Abstract
Introduction
Several studies have demonstrated that moderate exercise increases genital response to erotic stimuli in women. The increase in genital arousal could be the result of various changes that can occur in response to exercise including changes in hormone levels, neurotransmitter levels, mood, and autonomic nervous system activity.
Aim
The present study was an attempt to shed light on two such mechanisms through which exercise enhances sexual arousal.
Method
Sixteen participants came into the lab on two separate occasions: during one visit, they filled out questionnaires for 20 minutes, and during the other visit, they exercised on a treadmill for 20 minutes. The questionnaires and exercise were both followed by the presentation of a neutral then erotic film during which the women’s physiological sexual arousal was measured. Saliva samples were taken at baseline, prefilm, and postfilm.
Main Outcome Measures
Subjective arousal was measured using a self-report questionnaire, and genital arousal was measured by a vaginal photoplethysmograph. Testosterone and α-amylase (a marker of sympathetic nervous system [SNS] activity) were measured via saliva assays.
Results
Findings replicated previous studies showing a significant increase in physiological sexual arousal with exercise. There was a significant increase in α-amylase across the study in the exercise condition, but not in the no-exercise condition. There were no differences in testosterone levels between the exercise and no-exercise conditions.
Conclusions
SNS activity is one mechanism through which exercise increases genital sexual arousal. Testosterone does not mediate the relationship between exercise and genital sexual arousal.
Keywords: Arousal, Testosterone, Sympathetic Nervous System, Exercise, Alpha-Amylase
Introduction
Dynamic, cardiovascular exercise has many well-documented health benefits. One of the lesser known benefits is the positive effect of acute exercise on female sexual arousal. In a series of studies, Meston and Gorzalka [1–3] demonstrated that moderate-intensity exercise increased vaginal blood volume (VBV) and vaginal pulse amplitude (VPA) responses to an erotic film. In the first such study, 35 sexually functional, premenopausal women participated in two counterbalanced conditions: exercise and no-exercise. In the exercise condition, they exercised on a stationary bicycle for 20 minutes at 70% of their VO2 max (maximum volume of oxygen consumed over time during intense full-body exercise). After the exercise, the women watched a film sequence that consisted of a 3-minute neutral film clip followed by a 3-minute erotic film clip. In the no-exercise condition, participants filled out questionnaires and watched a similar film. Both VBV and VPA were significantly higher during the erotic portion of the film in the exercise condition as compared with the no-exercise condition [1]. Using the same experimental paradigm, Meston and Gorzalka replicated the postexercise increase in physiological arousal among sexually functional women and women with low sexual desire, but not among anorgasmic women [2]. A further study examining the effects of exercise at 5-, 15-, and 30-minute postexercise suggested a curvilinear relationship: VPA was significantly increased at 15 minutes, marginally increased at 30 minutes, and decreased at 5-minute postexercise [3].
In these studies, the authors suggested that exercise was enhancing genital arousal via activation of the sympathetic nervous system (SNS). This assumption was based on the findings that indicate exercise at moderate to high levels increases SNS activity, as measured by heart rate [4] and by catecholamine release [5]. These studies were some of the first to suggest that increased SNS activity may have a facilitatory, as opposed to inhibitory, influence on female sexual arousal. A follow-up study showing that clonidine, an antihypertensive medication that blunts normal SNS responses, blocked the effects of exercise on genital arousal supported the authors’ speculation [6].
There are a number of limitations to the inferences made in these studies however. First, SNS activation was not directly measured; heart rate was used as an indirect marker of SNS activity. Because heart rate declines after exercise, and sexual arousal was measured at postexercise, it is impossible to know whether the SNS was in fact still active, or whether the parasympathetic nervous system (PNS) had become dominant. Second, in addition to increasing SNS activity, exercise affects several other mechanisms that are thought to be involved in sexual arousal. For example, exercise has been shown to increase both dopamine and serotonin in women [7], and depending on the site of action and the receptor subtype affected, both dopamine and serotonin could have either an inhibitory or facilitatory effect on female sexual arousal (for reviews, see dopamine [8] and serotonin [9]). In general, adequate levels of both serotonin and dopamine are needed for normal sexual functioning, but it is unclear whether an increase due to exercise would be helpful or harmful to sexual arousal.
