Abstract
Background and Purpose
Focal cerebral ischemia causes microvessel matrix degradation and generates proteases known to degrade this matrix. However, proof that the proteases generated do indeed degrade vascular matrix is lacking. Here we demonstrate that active proteases derived from ischemic tissue after middle cerebral artery occlusion (MCAO) and transferred to normal tissue can degrade vascular matrix.
Methods
In an ex vivo bioassay, the effects of supernatants from ischemic and normal basal ganglia of nonhuman primates, proteases, and control buffer on the immunoreactivity of vascular matrix constituents in normal brain tissue sections were quantified. Protease families were identified with specific inhibitors.
Results
Plasmin, active matrix metalloproteinase (MMP)-2, and active MMP-9 significantly reduced microvessel-associated collagen, laminin, and heparan sulfate proteoglycans (HSPG). The vascular HSPG perlecan was more sensitive than collagen or laminin in the bioassay and in the ischemic core 2 hours after MCAO. Two-hour and 7-day ischemic tissue samples significantly degraded matrix perlecan and collagen. Inhibitor studies confirmed that while active MMPs were generated, active cysteine proteases significantly degraded microvessel perlecan. The cysteine proteases cathepsins B and L were generated in the microvasculature and adjacent neurons or glial cells 2 hours after MCAO and decreased perlecan in the bioassay.
Conclusions
This is the first direct evidence that active proteases are generated in ischemic cerebral tissues that are acutely responsible for vascular matrix degradation. Degradation of vascular perlecan, the most sensitive matrix component thus far identified, may be due to cathepsins B and L, generated very rapidly after MCAO.
Keywords: cathepsins, brain ischemia, cysteine proteases, matrix metalloproteinases, microvessels
The extracellular matrix (ECM) of the cerebral microvessel basal lamina contributes to the permeability barrier and to cerebral microvessel integrity.1 Cerebral microvessel ECM consists of type IV collagen, laminin, fibronectin, heparan sulfate proteoglycans (HSPG), and other glycoproteins. Among these, the HSPG perlecan is located in the endothelial surface, microvascular ECM, and extravascular sites that support cell function.2–4 The gradual loss of microvessel type IV collagen, laminin, and fibronectin during middle cerebral artery occlusion (MCAO) in the nonhuman primate correlates with increased permeability and hemorrhagic transformation.1,5 In the basal ganglia, microvessel responses to focal cerebral ischemia and neuron injury occur simultaneously,6,7 suggesting that the loss of these ECM components can promote neuron injury.
Microvascular ECM degradation is postulated to result from matrix metalloproteinases (MMPs), including pro-MMP-2 and pro-MMP-9, plasmin, and plasminogen activators generated in cerebral microvessels and perivascular tissue during ischemia.8–10 However, with 1 exception,8 only the inactive forms of MMP-2 and MMP-9 appear after MCAO, raising the question of whether they indeed contribute to matrix degradation in the central nervous system. In addition, other protease systems, including cysteine and serine proteases, may be involved in vascular matrix dissolution. 11–13 However, there is no evidence to indicate that these proteases appear during focal cerebral ischemia and degrade ECM.
In this report we hypothesize that active proteases generated during focal cerebral ischemia differentially contribute to the degradation of select vascular matrix components. We demonstrate that cerebrovascular matrix perlecan is more sensitive to focal ischemia than collagen or laminin both ex vivo and in vivo and that there is a time-dependent appearance of active MMP and cysteine protease activities that is consistent with the loss of vascular perlecan and neuron injury in vivo.
Materials and Methods
The experimental procedures were approved by the institutional Animal Research Committee and performed according to standards published by the National Research Council and the US Department of Agriculture Animal Welfare Act.
Experimental Stroke Model
Samples of cerebral tissues from 30 adolescent male baboons (Papio anubis/cynocephalus) were used. The surgical approach to the awake nonhuman primate stroke model has been previously described.1,14 Before the experiments, all animals were allowed a 7-day procedure-free interval after surgical implantation of the MCA balloon. The animals underwent MCAO for 1 hour (n=3) or 2 hours (n=6); 3-hour MCAO with subsequent reperfusion for 1 hour (n=3) or 4 hours (n=3); or 1.5-hour MCAO with 24-hour reperfusion (n=3). Subjects (n=6) that did not undergo any preparation procedure and subjects (n=6) that suffered MCAO at surgical implantation (and had sustained hemiparesis for 7 days) were used as controls. Brain tissues were removed under thiopental Na+ after transcardiac perfusion with isosmotic heparinized perfusate and prepared for frozen and paraffin sections.1,14
Reagents
Well-characterized immunological reagents were used for detecting microvessel-associated matrix components.1,7 A murine anti-human monoclonal antibody (MoAb) against collagen type IV and a rabbit anti-human polyclonal antibody against laminin were obtained from Sigma. Murine MoAbs against HSPG (HSPG-1, clone A7L6, and HSPG-2; Chemicon International, Inc) recognize the human and primate perlecan core protein. Murine MoAbs against human cathepsin B (clone CA10) and cathepsin L (clone 22) were obtained from BD Transduction Laboratories.
