Summary
A hepatocyte stimulating activity (HSA) has been extracted from rats that had received an injection of a pharmacological dose of T3 20 hours earlier. The injection of HSA from T3-treated rats into different recipient rats that had previously had 40% of their liver removed resulted in a significant increase in hepatic DNA synthesis. The injection of saline or HSA from normal rat liver had little or no effect on hepatic DNA synthesis in recipient rats. HSA from the T3-treated rats also stimulated DNA synthesis in Novikoff hepatoma cells and primary hepatocytes in culture, and in isolated normal rat liver nuclei in a nuclear incorporating system. In further experiments in which the increased DNA synthesis that follows partial hepatectomy was blocked by adriamycin, HSA appeared in these non-regenerating livers. This latter observation had indicated that the development of HSA is not merely an accompaniment of DNA synthesis.
Keywords: Hepatocyte Stimulating Activity (HSA), T3, Hepatic DNA Synthesis, Rat
Introduction
Extracts from livers which have increased hepatocyte proliferation contain a hepatocyte stimulatory activity (HSA) which can cause or amplify a regeneration-like response in third party test animals, and which can be demonstrated by tissue culture assays. HSA has been demonstrated in weanling rat livers (LaBrecque and Bachur 1982), and in the regenerating liver remnants of normal rats (LaBrecque and Pesch 1975; Hatase, Fujii, Kuramitsu, Itano, Takahashi, Murakami and Nisida 1979) and dogs (Starzl, Terblanche, Porter, Jones, Usui and Mazzoni 1979; Terblanche, Porter, Starzl, Moore, Patzelt and Hayashida 1980) after partial hepatectomy.
T3 has been shown to cause a regeneration-like response in normal rat livers (Klein, Chou, Short and Ove 1981; Short, Klein, Kibert and Ove 1980). We report here evidence that the livers of rats treated with this hormone contain HSA. In further experiments in which the increased DNA synthesis that follows partial hepatectomy was blocked by adriamycin, HSA appeared in these non-regenerating livers. This latter observation has indicated that the development of HSA is not merely an accompaniment of DNA synthesis.
Materials and Methods
Animals
Male Sprague Dawley rats received food and water ad libitum. They were kept in a temperature and light-controlled room. Rats weighing less than 100 g were considered weanling rats. Partial hepatectomy was performed according to Higgins and Anderson (1931). In sham-operated animals, the liver was manually manipulated and returned into the abdomen. Operations were performed between 7:30 and 9:00 AM.
Materials
Leibovitz L-15, Swimms S-77, Dulbecco’s Modified Eagle’s Medium (DME) Antibiotic/Antimycotic (penicillin, streptomycin, fungizone), and calf serum were purchased from GIBCO, Grand Island, New York. Fetal Bovine Serum was obtained from KC Biologicals Inc., Lenexa, Kansas. Collagenase Type 1A, trypsin inhibitor, T3 (3,3′5-triiodothyronine), and the unlabelled deoxyribonucleoside triphosphates were from Sigma Chemical Company, St. Louis, Missouri. [3H] thymidine and [3H] thymidine 5′-triphosphate were obtained from ICN Pharmaceuticals, Inc., Irvine, California.
Determination of DNA synthesis in animals
To test for HSA in an ethanol precipitate of liver extracts, partially (40%) hepatectomized rats were used. Six hours after the operation they were injected (I.P.) with 5 ml of HSA substance (5 mg/ml of an ethanol precipitate as described under “preparation of HSA”). Eighteen hours after HSA injection, the animals were injected (I.P.) with 10 u Ci [3H] thymidine and killed 2 hours later. Livers were removed and citric acid nuclei prepared (Coetzee, Short, Klein and Ove 1982). DNA synthesis was determined as previously described (Ove, Coetzee and Morris 1971; Ove, Coetzee and Morris 1973). In some experiments, animals were not pulsed in vivo. Instead, the liver was removed and 400–600 mg liver slices were prepared and incubated in Krebs-Ringer solution pH 7.45 for 2 hours under 95% O2–5% CO2 at 37° in the presence of 2 u Ci [3H] thymidine. Slices were then homogenized in 0.1 M citric acid and citric acid nuclei were prepared followed by the determination of DNA synthesis as above.