Exercise has also been shown to affect a variety of hormones such as testosterone [10,11], cortisol [11,12], estrogen [10,13], prolactin [14], and oxytocin [15], which have been linked with sexual arousal in women. For the purposes of the present study, we are particularly interested in the effects of exercise on testosterone. Most studies have found moderate levels of exercise increase plasma testosterone [10,11], although some studies have found no change in testosterone from pre- to post-exercise [16]. In women, testosterone is most often linked to increased sexual desire and positive mood, but evidence is mounting that testosterone affects the genital tissues as well. Women administered with exogenous androgens show increased genital arousal [17], although the mechanisms of this change are not well understood [18]. Thus, exercise could exert its positive effects on sexual arousal by increasing testosterone.
Aims
The present study was designed to better elucidate the mechanism(s) by which intense acute exercise enhances genital blood flow in women. The study had two primary goals: (i) to provide a more direct assessment of whether, and to what degree, the SNS is activated with exercise and sexual arousal, and (ii) to examine whether salivary measures of testosterone change with acute exercise and, if so, whether they could account for the exercise-induced increase in genital engorgement. SNS activation was assessed via salivary measures of α-amylase, a metabolite that is highly correlated with plasma levels of norepinephrine (NE) [19]. Because of its relationship to NE, α-amylase is thought to be a good indicator of adrenergic activity, which is intimately linked to SNS activity. α-Amylase has been shown to increase in response to physical and psychological stress including exercise [20]. Salivary testosterone is highly correlated with plasma free testosterone, the bioavailable form of the hormone [21].
Method
Participants
Participants were 16 women recruited from a community sample (see Table 1 for detailed demographics). All women had engaged in sexual activity with men in the past month. Prior to their first visit, the participants were screened by telephone to determine their eligibility for participation. Study criteria were as follows: women aged 18–45 and not yet menopausal; free of sexual problems; free of any drugs or medical conditions or medications that could affect sexual arousal, SNS activity, or testosterone (except hormonal contraceptives, N = 4); and physically able to exercise. These criteria were also confirmed via the self-report questionnaires administered in the laboratory.
Table 1.
Participants’ demographic information
| Participants (N = 16) |
||
|---|---|---|
| M | SD | |
| Age | 27.5 | 8.2 |
| FSFI total score | 30.11 | 4.46 |
| FSFI subscale scores | ||
| Desire | 4.65 | 0.88 |
| Arousal | 4.81 | 0.87 |
| Lubrication | 5.55 | 0.57 |
| Orgasm | 4.45 | 1.51 |
| Satisfaction | 4.9 | 1.09 |
| Pain | 5.75 | 0.5 |
| N | % | |
| Education | ||
| At least some college | 13 | 81.25 |
| Household income | ||
| Less than $30,000/year | 13 | 81.25 |
| 30,000–50,000 | 3 | 18.75 |
| Relationship status | ||
| Single, not dating | 1 | 6.25 |
| Single, dating | 6 | 37.5 |
| In a committed relationship | 9 | 56.25 |
| Ethnicity | ||
| Caucasian | 12 | 75 |
| Hispanic/Latina | 1 | 6.25 |
| Black | 1 | 6.25 |
| Asian | 2 | 12.5 |
| Hormonal contraception | ||
| Currently using | 4 | 25 |
FSFI = Female Sexual Function Index.
Procedures
All procedures were approved by the University of Texas Institutional Review Board. The participants visited the lab for two 90-minute sessions between days 5–10 of their menstrual cycle. All visits took place between 2:00 PM and 6:00 PM to control for diurnal fluctuations in testosterone. One of the laboratory sessions involved 20 minutes of exercise before the film sequence, while the other involved 20 minutes of questionnaires before the film sequence. These two sessions were counterbalanced across the participants. Both sessions began with a female researcher explaining the use of the vaginal photoplethysmograph and the placement of the electrodes for the electrocardiograph (ECG). Following this, the participants were asked to provide the baseline saliva sample.
No-Exercise Condition
In the no-exercise condition, the researcher left the room after requesting the first saliva sample. For the duration of the study, the participants were alone in a locked room, and the researcher communicated with the participants via an intercom.
After providing the first saliva sample, the participants were asked to fill out a series of questionnaires that took approximately 20 minutes to complete. The participants then waited for 10 minutes to provide the second saliva sample. Ten minutes is an adequate time for testosterone changes to register in saliva [22]. The participants then inserted the vaginal photoplethysmograph and attached the ECG electrodes. Once they were comfortable and relaxed, they completed the prefilm film scale and then watched the film sequence. This was followed by the administration of the postfilm film scale. Ten minutes after the end of the film, the participants provided their final saliva sample.
