Abstract
In the title compound, C15H12ClN3O, the quinoline ring system is essentially planar, with a maximum deviation of 0.017 (1) Å. The crystal packing is stabilized by π–π stacking interactions between the quinoline rings of adjacent molecule, with a centroid–centroid distance of 3.5913 (8) Å. A weak C—H⋯π contact is also observed between molecules.
Related literature
For pyrimidine analogues, see: Svenstrup et al. (2008 ▶). For quinoline analogues, see: Roopan & Khan (2009 ▶); Khan et al. (2009 ▶, 2010a
▶,b
▶). For the biological activity and mode of action of alkylating agent, see: Singer (1986 ▶). For the synthesis and regioselective alkylation of 4(3H)-pyrimidone, see: Roopan et al. (2010 ▶). For bond-length data, see: Allen et al. (1987 ▶). For a structural discussion on hydrogen bonding, see: Bernstein et al. (1995 ▶).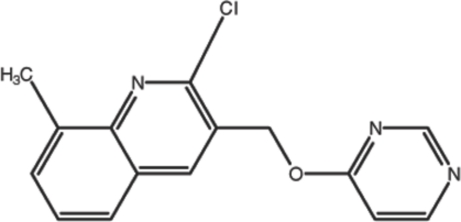
Experimental
Crystal data
C15H12ClN3O
M r = 285.73
Monoclinic,

a = 11.9975 (2) Å
b = 8.45037 (15) Å
c = 12.95869 (19) Å
β = 96.7619 (16)°
V = 1304.66 (4) Å3
Z = 4
Mo Kα radiation
μ = 0.29 mm−1
T = 295 K
0.23 × 0.18 × 0.15 mm
Data collection
Oxford Xcalibur Eos (Nova) CCD detector diffractometer
Absorption correction: multi-scan (CrysAlis PRO RED; Oxford Diffraction, 2009 ▶) T min = 0.936, T max = 0.958
13753 measured reflections
2564 independent reflections
2005 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.033
wR(F 2) = 0.089
S = 1.07
2564 reflections
182 parameters
H-atom parameters constrained
Δρmax = 0.18 e Å−3
Δρmin = −0.20 e Å−3
Data collection: CrysAlis PRO CCD (Oxford Diffraction, 2009 ▶); cell refinement: CrysAlis PRO CCD; data reduction: CrysAlis PRO RED (Oxford Diffraction, 2009 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 1997 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶) and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810011694/fj2289sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810011694/fj2289Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
Cg3 is the centroid of the C4–C9 ring.
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10—H10A⋯Cg3i | 0.97 | 2.72 | 3.5132 (15) | 140 |
Symmetry code: (i)  .
.
Acknowledgments
We thank the FIST program for the data collection at SSCU, IISc, Bangalore and Professor T. N. Guru Row, IISc, Bangalore, for his help with the data collection. FNK thanks the DST for Fast Track Proposal funding.
supplementary crystallographic information
Comment
Alkylating agents have been studied extensively both for their biological effects and for their mode of action (Singer et al., 1986). There have been over the past 25 years a veritable deluge of reviews, often focusing on a single aspect or agent or adduct. Direct alkylation of oxygens in pyrimidine nucleosides, under physiological conditions, has been known only since the middle 1970s. The pyrimidine analogues (Svenstrup et al., 2008) such as naturally occurring azacamptothecin based molecule have been focused of great interest by reason of their diversified biological activities. Thus, modifications of biologically active azacamptothecin synthons may lead to achieve the highly expected effective drugs. In connection with the program of synthesis and regioselective alkylation of 4(3H)-pyrimidone (Roopan et al., 2010), we report herein the synthesis of 2-chloro-8-methyl-3-[(pyrimidin-4-yloxy)methyl]quinoline.
In the molecule of the title compound, Fig. 1, bond lengths and angles are in normal ranges (Allen et al., 1987). The quinoline ring system (N1/C1–C9) is essentially planar, with a maximum deviation of -0.017 (1) Å for atom C1. The quinoline system (N1/C1–C9) makes a dihedral angle of 4.99 (6)° with the piyrimidone ring (N2/N3/C11–C14).