Human recombinant pro-MMP-2 and pro-MMP-9 were the kind gifts of Dr William G. Stetler-Stevenson (National Cancer Institute). Purified human glu-plasminogen, plasmin, and urokinase (uPA) were obtained from American Diagnostica Inc. Purified human cathepsins B and L were received from Calbiochem, and collagenase type-7, amiloride (a uPA inhibitor), 1,10-phenanthroline (a divalent cation chelator), and EDTA were obtained from Sigma. The MMP inhibitor GM6001 (Chemicon), serine protease inhibitor 4-amidinophenyl methanesulfonyl fluoride (APMSF) (Roche Diagnostics Corporation), and cysteine protease inhibitor E64 (Roche) were used.
Ex Vivo Vascular Matrix Assay
Samples derived from normal and ischemic brain tissue (50 µL) or purified reagents (100 µL) were applied to recipient tissues. The recipient tissues consisted of unfixed 10-µm-thick normal basal ganglia sections mounted on microscope slides.
Application of Proteases to Normal Recipient Tissues
PBS (pH 7.0) was used as the incubation buffer for type-7 bacterial collagenase. The MMPs and uPA/plasminogen were prepared in a buffer containing 90 mmol/L NaCl, 5 mmol/L KCl, 1.5 mmol/L MgCl2, 23 mmol/L Na gluconate, 27 mmol/L Na acetate, 10 mmol/L CaCl2, and 40 mmol/L ZnCl2, pH 7.4 (Baxter Health Care). Cathepsin B and cathepsin L were prepared in 200 mmol/L Na+ acetate containing 8 mmol/L dithiothreitol, 1 mmol/L EDTA, and 2.7 mmol/L l-cysteine, pH 6.0. Before application to recipient sections, plasminogen (5 U/mL) and uPA (5 U/mL) were incubated together at 37°C for 1 hour. Pro-MMP-2 (1 µg/mL) or pro-MMP-9 (1 µg/mL) was then added to this mixture and incubated for 1 hour. Then 100 µL of each mixture or buffer alone was incubated on the recipient sections for 5 hours (collagenase) or 18 hours (MMPs and cathepsins) at 37°C. The sections were then washed with PBS and fixed.
Donor Brain Tissue Specimen Preparation
Donor samples were derived from approximately 1.0-cm×1.0-cm×10-µm frozen sections. Ten consecutive 10-µm sections from the ischemic or normal basal ganglia were centrifuged for 30 seconds at 300g and then mixed by repeated gentle pipetting. Next, 50 µL of sample or PBS was applied to the recipient sections and incubated at 37°C for 18 hours. The recipient sections were then washed with PBS and fixed. Dilution experiments demonstrated that the matrix-altering activities of the tissue samples disappeared with 1:2 and 1:10 dilution in PBS.
Immunohistochemistry
Specific antigens were developed by immunoperoxidase methods as described.7 Acetone-fixed frozen sections were incubated with the primary antibody overnight at 4°C and developed for immunoperoxidase with 3,3′-diaminobenzidine tetrahydrochloride (Biomeda Corp). paraformaldehyde (PFA)-fixed paraffin-embedded sections were subject to the same procedures after deparaffination.
Region of Cellular dUTP Incorporation
dUTP incorporation into nuclear DNA was taken as evidence of nuclear DNA scission/repair and at 2-hour MCAO defined the ischemic core and peripheral regions of cellular neuronal injury.6 PFA-fixed cryosections were subject to the DNA polymerase I (Promega) method.6,7 All ischemic samples included ischemic core and ischemic peripheral regions.
Gelatin Zymography
Gelatin zymography to detect MMP-related activities was performed as previously described with a modification to increase sensitivity.9 Protease activities were identified by incubation of the gels in buffer containing GM6001 or APMSF.
Quantitative Analysis
The changes in microvessel matrix constituents on recipient tissues were automatically quantified as the percent total vascular surface area of immunoreactive microvessels (sample/buffer control) by computerized video imaging microscopy (Axiocam digital camera mounted on a Zeiss Invert S100 microscope, driven by KS 400 software). Data were acquired from stereotaxically identical 1.6-mm2 regions of interest (ROIs) at ×200.
Statistical Analysis
All data are presented as the mean and SD of multiple parallel determinations with separate samples. For each antigen, differences in time courses between ischemic and matched nonischemic samples were assessed by 2-way ANOVA or Student t test with Bonferroni corrections for multiple comparisons. Unless otherwise stated, each data point represents 3 separate animal subjects. Significance was set at 2P<0.05.
Results
Microvessel Matrix Degradation
Control experiments demonstrated the specificity of the bioassay for basal lamina and collagen IV but not laminin or perlecan degradation by collagenase. Incubation of uPA with plasminogen in vitro generated plasmin (Figure 1), which was inhibitable by APMSF (data not shown). Incubation of pro-MMP-2 or pro-MMP-9 with uPA+plasminogen generated active MMP-2 or active MMP-9, respectively.