Exercise Condition
In the exercise condition, after collecting the first saliva sample, a researcher took the participants’ blood pressure to ensure that it was within a normal range and they would not be put at risk when exercising. The researcher also took the participants’ pulse to calculate the target heart rate. Target heart rate was calculated using the Karvonen method, which uses an individual’s heart rate reserve in the calculation. The participants were asked to run or walk on a treadmill at their target heart rate (70% of their heart rate reserve) for 20 minutes. After demonstrating how to use the treadmill, the researcher left the room for the duration of the study. The participants wore chest band heart rate monitors that displayed their heart rate on the treadmill as they exercised. The participants adjusted the speed and incline of the treadmill in order to reach and maintain their target heart rate. The participants were told that they needed to reach their target heart rate in 5 minutes and once it was reached, they needed to exercise for 15 minutes at that level. After 20 minutes of exercise, the participants waited for 10 minutes to provide the second saliva sample. The rest of the session proceeded in the same manner as the no-exercise condition.
Materials
Film Sequences
The participants viewed two different 14-minute film sequences that consisted of 1-minute display of the word “relax” on a black screen, 3 minutes of a neutral film (a travel documentary), and 10 minutes of a woman-centered sexual film. The sexual films were drawn from the Female Sexual Psychophysiology Laboratory film library. All films in this library have been standardized in terms of length of different types of sexual scenes (i.e., foreplay, oral sex, and vaginal intercourse), and scenes depicting violence have been edited out. These clips were selected from sexual films produced and directed by women, and are intended to be woman-friendly erotica.
Demographic Questionnaire
We gathered demographic and medical screening information with a brief questionnaire that asked participants their age, level of education, relationship status, sexual orientation, ethnicity, and length of relationship with their current partner. We also asked them to report any menstrual cycle irregularities, current medication use, and any current medical conditions.
Female Sexual Function Index (FSFI)
To verify that participants were free of sexual problems, we administered the FSFI, a validated 19-item questionnaire designed to assess sexual functioning in women [23]. The FSFI measures sexual functioning in six domains: desire, arousal, lubrication, orgasm, satisfaction, and pain, plus a total score. A clinical cutoff score of 26.55 has been established to reliably discriminate between women with and without sexual dysfunction [24]. All of the participants had scores above the clinical cutoff.
Film Scale
Subjective responses to the erotic film were measured using the film scale, a 41-item questionnaire that examines four areas of subjective response: subjective experience of physiological sexual arousal, mental sexual arousal, positive affect, and negative affect [25]. The participants filled out the film scale immediately before and immediately after watching the film sequence, and the difference from prefilm and postfilm was assessed for each condition.
Main Outcome Measures
Vaginal Photoplethysmograph
Genital arousal in response to the neutral and erotic films was measured using a vaginal photoplethysmograph [26]. VPA was our measure of interest, and it was sampled 80 times/second. Results were measured in millivolts (mV). VPA was acquired using the software program Acq-Knowledge III, Version 3.7.3 (BIOPAC Systems, Inc., Santa Barbara, CA, USA) and a Model MP100WS data acquisition unit (BIOPAC Systems, Inc.) for analog/digital conversion. VPA data were reduced by calculating the total change in amplitude for each pulse wave. This was carried out by finding the peak and nadir for each pulse wave and computing the differences between the two to get the VPA for each pulse wave. Artifacts in the data were identified visually by the researcher and were removed manually. VPAs were averaged across the neutral portion of the film and the erotic portion of the film.
ECG
Heart rate was measured via ECG, which consists of three disposable electrodes that are attached to the participant’s body (upper right chest, lower left chest, and right ankle) and connected by cables to a BIOPAC Systems ECG100 module. The signal from the ECG100 module was recorded in real time using the AcqKnowledge software program. Heart rate data were calculated by finding the time change between each peak, inverting it, and multiplying it by 60 to determine the beats per minute (bpm).