Preparations of HSA
HSA was prepared as reported by LaBrecque and Pesch (1975). Livers or hepatoma tissue was homogenized in 35% (w/v) ice cold 0.15 M NaCl. Homogenates were heated at 65° for 15 min and centrifuged at 30,000 xg for 20 min. Six volumes of cold ethanol were added to the resulting supernatant and the mixture was stirred for 2 hours in ice and again centrifuged at 37,000 xg for 20 min. The pellet was re-dissolved in H2O, 5 mg/ml final concentration, and frozen if not used immediately. Protein was determined by the method of Lowry (Lowry, Rosebrough, Farr and Randall 1951).
Isolation of hepatocytes
Hepatocytes were isolated from male Sprague-Dawley rats, 150–200 g, by the in situ 2 step collagenase perfusion technique, essentially as described by Seglen (1976). The cells were dispersed with gentle mincing and agitation in 100 ml Leibovitz (L-15) medium and centrifuged at 50 xg for 3 min. The cells were washed twice and resuspended in L-15 medium, 20 mM Hepes pH = 7.4, 10 uM dexamethasone, 1 uM insulin, 1 uM T3, 0.2% bovine serum albumin, 5% fetal calf serum, 0.15% glucose, and 1% antibiotic/antimycotic. Viability was determined by the trypan blue exclusion test and cell number with a hemocytometer. Only preparations with a viability of over 75% were used.
Cells were distributed at a cell density of 2–2.5 × 106 cells per 60 mm diameter dish in 4.0 ml medium. The medium was changed at 4 hr and the cells maintained at 37°C.
At 24 hr the medium was replaced with a serum-free L-15 medium and again at 48 hr, at which time the HSA fractions to be tested were added. Cells were exposed for 16 hr to 2 u Ci [3H] thymidine per dish at 8 hr following fraction addition.
To determine DNA synthesis the cells were harvested by scraping the dishes and rinsing the dishes with 3.0 ml PBS. The rinse was combined with the cell suspension and centrifuged at 500 xg for 10 min. The pellet was dissolved in 1 ml 1 M NaOH and heated for 10 min at 80°C. Samples were cooled and trichloroacetic acid was added to a final concentration of 5% to precipitate the DNA. Incorporation of [3H] thymidine into DNA was determined as previously described (Ove, Coetzee and Morris 1973).
Novikoff hepatoma cell cultures
Stock cultures of Novikoff N 15167 hepatoma cells were maintained in 100 mm diameter tissue culture dishes. The medium, DME, was supplemented with 100 U/ml penicillin, 100 ug/ml streptomycin, and 5% calf serum. The atmosphere of the incubator was maintained at 5% CO2.
Cells for assays were set up in 60 mm diameter culture dishes at a cell density of 5 × 105 per dish in 4.0 ml DME-5% calf serum. When cells had grown to a density of 2 × 106 per dish, the medium was replaced with DME-0.1% calf serum. Twenty-four hr later medium was again changed (0.1% calf serum-DME) and the HSA fractions added. Cells were exposed for 2 hr to 0.25 uCi [3H] thymidine at 20–22 hr. Cells were harvested and thymidine incorporation determined as described for hepatocytes.
Isolation of nuclei and incorporation of [3H] TTP
Nuclei were isolated from rat liver as previously described (Ove, Coetzee and Morris 1973). The nuclei were washed once with 0.3 M sucrose and were immediately used for assay. An aliquot of the nuclear suspension was removed for determination of DNA (Burton 1968).
The reaction mixture contained (0.5 ml final volume) 50 umol Tris-HCl, pH 7.5; 2 umol MgCl2 4 umol 2-mercaptoethanol; 80 umo I KC1, 1 umol ATP; 0.04 umol each dATP, dCTP and dGTP and 0.02 umol [3H] TTP (50 uCi/umol). The reaction was stopped at times indicated by the addition of 1 ml 1 M NaOH and radioactivity determined as previously described (Ove, Coetzee and Morris 1973).
Results
DNA Synthesis in Donors of Extracts
The baseline DNA synthesis in weanling rats was more than double that in normal control animals that had received saline injections (Table 1). Synthesis was increased more than 8 fold by T3 injection in normal female rats, and 6 fold in normal males. The response to T3 was intermediate between that in controls and that after 70% hepatectomy.
