 2004). In addition, through pp90 ribosomal S6 kinase (p-RSK) family, p-ERK has been reported to result in cAMP response element-binding protein phosphorylation (p-CREB), a transcriptional factor that has been shown to regulate gene expression (Frodin and Gammeltoft 1999; Xing et al., 1998). Recently, studies have shown that prenatal cocaine treatment caused augmented p-RSKs and p-CREB protein levels in the heart tissue of neonatal rats (Sun and Quamina 2004).
Alternatively, dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa (DARPP-32), a cytosolic protein enriched in medium spiny neurons of CPu, is another important regulator in mediating cocaine-induced intracellular signaling (Greengard et al., 1999; Svenningsson et al., 2005). Once DARPP-32 is phosphorylated at Thr-34 site (p-Thr 34 DARPP-32) by means of protein kinase A (PKA), it becomes an inhibitor of protein phosphatase-1 (PP-1) thereby modulating several substrates (Hemmings et al., 1984; Nishi et al., 1997, 2000; Svenningsson et al., 2004). On the other hand, phosphoylation of DARPP-32 at the Thr75 site (p-Thr75 DARPP-32) inhibits PKA and attenuates its capability to increase p-Thr34 DARPP-32 (Bibb et al., 1999; Nairn et al., 2004). In vitro, dopamine D1 receptor activation has been shown to activate p-Thr34 and inhibit p-Thr75 DARPP-32 via PKA-activated protein phosphatase 2A (PP-2A; Nishi et al., 2000). In the mesocorticolimbic system and CPu, acute cocaine administration transiently increases p-Thr34 and decreases p-Thr75 DARPP-32 with correspondent elevation of extracellular dopamine levels (Nishi et al., 2000; Rauggi et al., 2005). Furthermore, studies have shown that DARPP-32 mutant mice display attenuated behavioral effects and blunted p-ERK in response to cocaine treatment, suggesting that DARPP-32 is an upstream regulator for ERK-mediated signaling (Fienberg et al., 1998; Valjent et al., 2005; Zachariou et al., 2006).
One possible candidate to link the DARPP-32- and ERK-mediated cascades is striatal-enriched protein tyrosine phosphatase (STEP), preferentially expressed in mesocorticolimbic system and CPu (Boulanger et al., 1995; Lombroso et al., 1993). Through direct interaction of a kinase-interacting motif, STEP and its non-neuronal homologues have been