Saliva Samples
Salivary assays are a relatively un-invasive way to examine biomarkers of interest that are often measured in blood. Although testosterone in saliva is not an accurate way to measure androgens for clinical diagnoses, it is appropriate for examining within-person changes among healthy women [27]. In this case, the objective levels of testosterone in the blood are not necessary because each women is her own control. The participants salivated without stimulation (e.g., gum) directly into untreated, polystyrene centrifuge tubes. Samples were frozen at −20°C until assay, at which point they were thawed and centrifuged for 10 minutes at 3,000 rpm (Salimetrics, State College, PA, USA). Testosterone and α-amylase assays were performed in-house using kits purchased from Salimetrics. All assays were run in duplicate. α-Amylase was measured via an enzymatic reaction; inter-assay C.V. was 6.34%, and intra-assay C.V. was 2.43%. Testosterone was measured via enzyme immunoassay; inter-assay C.V. was 8.43%, and intra-assay C.V. was 3.22%.
Data Analysis
Participants who showed a decrease in VPA from the neutral to erotic segments (N = 1) were excluded from the data analysis. All data were analyzed using SPSS Version 15.0 (SPSS Inc., Chicago, IL, USA). Because VPA raw data were not comparable across participants, in order to standardize scores for comparison across participants, we calculated the percent change over baseline for each participant’s VPA data. All other data analyses were conducted on raw scores. Overall effects were tested using repeated measures analysis of variance (ANOVA) or t-tests where appropriate.
Results
VPA
The mean percentage increase between neutral and erotic films for the no-exercise condition was 35.15% (SD = 44.54), which was a significant increase, t (14) = 3.06, P = 0.009. The mean percentage increase between neutral and erotic films for the exercise condition was 55.94% (SD = 32.36), which was also a significant increase, t (12) = 6.69, P < 0.001. These findings indicate the erotic films were effective in enhancing genital arousal. A repeated measures ANOVA was conducted on the VPA percent change scores with exercise/no-exercise condition as the within-subjects variable. Exercise led to a significantly larger percent increase in VPA compared with the no-exercise condition, F (1, 14) = 7.286, P = 0.02, indicating that the women had higher levels of genital arousal after exercise exposure.
Heart Rate
In the no-exercise condition, during the film sequence, the participants’ mean heart rate was 73.68 bpm (SD = 5.13) while watching the film sequence. The mean heart rate during the film sequence in the exercise condition was 87.44 (SD = 7.62). Results from a repeated measures ANOVA with condition as the within-subjects variable indicated that heart rate in the exercise condition was significantly higher than in the no-exercise condition, F (1, 14) = 4.65, P = 0.03.
α-Amylase
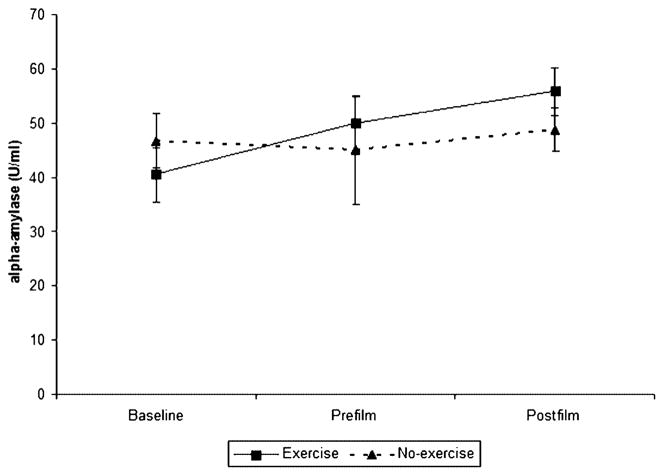
α-Amylase values were entered into a 2 × 3 (condition × time) repeated measures ANOVA. There was no significant main effect for condition, F (1, 14) = 0.17, P = 0.69, or time, F (2, 28) = 2.78, P = 0.11, but there was a significant interaction, F (2, 28) = 6.01, P = 0.02. Post hoc repeated measures ANOVA revealed that the change in α-amylase over time was significant for the exercise condition, F (2, 28) = 6.27, P = 0.02, but not for the no-exercise condition, F (2, 28) = 0.16, P = 0.74 (Figure 1). These results show that exercise increases α-amylase, a marker of SNS activity, and that it stays elevated after the erotic stimuli.
Figure 1.
α-Amylase change over time, by condition. α-Amylase increased significantly in the exercise condition, but not in the no-exercise condition.
