Abstract
The title compound C19H27N3O3S, crystallizes with two unique molecules in the asymmetric unit. The benzene ring of each benzothiazole unit carries a dipropylcarbamoyl substituent in the 6-position and a tert-butyl carbamate unit on each thiazole ring. In the crystal structure, intermolecular N—H⋯N and weak C—H⋯O hydrogen bonds form centrosymmetric dimers. Additional C—H⋯O contacts construct a three-dimensional network. A very weak C—H⋯π contact is also present.
Related literature
For benzothiazole derivatives with anti-tumor activity, see: Brantley et al. (2004 ▶); Ćaleta et al. (2009 ▶); Mortimer et al. (2006 ▶) and for benzothiazolines with anti-tuberculous properties, see: Palmer et al. (1971 ▶). For related benzothiazole structures, see: Lynch (2002 ▶); Matković-Čalogović et al. (2003 ▶); Lei et al. (2010 ▶).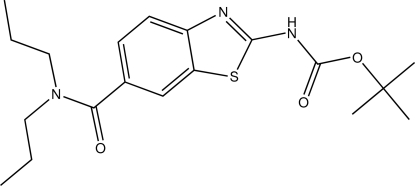
Experimental
Crystal data
C19H27N3O3S
M r = 377.50
Orthorhombic,

a = 14.068 (3) Å
b = 20.942 (4) Å
c = 26.515 (5) Å
V = 7812 (3) Å3
Z = 16
Mo Kα radiation
μ = 0.19 mm−1
T = 113 K
0.45 × 0.35 × 0.23 mm
Data collection
Rigaku Saturn 724 CCD area-detector diffractometer
Absorption correction: numerical (NUMABS; Higashi, 2000 ▶) T min = 0.993, T max = 0.995
62834 measured reflections
8937 independent reflections
8783 reflections with I > 2σ(I)
R int = 0.049
Refinement
R[F 2 > 2σ(F 2)] = 0.055
wR(F 2) = 0.114
S = 1.27
8937 reflections
469 parameters
H-atom parameters constrained
Δρmax = 0.33 e Å−3
Δρmin = −0.23 e Å−3
Data collection: CrystalClear (Rigaku, 2007 ▶); cell refinement: CrystalClear; data reduction: CrystalClear; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEX (McArdle, 1995 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053681001528X/sj2775sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681001528X/sj2775Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg is the centroid of the C26–C31 benzene ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1—H1⋯N5 | 0.86 | 2.12 | 2.963 (2) | 168 |
| N4—H4⋯N2 | 0.86 | 2.16 | 3.006 (2) | 167 |
| C8—H8⋯O4 | 0.93 | 2.59 | 3.461 (2) | 157 |
| C27—H27⋯O1 | 0.93 | 2.61 | 3.321 (2) | 134 |
| C11—H11⋯O6i | 0.93 | 2.38 | 3.161 (2) | 141 |
| C28—H28⋯O3ii | 0.93 | 2.61 | 3.292 (2) | 131 |
| C37—H37A⋯O6iii | 0.97 | 2.56 | 3.375 (3) | 142 |
| C16—H16B⋯O3iv | 0.96 | 2.44 | 3.397 (3) | 177 |
| C20—H20C⋯O2v | 0.96 | 2.61 | 3.460 (3) | 147 |
| C22—H22A⋯Cgvi | 0.96 | 2.98 | 3.942 (3) | 176 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  .
.
Acknowledgments
The authors gratefully acknowledge financial support from the Fujian Institute of Research on the Structure of Matter, State Key Laboratory of Structural Chemistry, Chinese Academy of Sciences (Nos. SZD08003 and NSFC– 30811130467), the Fujian Natural Science Foundation (No. 2008 J0330) and the Fujian Terms of Science and Technology (Nos. 2008 F5033, 2008 J1005 and 2009I0016).
supplementary crystallographic information
Comment
A number of benzothiazole derivatives have anti-tuberculous (Brantley et al., 2004; Mortimer et al., 2006; Ćaleta et al., 2009) or anti-microbial activities (Palmer et al., 1971). During our development of 2-aminobenzothiazole-based Urokinase-Type Plasminogen Activator (uPA) inhibitors, the title compound was synthesized as an intermediate while its activity was not tested because it is only a fragment of our target molecule.
There are two benzothiazole molecules in one crystallographically independent unit. The benzothiazole units are similar to previously reported benzothiazole compounds (Lynch, 2002; Matković-Čalogović et al., 2003), except that the two molecules are slightly distorted from a planar conformation with the angles between thiazole and benzene rings of 1.19 (7) ° for molecule 1 (C1 >> C19) and 4.01 (6) ° for molecule 2 (C20 >> C38), respectively. The dihedral angles between the carbonylamino group and the thiazole ring are 5.43 (15) ° for 1 and 18.19 (11) ° for 2, respectively. The dihedral angles between the dipropylcarbamoyl group and the benzene ring are 56.75 (16) ° for 1 and 54.0914 (1) \5 for 2, respectively.
In the crystal, molecules form pairs via N—H···N and C—H···O hydrogen bonds, Table 1. The dimers form a network through weak C—H···O hydrogen bonds. There is also a very weak C22—H22A···Cg contact (Cg is the centroid of the C26···C31 benzene ring). No π - π interactions are found in this structure, seemingly due to the steric hindrance of the dipropylcarbamoyl group. This is in contrast to what was found in the structure of ethyl 2-(tert-butoxycarbonylamino)-1,3-benzothiazole-6-carboxylate (Lei et al., 2010).