implicated in the dephosphorylation of p-ERK and prevention of its nuclear translocation (Nika et al., 2004; Zuniga et al., 1999). Stimulation of PKA has been reported to phosphorylate STEP (p-STEP) and decrease its capability to dephosphorylate p-ERK (Nika et al., 2004; Paul et al., 2000). Recently, Valjent et al. (2005) demonstrated that, in the CPu, acute amphetamine administration increases p-STEP and p-ERK in mice, whereas, similar activation is abolished in DARPP-32 mutant mice. Thus, psychostimulant-activated DARPP-32 signaling may control p-ERK signaling through activation of STEP.
On this basis, the aim of our study was to systematically investigate ERK- and DARPP-32-mediated signaling in the CPu, an area known to regulate cocaine treatment response, after acute cocaine administration.
2. Results
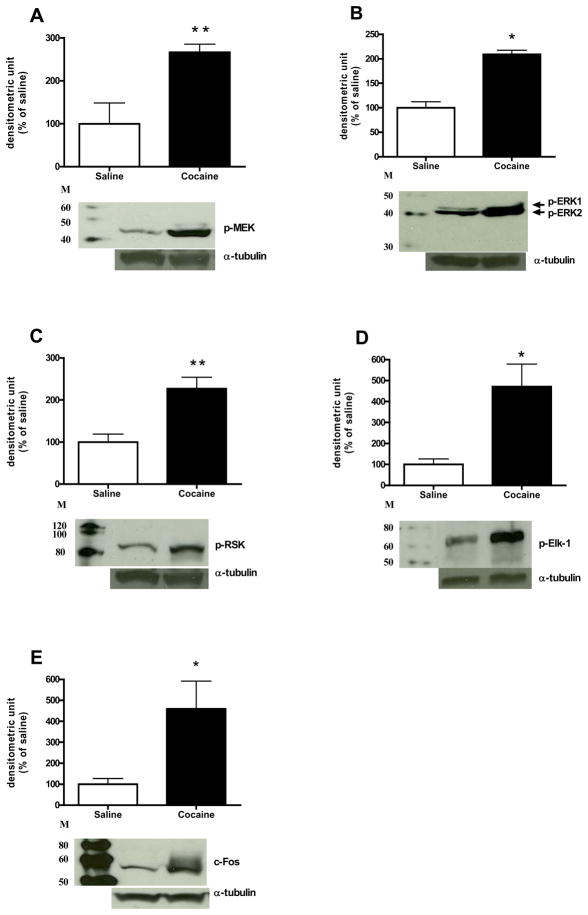
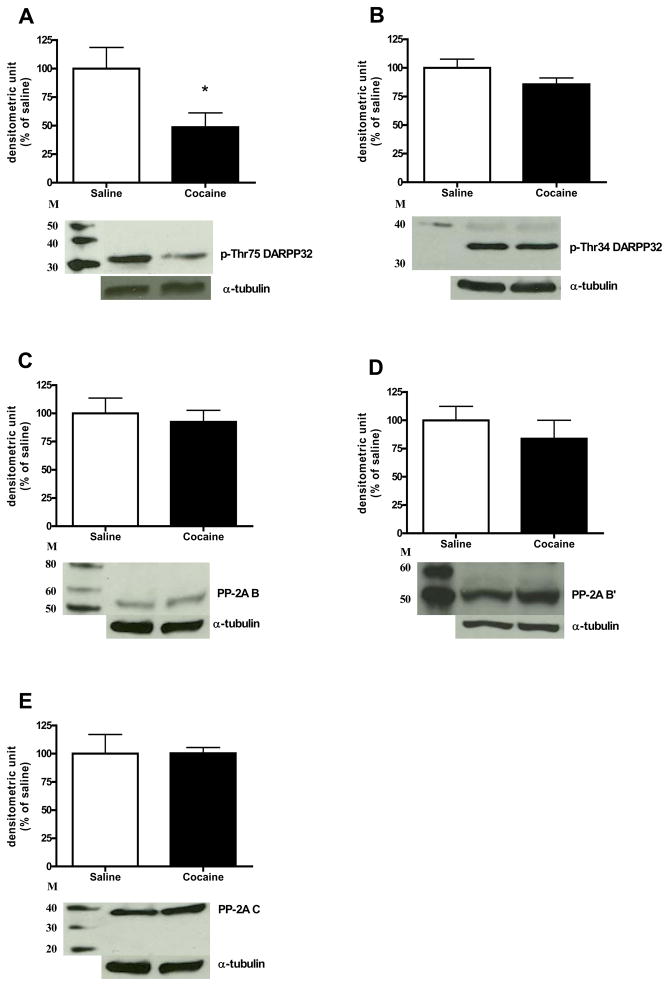
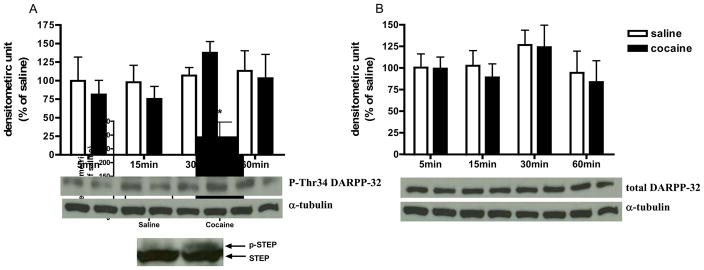
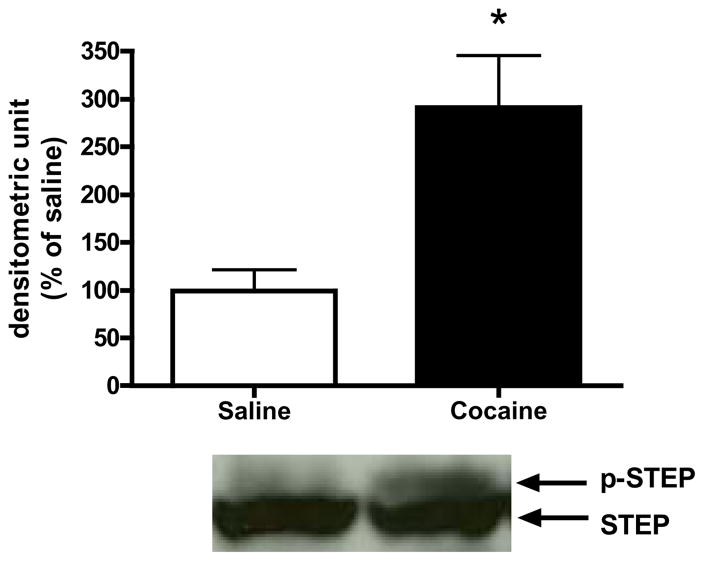
Comparison of control animals treated with saline to animals treated with acute cocaine demonstrated that the administration of 30 mg/kg cocaine significantly increased p-MEK and p-ERK protein levels [t(7) = 3.50, p< 0.01; t(7) = 7.57, p<0.0001; Fig. 1A and B, respectively]. In addition, cocaine also increased p-RSK, p-Elk-1, and c-Fos protein expression in the CPu [t(7) = 3.60, p< 0.01; t(7) = 2.99, p< 0.01; t(6) = 2.66, p< 0.05; Fig. 1C–E, respectively]. For the DARPP-32-mediated cascades, animals with cocaine administration had lower p-Thr75 DARPP-32 protein level compared to the saline control [t(7) = −2.37, p< 0.05; Fig. 2A]. However, the p-Thr34 DARPP-32 protein level was not significantly changed after cocaine (Fig. 2B). No significant changes were observed for PP-2A protein levels including its regulatory subunits, B and B′ subunits, and catalytic subunit, C-subunit (Fig. 2C–E, respectively). As shown in Table 1, the acute cocaine administration did not alter total protein levels for all phospho-proteins that we were measured. Furthermore, time course experiment demonstrated that acute cocaine injection did not alter p-Thr34 DARPP-32 or total DARPP-32 protein expression in the CPu compared to respective saline controls (Fig. 3A and B, respectively). Fig. 4 demonstrates that the phosphorylation of STEP as shown by the decrease of mobility of the 46-kDa STEP, the cytosolic isoform of STEP presented in the CPu (Lombroso et al., 1993; Valjent et al., 2005), was significantly increased after acute cocaine administration [t(6) = 3.27, p< 0.05].