Testosterone
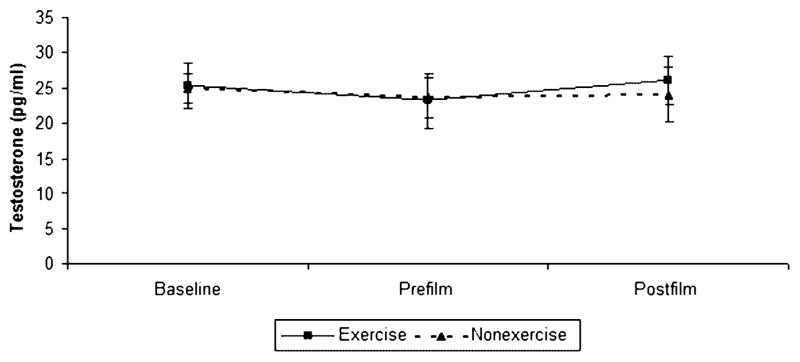
Three participants had at least one salivary testosterone value that was too low to be measured by our assay, so they were excluded from these analyses. Salivary testosterone values were entered into a 2 × 3 (condition × time) repeated measures ANOVA. There was no significant main effect for condition, F (1, 11) = 0.084, P = 0.78, or time, F (2, 22) = 0.52, P = 0.6, nor was there an interaction, F (2, 22) = 0.02, P = 0.97, between the two variables. There were no changes in salivary testosterone levels over the course of the study, and there were no differences in testosterone levels between the exercise and no-exercise conditions (Figure 2).
Figure 2.
Testosterone change over time, by condition. There were no changes in testosterone over the course of the study, nor were there any differences between the exercise and no-exercise conditions.
Subjective Measures
Each of the four subscales of the film scale (physiological sexual arousal, mental sexual arousal, positive affect, and negative affect) were entered into a repeated measures ANOVA to determine if there was a change from prefilm to postfilm, and if there were differences between the exercise and no-exercise conditions. There were significant increases over the course of the film in subjective ratings of physiological arousal, F (1, 13) = 36.76, P < 0.001, mental sexual arousal, F (1, 13) = 42.47, P < 0.001, and positive affect, F (1, 13) = 19.99, P < 0.001. There was no change in negative affect between conditions, F (1, 13) = 0.18, P = 0.67. These findings indicate the erotic films were effective in enhancing sexual arousal and positive but not negative mood. There were no significant differences between the exercise and no-exercise conditions in ratings of physiological sexual arousal, F (1, 14) = 0.08, P = 0.77, mental sexual arousal, F (1, 13) = 1.34, P = 0.27, positive affect, F (1, 14) = 0.71, P = 0.41, or negative affect, F (1, 14) = 0.12, P = 0.73.
Discussion
This study replicated previous studies [1–3], indicating intense acute exercise enhances physiological but not subjective sexual arousal in women. Our finding that there was no significant differences in subjective ratings of sexual arousal or mood between the exercise and no-exercise conditions supports the notion that exercise increases genital sexual arousal via physiological mechanisms, as opposed to feedback from cognitive arousal or alterations in mood that may have been a consequence of exercise.
The findings from this study extended past research of this nature by examining two of the mechanisms through which exercise could feasibly be enhancing genital engorgement in women—SNS activation and changes in testosterone. α-Amylase increased in response to exercise, and stayed elevated when measured 10 minutes after the erotic film, indicating that the SNS was still active during the erotic film. It appears that the increased SNS activity caused by exercise persists after the exercise is over, providing evidence that increased SNS activity is one feasible explanation for the increase in genital arousal reported in the literature. This information extends findings from previous studies that used heart rate as an indicator of SNS activity in that α-amylase is a more direct and reliable indicator of SNS. α-Amylase did not significantly change across the no-exercise condition, indicating that a visual erotic stimulus alone is not enough to significantly increase SNS activity. Although the SNS and PNS often increase and decrease their activity in opposition to one another, it is possible that they can also be active simultaneously. Unfortunately, α-amylase does not tell us if whether or not the PNS is active during the arousal phase of the female sexual response, thus, the findings do not rule out the potential additive influence of the PNS.
Contrary to predictions, testosterone did not increase in response to exercise or to the erotic film. Testosterone remained stable across all three time points for both the exercise and no-exercise conditions, and there were no differences between the conditions. Although testosterone often increases in response to exercise, it is not always the case [16], and at least one previous study has found that sexual stimuli does not increase plasma testosterone in women [28]. In the present study, testosterone response to the erotic films did not differ significantly between the exercise and no-exercise conditions, indicating a free testosterone is not playing a role in the increased genital arousal seen after exercise.
