Abstract
Systemic lupus erythematosus (SLE) is more common among women than men with a ratio of about 10 to 1. We undertook this study to describe familial male SLE within a large cohort of familial SLE. SLE families (two or more patients) were obtained from the Lupus Multiplex Registry and Repository. Genomic DNA and blood samples were obtained using standard methods. Autoantibodies were determined by multiple methods. Medical records were abstracted for SLE clinical data. Fluorescent in situ hybridization (FISH) was performed with X and Y centromere specific probes, and a probe specific for the toll-like receptor 7 gene on the X chromosome. Among 523 SLE families, we found five families in which all the SLE patients were male. FISH found no yaa gene equivalent in these families. SLE-unaffected primary female relatives from the five families with only-male SLE patients had a statistically increased rate of positive ANA compared to SLE-unaffected female relatives in other families. White men with SLE were 5 times more likely to have an offspring with SLE than were White women with SLE but there was no difference in this likelihood among Black men. These data suggest genetic susceptibility factors that act only in men.
Keywords: Systemic lupus erythematosus, men, autoantibodies, genetics
INTRODUCTION
An interplay between genetics and environmental factors has been implicated for human autoimmune diseases (1). Collectively, autoimmune diseases are one of the 10 leading causes of death among women less than 65 years of age in the US (2). In fact, autoimmune diseases predominately affect women, with an estimate that SLE, Sjogren's disease, systemic sclerosis and thyroiditis patients are at least 85% female, altogether (3).
Systemic lupus erythematosus (SLE) is an autoimmune disorder characterized by production of autoantibodies that react with nuclear components. (4,5). SLE has a female predominance of greater than 90% in general (3), and even more so in the childbearing years, where the female-to-male ratio has been reported as high as 12:1 (6). Men and women with SLE present with different clinical and serological profiles with men having more severe disease (7). The influence of hormones and chronobiology has been explored, but the non-hormonal influences of menstrual cycles and other unrecognized variables are only just being explored (8). Recent data suggest that number of X chromosomes rather than sex may be critically important for the sex predilection of SLE (9,10)
Familial aggregation of SLE has been demonstrated in the general population with 7-12% of SLE patients having a first degree relative with SLE in comparison to an average prevalence of 1 lupus patient per 2000 population in Europe (11). A high concordance rate of up to 20% among monozygotic twins also suggests a strong genetic component in the development of SLE (11,12). There are now multiple susceptibility genes identified by genetic linkage or association (13,14).
Genetics in animal models of lupus have also contributed to the knowledge of disease susceptibility. Multiple loci for susceptibility to lupus have been mapped for inbred strains of mice that consistently develop the disease (15). One such strain is BxSB, in which the lupus-like illness is both more common and more severe in males (16). Susceptibility to lupus in this strain has been mapped to the Y chromosome and the locus named yaa (y associated autoimmunity) since 1994. Recently, two independent reports have shown that an unequal crossover between the X and Y chromosome has resulted in a translocation of a syntenic 4 megabase region of the X chromosome to the Y chromosome, and this region contains the toll-like receptor 7 (TLR7) gene. Therefore, male mice of this strain have a 2-fold overexpression of TLR7, which was demonstrated sufficient to dysregulate TLR7-mediated activation of innate immune responses. Thus, these studies demonstrate that the yaa gene responsible for the susceptibility to a lupus-like illness in these mice is in fact an overexpression of TLR7. However, a recent investigation of 44 men and 55 women with SLE did not find an increased copy number of the TLR7 gene compared to matched controls (17).
Even though mouse models provide important insights into human immune function and disease, their mechanisms require careful validation, since many known immunological differences exist between the two species (18). TLR7 is located in a syntenic region of the X chromosome in humans and mice; thus, an unequal crossover between X and Y in humans could result in a yaa equivalent. The previous study of TLR7 copy number was in unrelated SLE patients (17). We hypothesized that if a yaa equivalent exists in humans with SLE, then the most likely scenario in which to find this putative susceptibility gene would be families where men sharing a Y chromosome had SLE.
Thus, we undertook this study to describe families in which the SLE patients are males. In particular, we wished to determine clinical differences in SLE among these men as well as determine the presence of a yaa gene equivalent.
METHODS
Patient collection methods
Families studied for this project were obtained from the collection of patients with SLE from the Lupus Family Registry and Repository (LFRR) based at the Oklahoma Medical Research Foundation (OMRF) (7,19,20). Recruitment is conducted following protocols approved by Institutional Review Boards of both OMRF and the University of Oklahoma Health Sciences Center. Informed consent is obtained from all participants before collection of relevant materials including medical charts and blood samples. A patient is recruited following a phone interview and an extensive review of medical records by a reviewer with a medical background. A patient thus enrolled must meet at least four of the 1982 American College of Rheumatology classification criterion to qualify (21,22). Recruiting efforts also involve enrolment of affected family members of the proband. To increase the capacity of studying genetic linkage or association, grandparents, parents, and siblings without lupus are also recruited. A blood sample is collected from all participants. As previously described by Moser et al (1998), genomic DNA is isolated by use of standard methods (20). A second set of families with SLE meeting the 1982 ACR criteria was studied as a confirmatory cohort (23).