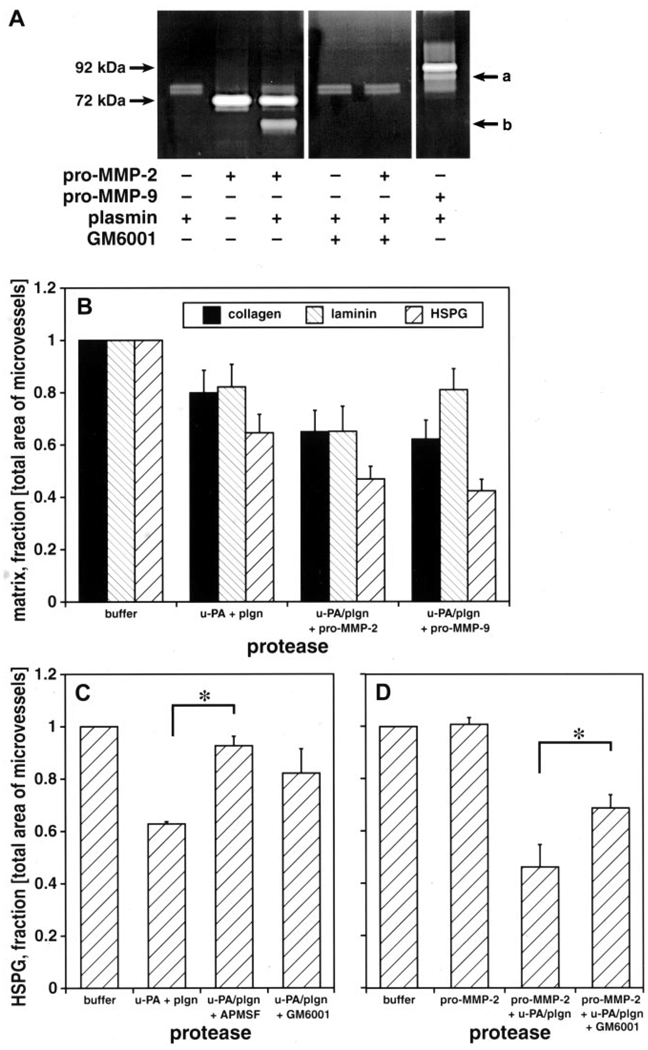
Figure 1.
A, Gelatinolytic activities of purified uPA, plasminogen, and recombinant pro-MMP-2 and pro-MMP-9. Plasminogen and uPA converted pro-MMP-2 to active MMP-2 (b) and converted pro-MMP-9 to active MMP-9 (a). GM6001 inhibited MMP-2 activity. B, Effect of uPA, plasminogen (plgn, 5 U/mL), and pro-MMP-2 (1 µg/mL) or pro-MMP-9 (1 µg/mL) with plasminogen+uPA on microvessel matrix collagen, laminin, and perlecan. C, D, Effect of protease inhibitors APMSF (100 µmol/L) and GM6001 (100 µmol/L) on perlecan. n=4 (B) or n=3 (C, D) animals each. *2P<0.05 (C, D).
The combination uPA+plasminogen (in the same molar ratio as above) significantly reduced collagen (20±9% reduction compared with buffer), laminin (18±9%), and perlecan (37±1%) immunoreactivity in the microvessel matrix (Figure 1). Pro-MMP-2 alone had no effect on matrix perlecan. Incubation of pro-MMP-2 with plasmin further reduced matrix collagen (35±8%), laminin (35±10%), and perlecan (54±9%) (Figure 1). Similarly, pro-MMP-9 incubated with plasmin significantly further diminished collagen and perlecan but not laminin immunoreactivity (Figure 1). Perlecan was the matrix constituent most sensitive to these proteases.
Perlecan degradation was significantly blocked by specific inhibitors. The HSPG-degrading activity of uPA+plasminogen was inhibited by APMSF and also GM6001 (Figures 1C, 2; P<0.05, multiple comparisons). GM6001 significantly inhibited the effect of pro-MMP-2+plasmin on perlecan (Figures 1C, 2; P<0.05). The MMP-2–related decrease in vascular perlecan was partly prevented by APMSF.
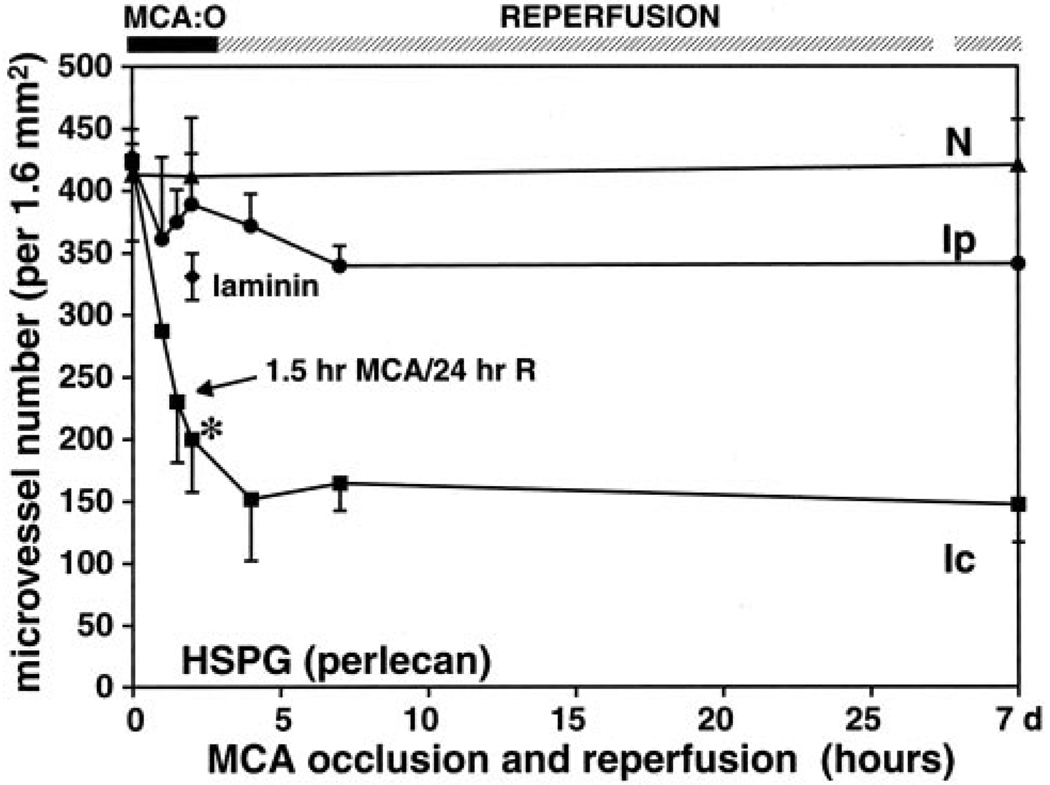
Figure 2.