It should be noted that a small percentage of women in this study were currently using hormonal contraceptives, which are known to elevate levels of sex hormone binding globulin and lower levels of free testosterone [29]. However, because the study used a within-subjects design and the participants served as their own controls, any differences in testosterone likely would have been present in both experimental conditions. Moreover, post hoc analyses indicated that baseline testosterone levels did not significantly differ between the four women on hormonal contraceptives and the 12 women who were not on oral contraceptives, t (15) = 1.23, P = 0.32.
Salivary testosterone is a reflection of the level of bioavailable testosterone in the body, but other androgens can also play a similar role to testosterone. The fact that we saw no change in testosterone in this study does not rule out the possibility that another androgen, such as dehydroepiandrosterone, is contributing to the increased genital arousal.
Other non-androgenic hormones that are related to both exercise and sexual arousal could also feasibly contribute to the exercise-induced enhancement of sexual arousal. For example, cortisol may be altered by exercise, but the direction of the change is still under debate as some studies find an increase in cortisol from pre- to postexercise [12], and some find a decrease [11]. If cortisol was to increase during exercise, it would likely have a negative effect on sexual arousal, given cortisol declines during sexual arousal [28].
Estrogen has been shown to increase after acute exercise [10], but decrease in response to long-term physical activity [13]. Estrogens in the peripheral nervous system are critical for the maintenance of vaginal tissue function and structure [18], and estrogen deficiency has been linked to various vaginal problems including reduced or delayed lubrication, reduced vaginal blood flow, and increased dyspareunia [30]. Studies in humans focus on the genomic effects of estrogen in the peripheral nervous system, but evidence is accumulating in animal research that the rapid effects of estrogen in the central nervous system (CNS) play a role in priming the CNS for arousability [31].
Oxytocin has been shown to increase in response to exercise in men [15]. Although orgasm and oxytocin are studied often, little research exists on the relationship between oxytocin and sexual arousal. One study in women found a linear increase in oxytocin with levels peaking after orgasm [32], and a more recent study has linked oxytocin levels to a well-validated measure of arousal and lubrication, but only in the luteal phase for cycling women and in the last week of pill-taking for women on hormonal contraceptives [33].
The increase in prolactin caused by exercise is well established [14]. High levels of prolactin, however, have a negative effect on sexual arousal as people with hyperprolactinemia have chronically low levels of sexual arousal and desire [34]. Pro-lactin increases after orgasm, and is theorized to be an “off switch” for sexual arousal [35]. The contradictory findings that exercise increases not only genital arousal, but also prolactin, which inhibits sexual arousal, are problematic and not well understood. Although prolactin increases after sex and after exercise, plasma levels are still much lower than in people who have hyperprolactinemia [34]. All of the studies on the effects of prolactin on sexual arousal have focused on chronically high levels of prolactin; it is feasible that the level of prolactin secreted immediately after exercise is not sufficient to impair arousal.
In addition to the biological changes that occur in response to exercise, there could be cognitive changes that may contribute to an increase in arousal. The subjective measures that we used in the present study did not reveal any differences between the exercise and no-exercise conditions, but these measures specifically targeted feelings of sexual arousal and mood. There are numerous cognitive factors that could also play a role in increasing arousal. For example, a recent study that manipulated women’s awareness of their bodies found that increased body awareness was linked with increased perceptions of arousal [36]. Exercise could also feasibly increase body awareness via increased bodily sensations (e.g., increased heart beat, muscle tension, etc.).
Conclusions
The findings from this study support the notion that exercise enhances physiological sexual arousal in women via increased SNS activation. Evidence for the role of testosterone in exercise-induced sexual arousal was not found. There are a number of additional mechanisms through which exercise could feasibly enhance genital engorgement that were not tested here. These include potential exercise-induced alterations in prolactin, dopamine, NE, serotonin, estrogen, oxytocin, and cortisol.
Statement of Authorship
Category 1
-
Conception and Design
Lisa Dawn Hamilton; Cindy Meston; Emily Fogle
-
Acquisition of Data
Lisa Dawn Hamilton; Emily Fogle
-
Analysis and Interpretation of Data
Lisa Dawn Hamilton; Cindy Meston
Category 2
-
Drafting the Article
Lisa Dawn Hamilton; Emily Fogle; Cindy Meston
-
Revising It for Intellectual Content
Lisa Dawn Hamilton; Cindy Meston
Category 3
-
Final Approval of the Completed Article
Lisa Dawn Hamilton; Emily Fogle; Cindy Meston
Footnotes
Conflict of Interest: None declared.