Experimental
A solution of ethyl 2-(tert-butoxycarbonylamino)benzo[d]thiazole-6-carboxylate (2.5 g, 7.76 mmol) was refluxed in a solution of EtOH (80 ml) and 2 N aq NaOH (50 ml) for 5 hours. Then the solution was cooled to 0oC and acidified with 1 N aq HCl solution. When pH < 2, white precipitate was collected, washed by water, and dried, afforded white solid of 2-(tert-butoxycarbonylamino)benzo[d]thiazole-6-carboxylic acid, N-Boc acid, (1.96 g, yield: 86%). 2-(1H-Benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate, HBTU (1138 mg, 3 mmol) and N,N-Diisopropylethylamine, DIEA, (310 mg, 2.4 mmol) were added to the solution of N-Boc acid (588 mg, 2 mmol) in dry DMF (20 ml) and stired for 8 hours at room temperature, then dipropylamine (303 mg, 3 mmol) was added dropwise and the reaction continued further for 12 hours. The reaction solution was treated with water (150 ml) and then the precipitate was collected and washed with water. The filter cake was dried to yield a yellow solid and purification was achieved by column chromatography (ethyl acetate/petroleum ether 1 : 2) to yield the final product as a white solid (600 mg, yield: 79.58%). The solid was dissolved again in DMF, and filtered. After the solvent evaporated slowly at room temperature for a week, colourless rhombic crystals suitable for X-ray structure analysis were separated from the solution.
Refinement
All H atoms bound to C and N atoms were refined as riding, with C—H distances in the range of 0.93 to 0.97 Å and N—H distances of 0.86 Å, with Uiso(H) = 1.2Ueq(C, N); 1.5Ueq(Cmethyl).
Figures
Fig. 1.
The asymmetric unit of the title compound with displacement ellipsoids for the non-hydrogen atoms drawn at the 50% probability level.
Crystal data
| C19H27N3O3S | F(000) = 3232 |
| Mr = 377.50 | Dx = 1.284 Mg m−3 |
| Orthorhombic, Pbca | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ac 2ab | Cell parameters from 28185 reflections |
| a = 14.068 (3) Å | θ = 3.0–27.5° |
| b = 20.942 (4) Å | µ = 0.19 mm−1 |
| c = 26.515 (5) Å | T = 113 K |
| V = 7812 (3) Å3 | Rhombic, colourless |
| Z = 16 | 0.45 × 0.35 × 0.23 mm |
Data collection
| Rigaku Saturn 724 CCD area-detector diffractometer | 8937 independent reflections |
| Radiation source: fine-focus sealed tube | 8783 reflections with I > 2σ(I) |
| graphite | Rint = 0.049 |
| Detector resolution: 28.5714 pixels mm-1 | θmax = 27.5°, θmin = 3.0° |
| dtprofit.ref scans | h = −15→18 |
| Absorption correction: numerical (NUMABS; Higashi, 2000) | k = −27→27 |
| Tmin = 0.993, Tmax = 0.995 | l = −30→34 |
| 62834 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.055 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.114 | H-atom parameters constrained |
| S = 1.26 | w = 1/[σ2(Fo2) + (0.0264P)2 + 6.2394P] where P = (Fo2 + 2Fc2)/3 |
| 8937 reflections | (Δ/σ)max = 0.001 |
| 469 parameters | Δρmax = 0.33 e Å−3 |
| 0 restraints | Δρmin = −0.23 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| S1 | 0.96277 (3) | 0.24066 (2) | 0.608747 (17) | 0.02135 (10) | |
| S2 | 0.93248 (3) | 0.50052 (2) | 0.403321 (17) | 0.02120 (10) | |
| O1 | 1.05224 (10) | 0.22536 (6) | 0.45079 (5) | 0.0225 (3) | |
| O2 | 1.03002 (10) | 0.17996 (6) | 0.52814 (5) | 0.0257 (3) | |
| O6 | 0.95635 (10) | 0.35162 (7) | 0.20407 (5) | 0.0278 (3) | |
