Abstract
The constraints to maximum walking speed and the underlying cause of the walk-run transition remains controversial. However, the motions of the body and legs can be reduced to a few mechanical principles, which, if valid, impose simple physics-based limits to walking speed. Bipedal walking may be viewed as a vaulting gait, with the centre of mass passing over a stiff stance leg (an ‘inverted pendulum’), while the swing leg swings forward (as a pendulum). At its simplest, this forms a ‘compass gait’ walker, which has a maximum walking speed constrained by simple mechanics: walk too fast, or with too high a step length, and gravity fails to keep the stance foot attached to the floor. But how useful is such an extremely reductionist model? Here, we report measurements on a range of duck breeds as example unspecialized, non-planar, crouch-limbed walkers, and contrast these findings with previous measurements on humans, using the theoretical framework of compass gait walking. Ducks walked as inverted pendulums with near-passive swing-legs up to relative velocities around 0.5, remarkably consistent with the theoretical model. In contrast, top walking speeds in humans cannot be achieved with passive swing legs: humans, while still constrained by compass gait mechanics, extend their envelope of walking speeds by using relatively high step frequencies. Therefore, the capacity to drive the swing leg forward by walking humans may be a specialization for walking, allowing near-passive vaulting of the centre of mass at walking speeds 4/3 that possible with a passive (duck-like) swing leg.
Keywords: Walk, run, gait, transition, inverted pendulum
Introduction
Walking animals use immensely complex combinations of muscle and nerve actions to drive and control their limbs. However, the mechanical principles underlying walking may be simple and general. Walking – in humans at least – is a relatively stiff-limbed gait without aerial phases, and is often described as acting as an ‘inverted pendulum’, with kinetic energy Ek at the beginning of each stance phase translating to gravitational potential energy Ep as the centre of mass CoM rises to its highest point near mid-stance, and returning as kinetic energy as the body falls towards the end of stance. In its simplest form, walking as an inverted pendulum can be described by a ‘compass gait’ model (McGeer, 1990; Alexander, 1995; Goswami et al., 1997; Garcia, 1998; see also Saunders et al., 1953 and Kuo, 2007). This model is planar, has completely rigid limbs, exactly one point-foot in contact with the ground at any time, and instantaneous transition between legs. The mechanically-imposed speed limits to walking with a compass gait are now well understood (Usherwood, 2005; Srinivasan and Ruina, 2006): at high speeds, especially at high step lengths, the acceleration due to gravity is insufficient to provide the centripetal acceleration requirements of vaulting in an arc over a stiff stance limb. But how generally can such an extremely simple model be useful? Does compass gait mechanics actually restrict walking speeds in humans? And is compass gait mechanics informative for unspecialized bipedal walkers with the relatively crouched limbs and ‘blurred’ walk-run transition typical of many birds (Gatesy and Biewener, 1991; Hancock et al., 2007)? Birds are living bipedal dinosaurs, and achieve walking and running gaits despite limb structures markedly different from the familiar mammalian bipeds, humans. Further, some birds walk despite specialization for swimming, resulting in widely spaced hips and foot placements, and further deviation from the assumptions of the compass gait model. Ducks therefore present a fascinating comparison with humans: is the mechanical approximation of a compass gait relevant to walking in such a non-specialized walker?
Here, we report forceplate-derived measurements of waddling, walking and running in three breeds of duck. We use the term ‘Energy Recovery’ (Cavagna et al., 1977) to describe the maximum potential for changes in CoM Ek and Ep to be passive, consistent with the inverted pendulum model of walking. We use Energy Recovery to distinguish between ‘walking’ (high ER) and ‘running’ (low ER) gaits. The importance of lateral motions – ‘waddling’ – to the potential passive qualities of walking is demonstrated by calculating Energy Recoveries both including and excluding the kinetic energy associated with lateral motions.