Fig. 1.
Effects of cocaine on ERK pathway. Results represent as protein levels over α-tubulin in the CPu expressed as percentage of saline control (4–5 animals per group). 10 min after rats were given injections: (A) p-MEK; (B) p-ERK (p-ERK1/2) and total ERK1/2; (C) p-RSK; (D) p-Elk-1. 45 min after rats were received injections: (E) c-Fos. M is the molecular marker in kDa. *p <0.05, **p <0.01 and ***p <0.001 as compared with saline group.
Fig. 2.
Effects of cocaine on DARPP-32 pathway. Results represent as protein levels over α-tubulin in the CPu expressed as percentage of saline control (4–5 animals per group). 10 min after rats were given injections: (A) p-Thr75 DARPP-32 and total DARPP-32; (B) p-Thr34 DARPP-32 and total DARPP-32; (C) PP-2A B; (D) PP-2A B′; (E) PP-2A C. M is the molecular marker in kDa. *p <0.05 as compared with saline group.
Table 1.
Effects of acute cocaine on total proteins in the rat CPu
| proteins/treatment | Saline | Cocaine | |
|---|---|---|---|
| MEK | 100.00±6.10 | 95.18±7.40 | 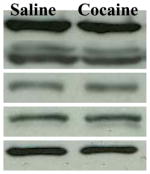 |
| ERK | 100.00±7.91 | 91.47±6.14 | |
| RSK | 100.00±12.39 | 102.69±8.26 | |
| Elk-1 | 100.00±6.74 | 124.38±16.48 | |
| DARPP-32 | 100.00±10.45 | 107.66±6.49 | |
Left: The ratio of total proteins over α-tubulin is expressed relative to saline controls which are set at 100%. Data represent mean ± SEM. Right: Representative Western blots for respective proteins after saline/cocaine injections.
Fig. 3.
The time course of cocaine effects on p-Thr34 DARPP-32. Results represent as protein levels over α-tubulin in the CPu expressed as percentage of saline control (4–6 animals per group). 5, 15, 30, or 60 min after rats were given injections: (A) p-Thr34 DARPP-32; (B) total DARPP-32.
Fig. 4.
Effects of cocaine on STEP. Result represents as p-STEP protein levels over STEP in the CPu expressed as percentage of saline control (4 animals per group) 10 min after injections. *p <0.05 as compared with saline group.