| O3 | 0.91447 (10) | 0.32656 (7) | 0.80962 (5) | 0.0270 (3) | |
| O5 | 0.84796 (11) | 0.56490 (7) | 0.47992 (5) | 0.0300 (3) | |
| O4 | 0.81103 (10) | 0.52117 (6) | 0.55639 (5) | 0.0242 (3) | |
| N2 | 0.92428 (11) | 0.35382 (7) | 0.57104 (6) | 0.0193 (3) | |
| N3 | 0.76025 (11) | 0.35404 (8) | 0.79978 (6) | 0.0229 (3) | |
| N5 | 0.92687 (11) | 0.38443 (7) | 0.43988 (6) | 0.0200 (3) | |
| N1 | 0.98199 (12) | 0.28194 (7) | 0.51118 (6) | 0.0216 (3) | |
| H1 | 0.9729 | 0.3096 | 0.4877 | 0.026* | |
| N4 | 0.88637 (11) | 0.46199 (7) | 0.49931 (6) | 0.0210 (3) | |
| H4 | 0.8936 | 0.4352 | 0.5236 | 0.025* | |
| N6 | 1.08841 (11) | 0.41277 (8) | 0.21075 (6) | 0.0218 (3) | |
| C1 | 1.18925 (15) | 0.15247 (12) | 0.45972 (8) | 0.0332 (5) | |
| H1A | 1.2308 | 0.1888 | 0.4604 | 0.050* | |
| H1B | 1.2216 | 0.1169 | 0.4446 | 0.050* | |
| H1C | 1.1711 | 0.1416 | 0.4935 | 0.050* | |
| C2 | 1.12919 (15) | 0.19117 (10) | 0.37669 (7) | 0.0279 (4) | |
| H2A | 1.1734 | 0.2259 | 0.3796 | 0.042* | |
| H2B | 1.0735 | 0.2053 | 0.3590 | 0.042* | |
| H2C | 1.1583 | 0.1567 | 0.3585 | 0.042* | |
| C3 | 1.03136 (16) | 0.11326 (10) | 0.42613 (8) | 0.0322 (5) | |
| H3A | 0.9773 | 0.1259 | 0.4064 | 0.048* | |
| H3B | 1.0110 | 0.1019 | 0.4595 | 0.048* | |
| H3C | 1.0616 | 0.0772 | 0.4106 | 0.048* | |
| C4 | 1.10119 (13) | 0.16828 (9) | 0.42915 (7) | 0.0217 (4) | |
| C5 | 1.02273 (13) | 0.22401 (9) | 0.49879 (7) | 0.0207 (4) | |
| C6 | 0.95554 (13) | 0.29683 (9) | 0.55966 (7) | 0.0190 (3) | |
| C7 | 0.90343 (13) | 0.35638 (9) | 0.62241 (7) | 0.0184 (3) | |
| C8 | 0.86796 (13) | 0.40970 (9) | 0.64792 (7) | 0.0215 (4) | |
| H8 | 0.8580 | 0.4480 | 0.6310 | 0.026* | |
| C9 | 0.84795 (14) | 0.40442 (9) | 0.69893 (7) | 0.0224 (4) | |
| H9 | 0.8225 | 0.4393 | 0.7159 | 0.027* | |
| C10 | 0.86521 (13) | 0.34754 (9) | 0.72555 (7) | 0.0202 (4) | |
| C11 | 0.90282 (13) | 0.29492 (9) | 0.70073 (7) | 0.0206 (4) | |
| H11 | 0.9161 | 0.2574 | 0.7181 | 0.025* | |
| C12 | 0.92015 (13) | 0.29959 (8) | 0.64920 (7) | 0.0191 (3) | |
| C13 | 0.84813 (13) | 0.34180 (9) | 0.78161 (7) | 0.0209 (4) | |
| C14 | 0.67510 (14) | 0.35806 (10) | 0.76828 (7) | 0.0251 (4) | |
| H14A | 0.6296 | 0.3264 | 0.7796 | 0.030* | |
| H14B | 0.6922 | 0.3478 | 0.7338 | 0.030* | |
| C15 | 0.62774 (16) | 0.42374 (10) | 0.76926 (8) | 0.0308 (5) | |
| H15A | 0.5810 | 0.4249 | 0.7961 | 0.037* | |
| H15B | 0.6753 | 0.4560 | 0.7763 | 0.037* | |
| C16 | 0.57946 (17) | 0.43906 (11) | 0.71957 (8) | 0.0336 (5) | |
| H16A | 0.5506 | 0.4805 | 0.7216 | 0.050* | |
| H16B | 0.5315 | 0.4076 | 0.7127 | 0.050* | |
| H16C | 0.6257 | 0.4388 | 0.6930 | 0.050* | |
| C17 | 0.74676 (15) | 0.35034 (10) | 0.85472 (7) | 0.0273 (4) | |
| H17A | 0.6920 | 0.3759 | 0.8640 | 0.033* | |
| H17B | 0.8020 | 0.3684 | 0.8713 | 0.033* | |
| C18 | 0.73213 (17) | 0.28227 (11) | 0.87350 (8) | 0.0346 (5) | |
| H18A | 0.7827 | 0.2553 | 0.8607 | 0.041* | |
| H18B | 0.6723 | 0.2660 | 0.8607 | 0.041* | |
| C19 | 0.7314 (2) | 0.27955 (14) | 0.93096 (9) | 0.0501 (7) | |
| H19A | 0.7220 | 0.2362 | 0.9417 | 0.075* | |
| H19B | 0.6808 | 0.3057 | 0.9436 | 0.075* | |
| H19C | 0.7911 | 0.2950 | 0.9437 | 0.075* | |
| C20 | 0.66237 (15) | 0.57762 (11) | 0.53708 (8) | 0.0319 (5) | |
| H20A | 0.6306 | 0.5371 | 0.5368 | 0.048* | |