Table 1.
Hepatic DNA synthesis in weanling, T3-injected and partially hepatectomized rats
| Treatment or Condition of Rat Incorporation | [3H] thymidine cpm/mg DNA | |
|---|---|---|
| Weanling rats (100 grams) | (7) | 2630 ± 420 |
| Normal rats-saline injection-(180–220 grams) | (21) | 1260 ± 340 |
| 200 μg T3 injection ♀ rats | (12) | 10310 ± 560 |
| 200 μg T3 injection ♂ rats | (12) | 7450 ± 380 |
| 70% hepatectomy + O | (21) | 17420 ± 1020 |
| 70% hepatectomy + 4 mg adriamycin | (4) | 1190± 200 |
| 70% hepatectomy + 6 mg adriamycin | (4) | 340 ± 110 |
| 70% hepatectomy + 8 mg adriamycin | (4) | 90 ± 20 |
The partially hepatectomized rats were injected with 10 μCi [3H] thymidine at 21 hours after the operation.
T3 injected rats were injected with the [3H] thymidine 23 hours after T3 injection.
T3 and adriamycin were injected IP.
Adriamycin was injected following partial hepatectomy.
[3H] thymidine was injected into the tail vein and animals were killed 1 hour later.
The numbers are the averages of the number of rats shown in parentheses ± SD.
The injection of adriamycin immediately after hepatectomy completely blocked the DNA response, and in high doses even suppressed the natural level of DNA synthesis (Table 1).
In Vitro Testing of Extracts
DNA synthesis twenty-four hours after 40% hepatectomy was double that found in normal rats. This elevated baseline was augmented slightly by liver extracts from sham-operated donors as shown in Table 2. DNA synthesis was increased 5 to 6 times by extracts from weanling or regenerating livers, and by extracts of livers from T3-injected rats. Even though DNA synthesis had been inhibited by adriamycin following hepatectomy in extract donors (Table 1), HSA was found in these non-regenerating livers. The results in Figure 1 show that DNA synthesis reaches a maximum around 24 hours after the injection of HSA. Maximal incorporation of [3H] thymidine into DNA also occurs around 24 hr following partial hepatectomy or the injection of T3.
Table 2.
Stimulation of hepatic DNA synthesis by different liver extracts
| Source of HSA | [3H] thymidine incorporation cpm/mg DNA | |
|---|---|---|
| Normal rat + Saline | (60) | 2480 ± 380* |
| Sham operated | (8) | 4040 ± 690 |
| Weanling rat | (16) | 13160 ± 1680 |
| 70% hepatectomy | (14) | 12480 ± 2240 |
| T3 injected rats | (8) | 11900 ± 1510 |
| 70% hepatectomy + 6 mg adriamycin | (4) | 7280 ± 520 |
This level of DNA synthesis is double that found in normal rats (see Normal rats, Table 1), and represents the response to 40% hepatectomy.
The rats used in these assays had 40% of their liver removed at 9 A.M. HSA from the indicated sources was injected (25 mg protein containing HSA in 5 ml H2O) IP six hours after the operation. At 9 A.M. the next day, animals were injected with 10μCi [3H] thymidine and killed 1 hour later. All animals weighed between 200 and 250 g. The numbers are the averages of the number of rats indicated in parentheses ± SD.
Fig. 1.