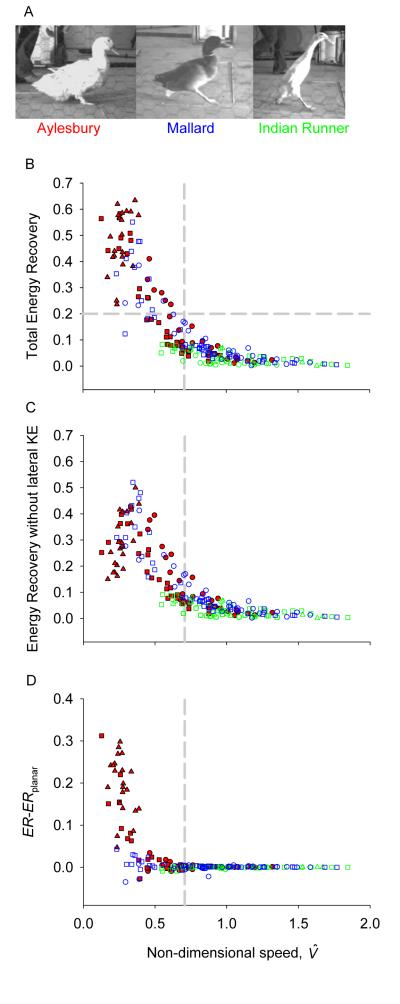
The three duck breeds, Aylesbury, Mallard and Indian Runner, are all derived from mallards Anas platyrhyncos, but have radically different forms (Fig. 1A). The Aylesbury is large, selected for the table. The Mallard used was close to wild-type, if overweight due to domestication. The Indian Runner or Penguin Duck has an upright form associated with highly reduced femora, and was bred for both egg-laying and a ‘peculiar’ shape and carriage (Ashton and Ashton, 2002). Although Aylesbury ducks were clearly not bred for walking efficiency, they demonstrate a reasonable proficiency at walking; historically, they were walked from Aylesbury to London for market, a distance of around 70km (>40 miles). The morphology of all ducks shows some degree of compromise between walking and swimming capability (Biewener and Corning, 2001). They were selected for this study precisely because they cannot be considered specialist cursors. Are these compromised, semi-aquatic animals capable of, and constrained by, the mechanics of inverted pendulum-like walking? And how do they contrast with relatively stiff-limbed humans, that are presumably more specialized for effective walking?
Figure 1.
Energy Recoveries for Aylesbury (red), mallard (blue) and Indian runner (green) ducks including (B) and excluding (C) the kinetic energy associated with lateral motions (the difference is displayed in D): an Energy Recovery of 1 indicates that changes in potential and kinetic energies of the centre of mass throughout a step has the potential to be completely passive, consistent with inverted-pendulum mechanics. Different symbols indicate different individuals. Vertical dashed lines indicate Froude number = 0.5 for reference; the horizontal dashed line (in A) shows the cut-off used to distinguish between walking and running for Fig. 3.
The compass gait boundary
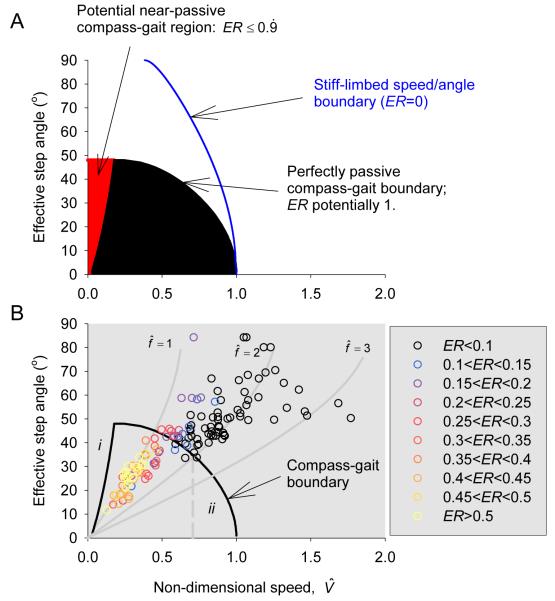
The range of possible relative velocities and step angles swept from the vertical Φ (see methods) possible while walking with a compass gait is dependent on the extent to which the vaulting of the body over the stiff stance limb can be considered passive. If the only condition is that sufficient gravity acts in line with the leg to provide enough centripetal acceleration to avoid a toe-drag or take-off (blue line, Fig. 2A) then step angles approaching 90° are theoretically possible. As higher instantaneous speeds are possible near midstance (gravity acts directly in line with the leg), mechanical energy has to be applied to both raise and accelerate the body throughout the first half of stance, and has to be removed throughout the second half; Energy Recovery = 0. In contrast, with completely passive vaulting mechanics (ER=1) lower maximum step angles are achievable. This is because the body moves most quickly (highest kinetic energy) at the extremes of leg angle (lowest potential energy). At this point, not only does a smaller component of gravity act in line with the leg, but the requirement for centripetal acceleration of the body about the foot is also highest (Usherwood, 2005). Achieving both passive body mechanics and ‘take-off’ avoidance results in a lower net horizontal velocity for a given leg angle than the constraints of take-off avoidance alone (Fig. 2A).