| H20B | 0.6838 | 0.5877 | 0.5036 | 0.048* | |
| H20C | 0.6191 | 0.6100 | 0.5485 | 0.048* | |
| C21 | 0.71580 (18) | 0.55301 (12) | 0.62447 (8) | 0.0382 (5) | |
| H21A | 0.6820 | 0.5133 | 0.6218 | 0.057* | |
| H21B | 0.6751 | 0.5848 | 0.6391 | 0.057* | |
| H21C | 0.7708 | 0.5473 | 0.6454 | 0.057* | |
| C22 | 0.80034 (16) | 0.63702 (11) | 0.57473 (10) | 0.0381 (5) | |
| H22A | 0.8531 | 0.6329 | 0.5975 | 0.057* | |
| H22B | 0.7586 | 0.6701 | 0.5865 | 0.057* | |
| H22C | 0.8234 | 0.6478 | 0.5417 | 0.057* | |
| C23 | 0.74685 (14) | 0.57458 (9) | 0.57230 (7) | 0.0238 (4) | |
| C24 | 0.84771 (13) | 0.52107 (9) | 0.50973 (7) | 0.0217 (4) | |
| C25 | 0.91377 (13) | 0.44426 (9) | 0.45142 (7) | 0.0194 (3) | |
| C26 | 0.95101 (13) | 0.37896 (9) | 0.38902 (7) | 0.0187 (3) | |
| C27 | 0.96320 (13) | 0.32156 (9) | 0.36315 (7) | 0.0211 (4) | |
| H27 | 0.9565 | 0.2827 | 0.3798 | 0.025* | |
| C28 | 0.98538 (13) | 0.32322 (9) | 0.31227 (7) | 0.0206 (4) | |
| H28 | 0.9914 | 0.2851 | 0.2945 | 0.025* | |
| C29 | 0.99895 (12) | 0.38148 (9) | 0.28707 (7) | 0.0191 (3) | |
| C30 | 0.98720 (13) | 0.43903 (9) | 0.31258 (7) | 0.0200 (4) | |
| H30 | 0.9967 | 0.4778 | 0.2962 | 0.024* | |
| C31 | 0.96087 (13) | 0.43706 (9) | 0.36330 (7) | 0.0196 (4) | |
| C32 | 1.01427 (13) | 0.38019 (8) | 0.23074 (7) | 0.0196 (4) | |
| C33 | 1.09844 (15) | 0.41331 (10) | 0.15547 (7) | 0.0270 (4) | |
| H33A | 1.0363 | 0.4072 | 0.1404 | 0.032* | |
| H33B | 1.1382 | 0.3777 | 0.1454 | 0.032* | |
| C34 | 1.14122 (19) | 0.47439 (11) | 0.13533 (9) | 0.0399 (5) | |
| H34A | 1.2048 | 0.4798 | 0.1489 | 0.048* | |
| H34B | 1.1031 | 0.5105 | 0.1461 | 0.048* | |
| C35 | 1.1456 (2) | 0.47286 (14) | 0.07794 (9) | 0.0505 (7) | |
| H35A | 1.1730 | 0.5119 | 0.0658 | 0.076* | |
| H35B | 1.0826 | 0.4682 | 0.0646 | 0.076* | |
| H35C | 1.1841 | 0.4375 | 0.0673 | 0.076* | |
| C36 | 1.17332 (14) | 0.42832 (10) | 0.24024 (8) | 0.0268 (4) | |
| H36A | 1.1567 | 0.4292 | 0.2757 | 0.032* | |
| H36B | 1.1952 | 0.4706 | 0.2309 | 0.032* | |
| C37 | 1.25318 (16) | 0.38125 (12) | 0.23238 (9) | 0.0386 (5) | |
| H37A | 1.3078 | 0.3947 | 0.2521 | 0.046* | |
| H37B | 1.2715 | 0.3816 | 0.1971 | 0.046* | |
| C38 | 1.2265 (2) | 0.31399 (13) | 0.24729 (12) | 0.0516 (7) | |
| H38A | 1.2795 | 0.2861 | 0.2415 | 0.077* | |
| H38B | 1.1733 | 0.3001 | 0.2274 | 0.077* | |
| H38C | 1.2096 | 0.3131 | 0.2824 | 0.077* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| S1 | 0.0280 (2) | 0.0193 (2) | 0.0168 (2) | 0.00554 (18) | 0.00337 (17) | 0.00088 (16) |
| S2 | 0.0278 (2) | 0.0174 (2) | 0.0184 (2) | 0.00189 (17) | 0.00406 (17) | 0.00045 (16) |
| O1 | 0.0295 (7) | 0.0212 (6) | 0.0169 (6) | 0.0050 (5) | 0.0044 (5) | −0.0015 (5) |
| O2 | 0.0333 (8) | 0.0220 (7) | 0.0217 (7) | 0.0055 (6) | 0.0063 (6) | 0.0019 (5) |
| O6 | 0.0283 (7) | 0.0346 (8) | 0.0204 (7) | −0.0086 (6) | −0.0001 (6) | −0.0030 (6) |
| O3 | 0.0263 (7) | 0.0340 (8) | 0.0207 (7) | 0.0040 (6) | −0.0011 (5) | 0.0021 (6) |
| O5 | 0.0414 (9) | 0.0227 (7) | 0.0259 (7) | 0.0060 (6) | 0.0078 (6) | 0.0032 (6) |
| O4 | 0.0314 (7) | 0.0238 (7) | 0.0174 (6) | 0.0085 (6) | 0.0028 (5) | −0.0022 (5) |
| N2 | 0.0209 (7) | 0.0198 (7) | 0.0171 (7) | 0.0013 (6) | 0.0015 (6) | −0.0005 (6) |
| N3 | 0.0233 (8) | 0.0284 (8) | 0.0169 (7) | 0.0039 (7) | 0.0020 (6) | −0.0011 (6) |