Effect of MCAO on microvessel-associated perlecan immunoreactivity compared with laminin. *2P<0.009 (t test) vs laminin at 2-hour ischemia. Ic indicates ischemic core; Ip, region peripheral to the core; and N, normal tissue.
Microvessel Perlecan Expression During Focal Cerebral Ischemia
The density of perlecan-immunoreactive microvessels after MCAO was examined with the use of 2 different antibodies, which gave identical results (Figure 2). Within the ischemic core region, the microvessel density significantly decreased 1 hour after MCAO and decreased further to 48.9±12.2% by 2 hours compared with the contralateral nonischemic striatum. No significant change in microvessel matrix perlecan was observed in the ischemic peripheral region. Notably, at 2-hour MCAO the decrease in perlecan expression significantly exceeded that of laminin in the ischemic core region (note that laminin-immunoreactive microvessel density decreased from 427.7±10.2 per ROI at 0 minutes to 331.0±18.7 per ROI). Reperfusion for 24 hours after 1.5-hour MCAO did not change the perlecan-immunoreactive microvessel density compared with 2-hour MCAO (57.3±10.8% versus 48.9±12.2%; 2P=0.846).
Ischemic Microvessel Matrix Degradation Is Transferable
Compared with normal tissue and controls, 2-hour and 7-day ischemic tissues significantly reduced microvessel matrix–associated collagen (15±9% and 19±8%, respectively) and perlecan (HSPG-2) (40±4% and 31±8%) (Figures 2, 3, 4A; P<0.05, multiple comparisons).
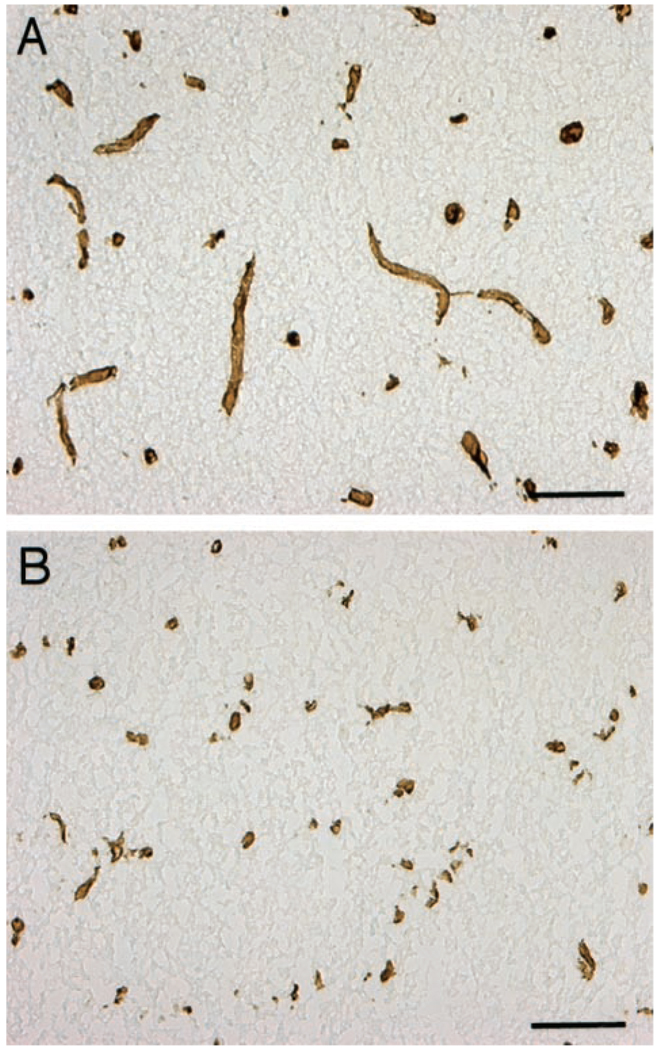
Figure 3.
Presence of perlecan in microvessels of normal basal ganglia after incubation with buffer (A) and donor 2-hour ischemic tissue (B). Perlecan immunoreactivity decreased on exposure to 2-hour tissue. Bar=10 µm.