Figure 2.
Step angle as a function of speed from theoretical compass gait predictions (A), and from forceplate observations of Mallard and Aylesbury ducks, coded by Energy Recovery (B). Perfectly passive (ER=1), stiff-limbed compass gait walking constrains both lower (i) and upper (ii) speeds (black area in A); with a small energy input (ER ≤ 0.9), the low-speed constraint is lost (red area); without any passive potential-kinetic energy interchange (ER=0) higher step angles and speeds are possible (blue line) until . Underlying grey curves (in B) indicate the contours associated with step frequencies normalized by the ideal pendular frequency of the swing leg with point mass at the foot. Ducks walk (with high Energy Recoveries, yellows and reds) with near-passive step frequencies, and transition to running (low Energy Recoveries, blues and black) at higher speeds, with higher-than-passive step frequencies.
For perfectly passive vaulting of the body over the foot, there is also a low-speed / high step angle boundary: very low speeds at high step angles (large step lengths) are impossible because there is insufficient kinetic energy to allow the CoM to rise passively over midstance (boundary i in Figs. 2B and 3) – the body would simply rock upwards and fall back again without ever making it completely over the stance foot. This boundary is probably of more theoretical than applied interest as, with an infinitesimally small energy input to hold, control and release the body at the top of the vault, very slow walking is feasible with large step angles (the region in red, Fig. 2A) with very nearly passive body motions.
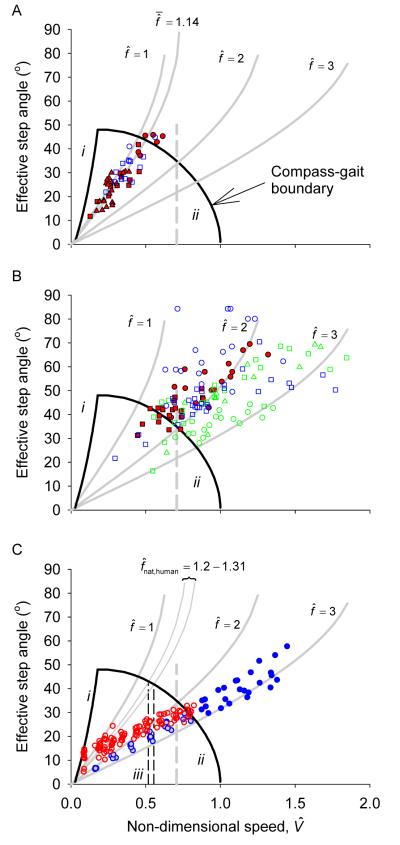
Figure 3.
Step angle as a function of speed for ‘walking’ ducks (A, determined as having ER greater than or equal to 0.2) or ‘running’ ducks (B, ER<0.2), and walking and running humans (C, walking in red (N=11 subjects), running in blue (N=5 subjects), with running at below maximum possible walking speed with grey fills). Ducks are coloured according to breed, with symbols denoting subject, as for Fig. 1. Underlying black and grey lines indicate the compass gait boundary and step frequencies described for Fig. 2. The vertical grey dashed line denotes (Fr=0.5), usually identified as the walk-run transition speed for humans. Vertical dashed black lines (C) mark the range in maximum speeds predicted if humans walked both with passive swing limbs and while constrained by compass gait mechanics.
With each speed and step angle combination, a step frequency is explicit. This is indicated in a relative form (Figs. 2B and 3), which gives the step frequency as a multiple of that expected for a swing leg acting as an ideal passive pendulum with mass focused at the foot (see methods).
