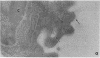
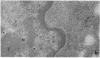
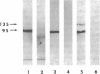
Abstract
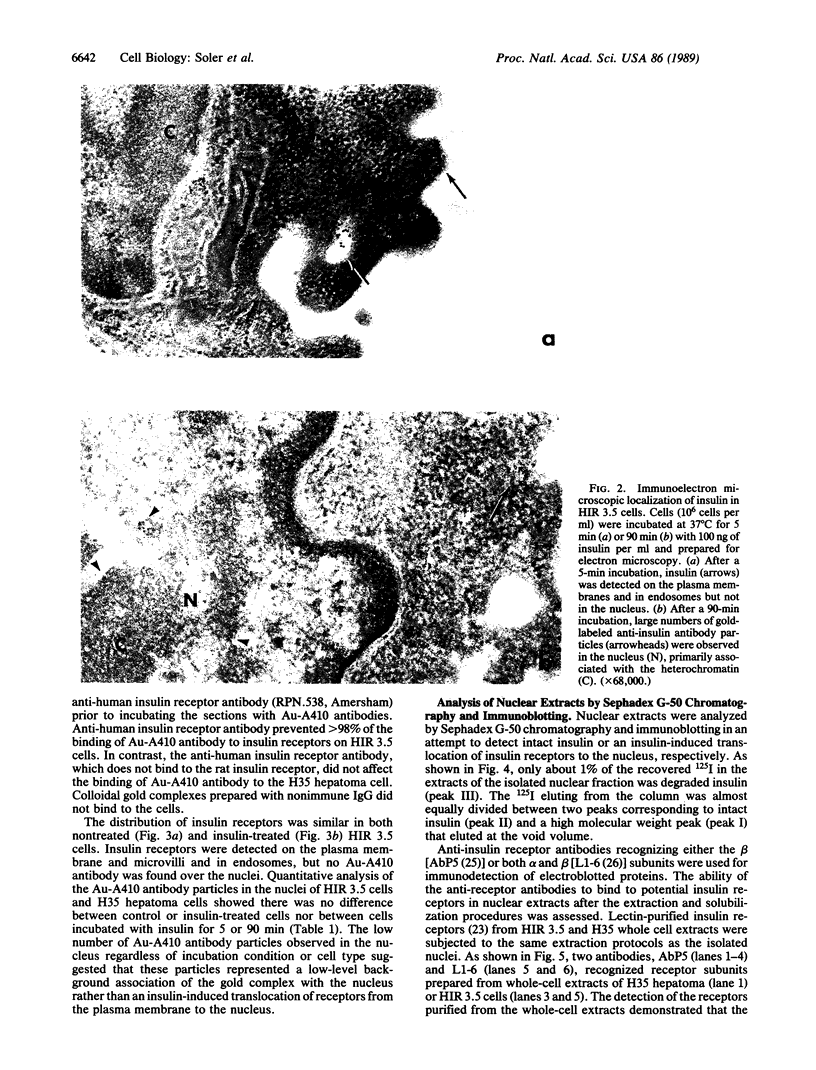
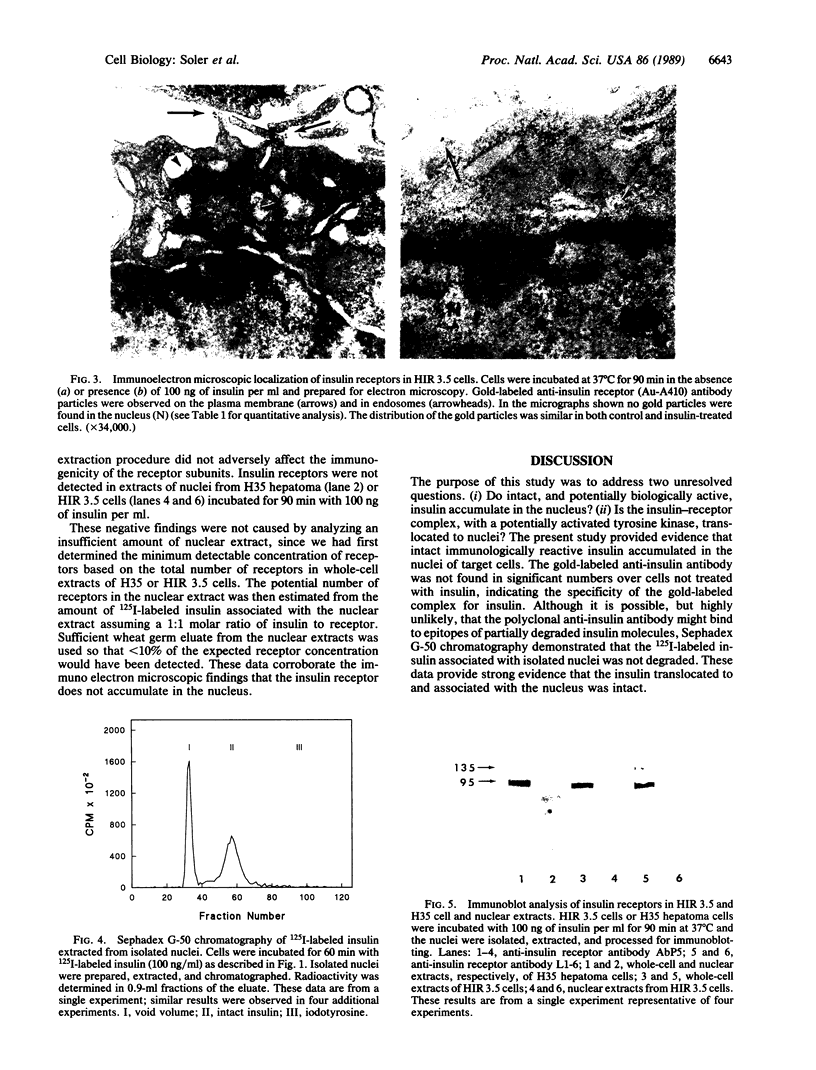
Although insulin is known to regulate nuclear-related processes, such as cell growth and gene transcription, the mechanisms involved are poorly understood. Previous studies suggested that translocation of insulin or its receptor to cell nuclei might be involved in some of these processes. The present investigation demonstrated that intact insulin, but not the insulin receptor, accumulated in nuclei of insulin-treated cells. Cell fractionation studies demonstrated that the nuclear accumulation of 125I-labeled insulin was time-, temperature-, and insulin-concentration-dependent. Electron microscopic immunocytochemistry demonstrated that the insulin that accumulated in the nucleus was immunologically intact and associated with the heterochromatin. Only 1% of the 125I-labeled insulin extracted from isolated nuclei was eluted from a Sephadex G-50 column as 125I-labeled tyrosine. Plasma membrane insulin receptors were not detected in the nucleus by immuno electron microscopy or when wheat germ agglutinin-purified extracts of the nuclei were subjected to PAGE, electrotransfer, and immunoblotting with anti-insulin receptor antibodies. These results suggested that internalized insulin dissociated from its receptor and accumulated in the nucleus without its membrane receptor. We propose that some of insulin's effects on nuclear function may be caused by the translocation of the intact and biologically active hormone to the nucleus and its binding to nuclear components in the heterochromatin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergeron J. J., Sikstrom R., Hand A. R., Posner B. I. Binding and uptake of 125I-insulin into rat liver hepatocytes and endothelium. An in vivo radioautographic study. J Cell Biol. 1979 Feb;80(2):427–443. doi: 10.1083/jcb.80.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpentier J. L., Gorden P., Barazzone P., Freychet P., Le Cam A., Orci L. Intracellular localization of 125I-labeled insulin in hepatocytes from intact rat liver. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2803–2807. doi: 10.1073/pnas.76.6.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Parikh I. Affinity chromatography of insulin receptors. Methods Enzymol. 1974;34:653–670. doi: 10.1016/s0076-6879(74)34088-8. [DOI] [PubMed] [Google Scholar]
- Duckworth W. C. Insulin degradation by liver cell membranes. Endocrinology. 1979 Jun;104(6):1758–1764. doi: 10.1210/endo-104-6-1758. [DOI] [PubMed] [Google Scholar]
- Fakan S., Puvion E. The ultrastructural visualization of nucleolar and extranucleolar RNA synthesis and distribution. Int Rev Cytol. 1980;65:255–299. doi: 10.1016/s0074-7696(08)61962-2. [DOI] [PubMed] [Google Scholar]