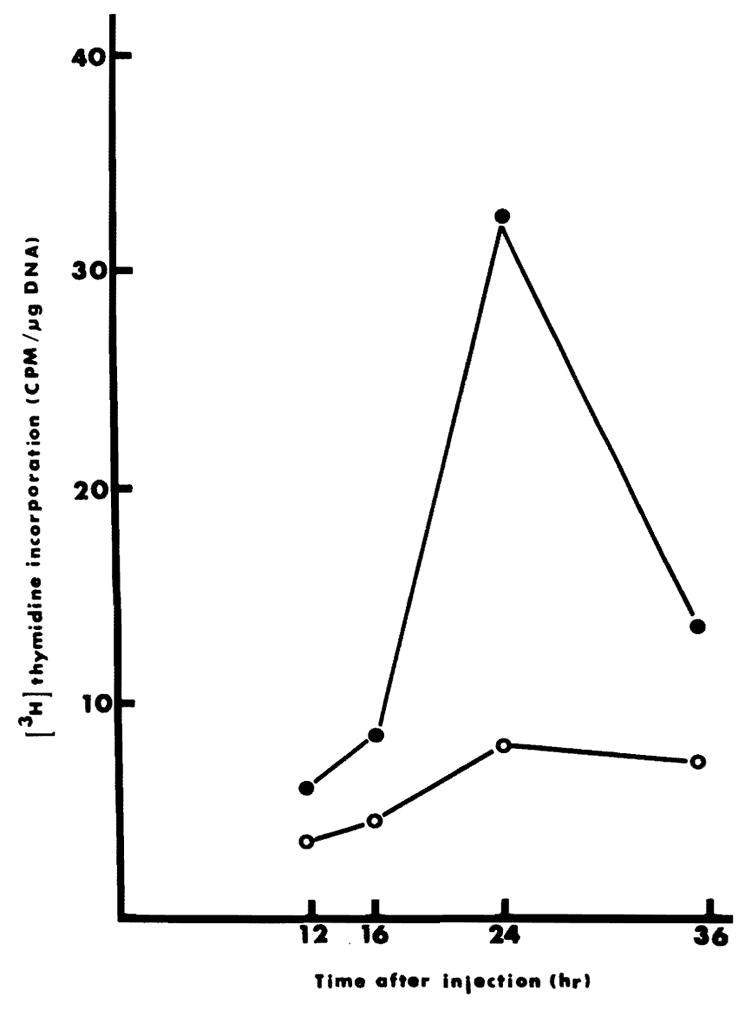
DNA synthesis at different times after HSA injection. Animals were injected with 25 mg protein containing HSA at zero time. At 10, 14, 22 and 34 hours, animals were injected with 10 μCi [3H] thymidine and killed 2 hours later. Incorporation of [3H] thymidine into hepatocytes was determined as described in “Methods”. Each point on the graph represents the average of two determinations. ●——●, injection of HSA prepared from 70% hepatectomized rats. ○——○, injection of saline.
In Vitro Testing of Extracts
Extracts from normal liver did not affect thymidine incorporation in Novikoff hepatoma cells indicating the absence or low levels of HSA. In contrast, HSA was present in the livers of weanling rats and liver remnants of animals which had been prepared with a 70% hepatectomy or an injection of T3 as shown in Table 3.
Table 3.
Stimulation of DNA synthesis in Novikoff hepatoma cells by HSA from various sources
| Source of Extract |
μg protein of HSA |
|||
|---|---|---|---|---|
| 50 | 100 | 200 | ||
| [3H] thymidine incorporation (cpm/106 cells) | ||||
| None | 640 ± 110 | |||
| Sham operated rats | 683 ± 84 | 627 ± 27 | 397 ± 62 | |
| Weanling rats | 966 ± 151 | 1222 ± 185 | 857 ± 45 | |
| 70% hepatectomy | 1402 ± 210 | 1856 ± 150 | 1229 ± 160 | |
| T3 injected rats | 774 ± 53 | 1030 ± 120 | 1325 ± 82 | |
| EtOH fractions from weanling rats | V | 928 ± 105 | 1139 ± 95 | 1990 ± 270 |
| VI | 986 ± 75 | 1594 ± 110 | 2048 ± 86 | |
The assay has been described in “Materials and Methods”. Cell density was between 1.5 and 2 × 106 at termination of experiment. Cells were exposed to 0.5 μCi [3H] thymidine per dish for 2 hours, 20 hours after addition of HSA in a total volume of 4 ml medium containing 0.1% calf serum. Numbers are the averages of 3 or more individual dishes ± S.D.
A partial fractionation of weanling liver HSA could be achieved by a stepwise ethanol precipitation. The fractions were prepared by adding 1 volume of ethanol to the 65° heated supernatant followed by stirring for 2 hours in ice and centrifugation at 37,000 xg for 20 min. This procedure was repeated 5 more times. The resulting precipitates were designated fractions I–VI. The activity was recovered in fractions V and VI with no significant stimulatory activity in fractions I–IV. For this procedure 20 mg protein of the 65° supernatant were precipitated with successive volumes of ethanol and 2.75 mg, 7.18 mg, 4.12 mg, 1.62 mg, 0.76 mg and 0.83 mg protein were recovered in fractions I–VI respectively.
