Abstract
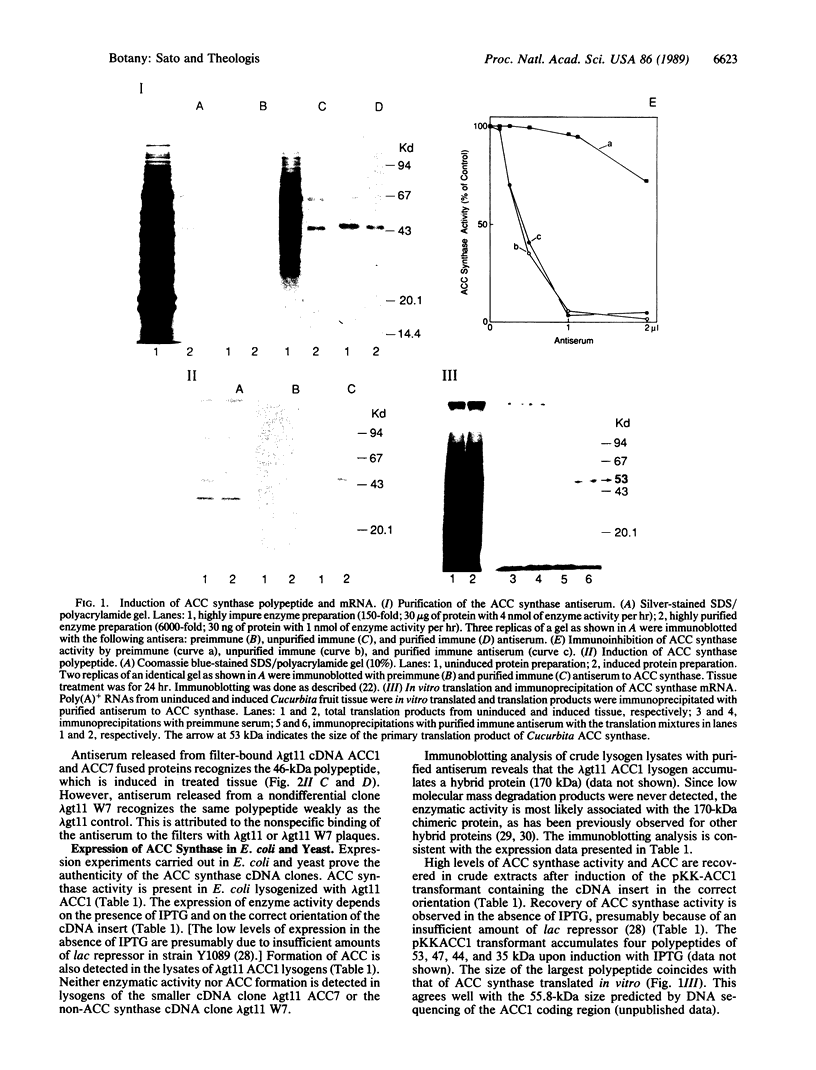
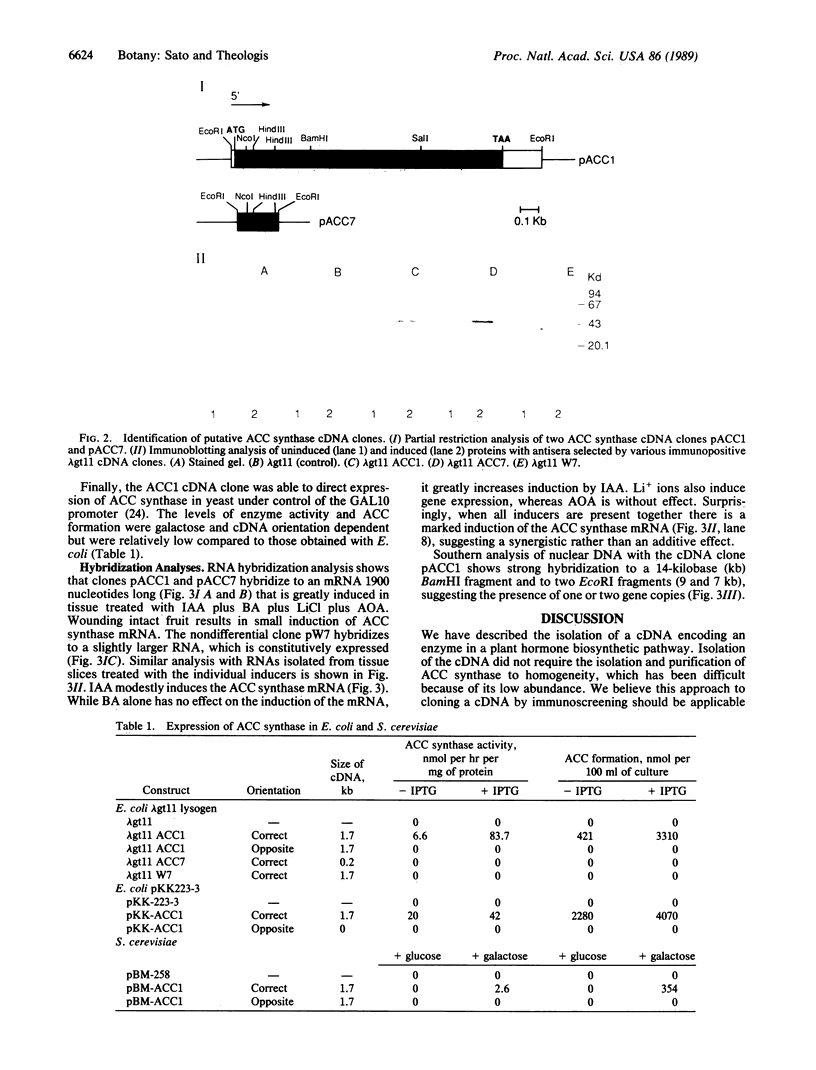
Ethylene is the plant hormone that controls several features of plant growth and development. The rate-limiting step in its synthesis is the formation of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC) from S-adenosylmethionine (AdoMet), catalyzed by ACC synthase. We have isolated a complementary DNA sequence encoding ACC synthase from zucchini (Cucurbita) fruits. The biological activity of the clone was confirmed by the ability of the cloned sequence to direct ACC synthase activity in Escherichia coli and yeast. In vivo studies using the ACC cDNA as probe showed that the ACC synthase gene is induced by a diverse group of inducers, including wounding, Li+ ions, and the plant hormone auxin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acaster M. A., Kende H. Properties and Partial Purification of 1-Aminocyclopropane-1-carboxylate Synthase. Plant Physiol. 1983 May;72(1):139–145. doi: 10.1104/pp.72.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleecker A. B., Kenyon W. H., Somerville S. C., Kende H. Use of monoclonal antibodies in the purification and characterization of 1-aminocyclopropane-1-carboxylate synthase, an enzyme in ethylene biosynthesis. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7755–7759. doi: 10.1073/pnas.83.20.7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlson J. R. A new means of inducibly inactivating a cellular protein. Mol Cell Biol. 1988 Jun;8(6):2638–2646. doi: 10.1128/mcb.8.6.2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker J. R., Davis R. W. Inhibition of gene expression in plant cells by expression of antisense RNA. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5372–5376. doi: 10.1073/pnas.83.15.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice L. C., Waxdal M. J., Weintraub B. D. Comparison of bovine and mouse pituitary glycoprotein hormone pre-alpha subunits synthesized in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4798–4802. doi: 10.1073/pnas.76.10.4798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Johnson L. M., Snyder M., Chang L. M., Davis R. W., Campbell J. L. Isolation of the gene encoding yeast DNA polymerase I. Cell. 1985 Nov;43(1):369–377. doi: 10.1016/0092-8674(85)90042-x. [DOI] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman D. L., McGinnis J. F., Krieger N. R., Tobin A. J. Brain glutamate decarboxylase cloned in lambda gt-11: fusion protein produces gamma-aminobutyric acid. Science. 