References
- 1.Meston CM, Gorzalka BB. The effects of sympathetic activation on physiological and subjective sexual arousal in women. Behav Res Ther. 1995;33:651–64. doi: 10.1016/0005-7967(95)00006-j. [DOI] [PubMed] [Google Scholar]
- 2.Meston CM, Gorzalka BB. Differential effects of sympathetic activation on sexual arousal in sexually dysfunctional and functional women. J Abnorm Psychol. 1996;105:582–91. doi: 10.1037//0021-843x.105.4.582. [DOI] [PubMed] [Google Scholar]
- 3.Meston CM, Gorzalka BB. The effects of immediate, delayed, and residual sympathetic activation on sexual arousal in women. Behav Res Ther. 1996;34:143–48. doi: 10.1016/0005-7967(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson BF, Epstein SE, Beiser GD, Braunwald E. Control of heart rate by the autonomic nervous system. Studies in man on the interrelation between baroreceptor mechanisms and exercise. Circ Res. 1966;19:400–11. doi: 10.1161/01.res.19.2.400. [DOI] [PubMed] [Google Scholar]
- 5.Christensen NJ, Galbo H. Sympathetic nervous activity during exercise. Annu Rev Physiol. 1983;45:139–53. doi: 10.1146/annurev.ph.45.030183.001035. [DOI] [PubMed] [Google Scholar]
- 6.Meston CM, Gorzalka BB, Wright JM. Inhibition of subjective and physiological sexual arousal in women by clondine. Psychosom Med. 1997;59:399–407. doi: 10.1097/00006842-199707000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Chalouloff F. Physical exercise and brain monoamines: A review. Acta Physiol Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- 8.Melis MR, Argiolas A. Dopamine and sexual behavior. Neurosci Biobehav Rev. 1995;19:19–38. doi: 10.1016/0149-7634(94)00020-2. [DOI] [PubMed] [Google Scholar]
- 9.Frohlich PF, Meston CM. Evidence that serotonin affects female sexual functioning via peripheral mechanisms. Physiol Behav. 2000;71:383–93. doi: 10.1016/s0031-9384(00)00344-9. [DOI] [PubMed] [Google Scholar]
- 10.Keizer HA, Kuipers H, de Haan J, Beckers E, Habets L. Multiple hormonal responses to physical exercise in eumenorrheic trained and untrained women. Int J Sports Med. 1987;8:139–50. doi: 10.1055/s-2008-1025720. [DOI] [PubMed] [Google Scholar]
- 11.Copeland JL, Consitt LA, Tremblay MS. Hormonal responses to endurance and resistance exercise in females aged 19–69 years. J Gerontol A Biol Sci Med Sci. 2002;57:B158–65. doi: 10.1093/gerona/57.4.b158. [DOI] [PubMed] [Google Scholar]
- 12.Cumming DC, Rebar RW. Exercise and reproductive function in women. Am J Int Med. 1983;4:113–25. [PubMed] [Google Scholar]
- 13.Boyden TW, Pamenter RW, Stanforth P, Rotkis T, Wilmore JH. Sex steroids and endurance running in women. Fertil Steril. 1983;39:629–32. doi: 10.1016/s0015-0282(16)47057-3. [DOI] [PubMed] [Google Scholar]
- 14.Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. Exercise and the stress system. Hormones. 2005;4:73–89. [PubMed] [Google Scholar]
- 15.Landgraf R, Häcker R, Buhl H. Plasma vasopressin and oxytocin in response to exercise during a day-night cycle in man. Endokrinologie. 1982;79:281–91. [PubMed] [Google Scholar]
- 16.Filaire E, Lac G. Dehydroepiandrosterone (DHEA) rather than testosterone shows saliva androgen responses to exercise in elite female handball players. Int J Sports Med. 2000;21:139–50. doi: 10.1055/s-2000-8851. [DOI] [PubMed] [Google Scholar]
- 17.Heard-Davison A, Heiman JR, Kuffel S. Genital and subjective measurement of the time course effects of an acute dose of testosterone vs. placebo in postmenopausal women. J Sex Med. 2007;4:209–17. doi: 10.1111/j.1743-6109.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 18.Traish AM, Kim N, Min K, Munarriz R, Goldstein I. Androgens in female genital sexual arousal function: A biochemical perspective. J Sex Marital Ther. 2002;28:233–44. doi: 10.1080/00926230252851366. [DOI] [PubMed] [Google Scholar]