In the title molecule, there is a weak intramolecular C—H···O interaction, generating an S(5) graph-set motif (Bernstein et al., 1995) (Table 1). The crystal packing is stabilized by π-π stacking interactions between the benzene rings of the quinoline ring system of the molecules related by the symmetry operator (1-x, 1-y, -z) [centroid-to-centroid distance = 3.5913 (8) Å]. In addition, a weak C—H···π contact is also observed between molecules (Table 1). The packing diagram viewing down the b-axis is shown in Fig. 2.
Experimental
To a mixed well solution of 4(3H)-pyrimidone (96 mg, 1 mmol, in 5 ml DMSO), NaH (25 mg, 1 mmol) and 2-chloro-3-(chloromethyl)-8-methylquinoline (225 mg, 1 mmol) were added and the resulting mixture was refluxed for 1 h. Completion of the reaction was monitored by TLC. After the completion of the reaction, cooled and removed the excess of solvent under reduced pressure. Crushed ice was mixed with the residue. White solid was formed, filtered and dried, purified by column chromatography using hexane and ethylacetate as the eluant. The low polar compound was subjected into crystallization by solvent evaporation from a solution of the compound in chloroform.
Refinement
All H atoms were placed in geometrically idealised positions and constrained to ride on their parent atoms (C—H = 0.93, 0.96 and 0.97 Å) and Uiso(H) values were taken to be equal to 1.2 Ueq(C) for aromatic and methylene H atoms and 1.5Ueq(C) for methyl H atoms.
Figures
Fig. 1.
The molecular structure of the title molecule with the atom-numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Packing diagram of the title compound viewed down b-axis. All H atoms have been omitted for clarity.
Crystal data
| C15H12ClN3O | F(000) = 592 |
| Mr = 285.73 | Dx = 1.455 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 1065 reflections |
| a = 11.9975 (2) Å | θ = 2.5–26.0° |
| b = 8.45037 (15) Å | µ = 0.29 mm−1 |
| c = 12.95869 (19) Å | T = 295 K |
| β = 96.7619 (16)° | Prism, colourless |
| V = 1304.66 (4) Å3 | 0.23 × 0.18 × 0.15 mm |
| Z = 4 |
Data collection
| Oxford Xcalibur Eos (Nova) CCD detector diffractometer | 2564 independent reflections |
| Radiation source: Enhance (Mo) X-ray Source | 2005 reflections with I > 2σ(I) |
| graphite | Rint = 0.031 |
| ω scans | θmax = 26.0°, θmin = 2.5° |
| Absorption correction: multi-scan (CrysAlis PRO RED; Oxford Diffraction, 2009) | h = −14→14 |
| Tmin = 0.936, Tmax = 0.958 | k = −10→10 |
| 13753 measured reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.033 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.089 | H-atom parameters constrained |
| S = 1.07 | w = 1/[σ2(Fo2) + (0.0503P)2] where P = (Fo2 + 2Fc2)/3 |
| 2564 reflections | (Δ/σ)max < 0.001 |
