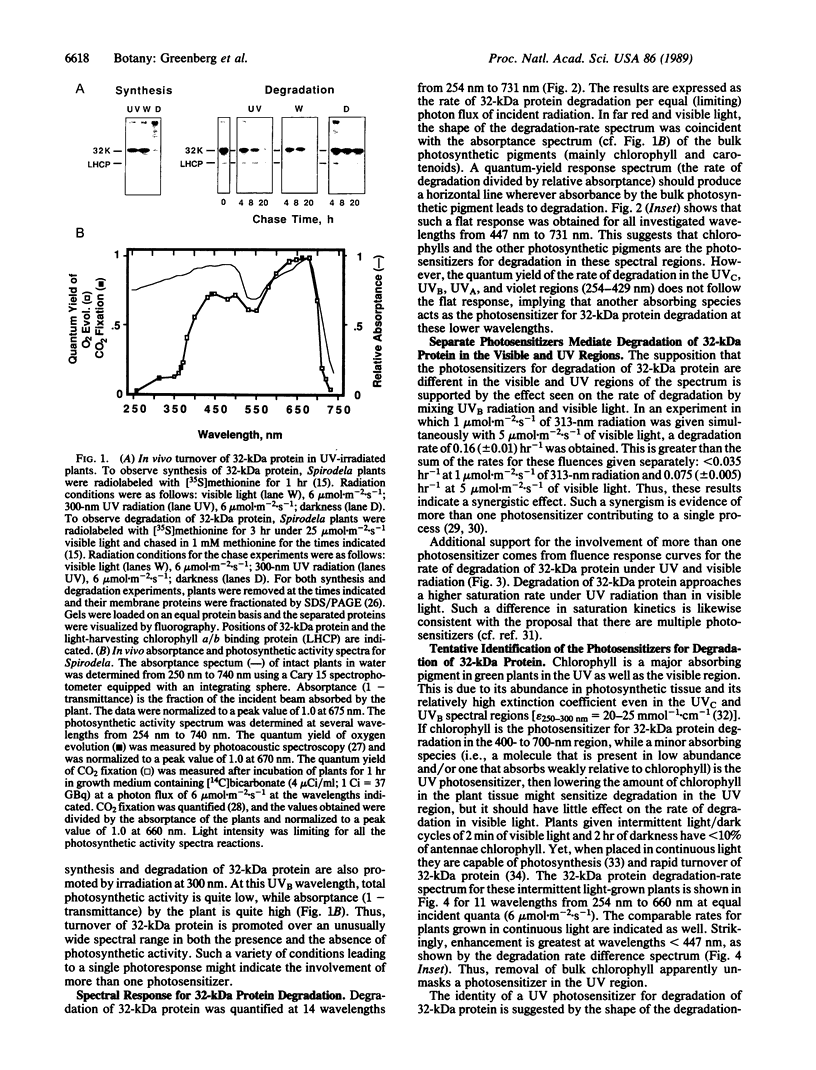
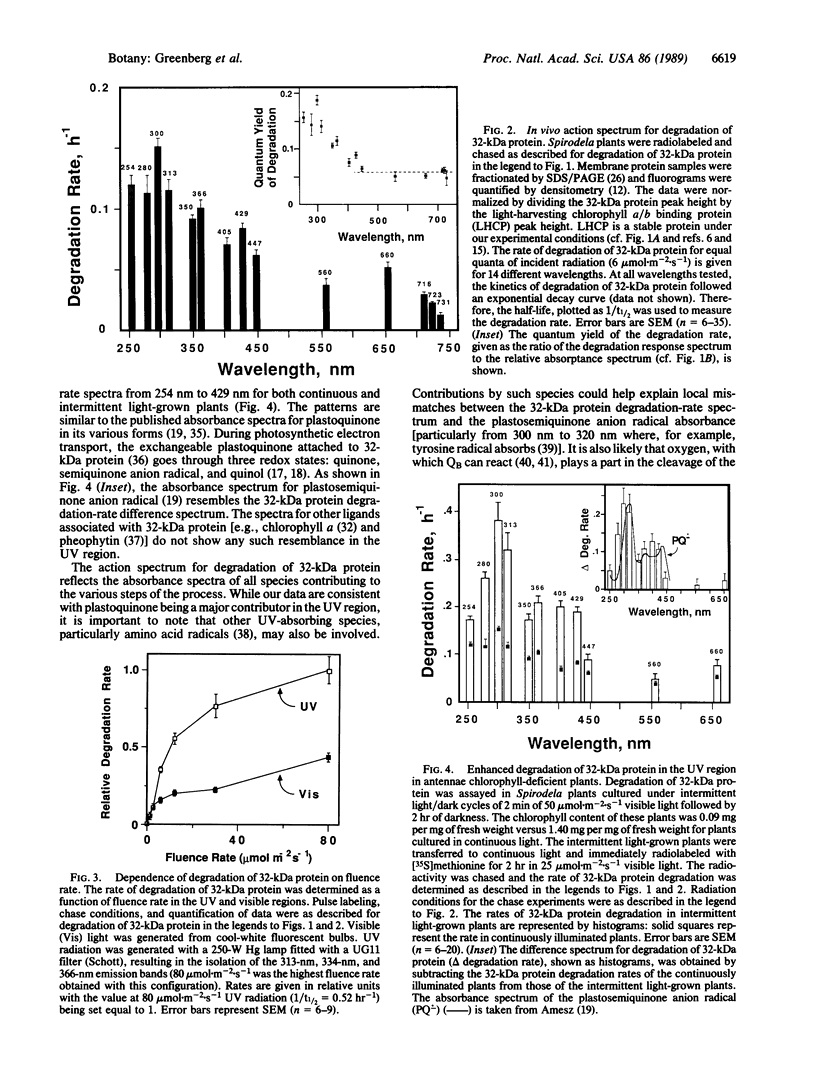
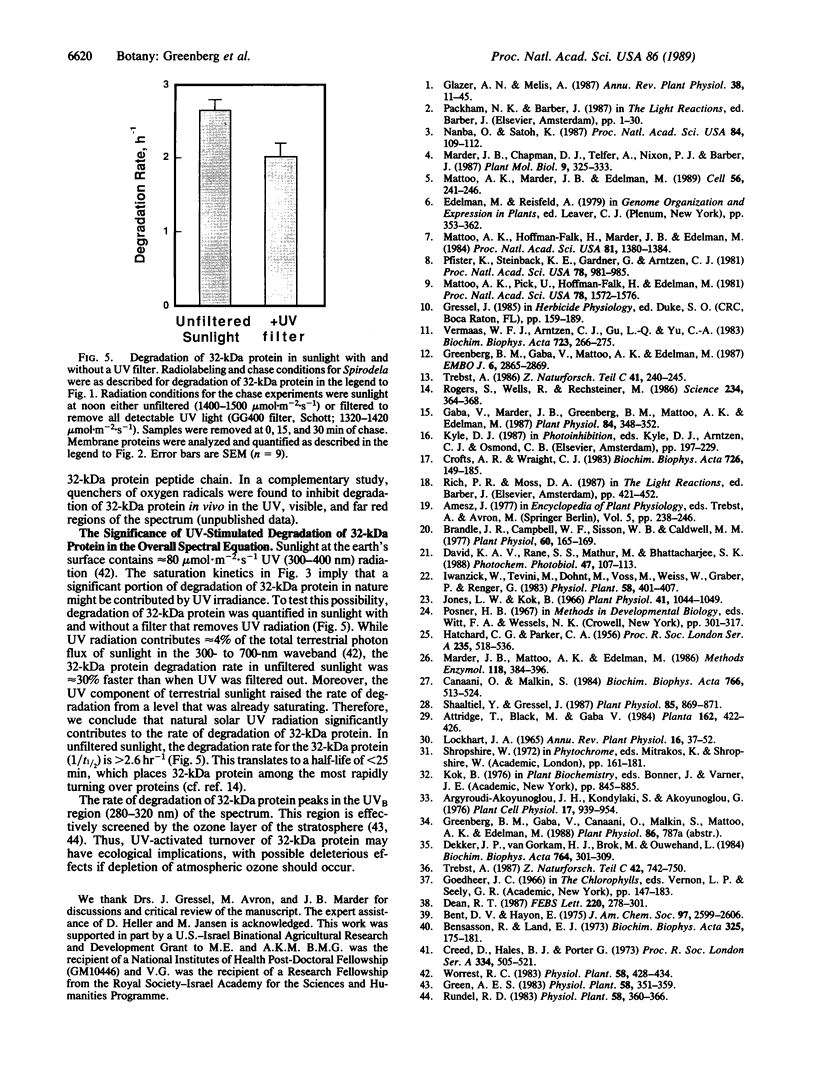
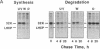Abstract
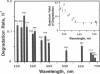
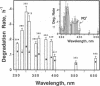
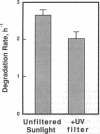
A component of the photosystem II reaction center, the 32-kDa protein, is rapidly turned over in the light. The mechanism of its light-dependent metabolism is largely unknown. We quantified the rate of 32-kDa protein degradation over a broad spectral range (UV, visible, and far red). The quantum yield for degradation was highest in the UVB (280-320 nm) region. Spectral evidence demonstrates two distinctly different photosensitizers for 32-kDa protein degradation. The data implicate the bulk photosynthetic pigments (primarily chlorophyll) in the visible and far red regions, and plastoquinone (in one or more of its redox states) in the UV region. A significant portion of 32-kDa protein degradation in sunlight is attributed to UVB irradiance.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bensasson R., Land E. J. Optical and kinetic properties of semireduced plastoquinone and ubiquinone: electron acceptors in photosynthesis. Biochim Biophys Acta. 1973 Oct 19;325(1):175–181. doi: 10.1016/0005-2728(73)90163-1. [DOI] [PubMed] [Google Scholar]