3. Discussion
In line with previous studies demonstrating that acute cocaine induced rapid and transient ERK activation in mice (Valjent et al., 2000, 2004), we further showed that p-MEK, p-ERK and p-Elk-1 protein levels were increased in the CPu of rats. After phosphorylation by p-MEK, p-ERK is able to translocate to the nuclear compartment, where it phosphorylates the ternary complex factor Elk-1 (Gille et al., 1992, 1995). Elk-1 and other ternary complex factors may associate with serum response factor (SRF), dimmerize with c-fos serum response element, and promote its transcription (Davis et al., 2000; Hill et al., 1993; Treisman 1996). Recently, we also demonstrated that the cocaine-induced ERK-mediated signaling is dependent on both dopamine D1 and glutamate NMDA receptors activation (Jenab et al., 2005). Thus, in the CPu, both dopamine and glutamate transmission may converge on the elevation of MEK/ERK/Elk-1 activation, resulting in c-Fos expression after acute cocaine administration.
Consistent with previous studies showing that prenatal cocaine exposure resulted in elevated p-RSK in neonatal heart tissue (Sun and Quamina 2004), p-RSK protein levels were also increased in the CPu after acute cocaine administration in the current study. Both in vitro and in vivo evidence have indicated that ERK activation is required for the phosphorylation of RSK (Alessi et al., 1995; Lazar et al., 1995; Sturgill et al., 1988). RSK has been shown to phosphorylate CREB (Pende et al., 1997; Xing et al., 1996) and up-regulate c-fos expression in an Elk-independent manner (Chen et al., 1993, 1996; De Cesare et al., 1998). Moreover, RSK and the CREB binding protein (CBP) physical interaction has been found in quiescent cells. After ERK activation, the RSK-CBP complex is dissociated allowing p-RSK to phosphorylate CREB, recruit CBP to p-CREB, and subsequently modulate underlying transcriptional mechanisms (Merienne et al., 2001). Together, it indicates that, instead of the ERK/Elk-1 signaling, ERK/RSK/CREB pathway may represent a distinct and/or redundant cascade to induce the c-Fos expression after acute cocaine administration.
Studies in PC12 and hippocampal neuronal cells have demonstrated that PKA-mediated signaling regulates ERK pathway activation (Impey et al., 1998; Roberson et al., 1999; Vossler et al., 1997). Recently, our laboratory and others have demonstrated that cocaine-induced p-ERK is dependent on the dopamine D1 receptor stimulation, which accumulates PKA through the activation of adenylyl cyclase (Jenab et al., 2005; Valjent et al., 2000; Zhang et al., 2004; Zhang and Xu 2006). To evaluate the influence of D1/PKA on ERK signaling, we systemically analyzed the DARPP-32 pathway in response to acute cocaine injections. Previous studies have shown that acute cocaine administration increases p-Thr34 DARPP-32 in the mice neostriatum or in the rat prefrontal cortex and nucleus accumbens (Nishi et al., 2000; Rauggi et al., 2005). However, we did not detect any changes in the dorsal stritum of Fischer rats. Recent study by D’Addario et al. (2007) demonstrated that acute cocaine (10 mg/kg) induced p-Thr34 DARPP-32 in Sprague-Dawley caudate extracts. However, in their study, rats received 5 days of vehicle injections before cocaine administration. In addition, they also used a different strain of rats, the Sprague-Dawley, which have been shown to differ in their response to cocaine than our Fischer rats (Kosten et al., 2007). Strains and/or cocaine injection schedule differences may contribute to the differential p-Thr34 DARPP-32 phosphorylations in the dorsal striatum of rats. On the other hand, the p-Thr75 DARPP-32 was decreased in response to acute cocaine administration. The PKA-activated PP-2A is the major protein phosphatase to downregulate p-Thr75 DARPP-32 in the striatum (Ahn et al., 2007; Nishi et al., 2000). Interestingly, in the current study, the PP-2A protein levels were not changed in the CPu, suggesting that during the early stage after acute cocaine (e.g., 10 min) the reduction of p-Thr75 DARPP-32 is not mediated by PKA activation. However, the inhibitory effect of PP-2A on p-Thr75 DARPP-32 at later time point after acute cocaine cannot be excluded. In addition, other protein phosphatases including PP-1 and PP-2C have been implicated in the dephosphorylation of p-Thr75 DARPP-32 in virto and neostriatal slice (Nishi et al., 2000). The activation of NMDA receptors is essential for acute cocaine-mediated ERK phopsphorylation; however, such receptors activation may dephosphorylate p-Thr75 DARPP-32 in Ca2+-dependent mechanisms (Jenab et al., 2005; Nishi et al., 2002). Thus, the inhibitory effects of NMDA-mediated Ca2+ influx and other protein phosphatases on p-Thr75 DARPP-32 should be further elucidated in the CPu after acute cocaine administration. Taken together, since p-Thr75 DARPP-32 is constitutively activated and has been shown to be a potential inhibitor of PKA, it is reasonable to postulate that disinhibition on PKA activity by attenuated p-Thr75 DARPP-32 may contribute to the ERK-dependent transcription after acute cocaine administration.