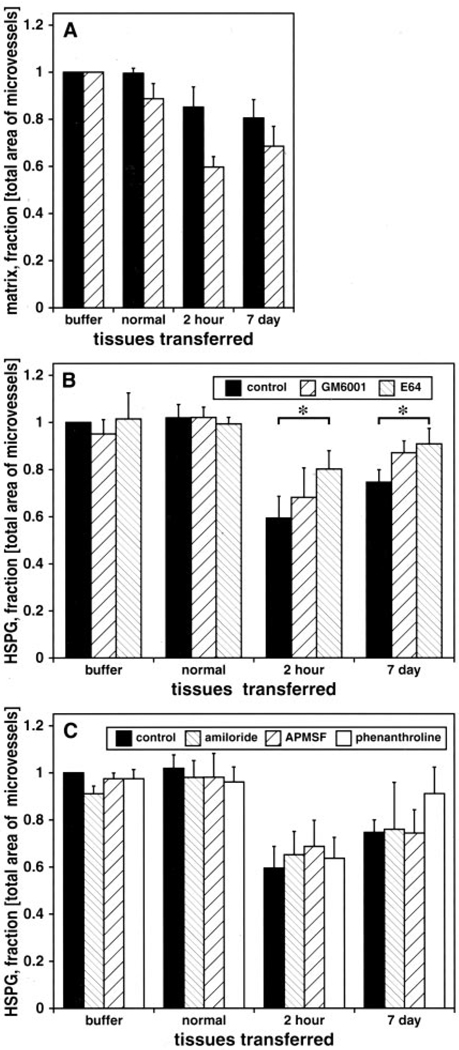
Figure 4.
Effect of 2-hour and 7-day MCAO donor tissue on normal basal ganglia microvessel collagen (solid bars) or perlecan (hatched bars) (A), perlecan with or without GM6001 (100 µmol/L) or E64 (100 µmol/L) (B), and perlecan with or without amiloride (5 mmol/L), APMSF (100 µmol/L), and 1,10-phenanthroline (5 mmol/L) (C). 2P<0.05 for group and time differences, compared with normal. *2P<0.05 (t test).
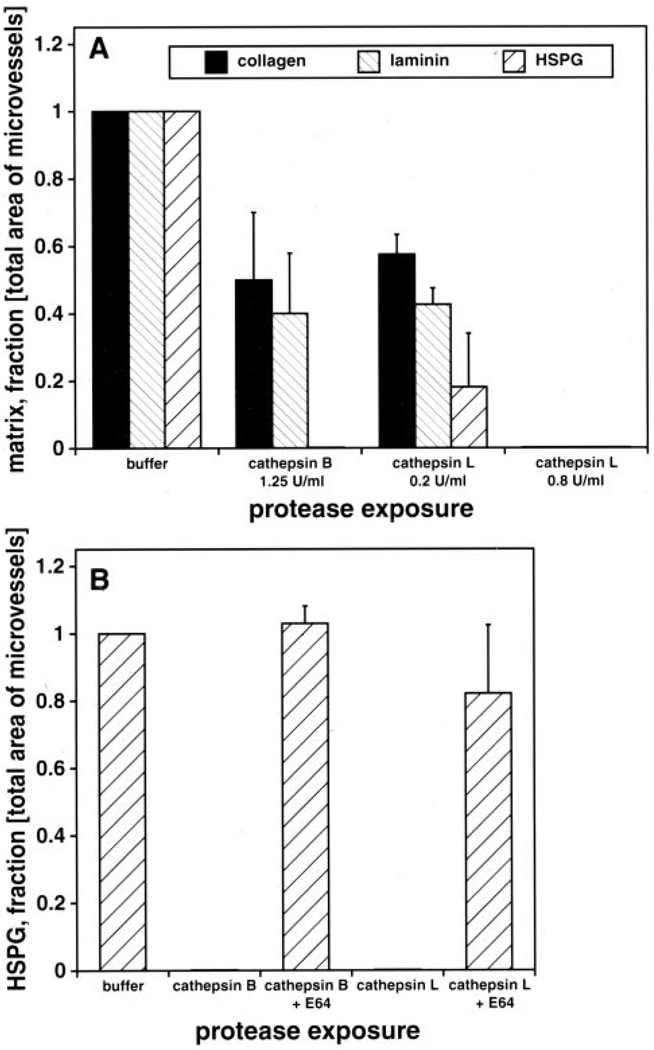
Inhibitor studies demonstrated significant group and time effects at 2 hours and 7 days compared with control and normal tissues (2P<0.05, multiple comparisons) (Figure 4B). The decrease in microvessel-associated perlecan (HSPG-1) after application of 2-hour or 7-day ischemic tissue was significantly attenuated by the cysteine protease inhibitor E64 (2P<0.05) but not by GM6001 (Figure 4B). However, GM6001 reduced matrix degradation. Serine proteases had no detectable effect on microvessel perlecan degradation (Figure 4C).
Cysteine Protease Effects
Among the cysteine proteases expressed in the central nervous system, cathepsins B and L can degrade HSPG.15 Both cathepsins significantly decreased microvessel collagen, laminin, and perlecan (Figure 5). Perlecan was completely eliminated by either cathepsin B or L, which was inhibited by E64.