The results with Novikoff tumor cell cultures were confirmed in normal hepatocytes, although there were variations in the degrees of response (Table 4) and again, the preparation, especially from weanling rats, was more effective at a lower than a higher concentration.
Table 4.
Stimulation of DNA synthesis in primary cultures of hepatocytes by liver extracts
| Source of HSA | Protein Added (μg) | [3H] thymidine Incorporation CPM/106 cells | % Increase |
|---|---|---|---|
| None | 0 | 1630 ± 95 | – |
| Sham hepatectomy | 50 | 1862 ± 167 | 14 |
| 100 | 1741 ± 220 | 7 | |
| Weanling rat | 50 | 3717 ± 960 | 128 |
| 100 | 2633 ± 325 | 62 | |
| Adult 70% hepatectomy | 50 | 1891 ± 41 | 16 |
| 100 | 2266 ± 196 | 39 | |
| T3 injected rat | 50 | 2103 ± 253 | 29 |
| 100 | 2543 ± 211 | 56 |
Hepatocytes were plated at a cell density of 2 × 106 cells per 60 mm dish in 4 ml medium. Medium was changed at 4 and 24 hours. Additions were made as indicated at 24 hours and cells were pulsed for 16 hours with 2 μCi [3H] thymidine per dish. Label was added 8 hours after HSA additions. Numbers are the averages of 3 determinations ± S.D.
The hepatocyte nuclei isolated from normal unoperated rats which were given an intraperitoneal saline injection 24 hours prior to sacrifice had low incorporation of [3H] TTP (Figure 2A). Incorporation was increased moderately (P ≤ 0.10) when the pretreatment was with weanling extract, and markedly (P ≤ 0.05) when the treatment was with T3. P values were determined by the Student’s t-test.
Fig. 2.
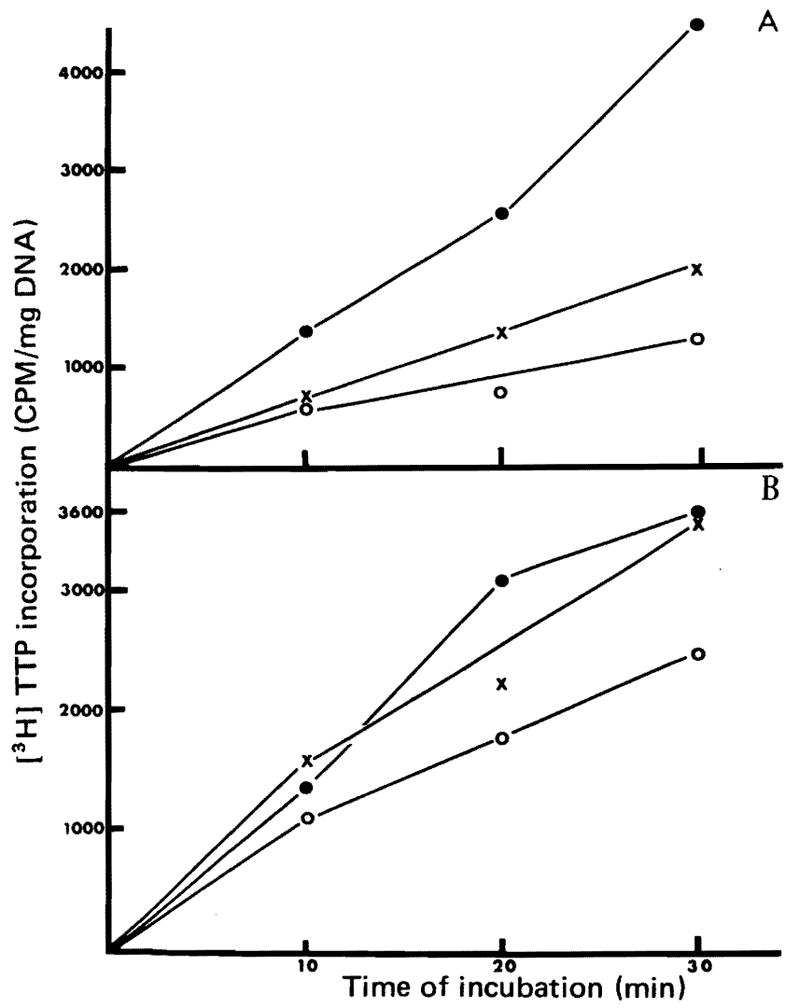
Stimulation of DNA synthesis in nuclei isolated from rats injected with HSA. Animals were injected with HSA, T, or saline and nuclei were isolated 20 hours later. [3H] TTP incorporation was determined as described in “Methods”.