By direct protein-protein interaction, STEP has been shown to dephosphorylate p-ERK providing a time-limited activation of ERK (Paul et al., 2000, 2003; Pulido et al., 1998). Phosphorylation of STEP reduces its affinity to p-ERK and causes dissociation between them via activation of D1 receptor and PKA (Blanco-Aparicio et al., 1999; Paul et al., 2000). Veljent et al. (2005) demonstrated that acute amphetamine injections increased p-STEP protein level, the decreased mobility of the 46-kDa isoform of STEP, with the activation of ERK in the CPu of mice. In the present study, we also showed that acute cocaine induced elevated p-STEP protein level, indicating that release of the inhibitory effect of STEP may promote the ERK phosphorylation stimulated by psychostimulants.
Finally, previous studies indicated that the induction of p-STEP is presumably dependent on the activation of p-Thr34 DARPP-32/PP-1 pathway (Girault et al., 2006; Valjent et al., 2005). However, in our analysis of CPu extracts, we did not detect any changes in p-Thr34 DARPP-32, suggesting that other molecular signaling cascades may contribute to STEP phosphorylation. In addition, application of protein phosphatase including PP-2A has been shown to dephosphorylate hematopic protein tyrosine phosphatase, a non-neuronal homolog of STEP, in T-cells (Nika et al., 2004). Thus, the unaltered PP-2A protein levels after acute cocaine administration in CPu could be partially explained by the net balance between the PKA-driven PP-2A elevation (an inhibitory effect on p-Thr75 DARPP-32) and reduction of PP-2A inhibitory effects on p-STEP.
In summary, at least two intracellular signaling pathways, the PKA/DARPP-32 and ERK pathways, have been shown to participate in the regulation of locomotor behavior by dopamine transmission (Greengard et al., 1999; Valjent et al., 2005). Here, we demonstrated that both pathways were modulated by acute cocaine administration and correlated immediate early gene expression was also enhanced in the CPu which may contribute to the initiation of cocaine induced behavioral effects.
4. Method
4.1. Animals
60-day-old male Fischer rats (Charles River, Raleigh, NC) were individually housed in Plexiglas chambers (20 × 20 × 41 cm). Rats were maintained on a 12-hour light/dark cycle (lights on at 9:00 a.m.) with free access to food and water. Animal care and use was in accordance with the Guide for the Care and Use of Laboratory Animals (NIH publication 85-23, Bethesda, MD) and approved by the Institutional Animal Care and Use Committee of Hunter College.
4.2. Drug and antibodies
Cocaine hydrochloride was purchased from Sigma chemical Co. (St. Louis, MO). Primary antibodies of p-Thr34 and p-Thr75 DARPP-32 were purchased from Phosphosolutions (Aurora, CO). Antibodies for p-MEK, MEK, p-ERK, ERK, DARPP-32, PP-2A B (PP-2A regulatory B subunit), PP-2A C (PP-2A catalytic subunit), p-RSK, RSK, p-Elk-1, Elk-1 and c-Fos were bought from Cell Signaling Technologies (Beverly, MA). Antibodies against STEP and PP-2A B′ (PP-2A regulatory B′ subunit) were from Upstate Group Inc. (Waltham, MA). α-tubulin antibody was purchased from Santa Cruz Technologies (Santa Cruz, CA). Both horseradish peroxidase-conjugated anti-rabbit IgG and anti-mouse IgG were purchased from Amersham Pharmacia (Piscataway, NJ).
4.3. Drug administration
Cocaine solutions were prepared by dissolution in physiological saline (0.9%) and injected intra-peritoneally (i.p.). Rats received an injection of saline (1 ml/kg) or cocaine (30 mg/kg) and sacrificed 5, 10, 15, 30, 45, or 60 min later. P-Thr34 DARPP-32 protein levels were evaluated by the time course design. c-Fos protein expression was determined 45 min after cocaine administration. The 10 min samples were used for detecting all the other proteins.
4.4. Protein preparation and measurement
After decapitation (following a brief 20 s exposure to CO2), rat brains were removed, flash frozen in 2-methylbutane (−40° C), and stored at −80° C until used. The coronal slices (1mm thick) were cut out in a matrix (ASI instruments, Warren, MI) and CPu was dissected out on a cold glass plate. CPu was homogenized by using a Polytron handheld homogenizer (Kinematica, Luzern, Switzerland) in lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 10% Glycerol, 1% Triton X-100, 1% Igepal CA-630, 1% sodium dexycholic acid) containing phosphatase inhibitors mixture. After 30 min incubation, homogenates were centrifuged at 13,000 rpm for 15 min at 4° C. Supernatants were then collected and stored at −80° C until used. Total protein content was determined using a Bradford kit from Bio-Rad Laboratories (Hercules, CA).