| 182 parameters | Δρmax = 0.18 e Å−3 |
| 0 restraints | Δρmin = −0.20 e Å−3 |
Special details
| Geometry. Bond distances, angles etc. have been calculated using the rounded fractional coordinates. All su's are estimated from the variances of the (full) variance-covariance matrix. The cell esds are taken into account in the estimation of distances, angles and torsion angles |
| Refinement. Refinement on F2 for ALL reflections except those flagged by the user for potential systematic errors. Weighted R-factors wR and all goodnesses of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The observed criterion of F2 > σ(F2) is used only for calculating -R-factor-obs etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cl1 | 0.52448 (3) | 0.22861 (5) | −0.21533 (3) | 0.0452 (2) | |
| O1 | 0.35909 (9) | 0.13571 (11) | 0.06428 (7) | 0.0413 (4) | |
| N1 | 0.66190 (10) | 0.38546 (12) | −0.08362 (8) | 0.0315 (4) | |
| N2 | 0.22497 (11) | −0.02532 (14) | −0.02386 (9) | 0.0398 (4) | |
| N3 | 0.08616 (12) | −0.14701 (16) | 0.06771 (13) | 0.0569 (6) | |
| C1 | 0.57162 (12) | 0.30022 (16) | −0.09125 (10) | 0.0297 (4) | |
| C2 | 0.50756 (12) | 0.25855 (14) | −0.01007 (10) | 0.0282 (4) | |
| C3 | 0.54805 (12) | 0.31329 (15) | 0.08638 (10) | 0.0299 (4) | |
| C4 | 0.69272 (13) | 0.46034 (15) | 0.20041 (11) | 0.0365 (5) | |
| C5 | 0.78847 (14) | 0.54796 (16) | 0.20983 (12) | 0.0418 (5) | |
| C6 | 0.84248 (13) | 0.58407 (16) | 0.12263 (12) | 0.0399 (5) | |
| C7 | 0.80215 (13) | 0.53220 (16) | 0.02491 (11) | 0.0345 (5) | |
| C8 | 0.70266 (12) | 0.43947 (15) | 0.01381 (10) | 0.0291 (4) | |
| C9 | 0.64712 (12) | 0.40438 (15) | 0.10159 (10) | 0.0296 (4) | |
| C10 | 0.40366 (12) | 0.16051 (16) | −0.03229 (10) | 0.0327 (5) | |
| C11 | 0.26755 (13) | 0.04265 (16) | 0.06302 (11) | 0.0344 (5) | |
| C12 | 0.22415 (15) | 0.02264 (18) | 0.15667 (12) | 0.0459 (6) | |
| C13 | 0.13310 (16) | −0.0742 (2) | 0.15376 (15) | 0.0551 (7) | |
| C14 | 0.13543 (15) | −0.11672 (19) | −0.01572 (14) | 0.0514 (6) | |
| C15 | 0.86040 (14) | 0.57135 (18) | −0.06845 (13) | 0.0465 (6) | |
| H3 | 0.50970 | 0.29010 | 0.14280 | 0.0360* | |
| H4 | 0.65740 | 0.43730 | 0.25880 | 0.0440* | |
| H5 | 0.81850 | 0.58440 | 0.27500 | 0.0500* | |
| H6 | 0.90750 | 0.64490 | 0.13120 | 0.0480* | |
| H10A | 0.34910 | 0.21490 | −0.08100 | 0.0390* | |
| H10B | 0.42140 | 0.05980 | −0.06240 | 0.0390* | |
| H12 | 0.25520 | 0.07210 | 0.21750 | 0.0550* | |
| H13 | 0.10170 | −0.09080 | 0.21520 | 0.0660* | |
| H14 | 0.10390 | −0.16500 | −0.07680 | 0.0620* | |
| H15A | 0.92390 | 0.63810 | −0.04780 | 0.0700* | |
| H15B | 0.80920 | 0.62550 | −0.11900 | 0.0700* | |
| H15C | 0.88530 | 0.47550 | −0.09820 | 0.0700* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cl1 | 0.0438 (3) | 0.0642 (3) | 0.0278 (2) | −0.0083 (2) | 0.0045 (2) | −0.0068 (2) |