- 19.Rohleder N, Nater UM, Wolf JM, Ehlert U, Kirschbaum C. Psychosocial stress-induced activation of salivary a-amylase. An indicator of sympathetic activity? Ann NY Acad Sci. 2004;1032:258–63. doi: 10.1196/annals.1314.033. [DOI] [PubMed] [Google Scholar]
- 20.Chatterton RT, Vogelsong KM, Lu Y, Ellman AB, Hudgens GA. Salivary α-amylase as a measure of endogenous adrenergic activity. Clin Physiol. 1996;16:433–48. doi: 10.1111/j.1475-097x.1996.tb00731.x. [DOI] [PubMed] [Google Scholar]
- 21.Wang C, Plymate S, Nieschlag E, Paulsen CA. Salivary testosterone in men: Further evidence of a direct correlation with free serum testosterone. J Clin Endocrinol Metab. 1981;53:1021–24. doi: 10.1210/jcem-53-5-1021. [DOI] [PubMed] [Google Scholar]
- 22.Malamud D, Tabak L. Saliva as a diagnostic fluid. New York, NY: Academy of Sciences; 1993. [Google Scholar]
- 23.Rosen R, Brown C, Heiman J, Leiblum S, Meston CM, Shabsigh R, Ferguson D, D’Agostino R., Jr The Female Sexual Function Index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 24.Wiegel M, Meston CM, Rosen RC. The Female Sexual Function Index (FSFI): Cross-validation and development of clinical cutoff scores. J Sex Marital Ther. 2005;31:1–20. doi: 10.1080/00926230590475206. [DOI] [PubMed] [Google Scholar]
- 25.Heiman JR, Rowland DL. Affective and physiological sexual response patterns: The effects of instructions on sexually functional and dysfunctional men. J Psychosom Res. 1983;27:105–16. doi: 10.1016/0022-3999(83)90086-7. [DOI] [PubMed] [Google Scholar]
- 26.Sintchak G, Geer JH. A vaginal plethysmograph system. Psychophysiology. 1975;12:113–5. doi: 10.1111/j.1469-8986.1975.tb03074.x. [DOI] [PubMed] [Google Scholar]
- 27.van Anders SM, Hamilton LD, Schmidt N, Watson NV. Associations between testosterone secretion and sexual activity in women. Horm Behav. 2007;51:477–82. doi: 10.1016/j.yhbeh.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 28.Exton NG, Truong TC, Exton MS, Wingenfeld SA, Leygraf N, Saller B, Hartmann U, Schedlowski M. Neuroendocrine response to film-induced sexual arousal in men and women. Psychoneuroendocrinology. 2000;25:187–99. doi: 10.1016/s0306-4530(99)00049-9. [DOI] [PubMed] [Google Scholar]
- 29.Panzer C, Wise S, Fantini G, Kang D, Munarriz R, Guay A, Goldstein I. Impact of oral contraceptives on sex hormone-binding globulin and androgen levels: A retrospective study in women with sexual dysfunction. J Sex Med. 2006;3:104–13. doi: 10.1111/j.1743-6109.2005.00198.x. [DOI] [PubMed] [Google Scholar]
- 30.Sarrel PM. Effects of hormone replacement therapy on sexual psychophysiology and behavior in post-menopause. J Womens Health Gend Based Med. 2000;9(1 suppl):25–32. doi: 10.1089/152460900318830. [DOI] [PubMed] [Google Scholar]
- 31.Caldwell JD. A sexual arousability model involving steroid effects at the plasma membrane. Neurosci Biobehav Rev. 2002;26:13–30. doi: 10.1016/s0149-7634(01)00035-5. [DOI] [PubMed] [Google Scholar]
- 32.Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;67:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- 33.Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47:164–9. doi: 10.1016/j.yhbeh.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Krüger THC, Haake P, Hartmann U, Schedlowski M, Exton MS. Orgasm-induced prolactin secretion: Feedback control of sexual drive? Neurosci Biobehav Rev. 2002;26:31–44. doi: 10.1016/s0149-7634(01)00036-7. [DOI] [PubMed] [Google Scholar]
- 35.Levin RJ. Is prolactin the biological “off switch” for human sexual arousal? Sex Relationship Ther. 2003;18:237–43. [Google Scholar]
- 36.Seal BN, Meston CM. The impact of body awareness on sexual arousal in women with sexual dysfunction. J Sex Med. 2007;4:990–1000. doi: 10.1111/j.1743-6109.2007.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]