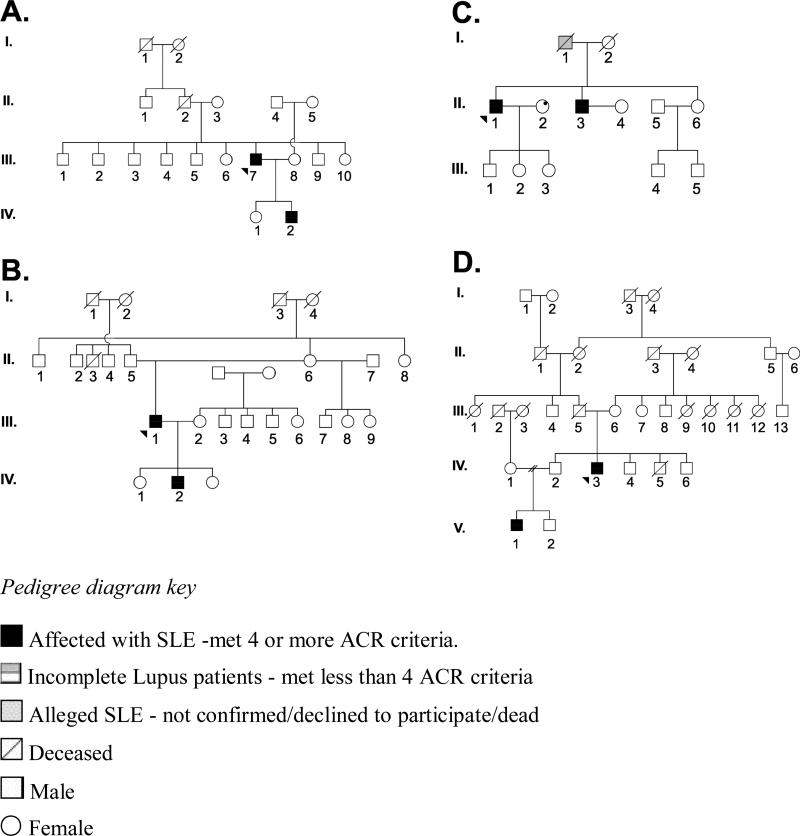
For this project, we identified all families in which the SLE affected individuals were males and only males, and where there were at least two SLE affected male patients. An alleged SLE-affected patient is one for whom the diagnosis could not be confirmed. In the case Family C in Figure 1, the alleged SLE patient, who was a man, was deceased when the proband was recruited to the study; thus, classification as a confirmed SLE could not be obtained. The Institutional Review Board of the OMRF has approved the use of the family tree diagrams of these families for this paper. In addition to these male only families, we have also studied families with affected male identical twins.
Figure 1.
Family Tree diagrams of families with male-only member SLE
Healthy family member in these families reported no known history of SLE and had no symptoms suggestive of the diagnosis of SLE as assessed by questionnaire.
Serology
Serology was performed at the OMRF Clinical Immunology Laboratory. Indirect imunofluorescence (24) was used to analyze ANA titers by use of a HEp-2 substrate. Anti-double stranded DNA was determined using Farr assay (25), and confirmed by Crithidia lucilliae kinetoplast immunofluorescence method (26). Antibodies to extractable nuclear antigens were determined by Ouchterlony agar gel immunodiffusion (27). ELISA was used to assay anticardiolipin IgM and IgG (28). Medical records were also abstracted for information about pre-existing laboratory results for these antibodies and used for determining the ACR criteria these patients met.
Autoantibody analysis was also done using the Bio-Rad BioPlex 2200 ANA (Bio-Rad, Hercules, CA), which is a fully automated clinical laboratory analysis tool. The BioPlex ANA Screen utilizes multiplex technology and dyed magnetic beads to simultaneously perform automated measurements of 13 autoantibodies. Undiluted sera samples were loaded onto the BioPlex 2200 following the recommendations of the manufacturer. This method was used to detect antibodies against any of the following antigens: 60 kD Ro (or SS-A 60), 52kD Ro (or SS-A52), La (or SS-B), SmRNP complex, Sm, RNP 68, RNP A, centromere B, Scl-70 (topoisomerase 1), Jo-1, chromatin, dsDNA, and ribosomal P. The centromere B, Jo-1, nRNP 68, nRNP A, Scl-70 (topoisomerase 1), and 52kD Ro were produced recombinantly, while dsDNA was synthesized by polymerase chain reaction, and the remaining antigens were affinity purified. The BioPlex 2200 ANA Screen reports a semi-quantitative value from 0-8, termed the antibody index (AI), for each autoantibody. The positive cut-off for each assay is established by the manufacturer to equal 1.0 AI for each assay, except for dsDNA which is a quantitative assay reporting IU/mL (normal cut-off is 10 IU/mL).
This analysis was also performed upon available sera of 12 healthy first degree female relatives from the male-only SLE families. Furthermore, these 12 healthy family members were matched on basis of ethnicity, gender and age within five years with four additional sets or 48 individual healthy family members not belonging to these male-only families.
FISH
Fluorescent in situ hybridization was performed with X and Y centromere specific probes and a probe specific for the toll-like receptor 7 gene on the X chromosome. (Vysis, Inc., Downers Grove, IL). Typing was done at 256 single nucleotide polymorphism (SNPs) from the non-pseudoautosomal regions of the X chromosomes, using the 10K GeneChip Array (Affymetrix Inc., Santa Clara, CA).