Methods
Three Aylesbury (77 steps, mass=3.61 (0.68) kg, leg length Lleg=0.163 (0.015) m, mean (s.d.) throughout), two near wild-type Mallard (70 steps, mass= 1.81 (1.6) kg; Lleg = 0.165 (0.01) m) and 3 Indian Runner ducks (54 steps, mass=1.12 (0.14), Lleg = 0.115 (0.02) m) were motivated to locomote along an array of 6 Kistler 9287B forceplates sampling at 500Hz, following procedures approved by The Royal Veterinary College. A range of speeds were elicited for each subject by clapping and waving. Fore-aft, lateral and vertical forces, along with centres of pressure were inspected to identify sequences in which locomotion was steady and in line with the forceplates (mean change in horizontal velocity over a step was <0.01; s.d.=0.10 ms−1). Individual steps were analyzed from midstance to midstance, identified by the crossing of the fore-aft forces from decelerative to accelerative. The forward progression of the centre of pressure from midstance to midstance provided step length Lstep, and, with the step period, the horizontal velocity V, required both as an integration constant in determining changes in speed and kinetic energy and for calculating relative velocity , where
Here, g is gravity (9.81ms−2) and Lleg the functional leg length, taken as the height from the ground to the hip during quiet standing. is equivalent to the square root of the most commonly used form of Froude number Fr in terrestrial animal locomotion studies. Relative step frequency is presented in a non-dimensional form :
indicating the step frequency as a proportion of the natural pendular frequency for a leg swinging about a stationary hip over small angles with the leg mass situated at the foot (an ‘ideal’ pendulum). Note that, as step (as opposed to stride) frequency is used, and the compass gait model assumes a duty factor of exactly 0.5, the period taken by a passively swinging limb to swing from foot off to foot on would be half that of a full pendulum cycle. This formulation intentionally errs on the side of familiarity and simplicity rather than realism: more detailed study of the passive nature of swing legs would have to consider jointed pendulums (see Mochon and McMahon, 1980), vaulting (or observed) hip motions, and duty factors deviating from 0.5. One layer of realism that can be added without an undue increase in complexity is the deviation from the point-mass aspect of the ‘ideal’ pendulum. In order to consider ‘real’ pendulums with distributed masses, the Effective Pendulum Length EPL is a useful concept: it gives the length an ideal pendulum would need to be in order to swing at the same frequency as the real pendulum.
where Ileg is the moment of inertia (second moment of mass) of the swing leg about its pivot (the hip), mleg is the mass of the swing leg, and LlegCoM the distance between pivot and swing leg centre of mass. The passive swing frequency is relatively insensitive to mass distribution. A constant (rod-like) leg mass distribution would result in EPL=2/3Lleg, and a passive step frequency of that of an ideal pendulum of the same length.
Fluctuations in gravitational potential energy Ep and kinetic energy Ek due to motions in each of the three axes were calculated from forceplate data following conventional methods pioneered by Cavagna (1975): measured forces (on known body masses) give centre of mass accelerations; when integrated, these give velocities (from which Ek is determined); which, when integrated, give motions of the CoM (changes in height allowing calculation of changes in Ep). Integration constants for determining velocities were based on the assumption that the gait was steady and symmetrical over a stride. The Energy Recovery ER was calculated:
where the prefix Δ+E denotes the sum of the positive increments of energy change (in a symmetrical bipedal gait, this is equivalent to the amplitude), and Em is the total external mechanical energy (Ek+Ep) of the body. To identify the importance of lateral, or ‘waddling’, motions to the potential passive nature of the Ek - Ep - Ek interchanges, Energy Recovery was also calculated excluding the kinetic energy associated with lateral motions, ERplanar. It should be remembered that ER is a measure only of the mechanically passive nature of the centre of mass: it provides no measure of ‘internal’ work, leaves the potential for considerable simultaneous, counteracting muscular work (see Donelan et al., 2002) and does not inform whether the Centre of Mass is vaulting following a path expected from relatively stiff limbs (see Usherwood et al., 2007).