| N5 | 0.0234 (8) | 0.0204 (7) | 0.0161 (7) | 0.0008 (6) | 0.0009 (6) | 0.0001 (6) |
| N1 | 0.0288 (8) | 0.0199 (8) | 0.0162 (7) | 0.0048 (6) | 0.0034 (6) | 0.0014 (6) |
| N4 | 0.0266 (8) | 0.0194 (7) | 0.0169 (7) | 0.0039 (6) | 0.0019 (6) | 0.0010 (6) |
| N6 | 0.0234 (8) | 0.0245 (8) | 0.0175 (8) | −0.0032 (6) | 0.0020 (6) | −0.0011 (6) |
| C1 | 0.0271 (10) | 0.0465 (13) | 0.0259 (10) | 0.0118 (9) | −0.0002 (8) | −0.0034 (9) |
| C2 | 0.0350 (11) | 0.0284 (10) | 0.0203 (9) | 0.0040 (8) | 0.0051 (8) | −0.0030 (8) |
| C3 | 0.0364 (12) | 0.0266 (10) | 0.0336 (11) | −0.0032 (9) | 0.0061 (9) | −0.0082 (9) |
| C4 | 0.0237 (9) | 0.0218 (9) | 0.0197 (9) | 0.0048 (7) | 0.0040 (7) | −0.0040 (7) |
| C5 | 0.0224 (9) | 0.0217 (9) | 0.0181 (8) | 0.0017 (7) | 0.0024 (7) | −0.0021 (7) |
| C6 | 0.0192 (8) | 0.0201 (8) | 0.0178 (8) | 0.0002 (7) | 0.0008 (7) | 0.0007 (7) |
| C7 | 0.0186 (8) | 0.0203 (8) | 0.0163 (8) | −0.0008 (7) | −0.0004 (6) | 0.0003 (7) |
| C8 | 0.0249 (9) | 0.0187 (8) | 0.0209 (9) | 0.0008 (7) | 0.0006 (7) | 0.0011 (7) |
| C9 | 0.0261 (9) | 0.0191 (9) | 0.0218 (9) | 0.0020 (7) | 0.0031 (7) | −0.0029 (7) |
| C10 | 0.0195 (8) | 0.0224 (9) | 0.0187 (9) | 0.0005 (7) | 0.0006 (7) | −0.0010 (7) |
| C11 | 0.0218 (9) | 0.0206 (9) | 0.0194 (9) | 0.0027 (7) | 0.0007 (7) | 0.0014 (7) |
| C12 | 0.0189 (8) | 0.0183 (8) | 0.0199 (9) | 0.0027 (7) | 0.0011 (7) | 0.0000 (7) |
| C13 | 0.0243 (9) | 0.0191 (8) | 0.0195 (9) | 0.0005 (7) | 0.0017 (7) | −0.0016 (7) |
| C14 | 0.0242 (9) | 0.0287 (10) | 0.0223 (9) | 0.0013 (8) | 0.0007 (7) | −0.0035 (8) |
| C15 | 0.0342 (11) | 0.0309 (11) | 0.0273 (10) | 0.0078 (9) | −0.0028 (9) | −0.0054 (8) |
| C16 | 0.0384 (12) | 0.0335 (11) | 0.0290 (11) | 0.0081 (9) | −0.0033 (9) | −0.0025 (9) |
| C17 | 0.0303 (10) | 0.0336 (11) | 0.0180 (9) | 0.0060 (8) | 0.0048 (8) | −0.0014 (8) |
| C18 | 0.0367 (12) | 0.0394 (12) | 0.0277 (11) | −0.0024 (10) | 0.0070 (9) | 0.0043 (9) |
| C19 | 0.0644 (18) | 0.0561 (16) | 0.0300 (12) | −0.0062 (14) | 0.0089 (12) | 0.0132 (11) |
| C20 | 0.0279 (11) | 0.0354 (11) | 0.0323 (11) | 0.0053 (9) | −0.0047 (8) | −0.0062 (9) |
| C21 | 0.0460 (13) | 0.0434 (13) | 0.0252 (10) | 0.0162 (11) | 0.0070 (9) | −0.0043 (9) |
| C22 | 0.0303 (11) | 0.0322 (11) | 0.0518 (14) | −0.0021 (9) | 0.0020 (10) | −0.0180 (10) |
| C23 | 0.0244 (9) | 0.0229 (9) | 0.0240 (9) | 0.0062 (7) | 0.0009 (7) | −0.0059 (7) |
| C24 | 0.0227 (9) | 0.0225 (9) | 0.0199 (9) | 0.0018 (7) | 0.0001 (7) | −0.0018 (7) |
| C25 | 0.0197 (8) | 0.0205 (8) | 0.0180 (8) | 0.0011 (7) | 0.0008 (7) | 0.0000 (7) |
| C26 | 0.0194 (8) | 0.0210 (8) | 0.0157 (8) | 0.0002 (7) | −0.0003 (6) | 0.0010 (7) |
| C27 | 0.0255 (9) | 0.0183 (8) | 0.0194 (9) | −0.0007 (7) | −0.0002 (7) | 0.0019 (7) |
| C28 | 0.0219 (9) | 0.0195 (9) | 0.0203 (9) | 0.0002 (7) | −0.0017 (7) | −0.0017 (7) |
| C29 | 0.0174 (8) | 0.0224 (9) | 0.0174 (8) | 0.0007 (7) | 0.0002 (6) | −0.0005 (7) |
| C30 | 0.0231 (9) | 0.0187 (8) | 0.0183 (8) | 0.0010 (7) | 0.0025 (7) | 0.0030 (7) |
| C31 | 0.0197 (8) | 0.0191 (8) | 0.0200 (9) | 0.0019 (7) | 0.0001 (7) | −0.0005 (7) |
| C32 | 0.0210 (9) | 0.0176 (8) | 0.0203 (9) | 0.0013 (7) | 0.0007 (7) | −0.0005 (7) |
| C33 | 0.0296 (10) | 0.0330 (11) | 0.0184 (9) | −0.0044 (8) | 0.0027 (7) | 0.0008 (8) |