1986 May 30;232(4754):1138–1140. doi: 10.1126/science.3518061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Marullo S., Delavier-Klutchko C., Eshdat Y., Strosberg A. D., Emorine L. Human beta 2-adrenergic receptors expressed in Escherichia coli membranes retain their pharmacological properties. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7551–7555. doi: 10.1073/pnas.85.20.7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta A. M., Jordan R. L., Anderson J. D., Mattoo A. K. Identification of a unique isoform of 1-aminocyclopropane-1-carboxylic acid synthase by monoclonal antibody. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8810–8814. doi: 10.1073/pnas.85.23.8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Privalle L. S., Graham J. S. Radiolabeling of a wound-inducible pyridoxal phosphate-utilizing enzyme: evidence for its identification as ACC synthase. Arch Biochem Biophys. 1987 Mar;253(2):333–340. doi: 10.1016/0003-9861(87)90186-x. [DOI] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theologis A., Huynh T. V., Davis R. W. Rapid induction of specific mRNAs by auxin in pea epicotyl tissue. J Mol Biol. 1985 May 5;183(1):53–68. doi: 10.1016/0022-2836(85)90280-3. [DOI] [PubMed] [Google Scholar]
- Thornburg R. W., An G., Cleveland T. E., Johnson R., Ryan C. A. Wound-inducible expression of a potato inhibitor II-chloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1987 Feb;84(3):744–748. doi: 10.1073/pnas.84.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai D. S., Arteca R. N., Bachman J. M., Phillips A. T. Purification and characterization of 1-aminocyclopropane-1-carboxylate synthase from etiolated mung bean hypocotyls. Arch Biochem Biophys. 1988 Aug 1;264(2):632–640. doi: 10.1016/0003-9861(88)90329-3. [DOI] [PubMed] [Google Scholar]
- Yu Y. B., Adams D. O., Yang S. F. 1-Aminocyclopropanecarboxylate synthase, a key enzyme in ethylene biosynthesis. Arch Biochem Biophys. 1979 Nov;198(1):280–286. doi: 10.1016/0003-9861(79)90420-x. [DOI] [PubMed] [Google Scholar]
- Yung K. H., Yang S. F., Schlenk F. Methionine synthesis from 3-methylthioribose in apple tissue. Biochem Biophys Res Commun. 1982 Jan 29;104(2):771–777. doi: 10.1016/0006-291x(82)90704-5. [DOI] [PubMed] [Google Scholar]
- de Boer H. A., Comstock L. J., Vasser M. The tac promoter: a functional hybrid derived from the trp and lac promoters. Proc Natl Acad Sci U S A. 1983 Jan;80(1):21–25. doi: 10.1073/pnas.80.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]