An effective step angle swept before and after the vertical, Φ was determined from the forceplate-derived step lengths and the measured leg length:
It should be noted that this may differ from any true kinematic angle, as it assumes a compass-like gait, with a single foot on the ground at any time and symmetry about the vertical.
Human data
Kinematic data from previous studies of humans walking (Bertram, 2005) or running (Gutmann et al., 2006) on treadmills were kindly provided by John Bertram. In these studies, the full range of speeds achievable during walking (N=11 subjects) and running (N=5 subjects) were measured. The highest walking speeds were moderately uncomfortable, and slightly above the preferred walk-run transition speed. The low running speeds were certainly unnatural, extending well below the preferred run-walk transition speed.
Results and Discussion
Waddling and walking
At low speeds, Energy Recovery (Fig. 1A) for Aylesbury and Mallard ducks is as high as 75%, close to that recorded in cursorial birds (Cavagna et al., 1977; Heglund et al., 1982), humans (Cavagna et al., 1977) and penguins (Griffin and Kram, 2000). At the lowest speeds, this ER is only achieved by the Aylesbury ducks if lateral, or ‘waddling’, kinetic energy is included (Fig. 1B, C): ERplanar indicates the Energy Recovery without consideration of lateral motions, and falls at the lowest speeds. This is NOT to suggest that waddling improves efficiency – side to side motions are presumably not the purpose of locomotion. A better view is that, at low speeds and given widely splayed foot placement (presumably due to artificial selection for size or natural selection for swimming capability), lateral forces, displacements and movements are inevitable. However, these (inevitable but ‘pointless’) motions have the potential to be achieved largely passively, though perhaps not without metabolic implications (Donelan et al., 2001).
Running
At (or Fr>0.5), Energy Recoveries are universally low – above these speeds, no duck maintains passive, inverted pendulum-like energy interchanges. Low ERs may be interpreted as suggesting a ‘bouncing’ or ‘running’ gait; however, this terminology must be treated with caution. It appears most unlikely that ducks are specialized for elastic energy storage and recovery. They have relatively short legs and thick tendons, likely reflecting some of the compromises in form associated with evolutionary pressures for swimming performance. Therefore, low ERs should be taken as departure from inverted pendulum behavior, perhaps as ‘pseudo-elastic’ bouncing (Srinivasan and Ruina, 2006; Ruina et al., 2005), but not necessarily involving extensive elastic storage and recoil. Nonetheless, a running gait, even without elastic energy storage, may be a gait choice that minimizes mechanical work and energetic costs at higher speeds under the fundamental constraints of legged bipedal locomotion in gravity (Srinivasan and Ruina, 2006).
Despite occasional slow trials, no steps of the Indian Runners displayed high ERs – ‘running’ gaits were maintained at low speeds (see also Fig. 3B). That this is mechanically possible is clear from observations of humans: it is perfectly possible to ‘run’ at no net forward speed (Fig. 3C). Given the ability of all three duck breeds tested to achieve ‘running’ mechanics (albeit predominantly ‘grounded running’, without aerial phases), but the failure of Indian Runners to display inverted-pendulum walking mechanics (high ER), perhaps a better, if distinctly awkward, classification of Indian Runners might be as ‘Indian Not-Walkers’. Both the Mallards and Indian Runners did achieve aerial phases (‘running’ distinguished by the traditional kinematic definition) at their highest speeds (movies 2 and 3, Supplementary Information). This differs from the findings of Abourachid (2001), presumably because the birds in the previous study achieved only up to 0.7. Running with an aerial phase was not observed by any of the Aylesbury ducks (movie 1 in Supplementary Information shows a typical gait).