| C34 | 0.0531 (15) | 0.0351 (12) | 0.0314 (12) | −0.0038 (11) | 0.0093 (10) | 0.0054 (10) |
| C35 | 0.0652 (18) | 0.0556 (16) | 0.0306 (12) | −0.0087 (14) | 0.0093 (12) | 0.0127 (11) |
| C36 | 0.0239 (10) | 0.0316 (10) | 0.0250 (10) | −0.0064 (8) | 0.0016 (8) | −0.0057 (8) |
| C37 | 0.0227 (10) | 0.0552 (15) | 0.0378 (12) | 0.0055 (10) | −0.0008 (9) | −0.0078 (11) |
| C38 | 0.0442 (15) | 0.0456 (14) | 0.0649 (18) | 0.0216 (12) | −0.0073 (13) | −0.0014 (13) |
Geometric parameters (Å, °)
| S1—C12 | 1.7416 (18) | C15—H15A | 0.9700 |
| S1—C6 | 1.7574 (18) | C15—H15B | 0.9700 |
| S2—C31 | 1.7469 (18) | C16—H16A | 0.9600 |
| S2—C25 | 1.7563 (19) | C16—H16B | 0.9600 |
| O1—C5 | 1.339 (2) | C16—H16C | 0.9600 |
| O1—C4 | 1.494 (2) | C17—C18 | 1.524 (3) |
| O2—C5 | 1.211 (2) | C17—H17A | 0.9700 |
| O6—C32 | 1.234 (2) | C17—H17B | 0.9700 |
| O3—C13 | 1.235 (2) | C18—C19 | 1.525 (3) |
| O5—C24 | 1.211 (2) | C18—H18A | 0.9700 |
| O4—C24 | 1.341 (2) | C18—H18B | 0.9700 |
| O4—C23 | 1.498 (2) | C19—H19A | 0.9600 |
| N2—C6 | 1.307 (2) | C19—H19B | 0.9600 |
| N2—C7 | 1.394 (2) | C19—H19C | 0.9600 |
| N3—C13 | 1.351 (2) | C20—C23 | 1.513 (3) |
| N3—C14 | 1.463 (2) | C20—H20A | 0.9600 |
| N3—C17 | 1.471 (2) | C20—H20B | 0.9600 |
| N5—C25 | 1.303 (2) | C20—H20C | 0.9600 |
| N5—C26 | 1.395 (2) | C21—C23 | 1.519 (3) |
| N1—C6 | 1.374 (2) | C21—H21A | 0.9600 |
| N1—C5 | 1.381 (2) | C21—H21B | 0.9600 |
| N1—H1 | 0.8600 | C21—H21C | 0.9600 |
| N4—C25 | 1.378 (2) | C22—C23 | 1.510 (3) |
| N4—C24 | 1.379 (2) | C22—H22A | 0.9600 |
| N4—H4 | 0.8600 | C22—H22B | 0.9600 |
| N6—C32 | 1.354 (2) | C22—H22C | 0.9600 |
| N6—C36 | 1.464 (2) | C26—C27 | 1.395 (2) |
| N6—C33 | 1.473 (2) | C26—C31 | 1.401 (2) |
| C1—C4 | 1.517 (3) | C27—C28 | 1.385 (3) |
| C1—H1A | 0.9600 | C27—H27 | 0.9300 |
| C1—H1B | 0.9600 | C28—C29 | 1.404 (3) |
| C1—H1C | 0.9600 | C28—H28 | 0.9300 |
| C2—C4 | 1.523 (3) | C29—C30 | 1.392 (3) |
| C2—H2A | 0.9600 | C29—C32 | 1.509 (2) |
| C2—H2B | 0.9600 | C30—C31 | 1.396 (2) |
| C2—H2C | 0.9600 | C30—H30 | 0.9300 |
| C3—C4 | 1.516 (3) | C33—C34 | 1.511 (3) |
| C3—H3A | 0.9600 | C33—H33A | 0.9700 |
| C3—H3B | 0.9600 | C33—H33B | 0.9700 |
| C3—H3C | 0.9600 | C34—C35 | 1.523 (3) |
| C7—C8 | 1.398 (2) | C34—H34A | 0.9700 |
| C7—C12 | 1.405 (2) | C34—H34B | 0.9700 |
| C8—C9 | 1.386 (3) | C35—H35A | 0.9600 |
| C8—H8 | 0.9300 | C35—H35B | 0.9600 |
| C9—C10 | 1.406 (3) | C35—H35C | 0.9600 |
| C9—H9 | 0.9300 | C36—C37 | 1.509 (3) |
| C10—C11 | 1.388 (3) | C36—H36A | 0.9700 |
| C10—C13 | 1.511 (2) | C36—H36B | 0.9700 |
| C11—C12 | 1.391 (2) | C37—C38 | 1.511 (4) |
| C11—H11 | 0.9300 | C37—H37A | 0.9700 |
| C14—C15 | 1.528 (3) | C37—H37B | 0.9700 |
| C14—H14A | 0.9700 | C38—H38A | 0.9600 |
| C14—H14B | 0.9700 | C38—H38B | 0.9600 |
| C15—C16 | 1.517 (3) | C38—H38C | 0.9600 |
| C12—S1—C6 | 87.81 (9) | C17—C18—H18B | 109.4 |
| C31—S2—C25 | 87.99 (9) | C19—C18—H18B | 109.4 |
| C5—O1—C4 | 119.41 (14) | H18A—C18—H18B | 108.0 |
| C24—O4—C23 | 119.54 (14) | C18—C19—H19A | 109.5 |
| C6—N2—C7 | 109.36 (15) | C18—C19—H19B | 109.5 |
| C13—N3—C14 | 123.81 (16) | H19A—C19—H19B | 109.5 |
| C13—N3—C17 | 117.46 (16) | C18—C19—H19C | 109.5 |
| C14—N3—C17 | 117.57 (16) | H19A—C19—H19C | 109.5 |
| C25—N5—C26 | 109.89 (15) | H19B—C19—H19C | 109.5 |