| O1 | 0.0385 (7) | 0.0543 (6) | 0.0322 (6) | −0.0162 (5) | 0.0092 (5) | −0.0009 (5) |
| N1 | 0.0286 (7) | 0.0364 (6) | 0.0301 (6) | 0.0026 (5) | 0.0059 (5) | 0.0019 (5) |
| N2 | 0.0355 (8) | 0.0428 (7) | 0.0406 (7) | −0.0044 (6) | 0.0027 (6) | 0.0005 (6) |
| N3 | 0.0425 (10) | 0.0507 (9) | 0.0792 (11) | −0.0086 (7) | 0.0143 (8) | 0.0065 (8) |
| C1 | 0.0293 (8) | 0.0340 (7) | 0.0259 (7) | 0.0036 (6) | 0.0034 (6) | −0.0007 (5) |
| C2 | 0.0268 (8) | 0.0278 (7) | 0.0303 (7) | 0.0045 (6) | 0.0046 (6) | 0.0023 (6) |
| C3 | 0.0307 (9) | 0.0319 (7) | 0.0280 (7) | 0.0033 (6) | 0.0078 (6) | 0.0029 (6) |
| C4 | 0.0409 (10) | 0.0368 (8) | 0.0313 (8) | 0.0009 (7) | 0.0025 (7) | 0.0009 (6) |
| C5 | 0.0469 (11) | 0.0396 (8) | 0.0362 (8) | 0.0005 (7) | −0.0062 (7) | −0.0046 (6) |
| C6 | 0.0315 (9) | 0.0359 (8) | 0.0507 (9) | −0.0041 (7) | −0.0015 (7) | −0.0012 (7) |
| C7 | 0.0306 (9) | 0.0316 (8) | 0.0418 (8) | 0.0017 (6) | 0.0060 (7) | 0.0014 (6) |
| C8 | 0.0279 (8) | 0.0285 (7) | 0.0309 (7) | 0.0037 (6) | 0.0035 (6) | 0.0006 (5) |
| C9 | 0.0297 (8) | 0.0279 (7) | 0.0310 (7) | 0.0037 (6) | 0.0029 (6) | 0.0021 (5) |
| C10 | 0.0310 (9) | 0.0386 (8) | 0.0288 (7) | −0.0009 (6) | 0.0054 (6) | 0.0011 (6) |
| C11 | 0.0295 (9) | 0.0343 (8) | 0.0399 (8) | −0.0010 (6) | 0.0063 (7) | 0.0045 (6) |
| C12 | 0.0498 (11) | 0.0489 (9) | 0.0417 (9) | −0.0058 (8) | 0.0165 (8) | 0.0004 (7) |
| C13 | 0.0533 (12) | 0.0523 (10) | 0.0647 (12) | −0.0042 (9) | 0.0281 (10) | 0.0087 (9) |
| C14 | 0.0422 (11) | 0.0501 (10) | 0.0605 (11) | −0.0089 (8) | 0.0008 (9) | −0.0019 (8) |
| C15 | 0.0387 (10) | 0.0498 (10) | 0.0526 (10) | −0.0108 (8) | 0.0119 (8) | 0.0019 (7) |
Geometric parameters (Å, °)
| Cl1—C1 | 1.7485 (14) | C7—C8 | 1.421 (2) |
| O1—C10 | 1.4330 (16) | C7—C15 | 1.504 (2) |
| O1—C11 | 1.3493 (18) | C8—C9 | 1.4158 (19) |
| N1—C1 | 1.2948 (18) | C11—C12 | 1.386 (2) |
| N1—C8 | 1.3770 (17) | C12—C13 | 1.362 (3) |
| N2—C11 | 1.3126 (18) | C3—H3 | 0.9300 |
| N2—C14 | 1.337 (2) | C4—H4 | 0.9300 |
| N3—C13 | 1.338 (2) | C5—H5 | 0.9300 |
| N3—C14 | 1.317 (2) | C6—H6 | 0.9300 |
| C1—C2 | 1.4184 (19) | C10—H10A | 0.9700 |
| C2—C3 | 1.3672 (18) | C10—H10B | 0.9700 |
| C2—C10 | 1.496 (2) | C12—H12 | 0.9300 |
| C3—C9 | 1.410 (2) | C13—H13 | 0.9300 |
| C4—C5 | 1.360 (2) | C14—H14 | 0.9300 |
| C4—C9 | 1.4134 (19) | C15—H15A | 0.9600 |
| C5—C6 | 1.401 (2) | C15—H15B | 0.9600 |
| C6—C7 | 1.373 (2) | C15—H15C | 0.9600 |
| Cl1···C15i | 3.5264 (17) | C5···H10Avi | 2.9800 |
| Cl1···H10A | 2.8900 | C6···H10Avi | 2.8600 |
| Cl1···H10B | 2.8400 | C7···H10Avi | 2.9500 |
| Cl1···H14ii | 3.0800 | C10···H10Bv | 2.9600 |
| O1···H3 | 2.3600 | C11···H15Bvi | 3.0700 |