The walk-run transition in ducks
While discontinuities in kinematics with increasing speed suggest gait transition in some birds (Hayes and Alexander, 1983; Gatesy and Biewener, 1991; Gatesy, 1999), the gait transition of ducks from a slow, vaulting, or mechanically-defined ‘walking’ gait (using a mechanical definition) to a faster ‘running’ gait is blurred (Fig. 1B), typical of a number of bird species. In contrast to humans and most mammalian quadrupeds, there is no discontinuity in mechanics apparent with increasing speed. Although a discontinuity in stride parameters is reported in the relatively small and crouched painted quail, between relative velocities of 0.6 to 0.8 (Gatesy and Biewener, 1991), such a transition is difficult to determine for ducks. However, when step angle and Energy Recovery are considered together, especially when viewed with the boundary determined by compass gait mechanics (Fig. 2B), a distinction between walking and running gaits can be perceived. Walking (high Energy Recovery) is achieved with near-passive swing or step frequencies; running, with low ER and beyond the boundary determined by compass gait mechanics, is achieved with above-passive step frequencies. Taking any observed ER value as the sole distinguishing boundary between walking and running – especially in cases where ER varies without discontinuities with speed – is certainly arbitrary; however, in order to highlight the relationship between speed, step frequency and Energy Recovery displayed in Figure 2B, ER ≥ 0.2 is used as an objective, quantitative condition for walking, (Fig. 3A). For steps at ER ≥ 0.2 the mean step frequency is . If the swing leg was passive, this would equate to an Effective Pendulum Length (see methods) EPL of 0.78 (Fig. 4A). Considering a real pendulum of even mass distribution results in a passive frequency of , swing-leg mechanics in walking ducks can be considered near-passive. Therefore, ducks do not walk above a speed consistent with both compass gait and passive swing-leg mechanics.
Figure 4.
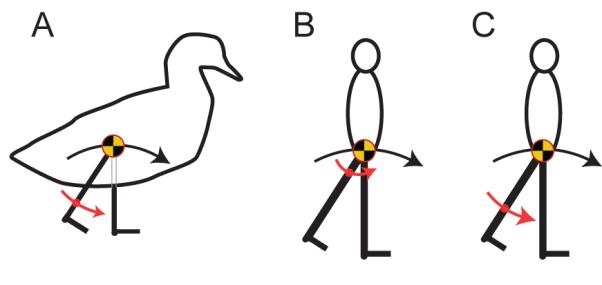
CoM trajectories (black arrows) for compass gait walking and motions of the Effective Pendulum Length (red arrows) for the swing leg for A) walking ducks assuming the swing leg is passive, and with EPL appropriate for mean observed walking frequencies; B) a walking human with passive swing leg if the mass distribution were such that the step frequency was that observed at high speeds ( EPL=Lleg/4); C) a walking human with EPL appropriate for reported mass distributions. Fast human walking cannot be achieved with a passive swing leg.
The walk-run transition in humans
That compass gait walking above is impossible has been understood for many years (e.g. Alexander, 1989): above this speed, gravity cannot keep the centre of mass connected to the stance foot (in contact with the ground) with a stance limb in compression. More recently, similar constraints have been identified, but for finite stance angles (Usherwood, 2005; Srinivasan and Ruina, 2006). Combined with some constraint on step frequency, this suggested an account for the observed walk-run transition speed of humans, at around (Fr=0.5) (Usherwood, 2005). Since this, research on human swing limb mechanics (Doke et al., 2005; Doke and Kuo, 2007) highlights that fast human walking is not actually achieved with passive swing-limb mechanics (see also Mochon and McMahon, 1980). Indeed, if humans were constrained to walking with a passive (duck-like) swing-leg, would be of the range (derived from Doke et al., (2005)) to (from Doke and Kuo (2007)) – close to that for a leg of even mass distribution, of (see Fig. 4B,C). In order to achieve passive swing-leg mechanics at observed step frequencies near top walking speeds (), EPL would have to be a quarter of the leg length – most unlikely, even with considerable leg flexion. If humans did walk with step frequencies determined by passive swing-leg mechanics, and were constrained to walking with compass gait mechanics, they (like ducks) would be limited to maximum walking speeds of the observed transition speed (Fig. 3C). Instead, humans increase the range of their walking speeds, while still complying with the constraints of compass gait CoM mechanics, by driving their swing limbs at well above their natural pendular frequencies. High step frequencies (short step lengths, or small step angles) have previously been identified as effective in reducing the energetic losses due to suddenly redirecting the centre of mass at the start of each step (Kuo, 2001, 2002; Donelan et al., 2002); the increased potential walking speed with forced swing legs described here provides an alternative, though certainly not mutually exclusive, account for the relatively high step frequencies of walking humans.