- Bent D. V., Hayon E. Excited state chemistry of aromatic amino acids and related peptides. I. Tyrosine. J Am Chem Soc. 1975 May 14;97(10):2599–2606. doi: 10.1021/ja00843a002. [DOI] [PubMed] [Google Scholar]
- Brandle J. R., Campbell W. F., Sisson W. B., Caldwell M. M. Net Photosynthesis, Electron Transport Capacity, and Ultrastructure of Pisum sativum L. Exposed to Ultraviolet-B Radiation. Plant Physiol. 1977 Jul;60(1):165–169. doi: 10.1104/pp.60.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean R. T. A mechanism for accelerated degradation of intracellular proteins after limited damage by free radicals. FEBS Lett. 1987 Aug 17;220(2):278–282. doi: 10.1016/0014-5793(87)80829-3. [DOI] [PubMed] [Google Scholar]
- Forest M. G., Mollard P., David M., Morel Y., Bertrand J. Syndrome d'insensibilité incomplète aux androgènes. Difficultés du diagnostic et de la conduite à tenir. Arch Fr Pediatr. 1990 Feb;47(2):107–113. [PubMed] [Google Scholar]
- Gaba V., Marder J. B., Greenberg B. M., Mattoo A. K., Edelman M. Degradation of the 32 kD Herbicide Binding Protein in Far Red Light. Plant Physiol. 1987 Jun;84(2):348–352. doi: 10.1104/pp.84.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg B. M., Gaba V., Mattoo A. K., Edelman M. Identification of a primary in vivo degradation product of the rapidly-turning-over 32 kd protein of photosystem II. EMBO J. 1987 Oct;6(10):2865–2869. doi: 10.1002/j.1460-2075.1987.tb02588.