| N1···H15B | 2.7600 | C12···H15Bvi | 3.0300 |
| N1···H15C | 2.8100 | H3···O1 | 2.3600 |
| N2···H10A | 2.6700 | H3···H4 | 2.5100 |
| N2···H10B | 2.5700 | H4···H3 | 2.5100 |
| N2···H4iii | 2.9300 | H4···N2ix | 2.9300 |
| N3···H15Aiv | 2.9400 | H5···C3x | 2.9800 |
| N3···H15Cv | 2.8200 | H6···H15A | 2.3500 |
| C1···C3vi | 3.5712 (19) | H10A···Cl1 | 2.8900 |
| C1···C11v | 3.476 (2) | H10A···N2 | 2.6700 |
| C2···C8vi | 3.5842 (19) | H10A···C5vi | 2.9800 |
| C2···C9vi | 3.5278 (18) | H10A···C6vi | 2.8600 |
| C3···C1vi | 3.5712 (19) | H10A···C7vi | 2.9500 |
| C7···C14v | 3.595 (2) | H10B···Cl1 | 2.8400 |
| C7···C10vi | 3.592 (2) | H10B···N2 | 2.5700 |
| C8···C2vi | 3.5842 (19) | H10B···C2v | 2.9400 |
| C8···C14v | 3.347 (2) | H10B···C10v | 2.9600 |
| C9···C2vi | 3.5278 (18) | H10B···H10Bv | 2.5400 |
| C10···C7vi | 3.592 (2) | H14···Cl1xi | 3.0800 |
| C10···C10v | 3.598 (2) | H15A···N3xii | 2.9400 |
| C11···C1v | 3.476 (2) | H15A···H6 | 2.3500 |
| C14···C8v | 3.347 (2) | H15B···N1 | 2.7600 |
| C14···C7v | 3.595 (2) | H15B···C11vi | 3.0700 |
| C15···Cl1vii | 3.5264 (17) | H15B···C12vi | 3.0300 |
| C2···H10Bv | 2.9400 | H15C···N1 | 2.8100 |
| C3···H5viii | 2.9800 | H15C···N3v | 2.8200 |
| C10—O1—C11 | 117.49 (10) | N3—C13—C12 | 123.83 (17) |
| C1—N1—C8 | 117.26 (11) | N2—C14—N3 | 128.27 (16) |
| C11—N2—C14 | 114.85 (13) | C2—C3—H3 | 119.00 |
| C13—N3—C14 | 114.15 (15) | C9—C3—H3 | 119.00 |
| Cl1—C1—N1 | 116.09 (10) | C5—C4—H4 | 120.00 |
| Cl1—C1—C2 | 116.83 (10) | C9—C4—H4 | 120.00 |
| N1—C1—C2 | 127.08 (12) | C4—C5—H5 | 120.00 |
| C1—C2—C3 | 115.39 (12) | C6—C5—H5 | 120.00 |
| C1—C2—C10 | 120.44 (11) | C5—C6—H6 | 119.00 |
| C3—C2—C10 | 124.17 (12) | C7—C6—H6 | 119.00 |
| C2—C3—C9 | 121.02 (12) | O1—C10—H10A | 110.00 |
| C5—C4—C9 | 119.75 (14) | O1—C10—H10B | 110.00 |
| C4—C5—C6 | 120.81 (14) | C2—C10—H10A | 110.00 |
| C5—C6—C7 | 121.88 (14) | C2—C10—H10B | 110.00 |
| C6—C7—C8 | 118.03 (13) | H10A—C10—H10B | 108.00 |
| C6—C7—C15 | 121.68 (14) | C11—C12—H12 | 122.00 |
| C8—C7—C15 | 120.29 (13) | C13—C12—H12 | 122.00 |
| N1—C8—C7 | 118.62 (12) | N3—C13—H13 | 118.00 |
| N1—C8—C9 | 121.15 (12) | C12—C13—H13 | 118.00 |
| C7—C8—C9 | 120.23 (12) | N2—C14—H14 | 116.00 |
| C3—C9—C4 | 122.63 (12) | N3—C14—H14 | 116.00 |
| C3—C9—C8 | 118.08 (12) | C7—C15—H15A | 109.00 |
| C4—C9—C8 | 119.29 (13) | C7—C15—H15B | 109.00 |
| O1—C10—C2 | 107.52 (10) | C7—C15—H15C | 109.00 |
| O1—C11—N2 | 119.96 (13) | H15A—C15—H15B | 109.00 |
| O1—C11—C12 | 116.72 (13) | H15A—C15—H15C | 109.00 |
| N2—C11—C12 | 123.31 (14) | H15B—C15—H15C | 109.00 |
| C11—C12—C13 | 115.58 (15) | ||
| C11—O1—C10—C2 | −176.75 (11) | C2—C3—C9—C4 | −178.71 (13) |
| C10—O1—C11—N2 | 2.21 (19) | C2—C3—C9—C8 | 0.9 (2) |
| C10—O1—C11—C12 | −178.68 (13) | C9—C4—C5—C6 | 0.2 (2) |