RESULTS
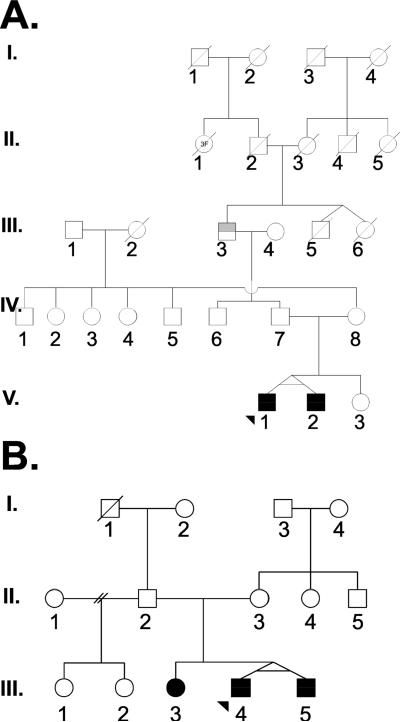
Over the last almost 20 years, we have been identifying and collecting material on families with two or more SLE patients as part of the Lupus Family Registry and Repository (LFRR) (20). Among 523 of these families, there are 1146 SLE patients of whom 115 (10%) are males. There are 245 families consisting of SLE-affected siblings, while the remaining families are multigenerational. Of the 523 families, we find that 5 (0.96%) are made up of only male SLE patients (Figure 1 and 2). Three of the families are multigenerational while 2 contain sibling brothers (Figure 1), one of which contains identical twins (Figure 2). Three of the male-only SLE families were self identified as White Americans, one as Black Americans, and one as American Indian.
Figure 2.
Families with identical male twins affected with SLE
We have also examined male twins in our collection of SLE families. There is one male-only SLE family that consists of identical twin brothers, while there is a second family with identical twin brothers as well as an SLE-affected sister (Figure 2). Of note, these are the only sets of identical twin boys with SLE in our cohort, and both sets are concordant for disease. There are 13 sets of female identical twins in which at least one of the two met criteria for SLE. Among these 13 sets of twins, six are concordant for SLE and seven are discordant for disease. All previously reported identical twins with SLE are female and the concordance rate is about 15% on aggregate (29).
We have examined another set of families with 2 or more SLE patients for families in which only males are affected with SLE. Among a total of 428 families, there are four where all the SLE patients were male. This included two sets of SLE-affected brothers and two father-son pairs. Thus, in this second group of SLE families, approximately 1% contained male-only SLE-affected members. This finding is similar to that in our cohort where 5 (0.96%) of 523 families had only males affected with SLE.
We next examined the clinical features of SLE among the patients and their relatives in the all-male families. The tabulated clinical features of disease (Table 1) demonstrate no clear pattern of more serious or worse disease among these men compared to men from families with SLE-affected women. Autoantibodies were determined by use of an automated analysis procedure, as well as by traditional methods, in the female first degree relatives in the five all-male SLE families. We found that all 12 of these female first-degree relatives without SLE had a positive ANA by indirect immunofluorescence. Among 48 age and ethnicity matched SLE-unaffected female first degree relatives from families with female SLE patients, only 19 had a positive ANA (χ2=11.7, p=0.0006). Antibodies to specific extractable nuclear antigens were uncommon in both groups and there was no difference between the female first-degree relatives from the all-male SLE families and the families with female SLE. Among the male SLE patients in these families anti-dsDNA was very common, while renal disease was found in only two of these men (Table 1).
Table 1.
Clinical features of SLE-affected subjects in the all-male SLE families.
| Family ID | Individual | Clinical features of pedigree members with SLE |
|---|---|---|
| 1 A | III 7 | Discoid rash, photosensitivity, arthritis, hematologic disorder immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA, anti-Sm, anti-Ro, anti-La, anti-RNP and anti-phospholipid. |
| IV 2 | Malar rash, oral ulcers, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA, anti-Sm and anti-RNP. | |
| 1 B | III 1 | Malar rash, photosensitivity, hematologic disorder, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA, anti-Sm, anti-Ro, anti-RNP and anti-phospholipid. |
| IV 2 | Serositis, hematologic disorder, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA and anti-phospholipid. | |
| 1 C | II 1 | Photosensitivity, arthritis, serositis, hematologic disorder immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA, anti-Ro and anti-La. |
| II 3 | Malar rash, discoid rash, photosensitivity, arthritis, serositis hematologic disorder, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA, anti-Ro, anti-La and anti-phospholipid. | |
| 1 D | IV 3 | Malar rash, photosensitivity, arthritis, serositis, hematologic disorder, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-Sm, anti-Ro, anti-La, anti-RNP and anti-phospholipid. |
| V 1 | Malar rash, arthritis, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA and anti-phospholipid. | |
| 2 A | III 3 | Photosensitivity, arthritis. |
| V 1 | Malar rash, photosensitivity, renal disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA and anti-phospholipid. | |
| V 2 | Malar rash, photosensitivity, arthritis, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA and anti-phospholipid. | |
| 2 B | III 3 | Arthritis, renal disorder, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA and anti-phospholipid. |
| III 4 | Oral ulcers, renal disorder, hematologic disorder, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA, anti-Sm and anti-phospholipid. | |
| III 5 | Malar rash, oral ulcers, renal disorder, immunologic disorder, antinuclear antibodies. Antibodies positive are anti-dsDNA and anti-phospholipid. |
Next, using the entire LFRR cohort of families with more than one SLE patient, we examined parent-offspring sets to determine sex differences. There were 82 mother-daughter pairs, 10 mother-son pairs, 17 father-daughter pairs, and 6 father-son pairs. In total, there were 924 patients of which 78 had a child with SLE. Fifteen of these were fathers and 53 of these were mothers. Thus, 22% of SLE patients who had children with SLE were fathers. White fathers are 4.9 times more likely to have a child with SLE compared to white women but there was no difference between American Black men and women (Table 2). Also, there is a trend toward fathers with SLE having sons with SLE compared to daughters in that of 23 offspring of SLE men, 6 of 23 (26.7%) are male. These data demonstrate that men are over-represented as parents of SLE patient, and tend to have male children with SLE.