Figure 5.
A, Effect of cathepsins B and L on microvessel collagen, laminin, and perlecan in normal basal ganglia. B, Effect of E64 (100 µm) on microvessel perlecan degradation by cathepsin B (1.25 U/mL) or cathepsin L (0.2 or 0.8 U/mL). All deficiencies are 2P<0.05 (t test).
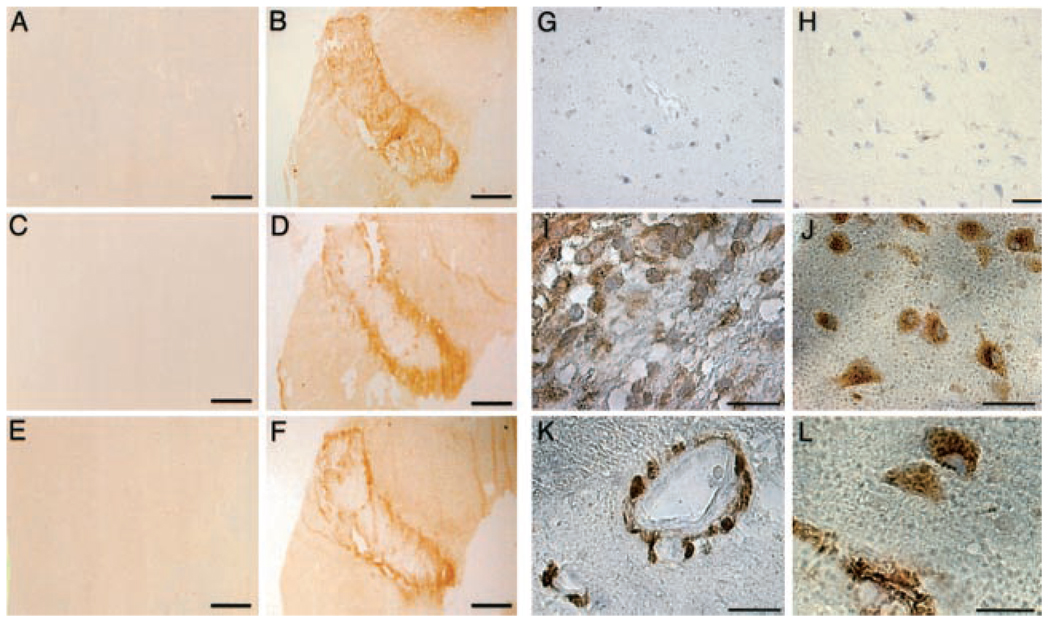
Immunohistochemical studies confirmed the absence of cathepsin B and cathepsin L in normal basal ganglia (Figure 6G, 6H, respectively). However, cathepsin B appeared in the ischemic core/ischemic peripheral boundary by 2 hours after MCAO (Figure 6D). Fixed embedded sections demonstrated cathepsin B in microvessel-associated pericytes adjacent to glial cells in the ischemic core region (Figure 6I, 6K). Cathepsin L was also found in the ischemic core/ischemic peripheral boundary (Figure 6F), where it was selectively expressed by microvessel-associated pericytes/matrix and adjacent neurons (Figure 6J, 6L).
Figure 6.
Expression of cathepsins in normal basal ganglia (A, C, E, G, H) and ischemic basal ganglia at 2-hour MCAO (B, D, F, I to L) in frozen (A to F) and paraffin sections (G to L). dUTP uptake is seen in ischemic issue (B) but not normal tissue (A). Cathepsin B (C, D, G, I, K) and cathepsin L (E, F, H, J, L) expression are demonstrated. A to F, Both cathepsin B and L immunoreactivity are seen in the border of the ischemic core region but not in normal tissue. G to L, Cathepsin B is observed on both glial cells (I) and on nearby microvessels (K) in the ischemic core/ischemic peripheral border, while cathepsin L is observed on neurons (J) and adjacent microvessels (L) in the same region. Bars=2 mm (A to F), 10 µm (G, H), 5 µm (I, J), and 2.5 µm (K, L).
Discussion
Focal cerebral ischemia causes degradation of the basal lamina of microvessels within the ischemic core and generates proteases known to degrade ECM constituents. Formerly, proteases thought to be associated with the loss of vascular matrix have been limited to latent MMPs and plasminogen activators. However, there has been no direct evidence that the active forms of these proteases contribute to cerebral vascular ECM degradation. In this study vascular matrix–protease activities generated after MCAO were identified by transfer of these activities from ischemic to normal tissues in an ex vivo assay. The specificity of this approach was demonstrated by the ability of collagenase to eliminate collagen but not other microvessel ECM components; plasmin, MMP-2, and MMP-9 activities degraded collagen and perlecan, but laminin was degraded only by plasmin and MMP-2. Vascular perlecan was significantly more sensitive to protease digestion than laminin or collagen in the bioassay. In view of the sensitivity of vascular HSPG, the impact of focal ischemia on microvessel perlecan was examined. Microvessel-associated perlecan within the primate striatum was more sensitive to ischemia than other major vascular matrix components (Figure 2).1 Temporally, the loss in vascular perlecan coincided with the earliest appearance of neuron injury.6,7 This is in accordance with the appearance of cysteine proteases immediately after ischemia and the more gradual degradation of vascular laminin and collagen.