| C8—N1—C1—Cl1 | −178.15 (10) | C5—C4—C9—C3 | −179.87 (13) |
| C8—N1—C1—C2 | 1.6 (2) | C5—C4—C9—C8 | 0.6 (2) |
| C1—N1—C8—C7 | 179.47 (12) | C4—C5—C6—C7 | −0.5 (2) |
| C1—N1—C8—C9 | −0.69 (19) | C5—C6—C7—C8 | 0.0 (2) |
| C14—N2—C11—O1 | 179.03 (13) | C5—C6—C7—C15 | 179.97 (14) |
| C14—N2—C11—C12 | 0.0 (2) | C6—C7—C8—N1 | −179.40 (12) |
| C11—N2—C14—N3 | −0.5 (2) | C6—C7—C8—C9 | 0.8 (2) |
| C14—N3—C13—C12 | −0.3 (2) | C15—C7—C8—N1 | 0.6 (2) |
| C13—N3—C14—N2 | 0.6 (3) | C15—C7—C8—C9 | −179.20 (13) |
| Cl1—C1—C2—C3 | 178.55 (10) | N1—C8—C9—C3 | −0.48 (19) |
| Cl1—C1—C2—C10 | −1.18 (17) | N1—C8—C9—C4 | 179.12 (12) |
| N1—C1—C2—C3 | −1.2 (2) | C7—C8—C9—C3 | 179.36 (12) |
| N1—C1—C2—C10 | 179.08 (13) | C7—C8—C9—C4 | −1.0 (2) |
| C1—C2—C3—C9 | −0.14 (19) | O1—C11—C12—C13 | −178.79 (14) |
| C10—C2—C3—C9 | 179.58 (12) | N2—C11—C12—C13 | 0.3 (2) |
| C1—C2—C10—O1 | 178.90 (11) | C11—C12—C13—N3 | −0.1 (3) |
| C3—C2—C10—O1 | −0.80 (18) |
Symmetry codes: (i) −x+3/2, y−1/2, −z−1/2; (ii) −x+1/2, y+1/2, −z−1/2; (iii) x−1/2, −y+1/2, z−1/2; (iv) x−1, y−1, z; (v) −x+1, −y, −z; (vi) −x+1, −y+1, −z; (vii) −x+3/2, y+1/2, −z−1/2; (viii) −x+3/2, y−1/2, −z+1/2; (ix) x+1/2, −y+1/2, z+1/2; (x) −x+3/2, y+1/2, −z+1/2; (xi) −x+1/2, y−1/2, −z−1/2; (xii) x+1, y+1, z.
Hydrogen-bond geometry (Å, °)
| Cg3 is the centroid of the C4–C9 ring. |
| D—H···A | D—H | H···A | D···A | D—H···A |
| C3—H3···O1 | 0.93 | 2.36 | 2.7056 (17) | 102 |
| C10—H10A···Cg3vi | 0.97 | 2.72 | 3.5132 (15) | 140 |
Symmetry codes: (vi) −x+1, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FJ2289).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Khan, F. N., Mohana Roopan, S., Hathwar, V. R. & Ng, S. W. (2010a). Acta Cryst. E66, o200. [DOI] [PMC free article] [PubMed]
- Khan, F. N., Mohana Roopan, S., Hathwar, V. R. & Ng, S. W. (2010b). Acta Cryst. E66, o201. [DOI] [PMC free article] [PubMed]
- Khan, F. N., Subashini, R., Roopan, S. M., Hathwar, V. R. & Ng, S. W. (2009). Acta Cryst. E65, o2686. [DOI] [PMC free article] [PubMed]
- Oxford Diffraction (2009). CrysAlis PRO CCD and CrysAlis PRO RED Oxford Diffraction Ltd, Yarnton, England.
- Roopan, S. M. & Khan, F. N. (2009). ARKIVOC, xiii, 161–169.
- Roopan, S. M., Khan, F. N. & Mandal, B. K. (2010). Tetrahedron Lett. doi:org/10.1016/j.tetlet.2010.02.128.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Singer, B. (1986). Cancer Res.46, 4879–4885. [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Svenstrup, N., Kuhl, A., Ehlert, K. & Habich, D. (2008). Bioorg. Med. Chem. Lett.18, 3215–3218. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810011694/fj2289sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810011694/fj2289Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