Table 2.
Men with SLE are more likely than women with SLE to have SLE –affected offspring.
| A. All families | ||
|---|---|---|
| SLE-affected parent | Child with SLE | No child with SLE |
| Father | 15 | 71 |
| Mother | 53 | 785 |
| B. White families only | ||
|---|---|---|
| SLE-affected parent | Child with SLE | No child with SLE |
| Father | 12 | 35 |
| Mother | 29 | 413 |
χ2=14.4, p<0.0001
X2=19.9, p<0.00001,
odds ratio = 4.9 for men to have SLE-affected child compared to women
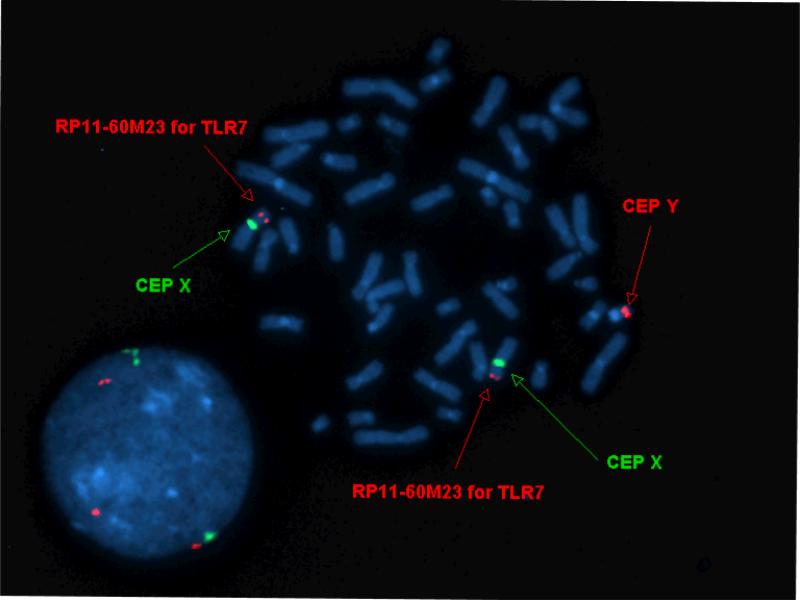
These data suggest a susceptibility factor specific for males. One such factor already defined in the BxSB mouse is the yaa gene as discussed above. We hypothesized that if such a yaa gene equivalent exists in human SLE, it would most likely be found in families with male SLE patients who share a Y chromosome. Therefore, we assessed the men in the all-male SLE families for translocation of the TLR7 gene on to the Y chromosome. For this purpose, we developed a FISH probe that hybridizes to the region of the X chromosome that contains the TLR7 gene. In fact, no patient had hybridization of the probe to the Y chromosome, while this probe did bind all X chromosomes studied (Figure 3). Thus, no male SLE patient in these all-male SLE families had the TLR7 gene translocated to the Y chromosome.
Figure 3.
Representative FISH in which one probe (RP11-60M23) hybridizes with TLR7 containing portion of the X chromosome. Meanwhile, the probes labelled CEP X and CEP Y hybridize with the X and Y chromosomes, respectively. Thus, this study demonstrates the presence of only one copy of the TLR7 gene found on the X chromosome while there is no evidence of a gene segment containing the TLR7 gene on the Y chromosome. This was the finding in all the SLE men studied.
DISCUSSION
Efforts to examine the genetics of SLE have resulted in large collections of families in which more than one person is affected with the disease. We have collected over 500 such families and our colleagues at UCLA have collected nearly 450 such families. Among both these cohorts of SLE families, about 1% consist of only SLE affected men. Considering the rarity of men with SLE, that there are families with only men is initially unexpected. On the other hand, in an idealized situation, if men represent 10% of SLE patients and each family has 2 SLE patients, then by chance alone one expects 1% of families to be male only. Nevertheless, even if not greater than expected by chance, study of families with only males affected by SLE may be fruitful.
In addition to the 5 male-only SLE families in the LFRR, we also found one family where we could confirm one SLE patient who had a brother with incomplete lupus (that is, less than 4 criteria), and yet another family with a confirmed SLE patient who had a brother with reported SLE,. This brother declined to participate in the study, however. No women in either family had member a putative or a confirmed diagnosis of SLE. While perhaps interesting, these two families with putative all-male SLE were not considered further, and were not included in the analyses.
SLE in men is different than that found in women, although all studies do not agree on this point (30). In general, SLE is more severe in men, with renal disease, serious haematological disease such as thrombocytopenia and autoimmune haemolytic anemia as well as neurological disease more likely in men than women (31-35). Several studies have found that SLE in men is more likely to present with serositis than SLE in women (33,36-39). An explanation of why SLE is more severe in men has not been achieved, but genetics may play a role. Stein and colleagues found that women with SLE who had a SLE-affected male relative had more severe disease than women without a SLE-affected male relative (7). This finding was in fact made in the LFRR cohort, which the present study has examined.