4.5. Western blot analysis
Protein samples were analyzed by using Western bolt as previously described (Jenab et al., 2005). Briefly, 40 μg of protein extracts were boiled in Lammeli buffer containing 1% β-mercaptoethanol for 5 min and ran on SDS-PAGE gels, transferred to PVDF membranes. Membranes were then blocked with 5% nonfat dry milk for 1hr at room temperature and incubated with antibodies of MEK (1:1000), p-MEK (1:500), ERK (1:1000), p-ERK (1:1000), RSK (1:1000), p-RSK (1:1000), Elk-1 (1:1000), p-Elk-1 (1:500), c-Fos (1:1000), DARPP-32 (1:1000), p-Thr34 DARPP-32 (1:1000), p-Thr75 DARPP-32 (1:1000), PP-2A B (1:2000), PP-2A B′ (1:1000), PP-2A C (1:5000), or STEP (1:2000) overnight at 4° C. After three washes with Tris-Tween-20 Buffer (TBST; pH = 7.4), membranes were incubated with their appropriate secondary antibodies (1:1000) for 1hr at room temperature followed by three more washes with TBST. Antibody binding was detected by using an enhanced chemiluminescence kit (ECL; Amersham Pharmacia, Piscataway, NJ). Intensity of protein bands was quantified with a computer densitometer and Image Quant Program (Molecular Dynamics). For normalization of protein levels, all membranes were re-probed with α-tubulin antibody (1:1000).
4.6. Statistical analysis
Protein levels were expressed as a ratio to α-tubulin levels. For p-STEP, the protein level was normalized by its non-phophospholated isoforms. Data was expressed as mean±SEM relative to saline controls, which were arbitrarily set at 100%. Student’s t-tests were used to determine differences between cocaine- and saline-treated animals. Determination of statistically significant differences was considered at 0.05 level.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56{delta} subunit. Proc Acad Sci USA. 2007;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Berhow MT, Nestler EJ. Regulation of ERK (extracellular signal regulated kinase) part of the neurotrophin signal transduction cascade, in the rat mesolimibic dopamine system by chronic exposure to morphine or cocaine. J Neurosci. 1996;16:4707–4715. doi: 10.1523/JNEUROSCI.16-15-04707.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Nairn AC, Greegard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Blanco-Aparicio C, Torres J, Pulido R. A novel regulatory mechanism of MAP kinases activation and nuclear translocation mediated by PKA and the PTP-SL tyrosine phosphatase. J Cell Biol. 1999;147:1129–1136. doi: 10.1083/jcb.147.6.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Abate C, Blenis J. Phosphorylation of the c-Fos transrepression domain by mitogen-activated protein kinase and 90-kDa ribosomal S6 kinase. Proc Acad Sci USA. 1993;90:10952–10956. doi: 10.1073/pnas.90.23.10952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen RH, Juo PC, Curran T, Blenis J. Phosphorylation of c-Fos at the C-terminus enhances its transforming activity. Oncogene. 1996;12:1493–1502. [PubMed] [Google Scholar]
- D’Addario C, Di Benedetto M, Candeletti S, Romualdi P. The kappa-opioid receptor agonist U-69593 prevents cocaine-induced phosphorylation of DARPP-32 at Thr(34) in the rat brain. Brain Res Bull. 2007;73:34–39. doi: 10.1016/j.brainresbull.2007.01.014. [DOI] [PubMed] [Google Scholar]
- Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element binding protein to control long term potentiation dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Cesare D, Jacquot S, Hanauer A, Sassone-Corsi P. Rsk-2 activity is necessary for epidermal growth factor-induced phosphorylation of CREB protein and transcription of c-fos gene. Proc Acad Sci USA. 1998;95:12202–12207. doi: 10.1073/pnas.95.21.12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SM, Fasano S, Yang P, Brambilla R, Robinson TE. Knockout of ERK1 enhances cocaine-evoked immediate early gene expression and behavioral plasticity. Neuropsychopharmacology. 2006;31:2660–2668. doi: 10.1038/sj.npp.1301014. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O’Callaghan JP, Miller DB, Cole DG, Corbett D, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: Regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–841. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Frodin M, Gammeltoft S. Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol Cell Endocrinol. 1999;151:65–77. doi: 10.1016/s0303-7207(99)00061-1. [DOI] [PubMed] [Google Scholar]
- Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb MH, Shaw PE. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gille H, Sharrocks AD, Shaw PE. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: A logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol. 2006;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Greegard P, Allen PB, Nairn AC. Beyond the dopamine receptor: The DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Hill CS, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: The neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloume JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Jenab S, Festa ED, Nazarian A, Wu HB, Sun WL, Hazim R, Russo SJ, Quinones-Jenab V. Cocaine induction of ERK proteins in dorsal striatum of Fischer rats. Brain Res Mol Brain Res. 2005;142:134–138. doi: 10.1016/j.molbrainres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. D1 receptors modulate glutamate transmission in the ventral tegmental area. J Neurosci. 1995;15:5379–5388. doi: 10.1523/JNEUROSCI.15-07-05379.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Zhang XY, Haile CN. Strain Differences in maintenance of cocaine self-administration and their relationship to novelty activity responses. Behav Neurosci. 2007;121:380–388. doi: 10.1037/0735-7044.121.2.380. [DOI] [PubMed] [Google Scholar]
- Lazar DF, Wiese RJ, Brady MJ, Mastick CC, Waters SB, Yamauchi K, Pessin JE, Cuatrecasas P, Saltiel AR. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- Lombroso PJ, Naegele JR, Sharma E, Lerner M. A protein tyrosine phosphatase expressed within dopaminoceptive neurons of the basal ganglia and related structures. J Neurosci. 1993;13:3064–3074. doi: 10.1523/JNEUROSCI.13-07-03064.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Kreek MJ. Acute tolerance to the dopamine response induced by a binge pattern of cocaine administration in male rats: An in vivo microdialysis study. J Pharmacol Exp Ther. 1994;268:916–921. [PubMed] [Google Scholar]
- Mattson BJ, Bossert JM, Simmons DE, Nozaki N, Nagarkar D, Kreuter JD, Hope BT. Cocaine-induced CREB phosphorylation in nucleus accumbens of cocaine-sensitized rats is enabled by enhanced activation of extracellular signal-related kinase, but not protein kinase A. J Neurochem. 2005;95:1481–1494. doi: 10.1111/j.1471-4159.2005.03500.x. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Vantaggiato C, Ciamei A, Fassano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pages G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouyssegur J, Brambilla R. Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal mediated learning and memory. Neuron. 2002;34:807–820. doi: 10.1016/s0896-6273(02)00716-x. [DOI] [PubMed] [Google Scholar]
- Merienne K, Pannetier S, Harel-Bellan A, Sassone-Corsi P. Mitogen-regulated RSK2-CBP interaction controls their kinase and acetylase activities. Mol Cell Biol. 2001;21:7089–7096. doi: 10.1128/MCB.21.20.7089-7096.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Marshall JF. Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron. 2005;47:873–884. doi: 10.1016/j.neuron.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Nairn AC, Svenningsson P, Nishi A, Fisone G, Girault JA, Greengard P. The role of DARPP-32 in the actions of drugs of abuse. Neuropharmacology. 2004;47(Suppl 1):14–23. doi: 10.1016/j.neuropharm.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Nika K, Hyunh H, Williams S, Paul S, Bottini N, Tasken K, Lombroso PJ, Mustelin T. Haematopoietic protein tyrosine phosphatase (HePTP) phosphorylation by cAMP-dependent protein kinase in T-cells: Dynamics and subcellular location. Biochem J. 2004;378:335–342. doi: 10.1042/BJ20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Matsuyama S, Hamada M, Higashi H, Nairn AC, Greengard P. Regulation of DARPP-32 dephosporylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: Distinct roles of calcineurin and protein phosphatase-2A. J Neurochem. 2002;81:832–841. doi: 10.1046/j.1471-4159.2002.00876.x. [DOI] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc Acad Sci USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S, Nairn AC, Wang P, Lombroso PJ. NMDA-mediated activation of the tyrosine phosphatase STEP regulates the duration of ERK signaling. Nat Neurosci. 2003;6:34–42. doi: 10.1038/nn989. [DOI] [PubMed] [Google Scholar]