- Forsayeth J., Maddux B., Goldfine I. D. Biosynthesis and processing of the human insulin receptor. Diabetes. 1986 Jul;35(7):837–846. doi: 10.2337/diab.35.7.837. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Ken R. Insulin stimulates incorporation of 32Pi into nuclear lamins A and C in quiescent BHK-21 cells. J Biol Chem. 1988 Jan 25;263(3):1103–1106. [PubMed] [Google Scholar]
- Goidl J. A. Insulin binding to isolated liver nuclei from obese and lean mice. Biochemistry. 1979 Aug 21;18(17):3674–3679. doi: 10.1021/bi00584a006. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y. Electron microscope autoradiographic analysis of [125I]iodoinsulin entry into adult rat hepatocytes in vivo: evidence for multiple sites of hormone localization. Endocrinology. 1981 May;108(5):1821–1828. doi: 10.1210/endo-108-5-1821. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Jones A. L., Hradek G. T., Wong K. Y., Mooney J. S. Entry of insulin into human cultured lymphocytes: electron microscope autoradiographic analysis. Science. 1978 Nov 17;202(4369):760–763. doi: 10.1126/science.715440. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Purrello F., Vigneri R., Clawson G. A. Insulin and the regulation of isolated nuclei and nuclear subfractions: potential relationship to mRNA metabolism. Diabetes Metab Rev. 1985;1(1-2):119–137. doi: 10.1002/dmr.5610010107. [DOI] [PubMed] [Google Scholar]
- Goldfine I. D., Smith G. J. Binding of insulin to isolated nuclei. Proc Natl Acad Sci U S A. 1976 May;73(5):1427–1431. doi: 10.1073/pnas.73.5.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R., Petruzzelli L., Thomas N., Bramson H. N., Kaiser E. T., Rosen O. M. An antipeptide antibody that specifically inhibits insulin receptor autophosphorylation and protein kinase activity. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7899–7903. doi: 10.1073/pnas.82.23.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvat A., Li E., Katsoyannis P. G. Cellular binding sites for insulin in rat liver. Biochim Biophys Acta. 1975 Apr 8;382(4):609–620. doi: 10.1016/0005-2736(75)90226-6. [DOI] [PubMed] [Google Scholar]
- Huecksteadt T., Olefsky J. M., Brandenberg D., Heidenreich K. A. Recycling of photoaffinity-labeled insulin receptors in rat adipocytes. Dissociation of insulin-receptor complexes is not required for receptor recycling. J Biol Chem. 1986 Jul 5;261(19):8655–8659. [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Coffey D. S., Shaper J. H. Considerations in the isolation of rat liver nuclear matrix, nuclear envelope, and pore complex lamina. Exp Cell Res. 1981 Mar;132(1):105–123. doi: 10.1016/0014-4827(81)90088-4. [DOI] [PubMed] [Google Scholar]