There are only a few past reports of male SLE families. Lahita and colleagues reported three families in which there were father-son pairs and no other SLE-affected members (40). In 1973, 2 sets of brother with SLE were described (41). While this report is prior to the 1982/1997 revised ACR classification criteria (21,22), upon review of this paper, these men have convincing SLE by present-day criteria (41). Arnett and Shulman's classic paper from 1976 described a father-son pair with SLE (42). One set of identical male twins, which were concordant for SLE, has been described (43). Thus, the twins reported herein bring the total to three sets, all of which are concordant for disease. While the number is low of course, this SLE concordance rate is far above that found for female identical twins.
SLE is a complex genetic disease where a large number of genes contribute to the risk but each individual one of these susceptibility genes imparts only a small increase in relative risk (reviewed in 44). In genetic association studies using single nucleotide polymorphism (SNP) typing, the relative risk for SLE associated with the presence of a specific SNP allele may be only 1.2 to 1.4 (see 45). The men in our families share a Y chromosome but we did not find a yaa gene equivalent (46-48) among these families (Figure 3). These results confirm a recent finding in a cohort of unrelated men with SLE where no significant concordance between the number of relative TLR7 gene copies and the SLE phenotype was found (17). Furthermore, this group did not find any difference in variation by ethnic group. These data suggest that the murine genomic segmental duplication in the TLR7 gene and the translocation of this segment to produce the yaa gene cannot be translated directly to humans with SLE. The contributory role of genetic variants in TLR7 gene on the human SLE phenotype has yet to be explored.
The genetics of autoimmune disease may act at several levels. This has been suggested by study of mice with a lupus-like illness in that an individual susceptibility gene may act by breaking tolerance to self-antigens, by affecting lymphocyte activity or by increasing likelihood of certain manifestations (49). The associations of HLA are stronger for specific autoantibodies than for SLE itself (see 50 and reviewed in 51). So, there may genetics for autoimmune disease in general, for specific diseases, for specific serological or clinical manifestations of a disease (19,50,52).
Could there be genetic factors that contribute to autoimmune disease that only operate in one sex or the other? The data are clear that this is the case for type 1 diabetes mellitus in which men with the disease are much more likely to have offspring with the disease than are women with type 1 diabetes (53). This effect may be mediated through genetic imprinting on chromosome 11 at the susceptibility locus IDDM2, for which allelic variations of the insulin-like growth factor II have been implicated (54).
Our data demonstrate that men with SLE are more likely to be the parents of children eventually affected by SLE than expected. There are several possible reasons to explain this observation. Women with SLE may have infertility problems because of recurrent spontaneous abortion associated with anti-phospholipid antibodies (55,56). Because of lupus nephritis, women may be told by the physicians to not have children, but this is obviously not the case for men with SLE. Conversely, there may be genetic causes for an increase in SLE-affected men fathering children with SLE. There is at least one example of an SLE genetic effect that operates in men but not in women (57). However, not all studies agree on which sex is affected by alleles within this gene (58). In a linkage study the effect was seen only in families with a male SLE patient (59). That men with SLE are more likely to be parents of SLE affected offspring than women may also be explained by the so-called Carter effect, which is defined as a polygenic inheritance model with a sexual dimorphic threshold such that the lesser affected sex is more likely to have children with the phenotype (60,61). The Carter effect is known to operate in multiple sclerosis (62).
Our data suggest that there are factors, genetic or otherwise, that contribute risk to SLE only for men. First, the SLE-unaffected women in the male-only SLE families almost universally had a positive ANA. Their rate of ANA positivity was higher than that found in SLE-unaffected relatives among families with female SLE patients. One can interpret this finding to mean that the break in tolerance that leads to autoimmunity is common between men and women in these male-only families. However, the factor or factors leading to disease are only acting in the men within these families. In addition, we have examined parent-offspring pairs with SLE in the LFRR collection of over 600 families with SLE. We find that men are more likely to be parents of SLE-affected offspring than are women, but that this difference was present only among American White men and not found among American Black men. It is possible that SLE in men is more genetic than in women. That is, men need to have more susceptibility genes than women because maleness is protective. One such protective factor might be the presence of one X chromosome instead of two (10). Determining that there are male-specific genetics may be a difficult task in that the number of men with SLE needed for such a study will be large. Notwithstanding, the data presented herein along with previous reports (57-59) suggest that genetic susceptibility factors act in a sex specific manner. Sorting this out in a genetically complex human disease will be challenging.
In summary, we report that in about 1% of families with SLE all the SLE patients are men. SLE-unaffected women in these families universally have autoantibodies. Along with data that SLE men tend to have children with SLE compared to women with SLE, these data imply that there are susceptibility factors operating in men but not in women in these families.
Acknowledgments
This work has been supported in part by NIH grants AR053734 and AR48204 to RHS.