- Paul S, Snyder GL, Yokakura H, Picciotto MR, Nairn AC, Lombroso PJ. The dopamine D1 receptor mediates the phosphorylation and inactivation of the protein tyrosine phosphatase STEP via a PKA-dependent pathway. J Neurosci. 2000;20:5630–5638. doi: 10.1523/JNEUROSCI.20-15-05630.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M, Fisher TL, Simpson PB, Russell JT, Blenis J, Gallo V. Neurotransmitter- and growth factor-induced cAMP response element binding protein phosphorylation in glial cell progenitors: role of calcium ions, protein kinase C, and mitogen-activated protein kinase/ribosomal S6 kinase pathway. J Neurosci. 1997;17:1291–1301. doi: 10.1523/JNEUROSCI.17-04-01291.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R, Zuniga A, Ullrich A. PTP-SL and STEP protein tyrosine phosphatases regulate the activation of the extracellular signal-regulated kinases ERK1 and ERK2 by association through a kinase interaction motif. EMBO J. 1998;17:7337–7350. doi: 10.1093/emboj/17.24.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauggi R, Scheggi S, Cassanelli A, De Montis MG, Tagliamonte A, Gambarana C. The mesolimbic dopaminergic response to novel palatable food consumption increases dopamine-D1 receptor-mediated signaling with complex modifications of the DARPP-32 phosphorylation pattern. J Neurochem. 2005;92:867–877. doi: 10.1111/j.1471-4159.2004.02920.x. [DOI] [PubMed] [Google Scholar]
- Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology. 1997;134:309–17. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Mo Q, Guo H, Kunko PM, Robinson S. Cocaine increases extraneuronal levels of aspartate and glutamate in the nucleus accumbens. Brain Res. 1995;683:264–269. doi: 10.1016/0006-8993(95)00383-2. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Weiss F. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 1999;22:521–527. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- Sturgill TW, Ray LB, Erikson E, Maller JL. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988;334:715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Sun LS, Quamina A. Extracellular receptor kinase and cAMP response element binding protein activation in the neonatal rat heart after perinatal cocaine exposure. Pediatr Res. 2004;56:947–952. doi: 10.1203/01.PDR.0000145279.42838.34. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Nairn AC, Greengard P. DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 2005;7:E353–360. doi: 10.1208/aapsj070235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Nishi A, Fisone G, Girault JA, Nairn AC, Greengard P. DARPP-32: An integrator of neurotransmission. Annu Rev Pharmacol Toxicol. 2004;44:269–296. doi: 10.1146/annurev.pharmtox.44.101802.121415. [DOI] [PubMed] [Google Scholar]
- Treisman R. Regulation of transcription by MAP kinase cascade. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- Valjent E, Corvol JC, Pages C, Besson JM, Maldanado R, Caboche J. Involvement of the extracellular signal regulated kinase cascade for cocaine rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pages C, Herve D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Valjent E, Pascoli V, Svenningsson P, Paul S, Herve E, Corvol JC, Stipanovich A, Caboche J, Lombroso PJ, Nairn AC, Greengard P, Herve D, Girault JA. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci USA. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossler MR, Yao H, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- Xing J, Ginty DD, Greenberg ME. Coupling of the RAS-MAPK pathway to gene activation by RSK2, a growth factor-regulated CREB kinase. Science. 1996;273:959–963. doi: 10.1126/science.273.5277.959. [DOI] [PubMed] [Google Scholar]
- Xing J, Kornhauser JM, Xia Z, Thiele EA, Greenberg ME. Nerve growth factor activates extracellular signal-regulated kinase and p38 mitogen-activated protein kinase pathways to stimulate CREB serine 133 phosphorylation. Mol Cell Biol. 1998;18:1946–1955. doi: 10.1128/mcb.18.4.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of DARPP-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zhang J, Xu M. Opposite regulation of cocaine-induced intracellular signaling and gene expression by dopamine D1 and D3 receptors. Ann NY Acad Sci. 2006;1074:1–12. doi: 10.1196/annals.1369.001. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Loonam TM, Noailles PA, Angulo JA. Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann NY Acad Sci. 2001;937:93–120. doi: 10.1111/j.1749-6632.2001.tb03560.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lou D, Jiao H, Zhang D, Wang X, Xia Y, Zhang J, Xu M. Cocaine induced intracellular signaling and gene expression are oppositly regulated by dopamine D1 and D3 receptors. J Neurosci. 2004;24:3344–3354. doi: 10.1523/JNEUROSCI.0060-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga A, Torres J, Ubeda J, Pulido R. Interaction of mitogen-activated protein kinases with the kinase interaction motif of the tyrosine phosphatase PTP-SL provides substrate specificity and retains ERK2 in the cytoplasm. J Biol Chem. 1999;274:21900–21907. doi: 10.1074/jbc.274.31.21900. [DOI] [PubMed] [Google Scholar]