2A Injections into normal rats: ●——●, injection of T3 200 μg/100 g; x——x, HSA from weanling rats; ○——○, saline.
2B Injections were made into animals that had 40% of their liver removed 4 hours before the injection. ●——●, HSA from T3,-injected rat; x——x, HSA from weanling rats; ○——○, saline.
In rats that were preconditioned by 40% hepatectomy a day before isolation of nuclei, the baseline level was higher (Figure 2B). Further increments in [3H] TTP incorporation were seen in the rats given injections of extracts from weanling (P ≤ 0.05) and T3 injected rats (P ≤ 0.05).
Nuclei were isolated from normal uninjected rats and were incubated with [3H] TTP in the presence or absence of HSA. As shown in Figure 3 incorporation was increased with extracts from weanling, 70% hepatectomy and T3-treated donors with no stimulation due to extracts from normal sham operated animals.
Fig. 3.
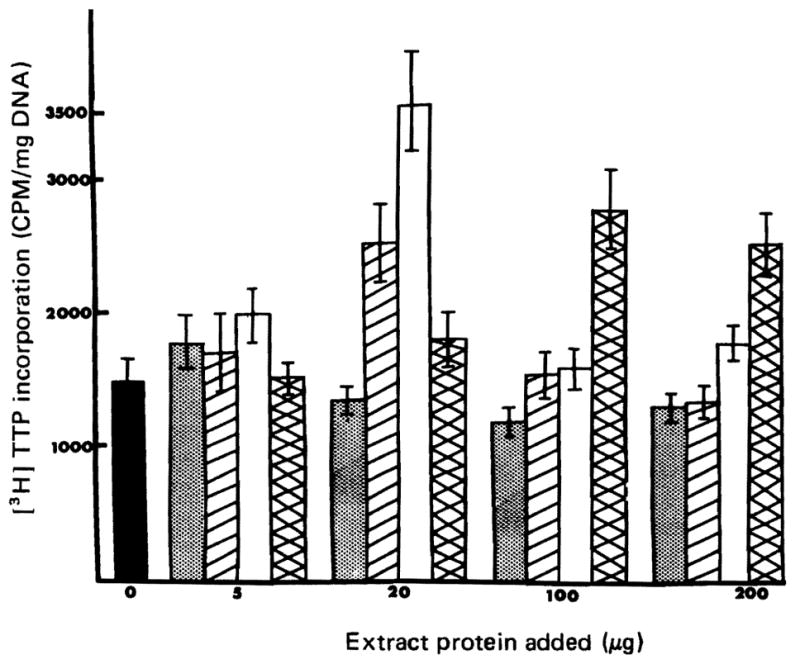
Stimulation of DNA synthesis in isolated nuclei by liver extracts. The assay procedure has been described in “Materials and Methods”. Control value is the average of 21 determinations and the values with extracts added are from 3 determinations each.
■ = Control
 = Sham operated adult rat extract
= Sham operated adult rat extract
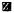 = 70% partially hepatectomized rat HSA
= 70% partially hepatectomized rat HSA
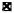 = T3 injected rat HSA
= T3 injected rat HSA
□ = Weanling HSA
Discussion
Evidence is provided that at least some events associated with liver regeneration are influenced by hormones (Starzl, Francavilla, Porter and Benichou 1978; Francavilla, Porter, Benichou, Jones and Starzl 1978). It has been shown before that a single injection of a pharmacological dose of T3 resulted in a dramatic increase in DNA synthesis in the liver (Klein et al. 1981; Short et al. 1980). Maximal DNA synthesis occurred between 20 and 24 hours after the injection, a time course similar to DNA synthesis induced by the removal of 70% of the liver. The mechanism of this stimulation has not been resolved. From our present data it seems that one of the mechanisms by which T3 induces hepatic DNA synthesis might be the induction or activation of a humoral factor (HSA) which in turn stimulates hepatic DNA synthesis.