Proteolysis of microvascular perlecan after ischemia is very rapid in the primate. There are important differences in the timing of vascular matrix degradation in response to cerebral ischemia between primates and rodents. For instance, the absence of pro-MMP-9 in murine genetic constructs can reduce cerebral infarct volume,16 whereas the expression in nonhuman primates of pro-MMP-2, but not pro-MMP-9, after MCAO is directly related to neuron injury. 9 uPA appears significantly later in rodents than primates. 6,8,9,16,17 Central to these studies is the peculiar sensitivity of basal ganglia to focal ischemia in humans and primates.14,17
These experiments are the first demonstration that ischemic brain tissue generates active proteases, which significantly and rapidly decrease microvessel matrix both early and late after MCAO. The inhibitor studies suggest that active cysteine proteases are responsible for perlecan degradation. However, perlecan-degrading activities of MMPs appear to overlap those of the cysteine proteases. Perlecan core protein can be degraded in vitro by active MMP-2, MMP-3, MMP-7, MMP-9, and MMP-13.18,19 GM6001 inhibited the effects of plasmin on vascular perlecan, implying that pro-MMP-2 or other pro-MMPs expressed within normal tissue can be activated via a plasmin-dependent pathway.9 Since pro-MMP-2 and uPA are coordinately induced in microvascular matrix 2 hours after MCAO, active MMP-2 could contribute to the loss of microvessel HSPG. However, active MMP-2 accounts for only approximately 1% of the total MMP-2 activity detected in the nonhuman primate.9 It is unclear whether this amount of active MMP-2 contributes to matrix degradation.
Perlecan consists of a 400- to 450-kDa proteoglycan core of 5 domains, with sulfated glycosaminoglycan side chains.2,4 It plays roles in hydration, charge-selective permeability, and integrin-dependent adhesion of vascular smooth muscle and endothelial cells.2,4 Acting as a reservoir for heparin-binding growth factors, perlecan can accumulate vascular endothelial growth factor, heparin-binding endothelial growth factor, fibroblast growth factor, and transforming growth factor-β. Thus, rapid degradation of this HSPG could cause profound alterations in cerebral microvessel integrity and dissociate other microvessel matrix components, thereby contributing to hemorrhagic transformation.
Among cysteine proteases, cathepsins B and L degrade HSPG.15 Both cathepsins are associated with intracellular proteolysis and extracellular matrix remodeling. They play pivotal roles in maintenance of the central nervous system.20 In vitro, cathepsins B and L completely eliminated microvessel perlecan, which was inhibitable by E64. Microarray analysis has indicated that cathepsin L mRNA from primate basal ganglia is upregulated 4-fold compared with normal (G.J. del Zoppo, MD and J. Walker, PhD, unpublished data, 2003). Both cathepsins are seen within the ischemic core/ischemic peripheral border zone 2 hours after MCAO in microvessel matrix. Hence, both cathepsins could plausibly contribute to early degradation of vascular perlecan. It is very unlikely that cathepsins B and L are derived from leukocytes because this early time point seen at 2-hour MCAO precedes neutrophil and monocyte invasion. It is possible that intracellular proteases are contained in the samples from ischemic brain. However, both cathepsins were induced in microvessel-associated pericytes at the ischemic core/ischemic peripheral boundary, and were greater than those proteases associated with normal cells.
Previous reports have described the appearance of both cathepsins in cerebral neurons of monkeys or rats after focal or global (hippocampal) ischemia; no association with microvessel ECM was examined, however.11–13 The working hypothesis was that ischemia activates neuron calpain, thereby inducing lysosomal release of intraneuronal cathepsins and directly damaging neurons.11,12 In the primate, cathepsins B and L are upregulated in both microvessels and adjacent glial cells or neurons in the ischemic basal ganglia, coinciding with the appearance of vascular perlecan-degrading activity in these tissues. The difference in observations may be due to differences in species and/or regions studied. We hypothesize that cathepsins B and L are generated very early during focal ischemia and contribute to rapid microvascular matrix HSPG degradation, which dissociates other matrix components and diminishes microvascular integrity. We further postulate that neuron injury is a secondary consequence through the loss of vascular ECM and intercellular HSPG, which are required for neuron viability. Locally, active MMPs may also contribute to the degradation of major microvessel HSPG components, including perlecan. This does not preclude that other families of matrix-degrading proteases, including cathepsins B and L, rapidly promote altered microvascular responses and neuron injury. Hence, decreased microvessel and neuron injury after ischemia could be accomplished by blockade of cathepsin-induced HSPG degradation by specific inhibitors.