References
- 1.Castiblanco J, Anaya JM. The nature and nurture of common autoimmunity. Ann N Y Acad Sci. 2007;1109:1–8. doi: 10.1196/annals.1398.001. [DOI] [PubMed] [Google Scholar]
- 2.Walsh SJ, Rau LM. Autoimmune diseases: a leading cause of death among young and middle-aged women in the United States. Am J Public Health. 2000;90:1463–1466. doi: 10.2105/ajph.90.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev. 2003;2:119–125. doi: 10.1016/s1568-9972(03)00006-5. [DOI] [PubMed] [Google Scholar]
- 4.Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–1368. doi: 10.1056/NEJM199805073381906. 1998. [DOI] [PubMed] [Google Scholar]
- 5.Kurien BT, Scofield RH. Autoantibody determination in the diagnosis of systemic lupus erythematosus. Scand J Immunol. 2006;64:227–235. doi: 10.1111/j.1365-3083.2006.01819.x. [DOI] [PubMed] [Google Scholar]
- 6.Ramsey-Goldman R, Manzi S. Women and Health. Academic Press; 2000. Systemic lupus erythematosis. p. 704. [Google Scholar]
- 7.Stein CM, Olson JM, Gray-McGuire C, Bruner GR, Harley JB, Moser KL. Increased prevalence of renal disease in systemic lupus erythematosus families with affected male relatives. Arthritis Rheum. 2002;46:428–435. doi: 10.1002/art.10105. [DOI] [PubMed] [Google Scholar]
- 8.McMurray RW, May W. Sex hormones and systemic lupus erythematosus: review and meta-analysis. Arthritis Rheum. 2003;48:2100–2110. doi: 10.1002/art.11105. [DOI] [PubMed] [Google Scholar]
- 9.Smith-Bouvier DL, Divekar AA, Sasidhar M, Du S, Tiwari-Woodruff SK, King JK, Arnold AP, Singh RR, Voskuhl RR. A role for sex chromosome complement in the female bias in autoimmune disease. J Exp Med. 2008;205:1099–1108. doi: 10.1084/jem.20070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scofield RH, Bruner GR, Namjou B, Kimberly RP, Ramsey-Goldman R, Petri M, Reveille JD, Alarcon GS, Vila LM, Reid J, Harris B, Li S, Kelly JA, Harley JB, Danchenko N, Satia JA, Anthony MS. Klinefelter's syndrome (47,XXY) in male systemic lupus erythematosus patients: support for the notion of a gene-dose effect from the X chromosome. Arthritis Rheum. 2008;58:2511–7. doi: 10.1002/art.23701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Arthritis Rheum. 2008;58:2511–2517. [Google Scholar]
- 12.Deapen D, Escalante A, Weinrib L, Horwitz D, Bachman B, Roy-Burman P, Walker A, Mack TM. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992;35:311–318. doi: 10.1002/art.1780350310. [DOI] [PubMed] [Google Scholar]
- 13.Hom G, Graham RR, Modrek B, Taylor KE, Ortmann W, Garnier S, Lee AT, Chung SA, Ferreira RC, Pant PV, Ballinger DG, Kosoy R, Demirci FY, Kamboh MI, Kao AH, Tian C, Gunnarsson I, Bengtsson AA, Rantapaa-Dahlqvist S, Petri M, Manzi S, Seldin MF, Ronnblom L, Syvanen AC, Criswell LA, Gregersen PK, Behrens TW. Association of systemic lupus erythematosus with C8orf13-BLK and ITGAM-ITGAX. N Engl J Med. 2008;358:900–909. doi: 10.1056/NEJMoa0707865. 2008. [DOI] [PubMed] [Google Scholar]
- 14.Harley JB, arcon-Riquelme ME, Criswell LA, Jacob CO, Kimberly RP, Moser KL, Tsao BP, Vyse TJ, Langefeld CD, Nath SK, Guthridge JM, Cobb BL, Mirel DB, Marion MC, Williams AH, Divers J, Wang W, Frank SG, Namjou B, Gabriel SB, Lee AT, Gregersen PK, Behrens TW, Taylor KE, Fernando M, Zidovetzki R, Gaffney PM, Edberg JC, Rioux JD, Ojwang JO, James JA, Merrill JT, Gilkeson GS, Seldin MF, Yin H, Baechler EC, Li QZ, Wakeland EK, Bruner GR, Kaufman KM, Kelly JA. Genome-wide association scan in women with systemic lupus erythematosus identifies susceptibility variants in ITGAM, PXK, KIAA1542 and other loci. Nat Genet. 2008;40:204–210. doi: 10.1038/ng.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kono DH, Theofilopoulos AN. Genetics of SLE in mice. Springer Semin Immunopathol. 2006;28:83–96. doi: 10.1007/s00281-006-0030-7. [DOI] [PubMed] [Google Scholar]
- 16.Andrews BS, Eisenberg RA, Theofilopoulos AN, Izui S, Wilson CB, McConahey PJ, Murphy ED, Roths JB, Dixon FJ. Spontaneous murine lupus-like syndromes. Clinical and immunopathological manifestations in several strains. J Exp Med. 1978;148:1198–1215. doi: 10.1084/jem.148.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelley J, Johnson MR, Alarcon GS, Kimberly RP, Edberg JC. Variation in the relative copy number of the TLR7 gene in patients with systemic lupus erythematosus and healthy control subjects. Arthritis Rheum. 2007;56:3375–3378. doi: 10.1002/art.22916. [DOI] [PubMed] [Google Scholar]
- 18.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 19.Sestak AL, Shaver TS, Moser KL, Neas BR, Harley JB. Familial aggregation of lupus and autoimmunity in an unusual multiplex pedigree. J Rheumatol. 1999;26:1495–1499. [PubMed] [Google Scholar]