Humoral and cytoplasmic factors similar to HSA have been reported from a number of laboratories (LaBrecque and Bachur 1982; LaBrecque and Pesch 1975; Hatase et al. 1979; Starzl et al. 1979; Terblanche et al. 1980; Starzl and Terblanche 1979; Moolten and Bucher 1967). All of these have in common that they can be extracted only or in greater quantity from proliferating liver or from the serum of rats in which the liver cells are proliferating. Our results indicate that HSA is not just a byproduct of DNA synthesis. When DNA synthesis following partial hepatectomy was completely inhibited by the injection of adriamycin, HSA could still be extracted from liver remnants. Furthermore, HSA could be extracted from liver remnants of 70% hepatectomized animals 16 hours after the operation. These data are not shown in the results. An increased DNA synthesis is barely detectable at 16 hours and it is not until 20 hours after the operation that substantial DNA synthesis occurs.
At present we do not know whether HSA is newly synthesized in response to removal of part of the liver or in response to T3 injections, or whether activation of a dormant molecule is involved. Since identical extraction procedures resulted in stimulatory activity whether regenerating liver or liver from T3 injected animals were used, it appears that the same substance in both preparations causes the stimulations. This can only be confirmed once HSA has been substantially purified. Another possibility is that T3 causes a release of HSA from storage. Attempts to inject T3-treated or 70% hepatectomized rats with a dose of cyclohexamide that would inhibit protein synthesis, resulted in death of the animals before the time that we normally prepare the extracts. HSA does seem to be associated with a protein, however. Treatment of the ethanol precipitate containing HSA with protease or trypsin resulted in the loss of HSA.
In many instances increased [3H] thymidine incorporation was not proportional to the amount of HSA added. There are many reports indicating that the liver contains inhibitors of DNA synthesis and cell proliferation (Lieberman and Ove 1960; Kuramitsu, Matsui, Tokuda and Hatase 1982; Klein, Coetzee, Madhav and Ove 1979; McMahon and Iype 1980), one of which has been shown to be arginase (Lieberman and Ove 1960, Klein et al. 1979), others to be inhibitors of thymidine kinase (Klein et al. 1979). It is most likely that our HSA fractions contain, in addition to the stimulating activity, some inhibitors. Indeed our results with a stepwise ethanol precipitation indicate that it might be possible to remove such inhibitors from the stimulating activity. A better dose response could be shown with fractions V and VI than with most of the HSA preparations.
It is too early to speculate on the mechanism of action of HSA, but the results of the experiments with isolated nuclei suggest that it has possibly a direct effect on chromatin. T3 added to such nuclei does not stimulate [3H] TTP incorporation, nor does T3 stimulate cell proliferation if added in vitro.
The use of T3 to produce HSA in normal or adult rats seems superior to other methods that involve hepatic injury such as partial hepatectomy or administration of CCl4. We are now attempting the further purification of HSA. Only after considerable purification will we be able to identify the active component and can design experiments that will give us some insight into its mechanism and site of action.
Acknowledgments
This study was supported by grants from the Veterans Administration; by project grant AM 29961 from the National Institute of Health and grant 82/0031096 from Consiglio Nazionale Delle Ricerche, Italy.
Footnotes
This work was supported by National Institute of Health Grants AM 30183 and AM 29961, research grants from the Veterans Administration and grant 82/0031096 from Consiglio Nazionale Delle Ricerche, Italy.