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L. W., Kok B. Photoinhibition of Chloroplast Reactions. II. Multiple Effects. Plant Physiol. 1966 Jun;41(6):1044–1049. doi: 10.1104/pp.41.6.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Hoffman-Falk H., Marder J. B., Edelman M. Regulation of protein metabolism: Coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1380–1384. doi: 10.1073/pnas.81.5.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo A. K., Marder J. B., Edelman M. Dynamics of the photosystem II reaction center. Cell. 1989 Jan 27;56(2):241–246. doi: 10.1016/0092-8674(89)90897-0. [DOI] [PubMed] [Google Scholar]
- Mattoo A. K., Pick U., Hoffman-Falk H., Edelman M. The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the "proteinaceous shield" regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1572–1576. doi: 10.1073/pnas.78.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister K., Steinback K. E., Gardner G., Arntzen C. J. Photoaffinity labeling of an herbicide receptor protein in chloroplast membranes. Proc Natl Acad Sci U S A. 1981 Feb;78(2):981–985. doi: 10.1073/pnas.78.2.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Shaaltiel Y., Gressel J. Kinetic Analysis of Resistance to Paraquat in Conyza: Evidence that Paraquat Transiently Inhibits Leaf Chloroplast Reactions in Resistant Plants. Plant Physiol. 1987 Dec;85(4):869–871. doi: 10.1104/pp.85.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]