Acknowledgment
This study was supported by National Institutes of Health grants NS26945 and NS38710.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
References
- 1.Hamann GF, Okada Y, Fitridge R, del Zoppo GJ. Microvascular basal lamina antigens disappear during cerebral ischemia and reperfusion. Stroke. 1995;26:2120–2126. doi: 10.1161/01.str.26.11.2120. [DOI] [PubMed] [Google Scholar]
- 2.Forsberg E, Kjellén L. Heparan sulfate: lessons from knockout mice. J Clin Invest. 2001;108:175–180. doi: 10.1172/JCI13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nugent MA, Nugent HM, Iozzo RV, Sanchak K, Edelman ER. Perlecan is required to inhibit thrombosis after deep vascular injury and contributes to endothelial cell-mediated inhibition of intimal hyperplasia. Proc Natl Acad Sci U S A. 2000;97:6722–6727. doi: 10.1073/pnas.97.12.6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olson BR. Life without perlecan has its problems. J Cell Biol. 1999;147:909–911. doi: 10.1083/jcb.147.5.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamann GF, Okada Y, del Zoppo GJ. Hemorrhagic transformation and microvascular integrity during focal cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1996;16:1373–1378. doi: 10.1097/00004647-199611000-00036. [DOI] [PubMed] [Google Scholar]
- 6.Tagaya M, Liu K-F, Copeland B, Seiffert D, Engler R, Garcia JH, del Zoppo GJ. DNA scission after focal brain ischemia: temporal differences in two species. Stroke. 1997;28:1245–1254. doi: 10.1161/01.str.28.6.1245. [DOI] [PubMed] [Google Scholar]
- 7.Tagaya M, Haring H-P, Stuiver I, Wagner S, Abumiya T, Lucero J, Lee P, Copeland BR, Seiffert D, del Zoppo GJ. Rapid loss of microvascular integrin expression during focal brain ischemia reflects neuron injury. J Cereb Blood Flow Metab. 2001;21:835–846. doi: 10.1097/00004647-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Gasche Y, Fujimura M, Morita-Fujimura Y, Massengale J, Kawase M, Chan PH. Early appearance of activated matrix metalloproteinase-9 after focal cerebral ischemia in mice: a possible role in blood-brain barrier dysfunction. J Cereb Blood Flow Metab. 1999;19:1020–1028. doi: 10.1097/00004647-199909000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Heo JH, Lucero J, Abumiya T, Koziol JA, Copeland BR, del Zoppo GJ. Matrix metalloproteinases increase very early during experimental focal cerebral ischemia. J Cereb Blood Flow Metab. 1999;19:624–633. doi: 10.1097/00004647-199906000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg GA, Navratil M, Barone F, Feuerstein G. Proteolytic cascade enzymes increase in focal cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1996;16:360–366. doi: 10.1097/00004647-199605000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Kohda Y, Yamashima T, Sakuda K, Yamashita J, Ueno T, Kominami E, Yoshioka T. Dynamic changes of cathepsins B and L expression in the monkey hippocampus after transient ischemia. Biochem Biophys Res Commun. 1996;228:616–622. doi: 10.1006/bbrc.1996.1706. [DOI] [PubMed] [Google Scholar]
- 12.Seyfried DM, Veyna R, Han Y, Li K, Tang N, Betts RL, Weinsheimer S, Chopp M, Anagli J. A selective cysteine protease inhibitor is non-toxic and cerebroprotective in rats undergoing transient middle cerebral artery ischemia. Brain Res. 2001;901:94–101. doi: 10.1016/s0006-8993(01)02289-2. [DOI] [PubMed] [Google Scholar]
- 13.Tsuchiya K, Kohda Y, Yoshida M, Zhao L, Ueno T, Yamashita J, Yoshioka T, Kominami E, Yamashima T. Postictal blockade of ischemic hippocampal neuronal death in primates using selective cathepsin inhibitors. Exp Neurol. 1999;2:187–194. doi: 10.1006/exnr.1998.6988. [DOI] [PubMed] [Google Scholar]
- 14.del Zoppo GJ, Copeland BR, Harker LA, Waltz TA, Zyroff J, Hanson SR, Battenberg E. Experimental acute thrombotic stroke in baboons. Stroke. 1986;17:1254–1265. doi: 10.1161/01.str.17.6.1254. [DOI] [PubMed] [Google Scholar]
- 15.Guinec N, Dalet-Fumeron V, Pagano M. “In vitro” study of basement membrane degradation by the cysteine proteinases, cathepsin B, B-like and L. Biol Chem Hoppe-Seyler. 1993;374:1135–1146. doi: 10.1515/bchm3.1993.374.7-12.1135. [DOI] [PubMed] [Google Scholar]
- 16.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: effects of gene knockout and enzyme inhibition with BB-94. J Cereb Blood Flow Metab. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Hosomi N, Lucero J, Heo JH, Koziol JA, Copeland BR, del Zoppo GJ. Rapid differential endogenous plasminogen activator expression after acute middle cerebral artery occlusion. Stroke. 2001;32:1341–1348. doi: 10.1161/01.str.32.6.1341. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald M, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halpert I, Sires UI, Roby JD, Potter-Perigo S, Wight TN, Shapiro SD, Welgus HD, Wickline SA, Parks WC. Matrilysin is expressed by lipid-laden macrophages at sites of potential rupture in atherosclerotic lesions and localizes to areas of versican deposition, a proteoglycan substrate for the enzyme. Proc Natl Acad Sci U S A. 1996;93:9748–9753. doi: 10.1073/pnas.93.18.9748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felbor U, Kessler B, Mothes W, Goebel HH, Ploegh HL, Bronson RT, Olsen BR. Neuronal loss and brain atrophy in mice lacking cathepsins B and L. Proc Natl Acad Sci U S A. 2002;99:7883–7888. doi: 10.1073/pnas.112632299. [DOI] [PMC free article] [PubMed] [Google Scholar]