- Koontz J. W., Iwahashi M. Insulin as a potent, specific growth factor in a rat hepatoma cell line. Science. 1981 Feb 27;211(4485):947–949. doi: 10.1126/science.7008195. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. L., Isham K. R., Johnson A., Kenney F. T. Insulin enhances transcription of the tyrosine aminotransferase gene in rat liver. Arch Biochem Biophys. 1986 Aug 1;248(2):597–603. doi: 10.1016/0003-9861(86)90513-8. [DOI] [PubMed] [Google Scholar]
- Massagué J., Blinderman L. A., Czech M. P. The high affinity insulin receptor mediates growth stimulation in rat hepatoma cells. J Biol Chem. 1982 Dec 10;257(23):13958–13963. [PubMed] [Google Scholar]
- Miller D. S. Stimulation of RNA and protein synthesis by intracellular insulin. Science. 1988 Apr 22;240(4851):506–509. doi: 10.1126/science.2451860. [DOI] [PubMed] [Google Scholar]
- Podlecki D. A., Smith R. M., Kao M., Tsai P., Huecksteadt T., Brandenburg D., Lasher R. S., Jarett L., Olefsky J. M. Nuclear translocation of the insulin receptor. A possible mediator of insulin's long term effects. J Biol Chem. 1987 Mar 5;262(7):3362–3368. [PubMed] [Google Scholar]
- Purrello F., Burnham D. B., Goldfine I. D. Insulin regulation of protein phosphorylation in isolated rat liver nuclear envelopes: potential relationship to mRNA metabolism. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1189–1193. doi: 10.1073/pnas.80.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K., Granner D. K. Regulation of phosphoenolpyruvate carboxykinase gene transcription by insulin and cAMP: reciprocal actions on initiation and elongation. Proc Natl Acad Sci U S A. 1988 May;85(9):2954–2958. doi: 10.1073/pnas.85.9.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Smith R. M., Goldberg R. I., Jarett L. Preparation and characterization of a colloidal gold-insulin complex with binding and biological activities identical to native insulin. J Histochem Cytochem. 1988 Apr;36(4):359–365. doi: 10.1177/36.4.3279110. [DOI] [PubMed] [Google Scholar]
- Smith R. M., Jarett L. Ultrastructural evidence for the accumulation of insulin in nuclei of intact 3T3-L1 adipocytes by an insulin-receptor mediated process. Proc Natl Acad Sci U S A. 1987 Jan;84(2):459–463. doi: 10.1073/pnas.84.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R., Roy A., Dieter R., Koontz J. Insulin as a growth factor in rat hepatoma cells. Stimulation of proto-oncogene expression. J Biol Chem. 1987 Aug 5;262(22):10893–10897. [PubMed] [Google Scholar]
- Whittaker J., Okamoto A. K., Thys R., Bell G. I., Steiner D. F., Hofmann C. A. High-level expression of human insulin receptor cDNA in mouse NIH 3T3 cells. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5237–5241. doi: 10.1073/pnas.84.15.5237. [DOI] [PMC free article] [PubMed] [Google Scholar]