References
- Burton K. Determination of DNA concentrations with diphenylamine. Methods Enzymol. 1968;12B:163–166. [Google Scholar]
- Coetzee ML, Short J, Klein K, Ove P. Correlation of circulating levels of a serum protein with triiodothyronine levels and hepatoma growth. Cancer Res. 1982;42:155–160. [PubMed] [Google Scholar]
- Francavilla A, Porter KA, Benichou J, Jones AF, Starzl TE. Liver regeneration in dogs; morphologic and chemical changes. J Surg Res. 1978;25:403–413. doi: 10.1016/s0022-4804(78)80005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatase O, Fujii T, Kuramitsu M, Itano T, Takahashi F, Murakami T, Nishida I. Co-existence of inhibitory and stimulatory factors modulating cell proliferation in rat liver cytoplasm. Acta Med Okayama. 1979;33:73–80. [PubMed] [Google Scholar]
- Higgins GM, Anderson RM. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- Klein K, Chou R, Short J, Ove P. Amounts of triiodothyronine and a serum protein related to hepatic DNA synthesis in the rat. Horm Met Res. 1981;13:165–170. doi: 10.1055/s-2007-1019206. [DOI] [PubMed] [Google Scholar]
- Klein K, Coetzee ML, Madhav R, Ove P. Inhibition of tritiated thymidine incorporation in cultured cells by a kidney extract. J Natl Cancer Instit. 1979;62:1557–1564. [PubMed] [Google Scholar]
- Kuramitsu M, Matsui H, Tokuda M, Hatase O. Factors inhibiting cell proliferation in rat liver cytoplasm. Acta Med Okayarna. 1982;36:1–10. doi: 10.18926/AMO/30708. [DOI] [PubMed] [Google Scholar]
- LaBrecque DR, Bachur NR. Hepatic stimulator substance: Physicochemical characteristics and specificity. Am J Physiol. 1982;242:G281–G288. doi: 10.1152/ajpgi.1982.242.3.G281. [DOI] [PubMed] [Google Scholar]
- LaBrecque DR, Pesch LA. Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol. 1975;248:273–284. doi: 10.1113/jphysiol.1975.sp010973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman I, Ove P. Inhibition of growth of cultured mammalian cells by liver extracts. Biochim Biophys Acta. 1960;38:153. doi: 10.1016/0006-3002(60)91208-7. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- McMahon JB, Iype PT. Specific inhibition of proliferation of nonmalignant rat hepatic cells by a factor from rat liver. Cancer Res. 1980;40:1249–1254. [PubMed] [Google Scholar]
- Moolten FL, Bucher NLR. Regeneration of rat liver: Transfer of humoral agent by cross circulation. Science. 1967;158:272–274. doi: 10.1126/science.158.3798.272. [DOI] [PubMed] [Google Scholar]
- Ove P, Coetzee ML, Morris HP. DNA synthesis and the effect of sucrose in nuclei of host liver and Morris hepatomas. Cancer Res. 1971;31:1389–1395. [PubMed] [Google Scholar]
- Ove P, Coetzee ML, Morris HP. Separable DNA polymerase activities in host liver and Morris Hepatomas. Cancer Res. 1973;33:1272–1283. [PubMed] [Google Scholar]
- Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Short JA, Klein K, Kibert L, Ove P. Involvement of the iodethyronines in liver and hepatoma cell proliferation in the rat. Cancer Res. 1980;40:2417–2422. [PubMed] [Google Scholar]
- Starzl TE, Francavilla A, Porter KA, Benichou J. The effect upon the liver of evisceration with or without hormone replacement. Surg Gynecol Obstet. 1978;146:524–532. [PMC free article] [PubMed] [Google Scholar]
- Starzl TE, Terblanche J. Hepatotrophic substances. In: Popper H, Schaffner F, editors. Progress in liver diseases. Vol. 6. New York/N.Y: Grune and Stratton; 1979. pp. 135–151. [PubMed] [Google Scholar]
- Starzl TE, Terblanche J, Porter KA, Jones AF, Usui S, Mazzoni G. Growth-Stimulating factor in regenerating canine liver. Lancet. 1979;I:127–130. doi: 10.1016/s0140-6736(79)90519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terblanche J, Porter KA, Starzl TE, Moore J, Patzelt L, Hayashida N. Stimulation of hepatic regeneration after partial hepatectomy by infusion of a cytosol extract from regenerating dog liver. Surg Gynec Obstet. 1980;151:538–544. [PMC free article] [PubMed] [Google Scholar]


