Abstract
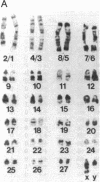
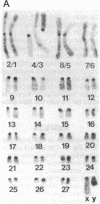
The observation of karyotypic uniformity in most species has led to the widespread belief that selection limits chromosomal change. We report an unprecedented amount of chromosomal variation in a natural population of the South American marsh rat Holochilus brasiliensis. This variation consists of four distinct classes of chromosomal rearrangements: whole-arm translocations, pericentric inversions, variation in the amount of euchromatin, and variation in number and kind of supernumerary (B) chromosomes. Twenty-six karyotypes are present among 42 animals. Observations of the natural population over a 7-year period and breeding experiments with captive animals indicate that heterozygous individuals suffer no detectable reduction in fitness. This is at odds with a central assumption in current models of chromosomal speciation and provides a firm rejection of the view that selection necessarily restricts chromosomal change.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Capanna E. Robertsonian numerical variation in animal speciation: Mus musculus, an emblematic model. Prog Clin Biol Res. 1982;96:155–177. [PubMed] [Google Scholar]
- Davisson M. T., Akeson E. C. An improved method for preparing G-banded chromosomes from mouse peripheral blood. Cytogenet Cell Genet. 1987;45(2):70–74. doi: 10.1159/000132432. [DOI] [PubMed] [Google Scholar]
- EVANS E. P., BRECKON G., FORD C. E. AN AIR-DRYING METHOD FOR MEIOTIC PREPARATIONS FROM MAMMALIAN TESTES. Cytogenetics. 1964;3:289–294. doi: 10.1159/000129818. [DOI] [PubMed] [Google Scholar]
- King M. Chromosomal rearrangements, speciation and the theoretical approach. Heredity (Edinb) 1987 Aug;59(Pt 1):1–6. doi: 10.1038/hdy.1987.90. [DOI] [PubMed] [Google Scholar]
- Koop B. F., Baker R. J., Genoways H. H. Numerous chromosomal polymorphisms in a natural population of rice rats (Oryzomys, Cricetidae). Cytogenet Cell Genet. 1983;35(2):131–135. doi: 10.1159/000131854. [DOI] [PubMed] [Google Scholar]
- Patton J. L. Chromosome studies of certain pocket mice, genus Perognathus (Rodentia: heteromyidae). J Mammal. 1967 Feb;48(1):27–37. [PubMed] [Google Scholar]
- Searle J. B. Meiotic studies of Robertsonian heterozygotes from natural populations of the common shrew, Sorex araneus L. Cytogenet Cell Genet. 1986;41(3):154–162. doi: 10.1159/000132220. [DOI] [PubMed] [Google Scholar]
- Stewart-Scott I. A., Bruère A. N. Distribution of heterozygous translocations and aneuploid spermatocyte frequency in domestic sheep. J Hered. 1987 Jan-Feb;78(1):37–40. doi: 10.1093/oxfordjournals.jhered.a110304. [DOI] [PubMed] [Google Scholar]
- White M. J. Models of speciation. New concepts suggest that the classical sympatric and allopatric models are not the only alternatives. Science. 1968 Mar 8;159(3819):1065–1070. doi: 10.1126/science.159.3819.1065. [DOI] [PubMed] [Google Scholar]
- White M. J. Rectangularity, speciation, and chromosome architecture. Prog Clin Biol Res. 1982;96:75–103. [PubMed] [Google Scholar]