- 20.Moser KL, Neas BR, Salmon JE, Yu H, Gray-McGuire C, Asundi N, Bruner GR, Fox J, Kelly J, Henshall S, Bacino D, Dietz M, Hogue R, Koelsch G, Nightingale L, Shaver T, Abdou NI, Albert DA, Carson C, Petri M, Treadwell EL, James JA, Harley JB. Genome scan of human systemic lupus erythematosus: evidence for linkage on chromosome 1q in African-American pedigrees. Proc Natl Acad Sci U S A. 1998;95:14869–14874. doi: 10.1073/pnas.95.25.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 22.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 23.Tsao BP, Cantor RM, Grossman JM, Kim SK, Strong N, Lau CS, Chen CJ, Shen N, Ginzler EM, Goldstein R, Kalunian KC, Arnett FC, Wallace DJ, Hahn BH. Linkage and interaction of loci on 1q23 and 16q12 may contribute to susceptibility to systemic lupus erythematosus. Arthritis Rheum. 2002;46:2928–2936. doi: 10.1002/art.10590. [DOI] [PubMed] [Google Scholar]
- 24.Holborow EJ, Weir DM, Johnson GD. A serum factor in lupus erythematosus with affinity for tissue nuclei. Br Med J. 1957;2:732–734. doi: 10.1136/bmj.2.5047.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pincus T, Schur PH, Rose JA, Decker JL, Talal N. Measurement of serum DNA-binding activity in systemic lupus erythematosus. N Engl J Med. 1969;281:701–705. doi: 10.1056/NEJM196909252811304. [DOI] [PubMed] [Google Scholar]
- 26.Aarden LA, de Groot ER, Feltkamp TE. Immunology of DNA. III. Crithidia luciliae, a simple substrate for the determination of anti-dsDNA with the immunofluorescence technique. Ann N Y Acad Sci. 1975;254:505–515. doi: 10.1111/j.1749-6632.1975.tb29197.x. [DOI] [PubMed] [Google Scholar]
- 27.Clark G, Reichlin M, Tomasi TB., Jr. Characterization of a soluble cytoplasmic antigen reactive with sera from patients with systemic lupus erythmatosus. J Immunol. 1969;102:117–122. [PubMed] [Google Scholar]
- 28.Harris EN. Antiphospholipid antibodies. Br J Haematol. 1990;74:1–9. doi: 10.1111/j.1365-2141.1990.tb02530.x. [DOI] [PubMed] [Google Scholar]
- 29.Block SR. A brief history of twins. Lupus. 2006;15:61–64. doi: 10.1191/0961203306lu2263ed. [DOI] [PubMed] [Google Scholar]
- 30.Ward MM, Studenski S. Systemic lupus erythematosus in men: a multivariate analysis of gender differences in clinical manifestations. J Rheumatol. 1990;17:220–224. [PubMed] [Google Scholar]
- 31.Andrade RM, Alarcon GS, Fernandez M, Apte M, Vila LM, Reveille JD. Accelerated damage accrual among men with systemic lupus erythematosus: XLIV. Results from a multiethnic US cohort. Arthritis Rheum. 2007;56:622–630. doi: 10.1002/art.22375. [DOI] [PubMed] [Google Scholar]
- 32.Garcia MA, Marcos JC, Marcos AI, Pons-Estel BA, Wojdyla D, Arturi A, Babini JC, Catoggio LJ, arcon-Segovia D. Male systemic lupus erythematosus in a Latin-American inception cohort of 1214 patients. Lupus. 2005;14:938–946. doi: 10.1191/0961203305lu2245oa. [DOI] [PubMed] [Google Scholar]
- 33.Arbuckle MR, James JA, Dennis GJ, Rubertone MV, McClain MT, Kim XR, Harley JB. Rapid clinical progression to diagnosis among African-American men with systemic lupus erythematosus. Lupus. 2003;12:99–106. doi: 10.1191/0961203303lu334oa. [DOI] [PubMed] [Google Scholar]
- 34.Aranow C, Del GJ, Barland P, Weinstein A. Systemic lupus erythematosus disease severity in men and women: a case-control study. J Rheumatol. 2002;29:1674–1677. [PubMed] [Google Scholar]
- 35.Poduval RD, Bananian S, Kumar KS, Fomberstein B. Systemic lupus erythematosus in males: a retrospective study with a review of the literature. J Gend Specif Med. 2000;3:29–32. [PubMed] [Google Scholar]
- 36.Koh WH, Fong KY, Boey ML, Feng PH. Systemic lupus erythematosus in 61 Oriental males. A study of clinical and laboratory manifestations. Br J Rheumatol. 1994;33:339–342. doi: 10.1093/rheumatology/33.4.339. [DOI] [PubMed] [Google Scholar]
- 37.Costallat LT, Coimbra AM. Systemic lupus erythematosus in 18 Brazilian males: clinical and laboratory analysis. Clin Rheumatol. 1993;12:522–525. doi: 10.1007/BF02231783. [DOI] [PubMed] [Google Scholar]
- 38.Aydintug AO, Domenech I, Cervera R, Khamashta MA, Jedryka-Goral A, Vianna JL, Hughes GR. Systemic lupus erythematosus in males: analysis of clinical and laboratory features. Lupus. 1992;1:295–298. [PubMed] [Google Scholar]
- 39.Font J, Cervera R, Navarro M, Pallares L, Lopez-Soto A, Vivancos J, Ingelmo M. Systemic lupus erythematosus in men: clinical and immunological characteristics. Ann Rheum Dis. 1992;51:1050–1052. doi: 10.1136/ard.51.9.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lahita RG, Chiorazzi N, Gibofsky A, Winchester RJ, Kunkel HG. Familial systemic lupus erythematosus in males. Arthritis Rheum. 1983;26:39–44. doi: 10.1002/art.1780260107. [DOI] [PubMed] [Google Scholar]