Limitations and future work
The compass gait model predictions of constraints to step angle and speed presented here are limited to the extreme cases of perfect, near-perfect and absent Energy Recovery (Fig. 2A), and are based on the assumption of completely stiff stance limbs, and consequently a duty factor of exactly 0.5. While this is an appealing and extremely reductionist abstraction allowing simple and intuitive predictions, real bipeds walk with intermediate Energy Recoveries and somewhat compliant limbs. In many cases, even the distinction between walking and running using Energy Recovery can be obscure or arbitrary. The extent to which additional layers of realism – for instance, a certain degree of limb compliance – can be added to the model before its generality and predictive nature is lost, remains to be determined.
Further forceplate measurements of walking bipeds are required to investigate whether it is the ducks or the humans (or both) that stand out as unusual. Also, the 4-legged analogue to the passive compass gait, a 4-bar linkage model (Usherwood et al., 2007) will be further developed to investigate whether similar constraints apply to quadrupeds.
Consequences and implications
The maximum speed of walking in both specialist (humans) and facultative (ducks) cursors appears constrained by compass gait mechanics. Humans increase their maximum walking speeds by driving their swing-limbs at above natural pendular frequencies. From the combination of the limitations imposed by compass gait mechanics and the cost of driving swing-limbs, predictions concerning maximum walking speeds can be made. Anything restricting the driving of the swing limb – for instance massive boots or massive lower limb prosthetics – would be predicted to reduce maximum walking speed. Conversely, anything enhancing driving of the swing-limb, for instance spring-like torques about the hip, or low-mass prosthetics, should permit higher walking speeds. Therefore, while the compass gait is an exceedingly reductionist model of bipedal walking, it is both effective in accounting for the walk-run transition speed in humans and ducks, and allows useful, intuitive predictions with application to prosthetic and orthotic designs.
Supplementary Material
Acknowledgements
We thank John Bertram for sharing his data on humans, and Bridget Brennan, the Van Hage Garden Company, and Sadie Rodgers for lending the ducks. JRU was funded by The Wellcome Trust.
References
- Abourachid A. Kinematic parameters of terrestrial locomotion in cursorial (ratites), swimming (ducks), and striding birds (quail and guinea fowl) Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;131:113–119. doi: 10.1016/s1095-6433(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Optimization and gaits in the locomotion of vertebrates. Physiol. Rev. 1989;69:1199–1227. doi: 10.1152/physrev.1989.69.4.1199. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Simple models of human movement. Appl. Mech. Rev. 1995;48:461–470. [Google Scholar]
- Ashton CA, Ashton MA. The Indian Runner Duck, a historical guide. Feathered World; Preston, UK: 2002. [Google Scholar]
- Bertram JE. Constrained optimization in human walking: cost minimization and gait plasticity. J. Exp. Biol. 2005;208:979–991. doi: 10.1242/jeb.01498. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Corning WR. Dynamics of mallard (Anas platyrhynchos) gastrocnemius function during swimming versus terrestrial locomotion. J. Exp. Biol. 2001;204:1745–1756. doi: 10.1242/jeb.204.10.1745. [DOI] [PubMed] [Google Scholar]
- Cavagna GA. Force platforms as ergometers. J. Appl. Physiol. 1975;39:174–179. doi: 10.1152/jappl.1975.39.1.174. [DOI] [PubMed] [Google Scholar]
- Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am. J. Physiol. 1977;233:R243–261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- Doke J, Kuo AD. Energetic cost of producing cyclic muscle force, rather than work, to swing the human leg. J. Exp. Biol. 2007;201:2390–2398. doi: 10.1242/jeb.02782. [DOI] [PubMed] [Google Scholar]