| C6—N1—C5 | 122.29 (15) | C23—C20—H20A | 109.5 |
| C6—N1—H1 | 118.9 | C23—C20—H20B | 109.5 |
| C5—N1—H1 | 118.9 | H20A—C20—H20B | 109.5 |
| C25—N4—C24 | 122.45 (16) | C23—C20—H20C | 109.5 |
| C25—N4—H4 | 118.8 | H20A—C20—H20C | 109.5 |
| C24—N4—H4 | 118.8 | H20B—C20—H20C | 109.5 |
| C32—N6—C36 | 122.09 (16) | C23—C21—H21A | 109.5 |
| C32—N6—C33 | 117.85 (16) | C23—C21—H21B | 109.5 |
| C36—N6—C33 | 116.85 (16) | H21A—C21—H21B | 109.5 |
| C4—C1—H1A | 109.5 | C23—C21—H21C | 109.5 |
| C4—C1—H1B | 109.5 | H21A—C21—H21C | 109.5 |
| H1A—C1—H1B | 109.5 | H21B—C21—H21C | 109.5 |
| C4—C1—H1C | 109.5 | C23—C22—H22A | 109.5 |
| H1A—C1—H1C | 109.5 | C23—C22—H22B | 109.5 |
| H1B—C1—H1C | 109.5 | H22A—C22—H22B | 109.5 |
| C4—C2—H2A | 109.5 | C23—C22—H22C | 109.5 |
| C4—C2—H2B | 109.5 | H22A—C22—H22C | 109.5 |
| H2A—C2—H2B | 109.5 | H22B—C22—H22C | 109.5 |
| C4—C2—H2C | 109.5 | O4—C23—C22 | 110.99 (16) |
| H2A—C2—H2C | 109.5 | O4—C23—C20 | 109.34 (15) |
| H2B—C2—H2C | 109.5 | C22—C23—C20 | 112.42 (18) |
| C4—C3—H3A | 109.5 | O4—C23—C21 | 101.98 (15) |
| C4—C3—H3B | 109.5 | C22—C23—C21 | 111.22 (18) |
| H3A—C3—H3B | 109.5 | C20—C23—C21 | 110.41 (18) |
| C4—C3—H3C | 109.5 | O5—C24—O4 | 127.02 (17) |
| H3A—C3—H3C | 109.5 | O5—C24—N4 | 123.22 (17) |
| H3B—C3—H3C | 109.5 | O4—C24—N4 | 109.76 (16) |
| O1—C4—C3 | 109.24 (15) | N5—C25—N4 | 121.01 (16) |
| O1—C4—C1 | 110.23 (15) | N5—C25—S2 | 116.96 (14) |
| C3—C4—C1 | 113.05 (18) | N4—C25—S2 | 122.03 (14) |
| O1—C4—C2 | 102.60 (14) | C27—C26—N5 | 125.16 (16) |
| C3—C4—C2 | 111.01 (17) | C27—C26—C31 | 119.80 (16) |
| C1—C4—C2 | 110.21 (17) | N5—C26—C31 | 115.03 (16) |
| O2—C5—O1 | 126.91 (17) | C28—C27—C26 | 119.02 (17) |
| O2—C5—N1 | 123.45 (17) | C28—C27—H27 | 120.5 |
| O1—C5—N1 | 109.64 (15) | C26—C27—H27 | 120.5 |
| N2—C6—N1 | 120.96 (16) | C27—C28—C29 | 121.05 (17) |
| N2—C6—S1 | 117.36 (14) | C27—C28—H28 | 119.5 |
| N1—C6—S1 | 121.68 (14) | C29—C28—H28 | 119.5 |
| N2—C7—C8 | 125.35 (16) | C30—C29—C28 | 120.34 (16) |
| N2—C7—C12 | 115.22 (16) | C30—C29—C32 | 120.88 (16) |
| C8—C7—C12 | 119.44 (16) | C28—C29—C32 | 118.35 (16) |
| C9—C8—C7 | 118.75 (17) | C29—C30—C31 | 118.30 (17) |
| C9—C8—H8 | 120.6 | C29—C30—H30 | 120.8 |
| C7—C8—H8 | 120.6 | C31—C30—H30 | 120.8 |
| C8—C9—C10 | 121.50 (17) | C30—C31—C26 | 121.38 (17) |
| C8—C9—H9 | 119.2 | C30—C31—S2 | 128.57 (14) |
| C10—C9—H9 | 119.2 | C26—C31—S2 | 110.02 (13) |
| C11—C10—C9 | 120.03 (17) | O6—C32—N6 | 121.92 (17) |
| C11—C10—C13 | 117.64 (16) | O6—C32—C29 | 118.79 (16) |
| C9—C10—C13 | 122.28 (16) | N6—C32—C29 | 119.22 (16) |
| C10—C11—C12 | 118.46 (17) | N6—C33—C34 | 113.35 (17) |
| C10—C11—H11 | 120.8 | N6—C33—H33A | 108.9 |
| C12—C11—H11 | 120.8 | C34—C33—H33A | 108.9 |
| C11—C12—C7 | 121.77 (16) | N6—C33—H33B | 108.9 |
| C11—C12—S1 | 127.97 (14) | C34—C33—H33B | 108.9 |
| C7—C12—S1 | 110.25 (13) | H33A—C33—H33B | 107.7 |
| O3—C13—N3 | 121.74 (17) | C33—C34—C35 | 110.6 (2) |
| O3—C13—C10 | 119.49 (17) | C33—C34—H34A | 109.5 |
| N3—C13—C10 | 118.77 (16) | C35—C34—H34A | 109.5 |
| N3—C14—C15 | 113.52 (16) | C33—C34—H34B | 109.5 |
| N3—C14—H14A | 108.9 | C35—C34—H34B | 109.5 |
| C15—C14—H14A | 108.9 | H34A—C34—H34B | 108.1 |
| N3—C14—H14B | 108.9 | C34—C35—H35A | 109.5 |
| C15—C14—H14B | 108.9 | C34—C35—H35B | 109.5 |