- 41.Spector DA, Jampol LM, Hayslett JP. Report of the familial occurrence of systemic lupus erythematosus in male siblings. Arthritis Rheum. 1973;16:221–224. doi: 10.1002/art.1780160213. [DOI] [PubMed] [Google Scholar]
- 42.Arnett FC, Shulman LE. Studies in familial systemic lupus erythematosus. Medicine. 1976;55:313–22. doi: 10.1097/00005792-197607000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Thai AC, Teoh PC, Feng PH. Systemic lupus erythematosus in a pair of male twins. Singapore Med J. 1980;21:775–777. [PubMed] [Google Scholar]
- 44.Rhodes B, Vyse TJ. General aspects of the genetics of SLE. Autoimmunity. 2007;40:550–559. doi: 10.1080/08916930701510657. [DOI] [PubMed] [Google Scholar]
- 45.Sestak AL, Nath SK, Sawalha AH, Harley JB. Current status of lupus genetics. Arthritis Res Ther. 2007;9:210. doi: 10.1186/ar2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Isitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 48.Krieg AM. The toll of too much TLR7. Immunity. 2007;27:695–697. doi: 10.1016/j.immuni.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 49.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 50.Scofield RH, Frank MB, Neas BR, Horowitz RM, Hardgrave KL, Fujisaku A, McArthur R, Harley JB. Cooperative association of T cell beta receptor and HLA-DQ alleles in the production of anti-Ro in systemic lupus erythematosus. Clin Immunol Immunopathol. 1994;72:335–341. doi: 10.1006/clin.1994.1150. [DOI] [PubMed] [Google Scholar]
- 51.Schur PH. Genetics of systemic lupus erythematosus. Lupus. 1995;4:425–437. doi: 10.1177/096120339500400603. [DOI] [PubMed] [Google Scholar]
- 52.Shoenfeld Y, Gilburd B, bu-Shakra M, Amital H, Barzilai O, Berkun Y, Blank M, Zandman-Goddard G, Katz U, Krause I, Langevitz P, Levy Y, Orbach H, Pordeus V, Ram M, Sherer Y, Toubi E, Tomer Y. The mosaic of autoimmunity: genetic factors involved in autoimmune diseases--2008. Isr Med Assoc J. 2008;10:3–7. [PubMed] [Google Scholar]
- 53.Warram JH, Krolewski AS, Gottlieb MS, Kahn CR. Differences in risk of insulin-dependent diabetes in offspring of diabetic mothers and diabetic fathers. N Engl J Med. 1984;311:149–152. doi: 10.1056/NEJM198407193110304. [DOI] [PubMed] [Google Scholar]
- 54.Polychronakos C, Kukuvitis A, Giannoukakis N, Colle E. Parental imprinting effect at the INS-IGF2 diabetes susceptibility locus. Diabetologia. 1995;38:715–719. doi: 10.1007/BF00401845. [DOI] [PubMed] [Google Scholar]
- 55.Salmon JE, Girardi G. Antiphospholipid antibodies and pregnancy loss: a disorder of inflammation. J Reprod Immunol. 2008;77:51–56. doi: 10.1016/j.jri.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carp HJ, Shoenfeld Y. Anti-phospholipid antibodies and infertility. Clin Rev Allergy Immunol. 2007;32:159–161. doi: 10.1007/s12016-007-0010-2. [DOI] [PubMed] [Google Scholar]
- 57.Han S, Guthridge JM, Harley IT, Sestak AL, Kim-Howard X, Kaufman KM, Namjou B, Deshmukh H, Bruner G, Espinoza LR, Gilkeson GS, Harley JB, James JA, Nath SK. Osteopontin and systemic lupus erythematosus association: a probable gene-gender interaction. PLoS ONE. 2008;3:e0001757. doi: 10.1371/journal.pone.0001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu AP, Bai J, Lu J, Liang YY, Li JG, Lai DY, Wan X, Huang HH. Osteopontin gene polymorphism in association with systemic lupus erythematosus in Chinese patients. Chin Med J (Engl) 2007;120:2124–2128. [PubMed] [Google Scholar]
- 59.Xing C, Gray-McGuire C, Kelly JA, Garriott P, Bukulmez H, Harley JB, Olson JM. Genetic linkage of systemic lupus erythematosus to 13q32 in African American families with affected male members. Hum Genet. 2005;118:309–321. doi: 10.1007/s00439-005-0061-5. [DOI] [PubMed] [Google Scholar]
- 60.Carter CO, Evans KA. Inheritance of congenital pyloric stenosis. J Med Genet. 1969;6:233–254. doi: 10.1136/jmg.6.3.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carter CO. The inheritance of congenital pyloric stenosis. Brit Med Bull. 1961;17:251–254. doi: 10.1093/oxfordjournals.bmb.a069918. [DOI] [PubMed] [Google Scholar]
- 62.Kantarci OH, Barcellos LF, Atkinson EJ, Ramsay PP, Lincoln R, Achenbach SJ, De Andrade M, Hauser SL, Weinshenker BG. Men transmit MS more often to their children vs women: the Carter effect. Neurol. 2006;67:305–310. doi: 10.1212/01.wnl.0000225070.13682.11. [DOI] [PubMed] [Google Scholar]