- Doke J, Donelan JM, Kuo AD. Mechanics and energetics of swinging the human leg. J. Exp. Biol. 2005;208:439–445. doi: 10.1242/jeb.01408. [DOI] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc. R. Soc. Lond. B. Biol. Sci. 2001;268:1985–1992. doi: 10.1098/rspb.2001.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J. Exp. Biol. 2002;205:3717–3727. doi: 10.1242/jeb.205.23.3717. [DOI] [PubMed] [Google Scholar]
- Garcia M, Chatterjee A, Ruina A, Coleman M. The simplest walking model: stability, complexity, and scaling. J. Biomech. Eng. 1998;120:281–288. doi: 10.1115/1.2798313. [DOI] [PubMed] [Google Scholar]
- Gatesy SM. Guineafowl hind limb function. I: Cineradiographic analysis and speed effects. J. Morphol. 1999;240:115–125. doi: 10.1002/(SICI)1097-4687(199905)240:2<115::AID-JMOR3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Gatesy SM, Biewener AA. Bipedal locomotion: effects of speed, size and limb posture in birds and humans. J. Zool. Lond. 1991;224:127–147. [Google Scholar]
- Goswami A, Espiau B, Keramane A. Limit cycles in a passive compass gait biped and passivity-mimicking control laws. Autonomous Robots. 1997;4:273–286. [Google Scholar]
- Griffin TM, Kram R. Penguin waddling is not wasteful. Nature. 2000;408:929. doi: 10.1038/35050167. [DOI] [PubMed] [Google Scholar]
- Gutmann A, Jacobi B, Butcher MT, Bertram JEA. Constrained optimization in human running. J. Exp. Biol. 2006;209:622–632. doi: 10.1242/jeb.02010. [DOI] [PubMed] [Google Scholar]
- Hancock JA, Stevens NJ, Biknevicius AR. Whole-body mechanics and kinematics of terrestrial locomotion in the elegant-crested tinamou Eudromia elegans. Ibis. 2007;149:605–614. [Google Scholar]
- Hayes G, Alexander RM. The hopping gaits of crows (Corvidae) and other bipeds. J. Zool. Lond. 1983;200:205–213. [Google Scholar]
- Heglund NC, Cavagna GA, Taylor CR. Energetics and mechanics of terrestrial locomotion III. Energy changes of the centre of mass as a function of speed and body size in birds and mammals. J. Exp. Biol. 1982;97:41–56. doi: 10.1242/jeb.97.1.41. [DOI] [PubMed] [Google Scholar]
- Kuo AD. A simple model of bipedal walking predicts the preferred speed-step length relationship. J. Biomech. Eng. 2001;123:264–269. doi: 10.1115/1.1372322. [DOI] [PubMed] [Google Scholar]
- Kuo AD. Energetics of actively powered locomotion using the simplest walking model. J. Biomech. Eng. 2002;124:113–120. doi: 10.1115/1.1427703. [DOI] [PubMed] [Google Scholar]
- Kuo AD. The six determinants of gait and the inverted pendulum analogy: A dynamic walking perspective. Human Movement Science. 2007;26:617–656. doi: 10.1016/j.humov.2007.04.003. [DOI] [PubMed] [Google Scholar]
- McGeer T. Passive dynamic walking. International Journal of Robotics Research. 1990;9:62–82. [Google Scholar]
- Mochon S, McMahon TA. Ballistic walking. J. Biomech. 1980;13:49–57. doi: 10.1016/0021-9290(80)90007-x. [DOI] [PubMed] [Google Scholar]
- Ruina A, Bertram JEA, Srinivasan M. A collisional model of the energetic cost of support work qualitatively explains leg sequencing in walking and galloping, pseudo-elastic leg behavior in running and the walk-to-run transition. J. Theor. Biol. 2005;237:170–192. doi: 10.1016/j.jtbi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Saunders J. B. d. M., Inman VT, Eberhart HD. The major determinants in normal and pathological gait. J. Bone Joint Surg. Am. 1953;35:543–558. [PubMed] [Google Scholar]
- Srinivasan M, Ruina A. Computer optimization of a minimal biped model discovers walking and running. Nature. 2006;439:72–75. doi: 10.1038/nature04113. [DOI] [PubMed] [Google Scholar]
- Usherwood JR. Why not walk faster? Biology Letters. 2005;1:338–341. doi: 10.1098/rsbl.2005.0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood JR, Williams SB, Wilson AM. Mechanics of dog walking compared with a passive, stiff-limbed, 4-bar linkage model, and their collisional implications. J. Exp. Biol. 2007;210:533–540. doi: 10.1242/jeb.02647. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.