| H14A—C14—H14B | 107.7 | H35A—C35—H35B | 109.5 |
| C16—C15—C14 | 111.76 (17) | C34—C35—H35C | 109.5 |
| C16—C15—H15A | 109.3 | H35A—C35—H35C | 109.5 |
| C14—C15—H15A | 109.3 | H35B—C35—H35C | 109.5 |
| C16—C15—H15B | 109.3 | N6—C36—C37 | 112.85 (17) |
| C14—C15—H15B | 109.3 | N6—C36—H36A | 109.0 |
| H15A—C15—H15B | 107.9 | C37—C36—H36A | 109.0 |
| C15—C16—H16A | 109.5 | N6—C36—H36B | 109.0 |
| C15—C16—H16B | 109.5 | C37—C36—H36B | 109.0 |
| H16A—C16—H16B | 109.5 | H36A—C36—H36B | 107.8 |
| C15—C16—H16C | 109.5 | C36—C37—C38 | 112.81 (19) |
| H16A—C16—H16C | 109.5 | C36—C37—H37A | 109.0 |
| H16B—C16—H16C | 109.5 | C38—C37—H37A | 109.0 |
| N3—C17—C18 | 112.97 (17) | C36—C37—H37B | 109.0 |
| N3—C17—H17A | 109.0 | C38—C37—H37B | 109.0 |
| C18—C17—H17A | 109.0 | H37A—C37—H37B | 107.8 |
| N3—C17—H17B | 109.0 | C37—C38—H38A | 109.5 |
| C18—C17—H17B | 109.0 | C37—C38—H38B | 109.5 |
| H17A—C17—H17B | 107.8 | H38A—C38—H38B | 109.5 |
| C17—C18—C19 | 111.2 (2) | C37—C38—H38C | 109.5 |
| C17—C18—H18A | 109.4 | H38A—C38—H38C | 109.5 |
| C19—C18—H18A | 109.4 | H38B—C38—H38C | 109.5 |
Hydrogen-bond geometry (Å, °)
| Cg is the centroid of the C26–C31 benzene ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1—H1···N5 | 0.86 | 2.12 | 2.963 (2) | 168 |
| N4—H4···N2 | 0.86 | 2.16 | 3.006 (2) | 167 |
| C8—H8···O4 | 0.93 | 2.59 | 3.461 (2) | 157 |
| C27—H27···O1 | 0.93 | 2.61 | 3.321 (2) | 134 |
| C11—H11···O6i | 0.93 | 2.38 | 3.161 (2) | 141 |
| C28—H28···O3ii | 0.93 | 2.61 | 3.292 (2) | 131 |
| C37—H37A···O6iii | 0.97 | 2.56 | 3.375 (3) | 142 |
| C16—H16B···O3iv | 0.96 | 2.44 | 3.397 (3) | 177 |
| C20—H20C···O2v | 0.96 | 2.61 | 3.460 (3) | 147 |
| C22—H22A···Cgvi | 0.96 | 2.98 | 3.942 (3) | 176 |
Symmetry codes: (i) x, −y+1/2, z+1/2; (ii) x, −y+1/2, z−1/2; (iii) x+1/2, y, −z+1/2; (iv) x−1/2, y, −z+3/2; (v) −x+3/2, y+1/2, z; (vi) −x+2, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: SJ2775).
References
- Brantley, E., Trapani, V., Alley, M. C., Hose, C. D., Bradshaw, T. D., Stevens, M. F. G., Sausville, E. A. & Stinson, S. F. (2004). Drug Metab. Disp.32, 1392–1401. [DOI] [PubMed]
- Ćaleta, I., Kralj, M., Marjanović, M., Bertoša, B., Tomić, S., Pavlović, G., Pavelić, K. & Karminski-Zamola, G. (2009). J. Med. Chem.52, 1744–1756. [DOI] [PubMed]
- Higashi, T. (2000). NUMABS Rigaku Corporation, Tokyo, Japan.
- Lei, C., Fang, X., Yu, H.-Y., Huang, M.-D. & Wang, J.-D. (2010). Acta Cryst. E66, o914. [DOI] [PMC free article] [PubMed]
- Lynch, D. E. (2002). Acta Cryst. E58, o1139–o1141.
- Matković-Čalogović, D., Popović, Z., Tralić-Kulenović, V., Racanè, L. & Karminski-Zamola, G. (2003). Acta Cryst. C59, o190–o191. [DOI] [PubMed]
- McArdle, P. (1995). J. Appl. Cryst.28, 65. [DOI] [PMC free article] [PubMed]
- Mortimer, C. G., Wells, G., Crochard, J., Stone, E. L., Bradshaw, T. D., Stevens, M. F. G. & Westwell, A. D. (2006). J. Med. Chem.49, 179–185. [DOI] [PubMed]
- Palmer, P. J., Trigg, R. B. & Warrington, J. V. (1971). J. Med. Chem.14, 248–251. [DOI] [PubMed]
- Rigaku (2007). CrystalClear. Rigaku Corporation, Tokyo, Japan.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S160053681001528X/sj2775sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053681001528X/sj2775Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



